Abstract
Background:
We examined the longitudinal association between sociodemographic factors and an expanded definition of underemployment among those with and without cancer history in the United States.
Methods:
Medical Expenditure Panel Survey data (2007–2013) were used in multivariable regression analyses to compare employment status between baseline and two-year follow-up among adults aged 25–62 years at baseline (n = 1,614 with and n = 39,324 without cancer). Underemployment was defined as becoming/staying unemployed, changing from full to part-time, or reducing part-time work significantly. Interaction effects between cancer history/time since diagnosis and predictors known to be associated with employment patterns, including age, gender/marital status, education, and health insurance status at baseline were modeled.
Results:
Approximately 25% of cancer survivors and 21% of individuals without cancer reported underemployment at follow-up (p = 0.002). Multivariable analyses indicated that those with a cancer history report underemployment more frequently (24.7%) than those without cancer (21.4%, p = 0.002) with underemployment rates increasing with time since cancer diagnosis. A significant interaction between gender/marital status and cancer history and underemployment was found (p = 0.0004). There were no other significant interactions. Married female survivors diagnosed >10 years ago reported underemployment most commonly (38.7%), and married men without cancer reported underemployment most infrequently (14.0%). A wider absolute difference in underemployment reports for married versus unmarried women as compared to married versus unmarried men was evident, with the widest difference apparent for unmarried versus married women diagnosed >10 years ago (18.1% vs. 38.7%).
Conclusion:
Cancer survivors are more likely to experience underemployment than those without cancer. Longer time since cancer diagnosis and gender/marital status are critical factors in predicting those at greatest risk of underemployment. The impact of cancer on work should be systematically studied across sociodemographic groups and recognized as a component of comprehensive survivorship care.
Keywords: cancer, employment, gender, marital status, neoplasms, oncology
Introduction
Cancer survivors face several issues upon completion of treatment, including maintaining employment and challenges related to returning to work (Amir, Neary, & Luker, 2008; Duijts et al., 2014; Farley Short, Vasey, & Moran, 2008; Mehnert, de Boer, & Feuerstein, 2013). Even years following a diagnosis, cancer survivors report lower full-time and part-time employment than those without a history of cancer, and the probability of returning to work for those survivors who have left the labor force decreases with time since diagnosis (Moran, Short, & Hollenbeak, 2011). Many cancer survivors face the need for schedule changes, a decrease in work hours and wages, and a decline in work ability (Mehnert, 2011). Approximately 62% of survivors in the American Cancer Society Study of Cancer Survivors reported one or more negative work-related outcomes within two years of diagnosis (Yu, Ferrucci, & McCorkle, 2012).
Evidence suggests that most cancer survivors who were working prior to diagnosis do return to work (Mehnert, 2011). The likelihood of returning to work is a function of the occupational demands relative to the flexibility of the employment environment to accommodate limitations in physical capabilities, internal and family pressures and preferences, and the physical and psychological impacts of the cancer, active treatment, and any long-term effects of treatment (Stergiou-Kita, Grigorovich, & Tseung, 2014). A higher likelihood of returning to work after diagnosis is associated with demographic factors such as higher educational attainment, male gender, and younger age at diagnosis; clinical factors such as receipt of less invasive surgery and experiencing fewer physical symptoms; and employment characteristics including provision of workplace accommodations such as flexible hours or telecommuting (Mehnert, 2011). Factors associated with reduced likelihood of returning to work include heavy physical labor, prior chemotherapy treatment, higher symptom burden, higher perceived problematic social interactions at work (Tevaarwerk, Lee, & Sesto, 2013) and female gender (Moran & Short, 2014; Zajacova, Dowd, Schoeni, & Wallace, 2015). However, the impact of cancer on employment and how the impact may vary across sociodemographic groups remains unclear, given limited data based on large longitudinal studies with age-matched controls without cancer.
Considering the many documented benefits of employment, including financial stability, identity, and maintaining an important dimension of self (McKay, Knott, & Del-fabbro, 2013), recovery, the symbolic “return to [a new] normal,” (van Muijen, Weevers, & Snels, 2013) and better quality of life (Mahar, BrintzenhofeSzoc, & Shields, 2008; Timperi et al., 2013), maintaining employment throughout and beyond cancer should be part of the goal of cancer care. Understanding sociodemographic factors that predispose survivors to challenges with employment after cancer can highlight those who are most in need of intervention. The objective of the current study was to characterize the risk of underemployment (sustained unemployment or significantly reduced employment) and to examine sociodemographic factors associated with underemployment in a nationally representative sample of adult cancer survivors and individuals without cancer over a two-year window. In addition, the study focused on sociodemo-graphic factors hypothesized to be associated with underemployment among cancer survivors, including age (Kiasuwa Mbengi, Otter, & Mortelmans, 2016), gender (Zajacova et al., 2015), marital status (Hollenbeak, Short, & Moran, 2011), educational attainment (Kiasuwa Mbengi et al., 2016; Taskila & Lindbohm, 2007), and health insurance coverage (Parsons, Harlan, & Lynch, 2012). We hypothesized that older survivors, married female survivors, and those with less educational attainment or lacking private health insurance coverage would be more likely to report underemployment than married men and those with a higher educational attainment and privately insured.
Methods
Data source
We used longitudinal data from the 2007–2013 Medical Expenditure Panel Survey (MEPS) (panels 12–17) Household Component files. The MEPS is an ongoing survey of healthcare expenditures, insurance, utilization, and access to care in the noninstitutionalized U.S population conducted by the Agency for Healthcare Research and Quality (Cohen, Cohen, & Banthin, 2009). Each panel is followed for two years with five rounds of in-person interviews. Combined overall panel response rates for 2007–2013 range from 54–59% (Agency for Healthcare Research and Quality 2015). These data were pooled to allow for a sufficient sample of cancer survivors in which to examine risk of underemployment between the baseline and two-year follow-up of the panel data collection.
