Abstract
Genistein is an isoflavone abundant in soybean and infants are exposed to high levels of genistein in soy-based formula. It is known that genistein mediates estrogen receptor (ER) signaling, and exposure during neonatal development could cause acute and long term endocrine effects. We assayed genistein’s impact on the neonatal mouse pituitary gland because it is an endocrine signaling hub and is sensitive to endocrine disruption during critical periods. Pituitary explant cultures, which actively proliferate and differentiate, were exposed to 0.06 μM to 36 μM genistein and assayed for mRNA and protein changes. Genistein induced mRNA expression of the ERα regulated gene, Cckar, to the same magnitude as estradiol (E2) but with less potency. Interestingly, 36 μM genistein strongly inhibited pituitary proliferation, measured by a reduction in mKi67 mRNA and phospho-Histone H3 immunostaining. Examining cell cycle dynamics, we found that 36 μM genistein decreased Ccnb1 (Cyclin B1) mRNA; while mRNA for the cyclin dependent kinase inhibitor Cdkn1a (p21) was upregulated, correlated with an apparent increase in p21 immunostained cells. Strikingly, we observed a robust onset of cellular senescence, permanent cell cycle exit, in 36 μM genistein treated pituitaries by increased senescence activated β-galactosidase staining. We also found that 36 μM genistein decreased Bcl2 mRNA levels, a gene protective against apoptosis. Taken together these data suggest that genistein exposure during the neonatal period could initiate senescence and halt proliferation during a time when the proper numbers of endocrine cells are being established for mature gland function.
Keywords: genistein, pituitary, proliferation, senescence
Graphical abstract
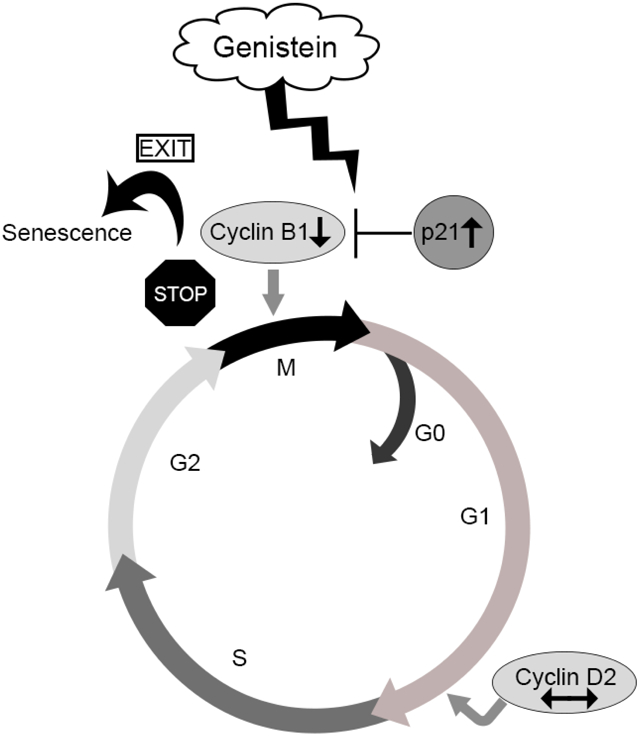
1. Introduction
Genistein is an isoflavone compound found in numerous species of plants, but is well known as the predominant flavonoid in soybean (Glycine max) (Patel et al., 2017; Rozman et al., 2006). Genistein can act as a botanical estrogen, or phytoestrogen, a naturally occurring plant compound that can mediate estrogen receptor (ER) signaling. As a selective estrogen receptor modulator (SERM) and endocrine disrupting chemical (EDC) genistein can bind directly to ERα and β isoforms as well as non-genomic ERs (Chang et al., 2008; Gong et al., 2014; Kuiper et al., 1998; Maggiolini et al., 2004; Oseni et al., 2008; Prossnitz and Barton, 2011), and regulate transcription of ER target genes. Genistein exposure is known to lead to steroid hormone changes and reproductive deficits (Jefferson et al., 2007, 2005; Medigović et al., 2012; Patel et al., 2017). Genistein can also impact proliferation of numerous cell types. It has been demonstrated to both enhance and repress proliferation, often in a concentration dependent fashion (Allred et al., 2001; Ju et al., 2001; Wang et al., 1996), and has been studied as an anticancer therapy (Hsiao et al., 2019; Li et al., 2013; Takimoto et al., 2003). Furthermore, genistein is known to be a tyrosine kinase inhibitor (Akiyama et al., 1987), an anti-angiogenic agent (Varinska et al., 2015) and can engage apoptosis in numerous contexts (Chen et al., 2015; Kabała-Dzik et al., 2018; Yanagihara et al., 1993; Zhou et al., 2017).
As it is highly concentrated in soybean, genistein is prevalent in the human diet due to the ubiquitous presence of soy-based foods. While obvious in such items such as soymilk and tofu, soybeans are also used as fillers in breads and breakfast cereals (Rozman et al., 2006). Moreover, soy formula is an increasingly popular choice particularly for infants intolerant to cow’s milk. It is reported that approximately 13% to 25% of infants consume soy formula (Badger et al., 2002; McCarver et al., 2010). With formula as a sole food source, this can lead to high circulating levels of genistein on the order of 1 to 5 μM or possibly higher (Badger et al., 2002; Barlow et al., 2007; Cao et al., 2009; Setchell et al., 1998). These levels are 100 to 700 fold higher than serum genistein levels in adult, non-vegetarian women (Jefferson and Williams, 2011). Because of this elevated exposure to genistein from soy infant formula, a number of studies have examined genistein’s effects in neonatal mammals including rats, mice and primates. These experiments have shown that environmentally relevant doses of genistein (0.5 to 50 mg/kg) cause irregular estrous cycles, subfertility/infertility, multioocyte follicles in the ovary and a decrease in thymus weight in neonatal mice (Cimafranca et al., 2010; Jefferson et al., 2005). Furthermore, postnatal day 1 to postnatal day 5 (P1 to P5) female rats dosed with 50 mg/kg genistein were predisposed to obesity later in life (Strakovsky et al., 2014). The neonatal period is a significant time in development of many organ systems and it is the time during which endocrine axes are being established (Milkovic et al., 1973; Pointis and Mahoudeau, 1976). The pituitary gland is considered to be a master regulator of the endocrine system receiving hormone signals from the brain and secreting hormones that regulate the function of numerous target organs such as the gonads, liver, adrenal glands and others (Treier and Rosenfeld, 1996). These target organs can in turn feedback upon the brain and pituitary (Keller-Wood and Dallman, 1984; Thorner M. et al., 1998). The pituitary is comprised of numerous ERα and ERβ positive cell types which are responsive to ER agonists and antagonists; for example, estrogens regulate prolactin and gonadotropin hormone synthesis and release (Lloyd et al., 1975; Oliveira et al., 1993; Sánchez-Criado et al., 2004). In addition, E2 and the EDC bisphenol A (BPA) given to pregnant mice have been shown to affect cell proliferation and differentiation in offspring (Brannick et al., 2012; Wu et al., 2008). Yet the influence of genistein on the pituitary gland during neonatal development, a critical exposure window when EDCs can impact pituitary maturation (Eckstrum et al., 2018, 2016), has not been fully assessed.
