Abstract
Background:
Chronic rhinosinusitis (CRS) is associated with bronchiectasis; however, this relationship has not been well studied in the United States (US) population. In this work we aimed to determine the prevalence of CRS among patients with bronchiectasis affiliated with a US tertiary medical center and identify which comorbid diseases are associated with the presence of CRS in patients with bronchiectasis.
Methods:
This was a retrospective cohort study in which data were obtained from a large database warehouse at a tertiary care center. Patients with bronchiectasis were identified from 2007 to 2017 using diagnosis codes from the the ninth and tenth revisions of the International Classification of Diseases (ICD-9/10) and confirmed by radiographic evidence of bronchiectasis on chest computed tomography (CT) scans. Patients were divided into cohorts based on presence or absence of concomitant CRS. Characteristics analyzed included demographics, comorbidities, peripheral eosinophil counts, and pulmonary function testing.
Results:
CRS was present in 45% (408 of 900) of patients with bronchiectasis. Females represented a majority of bronchiectasis patients, both with and without CRS (69% and 64%, respectively, p = 0.09). After controlling for demographic factors, asthma (p < 0.01), allergic rhinitis (p <0.01), gastroesophageal reflux disease (p < 0.01), and antibody deficiency (p < 0.01) were associated with the presence of CRS in patients with bronchiectasis.
Conclusion:
CRS had a high prevalence and was associated with numerous comorbid conditions in patients with bronchiectasis. These findings have clinical implications for the treatment of patients with bronchiectasis and future research.
Keywords: chronic rhinosinusitis, chronic disease, asthma
Bronchiectasis is a common lower respiratory tract disease characterized by the permanent dilation of proximal, medium-sized bronchi. Bronchiectasis is classically divided into cystic fibrosis (CF)-associated and non–CF-associated bronchiectasis, where the former affects a small, relatively homogeneous population of patients and the latter affects a heterogeneous population with diverse etiologies.1 Although the pathophysiology for most etiologies remains incompletely understood, the commonly accepted “vicious cycle” hypothesis states that there is a reinforcing cycle of inflammation producing airway damage, impaired mucociliary clearance, and frequent respiratory infections, which then cause further inflammation.2 Bronchiectasis remains an unresolved worldwide cause of impaired quality of life, increased mortality, and significant economic burden.3–5
Chronic rhinosinusitis (CRS) is an upper airway disease characterized by chronic inflammation of the nasal mucosa and paranasal sinuses that affects an estimated 3% to 6.4% of the United States (US) population.6 Recent literature has proposed the “united airways” hypothesis, which states that diseases of the upper and lower respiratory tract may be secondary to a similar pathogenic mechanism.7,8 Some studies have suggested that bronchiectasis patients with CRS have worse health-related quality of life, greater degree of bronchiectasis severity, elevated levels of inflammatory markers, and reduced time to first exacerbation of bronchiectasis than bronchiectasis patients without CRS.3,9,10 Nonetheless, the exact link between these 2 diseases remains speculative.10
Research into the relationship between CRS and bronchiectasis has predominantly stemmed from countries outside of the US.9–12 However, some evidence suggests that the etiology of bronchiectasis within the diverse US population may differ from that of other nations.4 Therefore, investigating the prevalence of CRS in patients with bronchiectasis and the nature of comorbidities associated with CRS and bronchiectasis specifically in the US population may offer better insight into the pathogenesis of bronchiectasis and potentially inform treatment strategies.13 We hypothesize that CRS is highly prevalent and associated with numerous comorbidities in patients with bronchiectasis. The primary objective of our study was to determine the prevalence of CRS and comorbidities associated with CRS in patients with non-CF bronchiectasis at a tertiary medical center in the US.
Materials and methods
Identification of subjects
This was a retrospective cohort study in which data were obtained from the Northwestern Medicine Enterprise Data Warehouse (EDW), an electronic repository of medical records of patients seen at Northwestern Medicine. Patients were included in our study if they had bronchiectasis as defined by the ninth or tenth edition of the International Classification of Diseases (ICD-9/10) diagnosis codes of bronchiectasis (494.0, 494.1, J47.9, J47.1, J47.0) and radiographic evidence of bronchiectasis on chest computed tomography (CT) scan. Patients were excluded if they had an ICD-9/10 diagnosis code for cystic fibrosis or primary ciliary dyskinesia (PCD). All data extracted were for patients seen between January 1, 2007 and December 31, 2017. Data were obtained for demographic information, which included age at which bronchiectasis was first documented in the electronic medical record, sex, race, body mass index (BMI), and smoking status. There were no age exclusions; however, as this institution is an adult care facility, all patients were ≥18 years of age.