Study population
Adults aged 25–62 years old upon MEPS entry comprised the study population. Younger adults were excluded because many are attending college or they may have unstable labor force participation. Adults aged > 62 years upon MEPS entry were excluded, because they would be aged 65 at Round 5 (two-year follow-up), a common retirement age in the United States. Cancer history was determined by the question: ever been told by a health professional that you had cancer or a malignancy of any kind? Participants who reported only nonmelanoma skin cancer were included in the “no cancer history” group. Participants were also asked the age (integer) they were diagnosed with their most recent cancer, and we subtracted this age from their age (integer) at survey to create the following categories of time since diagnosis: 1–5 years ago, 6–10 years ago, >10 years ago. Individuals with no cancer history were the comparison group. Employment status was assessed for each adult in the household, by job. If a respondent indicated that they were not working, then a “reasons why” follow-up question was asked and categorized into retired, on maternity/paternity leave, in school, or wanted time off.
Of the 46,550 age-eligible participants in MEPS panels 12–17, participants were excluded if: they had incomplete, missing, or ambiguous employment status at either interview (n = 3,479); their age at survey, site at diagnosis, or cancer status was unknown (n = 114); they had missing data on key covariates (n = 248); they were retired, on maternity/paternity leave, in school, or wanted time off at baseline (n = 1,103), or retired at two-year follow-up (n = 270). Given our focus on employment changes after cancer diagnosis, and that many patients take medical or disability leave during active treatment, individuals diagnosed within 12 months (same integer age at survey and at diagnosis) were excluded (n = 398). The final unweighted sample included 40,938 individuals: 1,614 cancer survivors and 39,324 individuals with no cancer history.
Employment change outcomes
Primary outcomes for the current study included a combination of employment status at baseline (Round 1) and at two-year follow-up (Round 5): full-time (≥35 hours per typical work week); (Bureau of Labor Statistics 2015) part-time (up to 34 hours per typical work week); or unemployed. Employment status at baseline and follow-up were compared to produce the following categories: sustained employment (maintained full-time or part-time status), increased employment (went from unemployed to part/full-time, part-time to full-time status, or if part-time in both with a 10+ hours increase), sustained unemployment (maintained unemployment) or decreased employment (went from part/full-time to unemployed or retired, or full-time to part-time, or if part-time with a 10+ hours reduction). For this study, we created an under-employment measure, defined as sustained unemployment or decreased employment status. We used this definition of underemployment, rather than the standard Bureau of Labor Statistics (BLS) definition (Sum & Khatiwada, 2010) to provide a comprehensive assessment of work status among cancer survivors at two-year follow-up.
Covariates
Sociodemographic factors included gender, race/ethnicity, and the following variables at baseline: age, educational attainment, marital status, and any minor children living in the home. Age was categorized into 25–44, 45–54, and 55–62 years to reflect young, middle, and older age groups. Gender and marital status were combined into four categories (male/married, male/not married, female/married, female/not married) to investigate differences in these combinations across cancer survivors and individuals without a cancer history, given general differences in employment patterns in these sociodemo-graphic groups. Given traditional associations of socioeconomic status and underemployment, additional covariates at baseline (metropolitan statistical area, household income as a percentage of the federal poverty level, and health insurance status [any private, public only, and no insurance]) were also included.
Statistical analysis
Models focused on a binary outcome measure, underemployed (sustained unemployment, decreased employment, or became retired) versus not underemployed (sustained or increased employment) at follow-up. Multivariable logistic regression modeling was used to examine the association of covariates with underemployment, with adjusted results presented using predicted marginals (Graubard & Korn, 1999). Weighted percentages represent the population proportion of each employment pattern group after covariate adjustment. Interaction effects were tested between cancer history and (1) age-group, (2) gender/marital status, (3) education, and (4) health insurance status at baseline. Only significant interaction terms were included in the final model.
Analyses were conducted using SAS-callable SUDAAN, release 11.0, (RTI International, Research Triangle Park, NC) to incorporate sampling weights and account for the complex sampling design. Statistical analyses were two sided and alpha was set at 0.05.
Results
Table 1 shows the number of cancer survivors and individuals without a cancer history according to sociodemographic factors. Compared to individuals without a cancer history, cancer survivors were older, and more likely to be non-Hispanic white. Men with cancer were less likely, but women with cancer were more likely, to be married. Cancer survivors were also more likely to be diagnosed with another chronic condition than those without a cancer history, less likely to be uninsured, and less likely to report excellent/very good health. The distribution of survivor-reported cancer sites in the current sample included female breast (16.1%), prostate (5.6%), colorectal (3.3%), lung (1.2%), all other (73.7%).
Table 1.
Characteristics for individuals with and without a cancer history.