The adult pituitary gland is comprised of 6 different hormone secreting cell types that facilitate a number of critical physiological processes such as reproduction, growth, lactation, metabolism and stress response. These cells are the gonadotropes, lactotropes, somatotropes, thyrotropes, corticotropes and melanotropes. Each of these mature endocrine cells was derived from a stem-like progenitor cell which differentiated into a hormone secreting cell through induction of lineage specifying transcription factors and engagement of developmental signaling cascades (Davis et al., 2016; Edwards and Raetzman, 2018; Fauquier et al., 2008; Jayakody et al., 2012; Raetzman et al., 2004).The pituitary gland undergoes two significant periods of cell proliferation and differentiation, embryonically and during the neonatal period. These processes are choreographed in part by cell cyclins, cyclin dependent kinases (CDK) and cyclin dependent kinase inhibitors (CDKI), which guide progression through and exit from the cell cycle. In addition to the mechanisms involved in endocrine cell proliferation and differentiation, the pituitary gland population can also be affected by cell death. For example, apoptotic machinery is activated in the pituitary during embryonic development to limit expansion of certain progenitor cells as part of the normal developmental program (Monahan et al., 2009; Noseda et al., 2004). However, gene mutation or exogenous compounds can induce apoptosis in the pituitary as well (Monahan et al., 2009; Weis and Raetzman, 2016). Another mechanism that can affect the complement of cells in the pituitary gland is cellular senescence, or permanent exit from the cell cycle. Senescence initiates an immune response intended to halt excessive proliferation that could result in pituitary tumors (Chesnokova et al., 2008, 2007; Collado et al., 2005). Paradoxically, onset of senescence in the developing pituitary gland that is accompanied by an overexpression of β-catenin, can actually promote formation of human pediatric tumors, namely adamantinomatous craniopharyngioma (ACP)(Gonzalez-Meljem et al., 2017; Gonzalez-Meljem and Martinez-Barbera, 2018). It is essential that the proper number and types of endocrine cells are formed and retained during critical developmental windows. Too few as well as too many of any cell type can prevent proper function of the adult pituitary or lead to endocrine disease (Asa and Ezzat, 2002; Hernández et al., 2007; Melmed, 1990; Wu et al., 1998). This balance of cells within the pituitary can be influenced by multiple factors including environmental exposures to EDCs.
Given the susceptibility of the developing pituitary to exogenous EDCs we assessed how genistein might impact the neonatal mouse pituitary gland. We employed pituitary explant cultures which provide an ideal platform to test chemical treatments on neonatal pituitary glands that are actively proliferating and undergoing differentiation (Weis and Raetzman, 2016). Using the pituitary organ cultures and genistein doses ranging from 0.06 μM to 36 μM, we determined genistein’s ability to activate ER regulated signaling, and examined its effects on cell proliferation and activation of cell death and senescence. Our results show that genistein acts as an ER agonist in the pituitary gland, and that 36 μM genistein induces cell senescence which may limit proliferation during a critical period of pituitary development.
2. Materials and Methods
2.1. Animals
CD-1 mice originally obtained from Charles River were bred in house and used for all experiments described. Mice were group housed and maintained in a 12 hr. light-dark environment. Cages contained corn cob bedding material, enriched with iso-Blox (Envigo), and covered with filtered lids. Teklad 8664 rodent diet (Envigo) and water were provided ad libitum. Pituitary glands were harvested from neonatal mice aged postnatal day 1 (PND1) for all culture experiments. Gender was confirmed by visual inspection and SRY genotyping using the primer sequences listed in Table 1. All procedures were approved by the University of Illinois, Urbana-Champaign, Institutional Animal Care and Use Committee.
Table 1.
PCR primers used in this study.
| Gene | Accession Number |
Forward Primer | Reverse Primer |
|---|---|---|---|
| mKi67 | X82786 | AGTAAGTGTGCCTGCCCGAC | ACTCATCTGCTGCTGCTTCTCC |
| Cckar | NM_009827.2 | AAGCGGCAGGATGGATGTGGTCG | CGTGATAACCAGCGTGTTCCC |
| Ccnb1 | NM_172301.3 | TTGAATTCTGACAGCCAGATGGG | TCCAGGTGGCATTACAAGACAGG |
| Ccnd2 | NM_009829 | ACACCGCACACATAGGCTTCTC | TAAGCATGCCGCAGCTGTTGAC |
| Cdkn1a | NM_007669.5 | TTGGAGTCAGGCGCAGATCCACA | CGCCATGAGCGCATCGCAATC |
| Cdkn1b | NM_009875 | TTCGGCCCGGTCAATCATGAAG | GCGCTGACTCGCTTCTTCCATATC |
| Cdkn1c | XM_006508467 | TCCATCACCAATCAGCCAGCAGAA | ATCGCTGGAGGCCAAGCGTTC |
| Gapdh | NM_001289726.1 | GGTGAGGCCGGTGCTGAGTATG | GACCCTTTTGGCTCCACCCTTC |
| Sry | U70642.1 | TGCAGCTCTACTCCAGTCTTG | GATCTTGATTTTTAGTGTTC |
| Bcl-2 | NM_009741.5 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| Bax | NM_007527.3 | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
| p53 | XM_006533157.3 | CCAGCCACTCCATGGCCC | TGCACAGGGCACGTCTTCGC |
2.2. Pituitary Explant Culture
Whole organ pituitary explant cultures were performed as described (Weis 2016, Eckstrum 2016). Briefly, pituitaries were harvested from mice at PND1, and 2 to 3 gender matched pituitaries were cultured on Millicell CM 24-well plate culture inserts (Millipore) in DMEM/F12 medium containing 10% charcoal stripped Fetal Bovine Serum (FBS, Sigma), and 10,000 IU Penicillin/10,000 μg/ml Streptomycin (Fisher Scientific). Treatments were applied to pituitary explants for 48 hrs., and individual pituitaries were harvested for qPCR or Immunohistochemistry (IHC) analysis. For dose response curves, the following ligands were used: 17β estradiol (E2, >99% purity, 10 pM to 100 nM, Tocris) and genistein (Gen, >98% purity, 0.06 μM to 36 μM, Botanical Research Center, University of Illinois). Vehicle for the E2 experiments was 0.1% ethanol and vehicle for genistein cultures was 0.075% DMSO. The concentrations of E2 that were chosen have been shown to induce ER mediated gene expression in a number of cell and tissue contexts (Eckert and Katzenellenbogen, 1981; Eckstrum et al., 2016). The genistein doses used include concentrations shown to mediate ER signaling and cause in vivoreproductive dysfunction as well as impairment of cell proliferation, cell cycle genes and steroidogenic enzyme activity in vitro. (Jefferson et al., 2005; Patel et al., 2016). Moreover, the 6 μM genistein dose is within the range of serum genistein levels measured in infants fed with soy formula (Badger et al., 2002; Barlow et al., 2007; Cao et al., 2009; Setchell et al., 1998). Vehicle controls were also added for each ligand: 0.1% ethanol for E2 and 0.075% DMSO for Gen. In combined treatments of Gen and ICI 182,780 (ICI, >99% purity, Tocris), Gen was co-treated with 10 μM ICI or 10 μM ICI alone was added to explants. Vehicle for the ICI co-treatment experiments consisted of 0.1% ethanol, 0.075% DMSO. Equal numbers of male and female mice were used for all pituitary explant culture experiments. We observed no sex-specific differences in any downstream assays so all data are represented as combined male and female pituitaries. Individual numbers for each experiment are listed in the Figure Legends. An average of 10 individual pituitaries were used per treatment for qPCR and 4 for IHC from 3 independent cultures.