This research was approved by the institutional review board of the Northwestern University Feinberg School of Medicine.
Identification of CRS
Patients were considered positive for CRS if they fulfilled at least 1 of the following criteria: Current Procedural Terminology (CPT) codes for previous sinus surgery; an ICD-9/10 diagnosis code for rhinosinusitis (473.xx, 461.xx, 477.9, 461.9, J32.9, J01.90, B96.89, J30.9, J01.90) and a documented visit to the institution’s allergy or otolaryngology (ENT) clinics that then confirmed the diagnosis of CRS; and/or radiographic evidence of sinus disease on a sinus CT scan.
Identification of asthma, allergic rhinitis, gastroesophageal reflux disease, chronic obstructive pulmonary disease, and peripheral eosinophil counts
The presence of asthma, allergic rhinitis, gastroesophageal reflux disease (GERD), and chronic obstructive pulmonary disease (COPD) was assessed via ICD-9/10 diagnosis codes. Peripheral eosinophil counts were examined in all patients who had undergone complete blood cell counts with differentials during the study period. For patients with multiple documented peripheral eosinophil counts, the value obtained closest to the date of first diagnostic chest CT for bronchiectasis was used for analysis.
Identification of autoimmune disease and antibody deficiency
The presence of autoimmune disease was considered positive if any of the following conditions were identified via ICD-9/10 diagnosis codes: rheumatoid arthritis; systemic lupus erythematosus; systemic sclerosis; or sarcoidosis. Quantitative immunoglobulins (total IgG, IgM, and IgA) and Streptococcus pneumoniae antibody titers were also analyzed. Patients were considered positive for antibody deficiency if they fulfilled at least 1 of the following criteria: hypogammaglobulinemia, defined as IgG < 600 mg/dL, IgM < 35 mg/dL, or IgA < 28 mg/dL; >50% of Streptococcus pneumoniae antibody titers of <1.3 μg/mL at least 4 to 6 weeks after 23-valent pneumococcal polysaccharide vaccination; and/or ICD-9/10 diagnosis codes for common variable immunodeficiency, antibody deficiency, or IgA deficiency.
Identification of pulmonary function data
Pulmonary function data, including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1 percent predicted, were collected for all patients who had undergone pulmonary function testing (PFT) or spirometry at this institution’s allergy or pulmonary clinics during the study period. For patients with multiple documented PFTs, the values gathered closest to the date of the first diagnostic chest CT for bronchiectasis were used for analysis.
Study endpoints
We examined associations in bronchiectasis patients with CRS compared with bronchiectasis patients without CRS and the following factors: asthma, allergic rhinitis, GERD, COPD, peripheral eosinophil counts, autoimmune disease, antibody deficiency, age, sex, race, ethnicity, BMI, smoking status, and pulmonary function based on FEV1 percent predicted. Of these factors, asthma, allergic rhinitis, GERD, COPD, autoimmune disease, and antibody deficiency were variables of particular interest as many of these are associated with bronchiectasis14–17; therefore, logistic regression analysis was performed to control for age, race, and sex as potential confounders. Associations of CRS with FEV1 percent predicted and peripheral eosinophil counts were also of interest; therefore, linear regression analysis was performed to control for age, sex, race, asthma, and COPD.