| Cancer history | No cancer history | ||||
|---|---|---|---|---|---|
| n | Weighted column % | n | Weighted column % | Wald F p-value | |
| Age* | |||||
| 25–44 | 428 | 24.0 | 21,975 | 54.3 | <0.0001 |
| 45–54 | 567 | 36.2 | 11,111 | 29.0 | |
| 55—62 | 619 | 39.9 | 6,238 | 16.7 | |
| Time since most recent cancer diagnosis (self-report) | |||||
| 1–5 years ago | 708 | 43.0 | — | — | — |
| 6–10 years ago | 347 | 20.2 | — | — | |
| >10 years | 559 | 36.8 | — | — | |
| Gender and marital status* | |||||
| Male and married | 309 | 22.7 | 11,639 | 30.7 | <0.0001 |
| Male and not married | 167 | 11.0 | 6,889 | 19.1 | |
| Female and married | 606 | 40.3 | 11,947 | 31.0 | |
| Female and not married | 532 | 26.0 | 8,849 | 19.1 | |
| Race/ethnicity | |||||
| Non-Hispanic white | 1,047 | 81.3 | 16,985 | 65.4 | <0.0001 |
| Other / multiple | 567 | 18.7 | 22,339 | 34.6 | |
| Educational attainment* | |||||
| High school or less | 756 | 38.4 | 19,467 | 40.2 | 0.22 |
| More than high school | 858 | 61.6 | 19,857 | 59.8 | |
| Family income as a percent of federal poverty line* | |||||
| <138% | 409 | 18.2 | 9,490 | 16.5 | 0.001 |
| 138% to <250% | 312 | 16.8 | 8,779 | 19.0 | |
| 250% to <400% | 311 | 19.6 | 8,597 | 23.3 | |
| 400% or more | 582 | 45.4 | 12,458 | 41.1 | |
| Any children <18 years old living at home* | |||||
| Yes | 484 | 27.0 | 18,517 | 42.3 | <0.0001 |
| No | 1,130 | 73.0 | 20,807 | 57.7 | |
| Number of known comorbidities (excluding cancer) reported* | |||||
| 0 | 438 | 28.1 | 19,634 | 49.2 | <0.0001 |
| 1 | 381 | 25.5 | 9,715 | 25.4 | |
| 2+ | 795 | 46.3 | 9,975 | 25.3 | |
| Metropolitan Statistical Area* | |||||
| Yes | 1,349 | 82.5 | 34,336 | 85.4 | 0.03 |
| No | 265 | 17.5 | 4,988 | 14.6 | |
| Health insurance coverage* | |||||
| Any private | 1,068 | 75.7 | 24,989 | 73.4 | <0.0001 |
| Public only | 332 | 13.3 | 5,130 | 9.1 | |
| Uninsured | 214 | 11.0 | 9,205 | 17.5 | |
| Perceived health status* | |||||
| Excellent / very good | 613 | 42.3 | 22,328 | 61.8 | <0.0001 |
| Good | 456 | 29.6 | 10,813 | 25.1 | |
| Fair/poor | 545 | 28.1 | 6,183 | 13.1 | |
| Panel Number | |||||
| 12 (2007–2008) | 205 | 14.6 | 4,940 | 16.4 | 0.34 |
| 13 (2008–2009) | 277 | 16.2 | 7,237 | 16.5 | |
| 14 (2009–2010) | 280 | 16.3 | 6,601 | 16.6 | |
| 15 (2010–2011) | 228 | 16.1 | 5,835 | 16.7 | |
| 16 (2011–2012) | 325 | 17.8 | 7,365 | 16.7 | |
| 17 (2012–2013) | 299 | 19.0 | 7,346 | 17.0 | |
Data source: Medical Expenditure Panel Survey, Panels 12–17 (2007–2013).
Columns add to 100%.
Measured at Round 1(baseline).
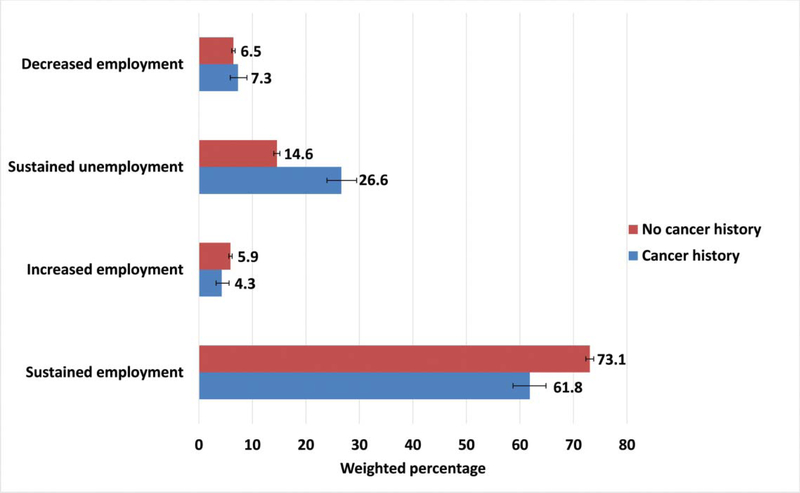
Over the two-year follow-up period, cancer survivors sustained employment less frequently than individuals without a cancer history (61.8% vs. 73.1%, p = 0.002) and more often remained unemployed than individuals without a cancer history (26.6% vs. 14.6%, p < 0.001) (Figure 1). The proportion with reduced employment over the two-year period with a cancer history was similar (7.3%, 95% CI [5.9– 9.0%]) to those without cancer (6.5%, 95% CI [6.2–6.8%]) (Figure 1). Subsequent analyses focus on those who sustained unemployment or reduced employment (underemployment to enhance statistical power).
Figure 1.
Weighted percentages of changes in employment status from baseline to two-year follow-up by cancer history. Bars represent weighted 95% confidence intervals.
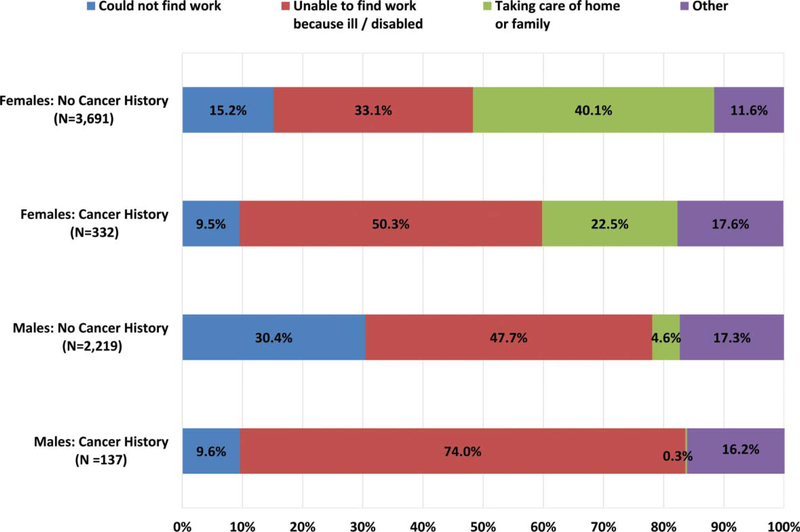
The reasons given for underemployment at follow-up differed between individuals with and without a cancer history and by gender (Figure 2), with the most common reason for three groups given as being ill or disabled (74.0% of male cancer survivors; 47.7% of men without cancer; 50.3% of female cancer survivors). Among women without cancer, the most common reason was taking care of the home or family (40.1%). Virtually no male survivors (0.3%) indicated that they were not working due to family or home responsibilities versus 22.5% of female survivors (30.6% of married, female survivors vs. 7.5% of unmarried female survivors, data not shown).