2.3. Quantitative Polymerase Chain Reaction (qPCR)
Following culture, individual pituitary explants were harvested for total RNA and reverse transcribed into cDNA as described previously (Eckstrum et al., 2016; Nantie et al., 2014). RNA purity was measured by spectrophotometry, with A260/A280 ratios routinely measuring 1.8-2.0. Oligonucleotide primers for mKi67, Cckar, Ccnd2, Ccnb1, Cdkn1a, Cdkn1b, Cdkn1c, p53, Bcl2 and Bax were used to amplify gene-specific transcripts by qPCR. Relative fold changes vs. controls were determined using the comparative ΔΔCT value method (Goldberg et al., 2011), normalized to Gapdh transcript. We chose Gapdh as an internal control as no genistein concentrations tested affected Gapdh mRNA levels. All primers were obtained from Life Technologies and sequences are listed in Table 1.
2.4. Immunohistochemistry (IHC)
Pituitary explants were fixed for 20 minutes in 3.7% formaldehyde/ phosphate buffered saline (PBS), cryoprotected in 30% sucrose/ PBS, flash frozen and sectioned to 12 μm using a cryostat (Leica). Immunostaining was performed using antibodies against SOX9, phospho-Histone H3, PIT1 and p21 as well as pituitary hormone antibodies against LHβ, TSHβ, ACTH and GH, described in Table 2. Briefly, slide-mounted sections were post-fixed for 5 minutes in 3.7% formaldehyde/PBS, and antigen retrieval was performed by immersion in 0.01M sodium citrate pH 6.0 at 95°C for 5-10 minutes depending on the primary antibody. Antigen retrieval was not done for the pituitary hormone antibodies. For 3-3’-diaminobenzidine staining (DAB), samples were treated with 5% hydrogen peroxide in PBS for 20 minutes and blocked for 1 hour with 5% normal donkey serum (Jackson ImmunoResearch), 3% bovine serum albumen (Jackson ImmunoResearch), and 0.5% Triton-X100 in PBS. Primary antibodies were applied to slides overnight at 4 °C at the concentrations indicated in Table 2. Sections were incubated with biotin conjugated anti-rabbit secondary antibodies (Jackson ImmunoResearch) for 1 hour at room temperature, followed by streptavidin-HRP amplification using the Vectastain Elite ABC kit (Vector) and visualization by DAB staining. For immunofluorescent staining, samples were blocked for 1 hour with 5% normal donkey serum (Jackson ImmunoResearch), 3% bovine serum albumen (Jackson ImmunoResearch), and 0.5% Triton-X100 in PBS. Primary antibodies were applied to slides overnight at 4 °C at the concentrations indicated in Table 2. Sections were incubated with cy3 or Alexa fluor 488 conjugated secondary anti-rabbit or anti-mouse antibodies (Jackson Immunoresearch), depending on the primary antibody, for 1 hour at room temperature. Where tertiary amplification was required, biotin conjugated anti-rabbit or anti-mouse secondary antibodies (Jackson ImmunoResearch) were used followed by incubation with streptavidin cy3, for 1 hour at room temperature. Slides were mounted using antifade mounting medium (0.1M Tris pH 8.5, 20% glycerol, 8% polyvinyl alcohol, 2.5% 1,4-diazabicyclo[2.2.2]octane) containing the nuclear stain 4’,6-Diamidino-2-Phenylindole, dihydrochloride (DAPI), and visualized with a fluorescent microscope (Leica). Cell senescence was assayed by senescence activated β-galactosidase staining (SA β-gal) using the β-gal staining kit (Invitrogen) with PBS buffer adjusted to pH 6.0 (Lee et al., 2006; Sabatino et al., 2015). Where SA β-gal was combined with antibody staining, the SA β-gal was performed first followed by DAB immunostaining. TUNEL staining was performed to assess apoptotic cell death using the fluorescein in situ cell death detection kit (Roche) according to the manufacturer’s instructions, and co-stained with DAPI to visualize cell nuclei. All IHC experiments were performed with control slides incubated without primary antibody, or enzyme for TUNEL and SA β-gal staining. No positive signal was detected on these controls.
Table 2.
Antibodies used for immunohistochemistry.
| Antibody | Dilution | Host | Source | Secondary | Tertiary | Detection |
|---|---|---|---|---|---|---|
| SOX9 | 1:2000 | Rabbit | Millipore | αRabbit 488 1:200 | N/A | Fluorescent |
| pH3 | 1:1000 | Rabbit | Millipore | αRabbit biotin 1:250 | Strep HRP | DAB |
| PIT1 | 1:1000 | Rabbit | Dr. Simon Rhodes | αRabbit 488 1:200 | N/A | Fluorescent |
| p21 | 1:500 | Mouse | Pharmingen | Mouse biotin 1:200 | Strep cy3 1:200 | Fluorescent |
| LHβ | 1:100 | Rabbit | National Hormone and Peptide Program | αRabbit biotin 1:250 | Strep HRP | DAB |
| TSHβ | 1:100 | Rabbit | National Hormone and Peptide Program | αRabbit biotin 1:250 | Strep HRP | DAB |
| GH | 1:100 | Rabbit | National Hormone and Peptide Program | αRabbit biotin 1:250 | Strep HRP | DAB |
| ACTH | 1:100 | Rabbit | DAKO | αRabbit biotin 1:250 | Strep HRP | DAB |
2.5. Quantification of IHC, Sβ-gal and TUNEL Staining
To quantify positively stained cells, 3-4 individual pituitary explants from 3 separate cultures were imaged at 40x magnification. The total number positive cells were quantified by cell counting using ImageJ software (NIH). One slide containing 2, 12 μm sections of the each pituitary gland was used to obtain two images (each half of the anterior lobe) from which cell counts were averaged for each n quantified. The number of positive cells was normalized to total area counted (pixels2 × 100,000). The average area counted for each slide was 1.8 × 106 pixels2. DAPI positive nuclei were quantified for multiple sections in each treatment group to ensure that the total cell number was not affected by genistein treatment.
2.6. Statistical analysis
All data are presented as mean +/− SEM. Statistical significance was determined using one-way ANOVA followed by Dunnett’s post-hoc test comparing treatments to a control group. P values less than 0.05 were considered significantly different from control values. All analyses were performed using GraphPad Prism 8.2.1.