Statistical analysis
We calculated descriptive statistics for all variables of interest. Categorical variables were summarized by frequency and percent, and continuous variables were summarized by mean and standard deviation or median and interquar-tile rank, as appropriate. The t test or Wilcoxon (Mann-Whitney U) rank sum test was used to determine statistical significance for continuous variables, whereas a chi-square test or Fisher’s exact test was used to determined statistical significance for categorical variables. Linear regression or logistic regression was used to calculate coefficients or odds ratios as appropriate and corresponding 95% confidence intervals when controlling for possible confounders such as age, sex, race, etc. Missing data were excluded from the analysis. p < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC) or R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ demographics
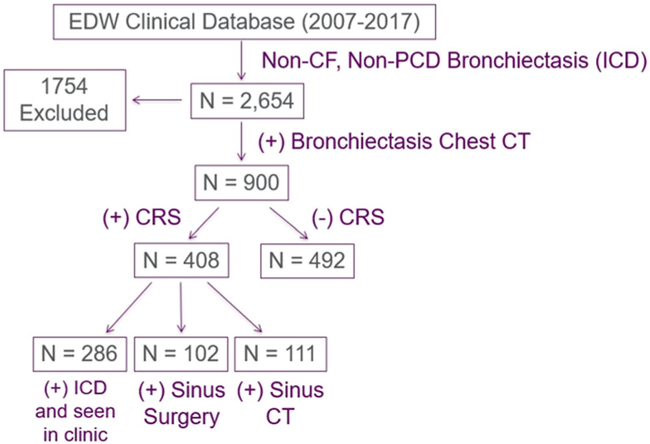
Using our algorithm, we identified 900 patients with bronchiectasis that were seen within our tertiary care center between 2007 and 2017. Of these, 408 (45%) had concomitant CRS, as defined by the inclusion criteria (Fig. 1). Sex, race, smoking status, and BMI did not significantly differ between bronchiectasis patients with and without CRS (Table 1), although bronchiectasis patients with CRS were younger than bronchiectasis patients without CRS (p = 0.03). The majority of bronchiectasis patients were female regardless of CRS status (p = 0.09).
FIGURE 1.
Algorithm for determining patient cohorts. CRS = chronic rhinosinusitus; CT = computed tomography; EDW = Enterprise Data Warehouse; ICD = International Classification of Diseases; PCD = primary ciliary dyskinesia.
TABLE 1.
Patients’ demographics
| Bronchiectasis (+)/CRS (+) | Bronchiectasis (+)/CRS (−) | p value | |
|---|---|---|---|
| Total number of subjects | 408 | 492 | |
| Age, in years (mean ± SD) | 64.31 ± 16.14 | 66.55 ± 14.48 | 0.03 |
| Female [n (%)] | 260 (63.7%) | 340 (69.1%) | 0.09 |
| Race [n (%)] | 0.68 | ||
| White | 266 (65.2%) | 307 (62.4%) | |
| Black | 54 (13.2%) | 68 (13.8%) | |
| Other | 88 (21.6%) | 117 (23.8%) | |
| Smoking status [n (%)] | 0.88 | ||
| Current/former smoker | 115 (39.3%) | 93 (38.6%) | |
| Never smoked | 178 (60.8%) | 148 (61.4%) | |
| Body mass index (mean ± SD) | 25.57 ± 5.59 | 25.42 ± 6.39 | 0.71 |
CRS = chronic rhinosinusitus; SD = standard deviation.
Association between comorbid disease and CRS in bronchiectasis
We next determined the prevalence of various comorbidities observed in patients with bronchiectasis and CRS and compared these findings with those observed in patients with bronchiectasis alone (Table 2). Asthma (p < 0.01), allergic rhinitis (p < 0.01), GERD (p < 0.01), and antibody deficiency (p = 0.01) were each independently associated with the presence of CRS in patients with bronchiectasis after controlling for age, sex, and race (Table 3). There was a trend toward a significant association between COPD and CRS in patients with bronchiectasis after controlling for age, sex, and race (p = 0.05).
TABLE 2.
Prevalence of comorbidities among bronchiectasis patients with and without CRS*
| Bronchiectasis (+)/CRS (+) | Bronchiectasis (+)/CRS (−) | p value | |
|---|---|---|---|
| Subjects (n) | 408 | 492 | |
| Allergic rhinitis/atopy | 97 (23.8%) | 65 (13.2%) | <0.01 |
| Antibody deficiency | 58 (14.2%) | 37 (7.5%) | <0.01 |
| Asthma | 147 (36.0%) | 129 (26.2%) | <0.01 |
| COPD | 108 (26.5%) | 104 (21.1%) | 0.06 |
| GERD | 178 (43.6%) | 167 (33.9%) | <0.01 |
| Autoimmune disease | 57 (14.0%) | 74 (15.0%) | 0.65 |
Data expressed as number (%), unless noted otherwise.