Figure 2.
Reasons given for not working for those unemployed at two-year follow-up by cancer history and gender.
Cancer history and underemployment
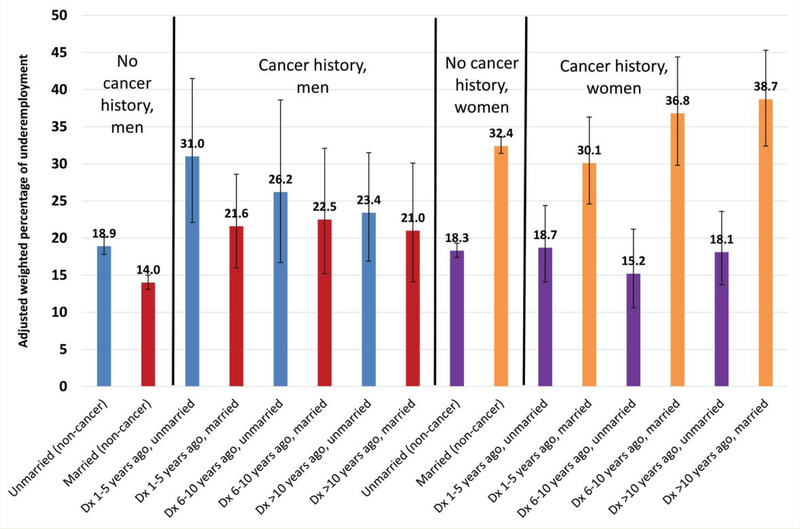
Multivariable analyses indicated that those with a cancer history report underemployment more frequently (24.7%) than those without cancer (21.4%, p = 0.002, data not shown), with underemployment rates increasing with time since cancer diagnosis (Table 2). The interaction between cancer history and gender/marital status was significant (p = 0.004). No other interaction terms with cancer history (age-group, education, and health insurance status at baseline) were significant, and thus excluded from further analysis. Figure 3 displays the subgroups of cancer history, time since diagnosis, and gender/marital status (See Supplemental Table 1 for parameter estimates and 95% confidence intervals).
Table 2.
Factors associated with underemployment in multivariable logistic regression analysis.
| Adjusted, Weighted Predicted Marginal | 95% CI | Wald F p-value | |
|---|---|---|---|
| Cancer history | |||
| No cancer history | 21.4 | 20.8–22.1 | — |
| Cancer, dx 1–5 years ago | 25.3 | 22.1–28.9 | |
| Cancer, dx 6–10 years ago | 25.9 | 22.0–30.2 | |
| Cancer, dx >10 years ago | 26.2 | 22.8–29.9 | |
| Gender/marital status* | — | ||
| Male/married | 14.4 | 13.5–15.4 | |
| Male/ not married | 19.3 | 18.2–20.5 | |
| Female/ married | 32.5 | 31.5–33.6 | |
| Female/ not married | 18.3 | 17.4–19.2 | |
| Cancer History * Gender/Marital Status | 0.004 | ||
| Age* | <0.0001 | ||
| 25–44 | 19.8 | 19.1–20.6 | |
| 45–54 | 19.9 | 18.9–20.9 | |
| 55–62 | 29.6 | 28.3–30.9 | |
| Race/Ethnicity | 0.001 | ||
| Non-Hispanic White | 22.1 | 21.4–22.9 | |
| Other/multiple | 20.8 | 19.9–21.6 | |
| Educational attainment* | <0.0001 | ||
| ≤High school | 23.1 | 22.3–23.9 | |
| High school+ | 20.3 | 19.6–21.1 | |
| Household income as % of FPL* | <0.0001 | ||
| <138% | 36.8 | 35.1–38.5 | |
| 138% to <250% | 23.7 | 22.6–24.8 | |
| 250% to <400% | 18.6 | 17.6–19.7 | |
| 400% or more | 14.6 | 13.8–15.4 | |
| Any minors in household* | 0.001 | ||
| Yes | 20.5 | 19.7–21.4 | |
| No | 22.4 | 21.5–23.2 | |
| Comorbidities (excluding cancer) * | <0.0001 | ||
| 0 | 19.8 | 19.1–20.6 | |
| 1 | 20.8 | 19.8–21.8 | |
| 2+ | 25.3 | 24.2–26.3 | |
| Metropolitan Statistical Area | 0.002 | ||
| Yes | 22.0 | 21.3–22.7 | |
| No | 19.9 | 18.6–21.2 | |
| Health insurance coverage* | <0.0001 | ||
| Any private | 16.4 | 15.8–17.1 | |
| Public only | 47.0 | 44.7–49.4 | |
| Uninsured | 25.6 | 24.2–27.0 | |
| Perceived health status* | <0.0001 | ||
| Excellent /very good | 18.9 | 18.2–19.6 | |
| Good | 20.7 | 19.8–21.7 | |
| Fair /poor | 32.4 | 31.0–33.8 | |
| Panel number | 0.002 | ||
| 12 (2007–2008) | 20.2 | 19.1–21.5 | |
| 13 (2008–2009) | 22.9 | 21.9–24.0 | |
| 14 (2009–2010) | 22.5 | 21.4–23.6 | |
| 15 (2010–2011) | 21.2 | 20.1–22.3 | |
| 16 (2011–2012) | 21.3 | 20.3–22.4 | |
| 17 (2012–2013) | 21.5 | 20.4–22.7 | |
Data source: Medical Expenditure Panel Survey, Panels 12–17 (2007–2013).