3. Results
3.1. Genistein Mediates Estrogen Receptor Signaling in the Pituitary Gland
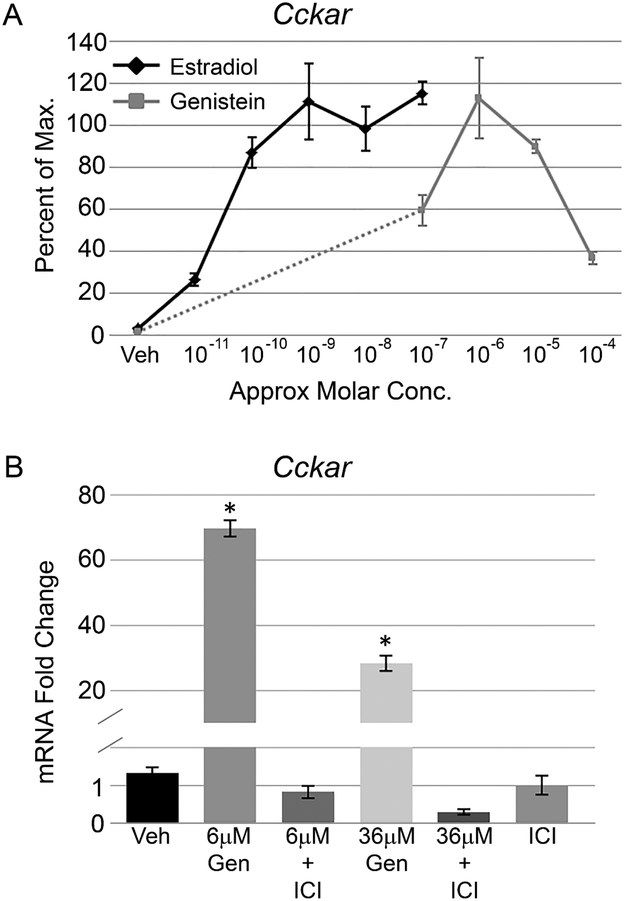
First we assessed the ability of genistein to activate estrogen receptor (ER) mediated transcription in the pituitary gland. Mouse pituitary explants were treated with genistein ranging in concentration from 0.06 μM to 36 μM. Equal numbers of male and female pituitary glands were pooled for each treatment, and no sex specific effects were seen in any of our experiments. We analyzed mRNA levels of cholecystokinin A receptor (Cckar), a gene which is strongly induced in the pituitary by estrogen via the ERα isoform (Kim et al., 2007). Cckar mRNA is robustly induced by genistein in a dose dependent manner, reaching peak levels at 0.6 μM (Figure 1 A). At 36 μM genistein, Cckar mRNA is still induced relative to vehicle, but to a lesser extent. We compared Cckar mRNA induction by genistein to a Cckar dose response curve with 17β estradiol treatment (E2) (E2 data from (Weis and Raetzman, 2016)). While both ligands increase Cckar gene expression to similar levels, genistein requires approximately 1000 fold higher concentration to reach maximal Cckar mRNA compared to E2. To confirm that the induction of Cckar by genistein is ER mediated, we co-treated cultured pituitaries with genistein and the ER antagonist ICI 182,780 (ICI). Cckar mRNA induced by either 6 μM or 36 μM genistein is completely blocked by the co-application of ICI, while ICI alone has no effect on Cckar levels (Figure 1B). These data indicate that genistein is an ERα agonist in the pituitary gland, but with substantially less potency than E2.
Figure1: Genistein can activate ERα regulated gene expression in the pituitary gland.
A) Cholecystokinin A receptor (Cckar) mRNA levels were measured by qPCR over a range of estradiol (E2) and genistein concentrations in cultured pituitary glands. Both E2 and genistein induce Cckar transcript to similar peak levels, but approximately 1000 fold more genistein is required for maximal Cckar activation relative to E2. Dashed line indicates genistein concentrations not tested. The graph represents the mean +/− SEM for 5-14 pituitaries per treatment. B) Pituitary explants were treated with 6 μM and 36 μM genistein alone or in combination with the antiestrogen ICI 182,780 (ICI). ICI completely antagonizes the genistein mediated induction of Cckar mRNA at both concentrations and ICI alone has no effect of Cckar levels. The graph represents the mean +/− SEM for 5-14 pituitaries per treatment. One way ANOVA P<0.0001, *P<0.05 by Dunnett’s post-hoc test.
3.2. Pituitary Cell Proliferation and Cell Cycle Progression Is Impaired by 36 μM Genistein
Genistein can be both pro- and anti-proliferative, depending on context. We examined the impact of genistein treatment on pituitary explant proliferation using 6 μM or 36 μM genistein doses. To monitor proliferation in the pituitary, we first measured mRNA levels for the active cell cycle gene mKi67 following genistein treatment. At 6 μM, genistein has no effect on mKi67 levels; however, 36 μM genistein reduces mKi67 mRNA by more than 60% relative to vehicle treated samples (Figure 2A). Moreover, co-treatment with the antiestrogen ICI does not reverse the anti-proliferative actions of 36 μM genistein, while ICI alone has no effect on mKi67 mRNA. These results suggest that 36 μM genistein restrains proliferation in the pituitary gland, independent of genistein’s ability to signal through ER. As another measure of proliferation we performed immunohistochemistry (IHC) for phospho-Flistone H3 (pH3) protein in pituitaries following vehicle, 6 μM genistein or 36 μM genistein application. Positive pH3 staining is indicative of actively mitotic cells and the pattern of staining can designate a particular phase of the cell cycle (Eguren et al., 2013). Vehicle and 6 μM genistein treated pituitary glands have pH3 positive cells throughout the anterior lobe (Figure 2B and C) with cells both in M phase (black arrows) as well as G2 phase (white arrows) of the cell cycle. We observe less overall pH3 immunostaining in 36 μM genistein treated pituitaries (Figure 2D), and most pH3 positive cells show a G2 phase pattern (white arrows). Quantification of pH3 positive cells demonstrates a significant decrease in cells exhibiting M phase morphology as well as the total number of pH3 stained cells in 36 μM genistein treated pituitaries (Figure 2E). Together, the decrease in mKi67 mRNA and pH3 protein suggests that 36 μM genistein might be interfering with cell cycle progression in the pituitary. To explore this further, we examined mRNA levels of cyclin genes, to see if genistein treatment directly impacts components of the cell cycle. The genes Cncd2 (CyclinD2) and Cncb1 (Cyclin B1) encode proteins that act at specific transitions in the cell cycle: G1/S for Cyclin D2 and G2/M for Cyclin B1 (Hirai et al., 1995; Kawamoto et al., 1997). Relative to vehicle treated pituitaries, 6 μM genistein causes a slight, but significant decrease in both Cncd2 and Cncb1 mRNA levels (Figure 2F). Interestingly, the higher dose of genistein, 36 μM, does not affect Cncd2 mRNA, but decreases Cncb1 mRNA 80% relative to vehicle control. Together these findings show that genistein halts proliferation and impairs cell cycle progress.
Figure 2: Genistein inhibits pituitary cell proliferation and cell cycle progression at 36 μM.
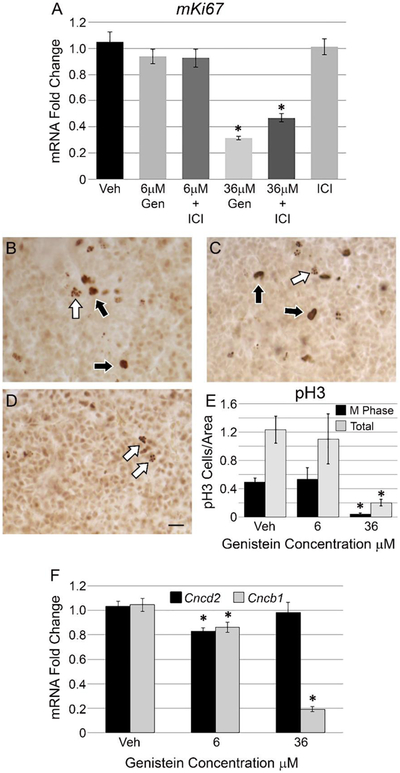
A) Transcript levels of the cell cycle marker mKi67 were measured by qPCR following genistein treatment in pituitary explants. Levels of mKi67 mRNA are not changed by 6 μM genistein, but 36 μM significantly represses mKi67 expression. Co-treatment with the antiestrogen ICI 182,780 (ICI) fails to reverse the 36 μM mediated downregulation of mKi67 mRNA, and ICI alone has no effect on mKi67. The graph represents the mean +/− SEM for 5-9 pituitaries per treatment. One way ANOVA P<0.0001, *P<0.05 by Dunnett’s post-hoc test. Immunohistochemistry (IHC) for phospho-histone H3 (pH3) was performed on pituitary explants following vehicle or genistein treatment at 6 μM and 36 μM. B) Vehicle and C) 6 μM genistein treated pituitaries show numerous pH3 positive cells in the anterior lobe displaying M phase pattern of staining (black arrows) as well as G2 pattern of staining (white arrows). D) In 36 μM genistein treated pituitary glands, there is far less pH3 detection in the anterior lobe and most cells exhibit the G2 phase pattern of staining (white arrows). E) Quantification of pH3 positive cells for vehicle, 6 μM and 36 μM genistein treated pituitary explants, showing a significant decrease in the number of cells with M phase staining and total pH3 positive cells in 36 μM genistein treated pituitaries relative to vehicle. Representative images for pH3 IHC, scale=50 μm, The graph represents the mean +/− SEM for 3 pituitaries per treatment. One way ANOVA for M phase cells P=0.02, one way ANOVA for total cells P=0.04. *P<0.05 by Dunnett’s post-hoc test. F) mRNA for Ccnd2 and Ccnb1 were assayed by qPCR in pituitary explants following genistein treatment. 36 μM genistein potently represses mRNA for Ccnb1, but has no effect on Ccnd2 levels. Both Ccnd2 and Ccnb1 show a slight but significant decrease in mRNA following 6 μM genistein treatment. The graph represents the mean +/− SEM for 9-15 pituitaries per treatment. One way ANOVA for Ccnd2, P=0.02, One way ANOVA for Ccnb1 P<0.0001, *P<0.05 by Dunnett’s post-hoc test.