COPD = chronic obstructive pulmonary disease; GERD = gastroesophageal reflux disease.
TABLE 3.
Adjusted ORs for comorbidities associated with the presence of CRS in patients with bronchiectasis
| OR (95% CI) | p value | N | |
|---|---|---|---|
| Asthma | |||
| Unadjusted | 1.58 (1.19–2.11) | <0.01 | 900 |
| Adjusted for age, sex, race | 1.56 (1.17–2.10) | <0.01 | 900 |
| GERD | |||
| Unadjusted | 1.51 (1.15–1.97) | <0.01 | 900 |
| Adjusted for age, sex, race | 1.56 (1.19–2.05) | <0.01 | 900 |
| Allergic rhinitis | |||
| Unadjusted | 2.05 (1.45–2.91) | <0.01 | 900 |
| Adjusted for age, sex, race | 1.99 (1.41–2.83) | <0.01 | 900 |
| Antibody deficiency | |||
| Unadjusted | 2.04 (1.32–3.17) | <0.01 | 900 |
| Adjusted for age, sex, race | 1.83 (1.18–2.88) | 0.01 | 900 |
| COPD | |||
| Unadjusted | 1.34 (0.99–1.83) | 0.06 | 900 |
| Adjusted for age, sex, race | 1.37 (1.01, 1.88) | 0.05 | 900 |
| Autoimmune disease | |||
| Unadjusted | 0.92 (0.63–1.33) | 0.65 | 900 |
| Adjusted for age, sex, race | 0.90 (0.61–1.33) | 0.60 | 900 |
CI = confidence interval; CRS = chronic rhinosinusitus; COPD = chronic obstructive pulmonary disease; GERD = gastroesophageal reflux disease; OR = odds ratio.
Peripheral eosinophil counts in patients with bronchiectasis
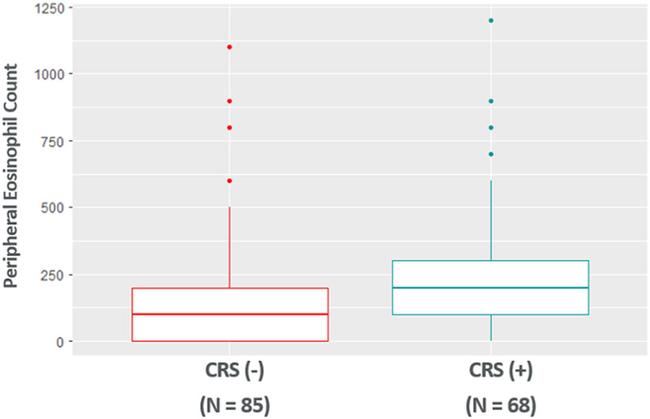
Peripheral eosinophil counts were available for 68 bronchiectasis patients with CRS and 85 bronchiectasis patients without CRS (Fig. 2). Peripheral eosinophil counts were significantly higher in patients with bronchiectasis and CRS (251.5 ± 252.4) than patients with bronchiectasis alone (165.9 ± 202.1) after controlling for age, sex, race, asthma, and COPD (p = 0.01).
FIGURE 2.
Peripheral eosinophil counts are elevated in patients with bronchiectasis and CRS. CRS = chronic rhinosinusitus.
Pulmonary function in patients with bronchiectasis
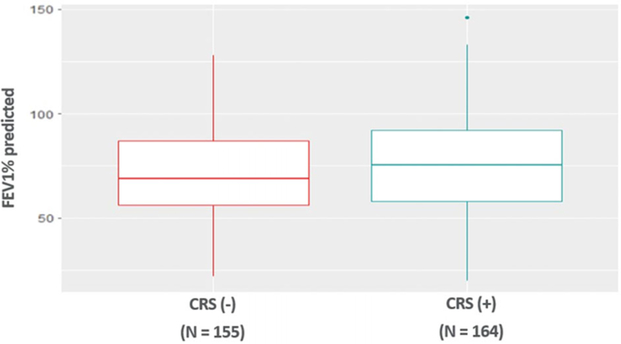
FEV1 percent predicted was available for 164 bronchiectasis patients with CRS and 155 bronchiectasis patients without CRS (Fig. 3). FEV1 percent predicted did not show a significant difference between patients with bronchiectasis and CRS (74.6 ± 22.3) and patients with bronchiectasis alone (71.1 ± 24.1) after controlling for age, sex, race, asthma, and COPD (p = 0.09).