Measured at Round 1 (baseline).
Figure 3.
Adjusted, weighted percentages of underemployment (sustained unemployment/ employment reductions) among individuals with and without cancer history. Bars represent weighted 95% confidence intervals. DX = diagnosed. Adjusted for age at survey, race/ethnicity, education, household income, minors in household, comorbidities, Metropolitan Statistical Area, and panel number (see Table 2).
Among men without cancer, the frequency of underemployment was higher for unmarried as compared to married men. Similar results were found for men with cancer who were within 10 years of diagnosis, with unmarried men underemployed most commonly. The pattern was the opposite for women. Among women with and without cancer, the underemployment was higher for married compared to unmarried women. Among married women with cancer, the prevalence of underemployment increased, although not significantly, in conjunction with time since cancer diagnosis (Figure 3). Married men without cancer reported the lowest underemployment levels (14.0%, 95% CI [13.1–15.0%]), significantly different from unmarried men without cancer (18.9%, 95% CI [17.8–20.1%]), and unmarried male survivors diagnosed 1–5 (31.0%, 95% CI [22.1–41.5.0%]) and 6–10 (26.2%, 95% CI [16.7–38.6%]) years ago. Married female cancer survivors, how-ever, reported the highest underemployment rates, ranging from 30.1% to 38.7%. These were not, however, significantly different from married women without a history of cancer (32.4%, 95% CI [31.4–33.6%]. Among unmarried women, there were no significant differences in underemployment between those without cancer (15.2%, 95% CI [10.6–21.2%]) and those with a history of cancer 1–5 years ago (18.7%, 95% CI [14.1–24.4%]), 6–10 (15.2%, 95% CI [10.6–21.2%]), or >10 (18.1%, 95% CI [13.7–23.6%]) years ago. The difference in underemployment rates in women appears to depend greatly on marital status, as opposed to cancer history, while the difference in underemployment in men appears to depend both on marital status and cancer history. As a whole, Figure 3 illustrates the adjusted, weighted percentages of underemployment among each gender-by-marital status subgroup, for individuals with and without cancer, and these differences may be compared within each stratum. Most notably, there is a wider difference in underemployment for married versus unmarried women as compared to married versus unmarried men, with the widest difference apparent for unmarried versus married women diagnosed >10 years ago.
We conducted additional sensitivity analyses to examine the robustness of these findings. We examined the impact of the interaction between cancer history and gender/marital status on underemployment directly, with no adjustment for health insurance and household poverty level in the model, and with those working part-time at either time point or those retired at Round 5 removed from the model. Overall distributions were similar with and without insurance and poverty level as well as whether or not individuals working part-time or retired at Round 5 were included when compared to Figure 3 (data not shown).
Discussion
In our longitudinal observational study of employment patterns in a nationally representative sample of cancer survivors and individuals without a cancer history, we found that cancer survivors were underemployed at higher rates than individuals without a cancer history. Though the absolute difference in underemployment between individuals with and without a cancer diagnosis was only about 3.5%, the absolute differences in substrata were far larger, as large as 20% between unmarried women without cancer and married women diagnosed over 10 years ago. Contrary to our hypotheses, cancer survivors who were older at interview, with lower educational attainment, and lacking private health insurance did not report underemployment more frequently than those without a cancer history. However, the association between cancer history and underemployment varied significantly by gender and marital status. Married female cancer survivors had more than three times the rates of underemployment than married men without a cancer history. Married women who had a longer time since cancer diagnosis reported underemployment more frequently than those more recently diagnosed. The current study is the first to our knowledge to examine the interaction between gender, marital status, and cancer history on underemployment. Consistent with previous literature, provider discussions about employment or referral to other disciplines, such as rehabilitation and social work during and after the completion of treatment, may be helpful for cancer survivors (Alfano, Kent, Padgett, Grimes, & de Moor, 2017; de Boer et al., 2011; Silver, Baima, Newman, Galantino, & Shockney, 2013). Our findings suggest that, in particular, married female cancer survivors may benefit from such discussions with providers given high underemployment found in our study. To the extent that these discussions are not occurring, our results high-light the potential need to develop tools to assist providers to initiate such conversation. Integrating discussions about the impact of cancer on employment into routine clinical practice may help cancer patients and their families set realistic expectations and plan for returning to work (Bradley, 2015).
At the end of the two-year observation period, the reasons given for underemployment also varied by gender and cancer history. Unlike previous research, we found that female gender alone is not clearly associated with underemployment; however, being married and female is associated with underemployment. Much of the underemployment evident in this study is concentrated among married women. However, there is a gradient in time since diagnosis, suggesting the longer the time since diagnosis the more likely a married woman will report underemployment.
Among cancer survivors, the most common reason for not working was illness/disability. In addition, virtually no male survivors indicated they were not working due to family or home responsibilities compared to about one third of female survivors. Further differences were evident among female cancer survivors by marital status, with four times as many married compared to unmarried female cancer survivors citing family/home responsibilities for not working. Thus, even among cancer survivors, household responsibilities appear to have a bigger impact on employment decisions among women, particularly married women, than men. Studies on the challenges of return-to-work and work retention suggest that the employment challenges stem from many sources, including residual symptom burden, fear of cancer disclosure, job-related concerns such as perceived low support and lack of job flexibility, and unfair labor practices that discriminate based on disability (Grunfeld & Cooper, 2012; Grunfeld, Drudge-Coates, Rixon, Eaton, & Cooper, 2013; Gunnarsdottir et al., 2013; Kim, Yun, & Chang, 2014; Koch, Wittekindt, Altendorf-Hofmann, Singer, & Guntinas-Lichius, 2015; Tevaarwerk et al., 2013).