3.3. Genistein Induces the Cyclin Dependent Kinase Inhibitor p21, Independently of p53 in the Pituitary
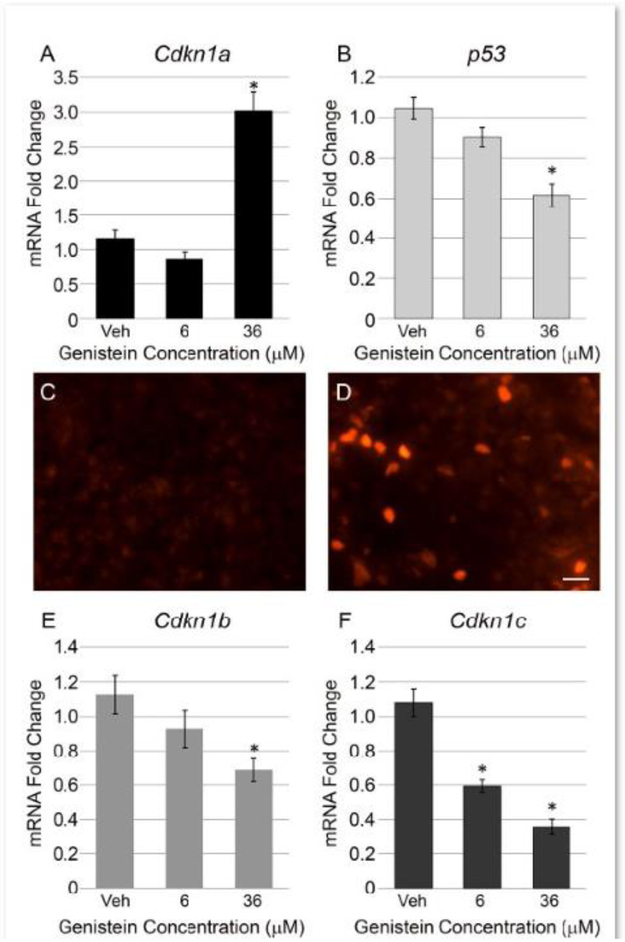
Because genistein appears to intervene in the cell cycle, we investigated its impact on other cell cycle effectors in the pituitary gland, namely, cyclin dependent kinase inhibitors (CDKIs). We first assessed the levels of Cdkn1a (p21) mRNA in pituitary explants treated with 6 μM and 36 μM genistein. Cdkn1a is an important regulator of pituitary proliferation and can serve as a predictor of cell death or senescence (Chesnokova et al., 2008; Kang et al., 1999; McConnell et al., 1998; Qiao et al., 2002). While 6 μM genistein does not alter Cdkn1a mRNA relative to vehicle control, 36 μM genistein increases Cdkn1a mRNA 3 fold over control levels (Figure 3A). An increase in Cdkn1a/p21 can often result from activation by the tumor protein p53 in many cell and tissue contexts including the pituitary gland (Chesnokova et al., 2013; Macleod et al., 1995). Therefore, we examined p53 mRNA levels in response to genistein treatment. Interestingly, p53 mRNA is significantly decreased by 36 μM genistein in pituitary explants (Figure 3B) suggesting that the genistein induction of Cdkn1a gene expression is not dependent upon p53 upregulation. Since mRNA for Cdkn1a is increased by genistein, we investigated whether p21 protein is also induced in the pituitary cultures. IHC with p21 antibody shows virtually no positive immunostaining for p21 in the anterior lobe cells of vehicle treated pituitary explants (Figure 3C). Upon treatment with 36 μM genistein, a strong induction of cells expressing p21 protein can be seen in the anterior pituitary (Figure 3D). There are a small number of p21 positive nuclei in the posterior lobe of both vehicle and 36 μM treated pituitary glands (data not shown) which would account for the basal levels of Cdkn1a mRNA seen in qPCR for vehicle treated explants. Finally, we examined the levels of two additional cyclin dependent kinase inhibitors, Cdkn1b (p27) and Cdkn1c (p57). In contrast to Cdkn1a, mRNA for Cdkn1b is significantly repressed by 36 μM genistein (Figure 3E); moreover, Cdkn1c mRNA levels are significantly reduced by both 6 μM and 36 μM genistein in a dose dependent manner (Figure 3F). These data demonstrate that genistein can differentially impact cyclin dependent kinase inhibitors in the pituitary gland, and strongly induces Cdkn1a/p21.
Figure 3: Genistein differentially impacts cyclin dependent kinase inhibitors in the neonatal pituitary, strongly inducing Cdkn1a/p21.
A) Cyclin dependent kinase inhibitor Cdkn1a mRNA was measured by qPCR in pituitary explant cultures following 6 μM and 36 μM genistein treatment. The 36 μM genistein treatment strongly induces Cdkn1a mRNA relative to vehicle and 6 μM genistein. The graph represents the mean +/− SEM for 10-15 pituitaries per treatment. One way ANOVA P<0.0001, *P<0.05 by Dunnett’s post-hoc test. B) Tumor protein p53 mRNA is significantly downregulated by 36 μM genistein in the pituitary gland. The graph represents the mean +/− SEM for 9-15 pituitaries per treatment. One way ANOVA P<0.0001, *P<0.05 by Dunnett’s post-hoc test. Immunohistochemistry (IHC) for p21 shows a noticeable increase in positively stained cells in 36 μM genistein treated pituitary anterior lobe (D) relative to vehicle treatment (C). Representative images of IHC for 3 pituitaries per sample, scale=50 μm. mRNA levels of the cyclin dependent kinase inhibitors Cdkn1b and Cdkn1c were assessed by qPCR. Pituitary explants treated with 36 μM genistein exhibit a significant decrease in Cdkn1b mRNA (E); while, both 6 μM and 36 μM genistein downregulate mRNA for Cdkn1c in the pituitary gland (F). The graph represents the mean +/− SEM for 10-15 pituitaries per treatment. One way ANOVA for Cdkn1b, P=0.02, One way ANOVA for Cdkn1c P<0.0001, *P<0.05 by Dunnett’s post-hoc test.