FIGURE 3.
No difference in FEV1 percent predicted in bronchiectasis patients with and without CRS. CRS = chronic rhinosinusitus; FEV1 forced expiratory volume in 1 second.
Discussion
This large retrospective study found that almost half of the patients with non-CF bronchiectasis had comorbid CRS. We found that allergic rhinitis, antibody deficiency, asthma, and GERD were significantly associated with the presence of CRS in patients with bronchiectasis. To our knowledge, this is the largest study to describe such an association between various comorbid diseases and CRS in patients with bronchiectasis within the US population. Future areas of research should include a replication study, preferably in a multicenter setting.
Our finding that CRS was present in 45% of patients with bronchiectasis is notably lower than other studies that have evaluated the prevalence of CRS in patients with bronchiectasis worldwide. A recent systematic review by Handley et al estimated the pooled prevalence of CRS in patients with bronchiectasis to be 62%.12 However, that review only highlighted studies from outside the US. One possible explanation for the lower prevalence of CRS specifically in US patients with bronchiectasis would be differences in environmental exposures. This lower prevalence may also be attributable to regional differences in the primary etiology of bronchiectasis,18 although this was not an endpoint of our study.
Asthma, allergic rhinitis, GERD, and antibody deficiency were significantly associated with the presence of CRS in patients with bronchiectasis. This finding is clinically relevant as past evidence suggests that CRS and other comorbidities contribute to increased exacerbation frequency and decreased quality of life in patients with bronchiectasis.7,19 It has been suggested that COPD and asthma are independently associated with a higher mortality risk in patients with bronchiectasis.19 Further research is warranted as to whether CRS, one of the most frequent concomitant conditions in bronchiectasis,9 may also be associated with an increased morbidity and possibly mortality.
Bronchiectasis is traditionally considered to be a neutrophil-mediated disease.20,21 However, we found significantly higher peripheral eosinophil counts in patients with bronchiectasis and CRS than in patients with bronchiectasis alone. It remains unclear whether the elevated peripheral eosinophil counts are simply a manifestation of CRS, a traditionally eosinophilic disorder, or if bronchiectasis patients with CRS have a different pathogenic mechanism that leads to a distinct eosinophil-driven endotype.22 If this endotype is confirmed, the use of biologic agents targeting type II inflammation in patients with bronchiectasis and CRS should be studied.23
A strength of our study is that we used a rigorous algorithm for defining bronchiectasis—by requiring both an ICD-9/10 diagnosis code and radiographic evidence of bronchiectasis, we can be certain that all patients included had radiographically significant bronchiectasis. Moreover, our institution is associated with a large multidisciplinary sinus center; therefore, our study has one of the best-characterized CRS groups as compared with other studies that investigated the prevalence of CRS in patients with bronchiectasis.
Limitations of our study include the retrospective design, which restricted our ability to draw causal inferences from the data. Furthermore, not all patients with ICD-9/10 diagnosis codes for CRS had available sinus imaging as this may have been performed at an outside institution. Therefore, documented evidence of CRS on sinus imaging was not required for inclusion in the bronchiectasis/CRS cohort. Similarly, not all patients had available data for pulmonary function testing or peripheral eosinophil counts thus limiting our analysis of these domains. Lastly, our analysis is from a large single tertiary academic medical care center with a multidisciplinary sinus center in an urban setting. Generalizability of our study findings beyond this patient population is thus limited.
In summary, we found that US patients with bronchiectasis had a high prevalence of CRS and that asthma, allergic rhinitis, GERD, and antibody deficiency were associated with CRS in patients with bronchiectasis.
Conclusion
Patients with bronchiectasis should be evaluated for CRS, especially if they have comorbid asthma, allergic rhinitis, GERD, or antibody deficiency. Patients with bronchiectasis and CRS should be promptly diagnosed and treated by a multidisciplinary team of allergists, otolaryngologists, and pulmonologists to minimize the likely increased burden associated with their concomitant disease.
Acknowledgements
The authors thank Anna Pawlowski for assistance with the Northwestern Medicine Enterprise Data Warehouse.