Challenges in return-to-work and work retention have been reported by other studies of cancer survivors, but much of the explanatory work has been conducted in cancer-site and gender-specific groups (Grunfeld & Cooper, 2012; Grunfeld et al., 2013; Timperi et al., 2013); thus, it may be hard to generalize. One study of the impact of cancer survivorship on spousal employment found that wives of cancer survivors had a lower probability of being employed 2–6 years after diagnosis, but if employed, a higher probability of working full-time (Hollenbeak et al., 2011). This finding, in concert with the findings from the current study, suggests the possibility of employment tradeoffs for the purposes of insurance coverage among married partners. A recent study using data from the Panel Study of Income Dynamics from 1990–2009 found that employment rates dropped by 20% in the first year after cancer diagnosis, but rebounded somewhat within four years of diagnosis (Zajacova et al., 2015). The effects among male cancer survivors were far more pronounced than among female survivors; most of the results in women were likely underpowered and not statistically significant. However, the study did not adjust for or examine effect modification of marital status, which proved to have a significant interaction with gender and cancer history in the current study.
Survivors’ work experiences after a cancer diagnosis can certainly be heterogeneous. However, the finding that sociodemographic factors may influence returning to work indicates important considerations for further research and for clinicians who interact with cancer survivors. Rates of underemployment among unmarried women were similar between cancer survivors and women without cancer and were actually lower than those of unmarried men with cancer. Whether this indicates some degree of “job-lock” in order to retain health insurance (Tunceli, Short, Moran, & Tunceli, 2009) with cancer is a speculative but plausible explanation for some unmarried individuals, especially considering comparisons to their married counterparts. Future research is needed to examine the reasons behind these patterns. Higher rates of return-to-work and retention have been reported among those with less paid sick leave (Mehnert, 2011) and among female cancer survivors who depend on their own jobs for health insurance (Bradley, Neumark, & Barkowski, 2013). For married women in the current study, not only were adjusted underemployment rates high, they were highest among long-term survivors. Better understanding of why more married women with a cancer history are remaining unemployed, becoming unemployed, or reducing their number of hours per week is critically important for designing appropriately targeted interventions to help these women meet work goals. It will also be important to follow trends in employment trajectories among cancer survivors by gender over time to determine whether the changes evident in these data are contemporary or persistent.
Despite the relatively large, population-based sample of adults with and without cancer, there are limitations to this study. Information about length of time with an employer and job type is unavailable and would greatly inform our understanding of job characteristics associated with underemployment. Sample sizes were too small to investigate differences across cancer sites, and the participation of individuals with short-survival cancers is likely limited. In addition, information on sexual orientation and gender identity was not available in the MEPS dataset for the years we included; thus, we could not investigate whether individuals identifying as a member of a sexual or gender minority had higher rates of underemployment. Even though our sample included both short- and long-term cancer survivors, the two-year observation window may be insufficient to determine the full impact cancer may have on employment. We chose to exclude individuals whose most recent cancer diagnosis was close to MEPS Round 1(baseline) date due to the lack of precision with dates and possibility of short-term employment changes that lessen with time. Although short-term work hour reductions, job loss, and absenteeism within the year after diagnosis are common (McGrath et al., 2017), the focus of the current study was on longer-term employment changes.
Previous research has reported differences in employment participation and experiences among male cancer survivors per cancer site (Gunnarsdottir et al., 2013), and this will be an important area for future work exploring the effects of marital status, particularly in cohorts of newly diagnosed cancer survivors. Moreover, there is a need to focus on the intersection of household and family responsibilities and employment and how that might have a differential impact on male and female cancer survivors. Indeed, a recent study of women with early-stage breast cancer reported that employees of more accommodating employers are more than twice as likely to retain their jobs after diagnosis (Blinder, Eberle, Patil, Gany, & Bradley, 2017). In addition, sample size limitations precluded complete investigation of reasons given for not working at Round 5 (follow-up) by gender/marital status categories. Cancer history, employment status, and hours worked were all based on self-report. In addition, the purpose of the study was to examine employment rates, rather than job quality, and thus we did not adjust for changes in job type that may have occurred over the observational period. The current study focused solely on employment outcomes, as other recent MEPS data analyses detected higher economic burden among cancer survivors than individuals without cancer in terms of annual medical expenditures and productivity losses (Guy, Ekwueme, & Yabroff, 2013; Zheng, Yabroff, & Guy, 2015). In order to have a sufficient sample to evaluate underemployment in cancer survivors, we had to pool multiple MEPS panels, including years during the economic downturn that affected employment in the United States. Our multivariable models adjusted for MEPS panels, which addresses this secular trend to some extent. Future research should examine the impact of the recession on employment among cancer survivors. Finally, given sample size constraints and lack of standards for comprehensive assessment of employment changes in clinical populations, we expanded the BLS definition of the underemployed to include the unemployed, thus measuring labor underutilization, which partially limits comparisons to other studies of underemployment.