3.4. Genistein Reduces mRNA for Bcl2 and increases Bax/Bcl2 Ratio in the Pituitary Gland
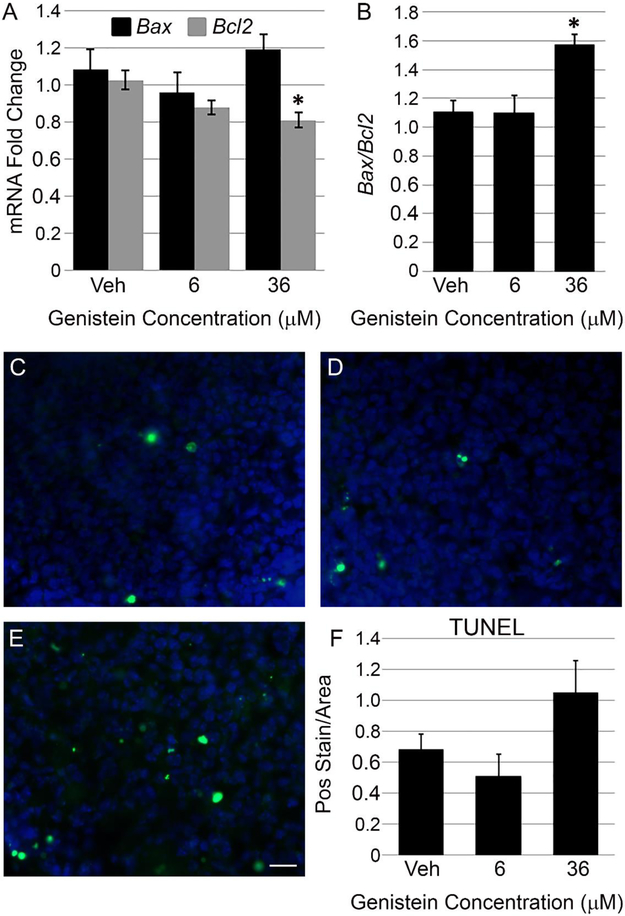
The potent upregulation of Cdkn1a/p21 that we observed following 36 μM genistein treatment lead us to question if genistein might activate cell death machinery in the pituitary gland. We initially looked at genes involved in apoptotic cell death, specifically the pro-apoptotic gene Bax and the anti-apoptotic gene Bcl2. In pituitary explants treated with vehicle, 6 μM genistein or 36 μM genistein we see no difference in Bax mRNA (Figure 4A) However, 36 μM genistein is able to significantly reduce mRNA for the cell protective gene Bcl2 relative to vehicle (Figure 4A) and increase the Bax/Bcl2 ratio (Figure 2B) compared to control pituitaries. We further assessed apoptosis in genistein treated pituitary cultures by TUNEL staining for DNA fragmentation. Whereas vehicle and 6 μM treated pituitary sections have similar staining by the TUNEL assay in the anterior lobe parenchyma (Figure 4C and D, the 36 μM genistein treatment shows a noticeable, but not statistically significant, increase in TUNEL staining relative to vehicle or the 6 μM dose (Figure 4E and F). These data show that 36 μM genistein potentially increases pro-apoptotic conditions in the pituitary gland.
Figure 4: Bcl2 mRNA is downregulated by genistein in the pituitary gland.
A) Levels of mRNA for the pro-apoptotic gene Bax and the anti-apoptotic gene Bcl2 were measured by qPCR in pituitary explant cultures following 6 μM and 36 μM genistein treatment. Genistein does not affect Bax mRNA levels, but both 6 μM and 36 μM genistein significantly reduce Bcl2 mRNA relative to vehicle control. The graph represents mean +/− SEM for 8-13 pituitaries per treatment. One way ANOVA for Bcl2 P=0.006, *P<0.05 by Dunnett’s post-hoc test. B) Bax/Bcl2 mRNA ratio of 36 μM genistein treated pituitaries is significantly increased relative to vehicle and 6 μM genistein treated cultures. The graph represents mean +/− SEM for 8-13 pituitaries per treatment. One way ANOVA P=0.01, *P<0.05 by Dunnett’s post-hoc test. TUNEL assay was performed on pituitary explants to monitor apoptosis following genistein exposure. Vehicle (C) and 6 μM genistein (D) treated pituitaries exhibit similar levels of DNA fragmented ends (green speckles) in the anterior lobe. Whereas, 36 μM genistein treated pituitary glands (E) show a noticeable increase in TUNEL staining relative to vehicle or 6 μM genistein. Cell nuclei are visualized with DAPI (blue stain). Representative images of TUNEL staining, scale=50 μm. F) Quantification shows increase in TUNEL staining of 36 μM genistein treated pituitaries is not significantly different from vehicle. Graph represents mean +/− SEM for 3 pituitaries per sample, One way ANOVA P=0.12.
3.5. Genistein Induces Cellular Senescence in the Pituitary Gland
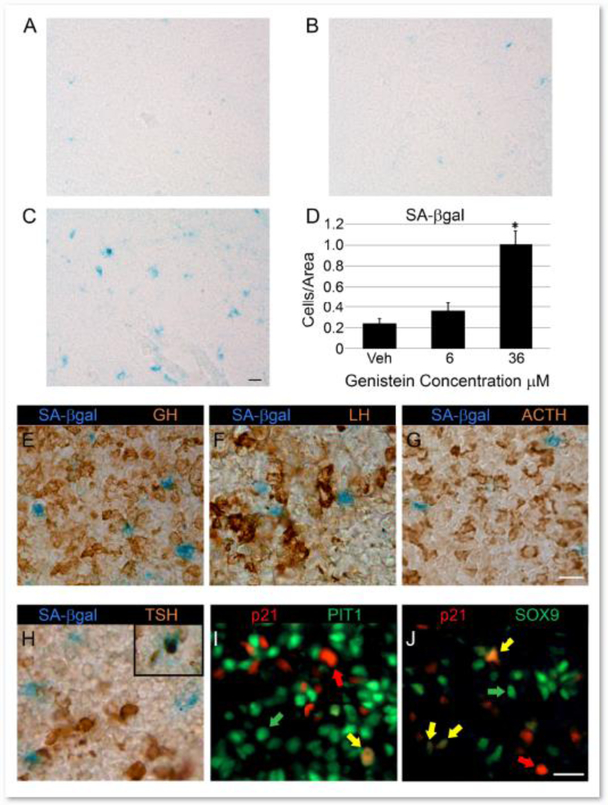
While it often precedes the initiation of apoptosis, an induction of Cdkn1a/p21 can also indicate the permanent exit of cells from the cell cycle or cellular senescence. We investigated if senescence was increased in pituitary explants following genistein exposure by performing senescence activated β-galactosidase (SA β-gal) staining. Few positively stained, blue cells can be seen in in the anterior lobe of vehicle and 6 μM genistein treated pituitaries (Figure 5A and B, respectively). Strikingly, we observe a potent increase in SA β-gal positive cells when pituitaries were treated with 36 μM genistein, indicative of an increase in senescence (Figure 5C,D). To determine which pituitary cell types are becoming senescent with genistein exposure, we co-stained 36 μM genistein treated pituitary explants with SA β-gal and a complement of pituitary hormone antibodies: growth hormone (GH), luteinizing hormone (LHβ), adrenocorticotropic hormone (ACTH) and thyroid stimulating hormone (TSHβ) (Figure 5E, F, G and H respectively). The vast majority of senescent cells throughout the pituitary anterior lobe fail to co-localize to hormone producing cells. We rarely find isolated co-stained cells for SA β-gal and TSHβ (inset Figure 5H). We were unable to perform IHC for lactotrope cells since prolactin (PRL) is not abundantly expressed at this age. We next examined cells in the pituitary which are positive for PIT1, a lineage specification transcription factor for somatotrope, thyrotrope and lactotrope cells. We performed co-immunostaining for PIT1 and p21 which we see induced in 36 μM genistein treated pituitary cells. The majority of PIT1 positive cells in the pituitary anterior lobe (Figure 5I, green arrows) fail to co-localize with p21 (Figure 5I, red arrows). However, we were able to identify cells that do co-immunostain for PIT1 and p21 (Figure 5I yellow arrows). Following IHC quantification we found PIT1/p21 positive cells represented 13.1% (SEM +/− 3.4) of the total population of p21 positive cells visualized in 36 μM treated pituitary explants. To examine pituitary progenitor cells, we co-immunostained p21 with antibody against the stem cell marker SOX9. While the majority of the SOX9 positive cells (Figure 5J, green arrow) fail to double-stain with p21 positive cells (Figure 5J, red arrow), we are able to identify a number of SOX9/p21 positive cells (Figure 5J, yellow arrows) in the anterior lobe parenchyma. SOX9/p21 IHC was quantified and we found that 43.7% (SEM +/−3.5) of p21 expressing cells were also positive for SOX9 in 36 μM treated pituitary glands. These results suggest that some pituitary progenitor cells might be more likely to undergo cell senescence than the mature hormone secreting cells.