Funding sources for the study: Division of Allergy and Immunology, Department of Medicine, Northwestern University Feinberg School of Medicine; Ernest Bazley Foundation.
Footnotes
Potential conflict of interest: None provided.
References
- 1.Costa JC, Machado JN, Ferreira C, et al. The bronchiectasis severity index and FACED score for assessment of the severity of bronchiectasis. Pulmonology. 2018;24:149–154. [DOI] [PubMed] [Google Scholar]
- 2.Cole PJ. Inflammation: a two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. [PubMed] [Google Scholar]
- 3.Guilemany JM, Angrill J, Alobid I, et al. United airways: the impact of chronic rhinosinusitis and nasal polyps in bronchiectasic patient’s quality of life. Allergy. 2009;64:1524–1529. [DOI] [PubMed] [Google Scholar]
- 4.McShane PJ, Naureckas ET, Strek ME. Bronchiectasis in a diverse US population: effects of ethnicity on etiology and sputum culture. Chest. 2012;1:159–167. [DOI] [PubMed] [Google Scholar]
- 5.Machado BC, Jacques PS, Penteado LP, et al. Prognostic factors in adult patients with non-cystic fibrosis bronchiectasis. Lung. 2018;196:691–697. [DOI] [PubMed] [Google Scholar]
- 6.Dietz de Loos D, Lourijsen ES, Wildeman MAM, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol. 2019;143:1207–1214. [DOI] [PubMed] [Google Scholar]
- 7.Guilemany JM, Alobid I, Angrill J, et al. The impact of bronchiectasis associated to sinonasal disease on quality of life. Respir Med. 2006;100:1997–2003. [DOI] [PubMed] [Google Scholar]
- 8.Philpott CM, McKiernan DC. Bronchiectasis and sino-nasal disease: a review. J Laryngol Otol. 2008;122:11–15. [DOI] [PubMed] [Google Scholar]
- 9.Guilemany JM, Angrill J, Alobid I, et al. United airways again: high prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy. 2009;64:790–797. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Xu Y, Jin J, et al. Chronic rhinosinusitis is associated with higher prevalence and severity of bronchiectasis in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W, Gao Y, Li H, et al. Impacts of co-existing chronic rhinosinusitis on disease severity and risks of exacerbations in Chinese adults with bronchiectasis. PLoS One. 2015;10:e0137348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handley E, Nicolson CH, Hew M, et al. Prevalence and clinical implications of chronic rhinosinusitis in people with bronchiectasis: a systematic review. J Allergy Clin Immunol Pract. 2019;7:2004–2012. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan VR, Ferril GR, Suh JD, et al. Upper and lower airways associations in patients with chronic rhinosinusitis and bronchiectasis. Int Forum Allergy Rhinol. 2013;3:921–927. [DOI] [PubMed] [Google Scholar]
- 14.Coman I, Pola-Bibián B, Barranco P, et al. Bronchiectasis in severe asthma: clinical features and outcomes. Ann Allergy Asthma Immunol. 2018;120:409–413. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodkinson JP, Bangs C, Wartenberg-Demand A, et al. Low IgA and IgM is associated with a higher prevalence of bronchiectasis in primary antibody deficiency. J Clin Immunol. 2017;37:329–331. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell MJ, O’Toole D, Ward C, et al. A qualitative synthesis of gastro-oesophageal reflux in bronchiectasis: current understanding and future risk. Respir Med. 2018;141:132–143. [DOI] [PubMed] [Google Scholar]
- 18.Lonni S, Chalmers JD, Goeminne PC, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12:1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell MJ, Aliberti SA, Goeminne PC, et al. Comorbidities and the risk of mortality in bronchiectasis patients: an international cohort study. Lancet Respir Med. 2016;4:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shteinberg M, Nassrallah N, Jrbashyan J, et al. Upper airway involvement in bronchiectasis is marked by early onset and allergic features. ERJ Open Res. 2018;4:00115–02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Miranda J, Traversi L, Polverino E. Bronchiectasis in severe asthma: a distinct phenotype? Curr Opin Pulm Med. 2019;25:71–78. [DOI] [PubMed] [Google Scholar]
- 22.Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328. [DOI] [PubMed] [Google Scholar]
- 23.Bachert C, Zhang N, Hellings PW, et al. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1543–1551. [DOI] [PubMed] [Google Scholar]