Implications for providers and policy
Our findings indicate differences in employment outcomes by gender and marital status among cancer survivors. Further in-depth research, in particular qualitative work, is needed to understand the nuances of why married women with cancer have the highest frequency of underemployment. Preliminary research indicates that employers may be ill-equipped to offer information, resources, and accommodations to their employees who experience cancer (Murphy, Markle, Nguyen, & Wilkinson, 2013). Several initiatives, however, have recently been launched to help employers address their employees’ needs after a cancer diagnosis in the workplace. One example is the National Business Group on Health: Employer’s Guide to Cancer Treatment and Prevention, which includes a set of tools to assist workplace benefit managers navigate myriad issues that arise after cancer, such as medical benefits, short-term disability, and employee assistance programs (National Business Group on Health 2011). Healthcare providers may also have a role to play in discussing expectations and decisions about cancer treatment and making appropriate referrals for rehabilitative, symptom management, and/or social work guided interventions. A recent nationally representative study of cancer survivors reported that less than half of cancer survivors report high quality discussions with providers, at any time following diagnosis, regarding late or long-term effects of cancer and treatment (Chawla, Blanch-Hartigan, & Virgo, 2016). Given the importance of work and the high levels of underemployment that many cancer survivors face, particularly married women, discussion about how cancer and its treatment may have an impact on work should be both systematically studied and recognized as a component of comprehensive survivorship care. The current study suggests the need for a comprehensive conceptualization of work for cancer survivors, such as proposed by the Mehnert, de Boer, and Feurstein model (Mehnert et al., 2013) that includes the assessment and evaluation of work-related skills and demands, skills training, and employer education and counseling.
Supplementary Material
Acknowledgments
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute, the Centers for Disease Control and Prevention, or the US Department of Health and Human Services.
References
- Agency for Healthcare Research and Quality. (2015). MEPS-HC Response Rates. Retrieved from: http://meps.ahrq.gov/mepsweb/survey_comp/hc_response_rate.jsp.
- Alfano CM, Kent EE, Padgett LS, Grimes M, & de Moor JS (2017). Making cancer rehabilitation services work for cancer patients: Recommendations for research and practice to improve employment outcomes. PM & R, 9, S398–S406. doi: 10.1016/j.pmrj.2017.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir Z, Neary D, & Luker K (2008). Cancer survivors’ views of work 3 years post diagnosis: A UK perspective. European Journal of Oncology Nursing, 12, 190–197. doi: 10.1016/j.ejon.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Blinder V, Eberle C, Patil S, Gany FM, & Bradley CJ (2017). Women with breast cancer who work for accommodating employers more likely to retain jobs after treatment. Health Affairs, 36, 274–281. doi: 10.1377/hlthaff.2016.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CJ (2015). Economic recovery: A measure of the quality of cancer treatment and survivorship? Cancer, 121, 4282–4285. doi: 10.1002/cncr.29511 [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Neumark D, & Barkowski S (2013). Does employer-provided health insurance constrain labor supply adjustments to health shocks? New evidence on women diagnosed with breast cancer. Journal of Health Economics, 32, 833–849. doi: 10.1016/j.jhealeco.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. (2015). Glossary. Retrieved from: http://www.bls.gov/bls/glossary.htm#F
- Chawla N, Blanch-Hartigan D, Virgo KS, Ekwueme DU, Han X, Forsythe L, … Yabroff KR (2016). Quality of patient-provider communication among cancer survivors: Findings from a nationally representative sample. Journal of Oncology Practice, 12, e964–e973. doi: 10.1200/JOP.2015.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JW, Cohen SB, & Banthin JS (2009). The medical expenditure panel survey: A national information resource to support healthcare cost research and inform policy and practice. Medical Care, 47, S44–50. doi: 10.1097/MLR.0b013e3181a23e3a [DOI] [PubMed] [Google Scholar]
- de Boer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, & Verbeek JH (2011). Interventions to enhance return-to-work for cancer patients. The Cochrane Data-base of Systematic Reviews, CD007569. [DOI] [PubMed] [Google Scholar]
- Duijts SF, van Egmond MP, Spelten E, van Muijen P, Anema JR, & van der Beek AJ (2014). Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psycho-Oncology, 23, 481–492. doi: 10.1002/pon.3467 [DOI] [PubMed] [Google Scholar]
- Farley Short P, Vasey JJ, & Moran JR (2008). Long-term effects of cancer survivorship on the employment of older workers. Health Services Research, 43, 193–210. doi: 10.1111/j.1475-6773.2007.00752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard BI, & Korn EL (1999). Predictive margins with survey data. Biometrics, 55, 652–659. doi: 10.1111/j.0006-341X.1999.00652.x [DOI] [PubMed] [Google Scholar]
- Grunfeld EA, & Cooper AF (2012). A longitudinal qualitative study of the experience of working following treatment for gynaecological cancer. Psycho-Oncology, 21, 82–89. doi: 10.1002/pon.1874 [DOI] [PubMed] [Google Scholar]
- Grunfeld EA, Drudge-Coates L, Rixon L, Eaton E, & Cooper AF (2013). “The only way I know how to live is to work”: A qualitative study of work following treatment for prostate cancer. Health Psychology, 32, 75–82. doi: 10.1037/a0030387 [DOI] [PubMed] [Google Scholar]
- Gunnarsdottir HK, Vidarsdottir H, Rafnsdottir GL, Tryggvadottir L, Olafsdottir EJ, & Lindbohm ML (2013). Employment participation and work experience of male cancer sur- vivors: A NOCWO study. Work, 46, 385–393. [DOI] [PubMed] [Google Scholar]