Figure 5: Pituitary cell senescence is induced by 36 μM genistein, predominantly in non-differentiated cells.
Senescence was assessed in the anterior lobes of pituitaries cultured with 6 μM and 36 μM genistein by senescence activated β-galactosidase (SA β-gal) staining. Vehicle and 6 μM genistein dosed pituitary explants have a few SA β-gal stained cells scattered throughout the gland (blue staining in A and B, respectively). However, pituitary glands treated with 36 μM genistein show numerous SA β-gal positive cells (C). D) Quantification of SA β-gal shows significant increase in senescent cells in 36 μM treated pituitary explants relative to vehicle controls. Representative images for SA β-gal, scale=50 μm. Graph represents mean +/− SEM for 4 pituitaries per sample. One way ANOVA P<0.0005, *P<0.05 by Dunnett’s post-hoc test. SA β-gal staining was carried out along with immunohistochemistry (IHC) for hormone antibodies against growth hormone (GH), luteinizing hormone (LHβ), adrenocorticotropic hormone (ACTH) and thyroid stimulating hormone (TSHβ) (E, F, G and H respectively) in pituitary explants cultured with 36 μM genistein. The absence of SA β-gal and hormone antibody co-staining indicates mature hormone expressing cells are not becoming senescent following genistein exposure. We see only rare SA β-gal/TSHβ co-positive cells (inset panel H). IHC was performed for p21 and PIT1 expressing cells (I) in 36 μM genistein treated pituitary glands. Anterior lobe sections show p21/PIT double stained cells (yellow arrow) relative to cells expressing only p21 (red arrow) or PIT1 (green arrow). We further examined p21 expression in SOX9 positive pituitary progenitors (J) in 36 μM genistein treated explants. We also see a p21/SOX9 co-stained progenitor cells (yellow arrows) in the pituitary anterior lobe relative to cells expressing p21 (red arrow) or SOX9 alone (green arrow) Representative images for SA β-gal and/or IHC, 3 pituitaries per sample, scale=50 μm.
4. Discussion
In this study we investigated the impacts of the soy isoflavone genistein in the context of the developing pituitary gland using whole organ pituitary explants. We demonstrated that genistein acts as an estrogen receptor (ER) α agonist in the pituitary based on its ability of induce the ERα regulated gene Cckar. Separate from its ER modulating function, we observed severely reduced cell proliferation in 36 μM genistein treated pituitary glands, and cell cycle impairment as seen by a reduction in mRNA of the cyclin gene Ccnb1 and pH3 immunostaining. We observed a coincident increase in the cyclin dependent kinase inhibitor (CDKI) Cdkn1a/p21 following pituitary genistein exposure at 36 μM as well as a decrease in Bcl-2 mRNA. Most surprisingly, we saw a robust onset of senescence in the pituitary gland after 36 μM genistein treatment. Notably, although 6 μM genistein did not cause overt changes in proliferation, cell death or senescence, we found decreases in cell cyclins Cncb1 and Cncd2 as well as Cdkn1c mRNA levels. Together our data reveal alterations in pituitary gene expression occurring at concentrations of genistein ranging from 0.06 μM to 36 μM. These doses are highly relevant to human exposures with the 6 μM concentration correlating with serum genistein levels measured in infants consuming a soy-formula diet (Badger et al., 2002; Barlow et al., 2007; Cao et al., 2009; Setchell et al., 1998). These results highlight impacts of early life exposure to soy-based diets during a sensitive window of pituitary gland development.
It has long been known that genistein acts as a selective estrogen receptor modulator (SERM) in multiple reproductively important tissues such as uterus, breast and endometrium (Carter et al., 1953; Heikaus et al., 2002; Hopert et al., 1998; Hsieh et al., 1998; Makela et al., 1994; Martin et al., 1978; Noteboom and Gorski, 1963; Santell et al., 1997). Less is known about genistein’s ability to regulate transcriptional activity in the ER positive cells of the intact pituitary gland, especially during development. We were able to demonstrate that genistein can induce mRNA of Cckar, an ERα regulated gene, maximally at 0.6 μM. The fact that genistein can activate ER signaling in the neonatal pituitary gland is significant as this is a critical developmental window when the pituitary gland is susceptible to endocrine disrupting chemical (EDC) exposures that could alter the course of pituitary cell differentiation (Eckstrum et al., 2018, 2016; Leffers et al., 2006). Compared to the potent ER agonist E2, approximately 1000 fold more genistein was required for maximal Cckar induction in the pituitary gland. This is consistent with published relative ER binding affinities for genistein which are 100 to 5000 fold less than E2 (Jiang et al., 2013; Kuiper et al., 1998; Stahl et al., 1998). However, the reduced potency of genistein relative to E2 does not preclude its ability to function as a powerful EDC in the pituitary. The concentration range that induced maximal estrogenic activity in the pituitary (0.06 μM to 6 μM) correlates with reported serum genistein measurements in humans and infants consuming soy foods or formula (Jefferson and Williams, 2011). Beyond its ability to mediate transcription though ERα, genistein could also engage signaling via ERβ or the G-coupled protein estrogen receptor (GPER, GPR30, (Hazell et al., 2009)), possibly with even higher efficacy. Therefore we cannot discount that genistein might facilitate endocrine disruption of the neonatal pituitary gland using multiple ER isoforms or non-genomic ER pathways.
Genistein has been shown to be both pro-proliferative and anti-proliferative depending on cell/tissue type and exposure levels. In the context of the mouse pituitary gland we found 36 μM genistein to be strongly anti-proliferative based on its ability to decrease mKi67 mRNA and pH3 protein staining. We did not observe any increase in proliferation in lower concentrations of genistein down to 0.06 μM (data not shown). This inhibition of pituitary cell proliferation by 36 μM genistein appears to be ERα and ERβ independent based on the failure of the antiestrogen ICI to fully restore mKi67 RNA levels. However, we cannot rule out non-genomic ER, such as GPER, as a mechanism genistein utilizes to attenuate proliferation. Consistent with our data, high concentrations of genistein (10-36 μM) have exhibited anti-proliferative properties in ER positive and negative breast cancer cell lines (Cappelletti et al., 2000; Hsieh et al., 1998; Peterson and Barnes, 1991; Wang et al., 1996), cultured ovarian antral follicles (Patel et al., 2016) and prostate cancer cell lines (Mahmoud et al., 2013). In vivo, neonatal mice injected with 100 mg/kg genistein for 10 days showed decreased ovary and uterine cell proliferation (Wu et al., 2018). While we do not know the mechanism by which 36 μM genistein inhibits pituitary cell proliferation, others have suggested that genistein’s anti-proliferative capacity at high concentration is due to its inherent tyrosine kinase inhibitory activity (Akiyama et al., 1987; Peterson and Barnes, 1991). Tyrosine kinases regulate a number of proteins that could alter cell division, including epidermal growth factor (EGF), protein kinase B (PKB), ERK/MAPK, vascular endothelial growth factor receptor (VEGFR) and others (Mahmoud et al., 2014; Yu et al., 2012). However, genistein can orchestrate a host of different mechanisms that might affect cell proliferation. For instance, genistein has been demonstrated to regulate mitosis via IGF-1, TGF-β and Wnt/β-catenin signaling pathways (Lee et al., 2012; Liss et al., 2010; Yu et al., 2005). We conducted a similar study on another plant isoflavone, isoliquiritigenin (ISL), in pituitary explant cultures, and found potent repression of pituitary cell proliferation at 200 μM ISL (Weis and Raetzman, 2016). It is not known if ISL and genistein limit pituitary proliferation by similar means, but interestingly, ISL has been shown to suppress VEGF/VEGFR2 signaling (Wang et al., 2013) and reduce ERK/MAPK phosphorylation (Jung et al., 2014; Wu et al., 2015), both of which are potential tyrosine kinase regulatory targets (Mahmoud et al., 2014).