- Guy GP Jr., Ekwueme DU, & Yabroff KR (2013). Economic burden of cancer survivor- ship among adults in the United States. Journal of Clinical Oncology, 31, 3749–3757. doi: 10.1200/JCO.2013.49.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeak CS, Short PF, & Moran J (2011). The implications of cancer survivorship for spousal employment. Journal of Cancer Survivorship, 5, 226–234. doi: 10.1007/s11764-011-0175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiasuwa Mbengi R, Otter R, & Mortelmans K (2016). Barriers and opportunities for return-to-work of cancer survivors: Time for action–rapid review and expert consultation. Systematic Reviews, 5, 35. doi: 10.1186/s13643-016-0210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YA, Yun YH, & Chang YJ (2014). Employment status and work-related difficulties in lung cancer survivors compared with the general population. Annals of Surgery, 259, 569–575. doi: 10.1097/SLA.0b013e318291db9d [DOI] [PubMed] [Google Scholar]
- Koch R, Wittekindt C, Altendorf-Hofmann A, Singer S, & Guntinas-Lichius O (2015). Employment pathways and work-related issues in head and neck cancer survivors. Head and Neck, 37, 585–593. doi: 10.1002/hed.23640 [DOI] [PubMed] [Google Scholar]
- Mahar KK, BrintzenhofeSzoc K, & Shields JJ (2008). The impact of changes in employment status on psychosocial well-being: A study of breast cancer survivors. Journal of Psychosocial Oncology, 26, 1–17. doi: 10.1080/07347330802115400 [DOI] [PubMed] [Google Scholar]
- McGrath C, Mihala G, Beesley VL, Lynch BM, Graves N, & Gordon LG (2017). “Cancer Put My Life on Hold”: Work-related challenges among middle-aged adults 12 months after a diagnosis of colorectal cancer. Cancer Nursing, 40(2), 160–167. https://protect-us.mimecast.com/s/PKSkCBB8n5t7RPM7ot6×3b5?domain=ncbi.nlm.nih.gov [DOI] [PubMed] [Google Scholar]
- McKay G, Knott V, & Delfabbro P (2013). Return to work and cancer: The Australian experience. Journal of Occupational Rehabilitation, 23, 93–105. doi: 10.1007/s10926-012-9386-9 [DOI] [PubMed] [Google Scholar]
- Mehnert A (2011). Employment and work-related issues in cancer survivors. Critical Reviews in Oncology/Hematology, 77, 109–130. doi: 10.1016/j.critrevonc.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Mehnert A, de Boer A, & Feuerstein M (2013). Employment challenges for cancer survivors. Cancer, 119, 2151–2159. doi: 10.1002/cncr.28067 [DOI] [PubMed] [Google Scholar]
- Moran JR, & Short PF (2014). Does cancer reduce labor market entry? Evidence for prime-age females. Medical Care Research and Review, 71, 224–242. doi: 10.1177/1077558713510359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JR, Short PF, & Hollenbeak CS (2011). Long-term employment effects of surviving cancer. Journal of Health Economics, 30, 505–514. doi: 10.1016/j.jhealeco.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Markle MM, Nguyen V, & Wilkinson W (2013). Addressing the employment-related needs of cancer survivors. Work, 46, 423–432. [DOI] [PubMed] [Google Scholar]
- National Business Group on Health. (2011). Launches major initiative to address cancer in the workplace. Journal of the National Comprehensive Cancer Network, 9, xlviii–lii. [PubMed] [Google Scholar]
- Parsons HM, Harlan LC, & Lynch CF (2012). Impact of cancer on work and education among adolescent and young adult cancer survivors. Journal of Clinical Oncology, 30, 2393–2400. doi: 10.1200/JCO.2011.39.6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JK, Baima J, Newman R, Galantino ML, & Shockney LD (2013). Cancer rehabilitation may improve function in survivors and decrease the economic burden of cancer to individuals and society. Work, 46, 455–472. [DOI] [PubMed] [Google Scholar]
- Stergiou-Kita M, Grigorovich A, & Tseung V (2014). Qualitative meta-synthesis of survivors’ work experiences and the development of strategies to facilitate return to work. Journal of Cancer Survivorship, 8, 657–670. doi: 10.1007/s11764-014-0377-z [DOI] [PubMed] [Google Scholar]
- Sum A, & Khatiwada I (2010). The Nation’s underemployed in the “Great Recession” of 2007–09. Monthly Labor Review, 3–15. [Google Scholar]
- Taskila T, & Lindbohm ML (2007). Factors affecting cancer survivors’ employment and work ability. Acta Oncologica, 46, 446–451. doi: 10.1080/02841860701355048 [DOI] [PubMed] [Google Scholar]
- Tevaarwerk AJ, Lee JW, & Sesto ME (2013). Employment outcomes among survivors of common cancers: The Symptom Outcomes and Practice Patterns (SOAPP) study. Journal of Cancer Survivorship, 7, 191–202. doi: 10.1007/s11764-012-0258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timperi AW, Ergas IJ, Rehkopf DH, Roh JM, Kwan ML, & Kushi LH (2013). Employment status and quality of life in recently diagnosed breast cancer survivors. Psychooncology, 22, 1411–1420. doi: 10.1002/pon.3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunceli K, Short PF, Moran JR, & Tunceli O (2009). Cancer survivorship, health insurance, and employment transitions among older workers. Inquiry, 46, 17–32. doi: 10.5034/inquiryjrnl_46.01.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Muijen P, Weevers NL, & Snels IA (2013). Predictors of return to work and employment in cancer survivors: A systematic review. European Journal of Cancer Care, 22, 144–160. doi: 10.1111/ecc.12033 [DOI] [PubMed] [Google Scholar]
- Yu M, Ferrucci LM, & McCorkle R (2012). Employment experience of cancer survivors 2 years post-diagnosis in the Study of Cancer Survivors-I. Journal of Cancer Survivorship, 6, 210–218. doi: 10.1007/s11764-011-0212-8 [DOI] [PubMed] [Google Scholar]
- Zajacova A, Dowd JB, Schoeni RF, & Wallace RB (2015). Employment and income losses among cancer survivors: Estimates from a national longitudinal survey of American families. Cancer, 121, 4425–4432. doi: 10.1002/cncr.29510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Yabroff KR, Guy GP Jr., Han X, Li C, Banegas MP, … Jemal A (2015). Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. Journal of the National Cancer Institute, 108. doi: 10.1093/jnci/djv382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.