Our study demonstrates that genistein appears to impair progress through the cell cycle in the neonatal pituitary. In addition to the overall reduction of pH3 staining in 36 μM genistein treated pituitary explants, we observed that the predominant pattern of pH3 staining was indicative of cells in the G2 phase of the cell cycle (Eguren et al., 2013). This suggests that 36 μM genistein prevents pituitary cell cycle progression beyond the G2/M transition. To lend support to this finding, we further found that genistein treatment of pituitary explants altered mRNA levels of cell cyclins. At 36 μM genistein mRNA for Cncd2 (Cyclin D2) was not affected in the pituitary; whereas, the same concentration repressed mRNA for Cncb1 (Cyclin B1). Cyclin D2 and Cyclin B1 aid progression through G1/S and G2/M transitions of the cell cycle respectively (Hirai et al., 1995; Kawamoto et al., 1997). The 80% repression of Cncb1 mRNA by 36 μM genistein, together with our pH3 data, support the conclusion that genistein likely halts pituitary cells at the G2/M transition of the cell cycle. Interestingly, we also observed a slight but significant decrease in mRNA levels for both Cncd2 and Cncb1 following 6 μM genistein treatment in the pituitary gland. This could indicate some interference with cell cycle progression by genistein even at a concentration where we do not observe any decrease in cell proliferation.
In the pituitary gland the Cip and Kip family CDKIs, Cdkn1a (p21), Cdkn1b (p27) and Cdkn1c (p57), serve critical functions in normal cell differentiation and tumor suppression, and generally function as inhibitors to cell cyclin/CDKs (Bilodeau et al., 2009; Chen et al., 1995; García-Fernández et al., 2011; Philipp-Staheli et al., 2004; Quereda and Malumbres, 2009; Xiong et al., 1993). In our study we found that 36 μM genistein induced mRNA for Cdkn1a and p21 protein relative to vehicle treated pituitaries. This increase in p21 is consistent with downregulation of Cncb1 as p21 can function as an inhibitory checkpoint of multiple cyclins/CDKs including cyclin B1. Conversely, genistein at 36 μM had a repressive effect on mRNA levels for Cdkn1b and Cdkn1c. In fact Cdkn1c mRNA was significantly decreased by 6 μM genistein as well. This differential effect on the pituitary CDKIs serves to highlight their distinct roles in the gland. In addition to serving as tumor suppressors, the CDKIs p27 and p57 are known to function in the developing pituitary gland to arrest proliferation of progenitor cells and allow for terminal differentiation of endocrine cells to occur (Bilodeau et al., 2009; Monahan et al., 2012). The repression of Cdkn1b mRNA by 36 μM genistein and Cdkn1c mRNA by both 6 μM and 36 μM genistein could indicate inappropriate exit of pituitary progenitor cells from the cell cycle, suggesting that genistein might dysregulate the differentiation process. On the other hand Cdkn1a/p21 is emerging as a pivotal repressor of pituitary cell proliferation as we observed with genistein, or in similar experiments with the plant compound ISL (Weis and Raetzman, 2016). These data suggest that p21 could be a primary effector used by flavonoids to restrain proliferation in the pituitary gland.
As a cell protective mechanism especially in tumor contexts, p21 induction can cause cells to adopt a senescent state or to initiate apoptosis (Chesnokova et al., 2008; Kang et al., 1999; McConnell et al., 1998; Qiao et al., 2002). In neonatal pituitary explant cultures, we found that 36 μM genistein was able to decrease mRNA of the cell protective gene Bcl-2 at 36 μM. This resulted in an increased Bax/Bcl2 ratio in 36 μM genistein treated pituitaries relative to vehicle treatment. An increased Bax/Bcl2 ratio can indicate initiation of apoptosis in cells. While we did not see a significant increase in TUNEL staining of the 36 μM genistein treated explants following the 48 hour duration of our culture period, the downregulation of Bcl2 mRNA could promote apoptotic cell death, possibly given more time. The apoptotic properties of genistein are well documented in the literature, and are generally observed at concentrations >10 μM (Chen et al., 2015; Kabała-Dzik et al., 2018; Yanagihara et al., 1993; Zhou et al., 2017). In a number of studies genistein has been shown to decrease mRNA and protein levels of Bcl-2 in cells treated with doses ranging from 15 μM to 120 μM (Constantinou et al., 1998; Li et al., 1999; Su et al., 2003; Yu et al., 2004). However when examining effects on both mRNA and protein, other researchers have seen no change in Bcl-2 levels following genistein treatment, inductions of Bcl-2 or effects on other Bcl family members such as the pro-apoptotic Bax gene (Chi et al., 2018; Leung and Wang, 2000; Li et al., 1999; Tophkhane et al., 2007). It would seem that the mechanism by which genistein initiates apoptosis can vary by cell or tissue type, but in the pituitary it appears that 36 μM genistein might create an environment conducive to cell death by downregulation of Bcl-2. This differs from what we observed with the plant flavonoid ISL where we found 200 μM ISL increased Bax mRNA with no change in Bcl-2 transcript leading to a potent increase in apoptosis (Weis and Raetzman, 2016).
Increased expression of p21 can also precede permanent exit of cells from the cell cycle or cellular senescence (Chesnokova et al., 2008; McConnell et al., 1998). Senescent cells cease to proliferate, but remain viable and metabolically active (Campisi, 2013), and activation of cellular senescence has long been thought to be a protective mechanism whereby excessively mitotic cells adopt a senescent state to avoid becoming tumors (Campisi, 2005; Chesnokova et al., 2010; Collado et al., 2005; Sabatino et al., 2015). We found that 36 μM genistein treatment of pituitary explant cultures induced senescence in anterior lobe cells. This is the first study we know of linking genistein exposure to initiation of senescence in the developing pituitary gland. Cellular senescence can be triggered by a host of different stimuli such as the expression of oncogenic proteins (Dhomen et al., 2009; Di Micco et al., 2006), DNA damage (Bartkova et al., 2006), or chemotoxic agents (Chang et al., 2002; Novakova et al., 2010). In the pituitary, p21 initiated senescence can occur by numerous mechanisms (Chesnokova et al., 2010), and in one example, cellular senescence prevents the activation of apoptosis through growth hormone upregulation and is believed to halt benign pituitary tumors from becoming carcinomas (Chesnokova et al., 2013). In this process p21 induction is dependent on activation of p53. However, p21 can also mediate cellular senescence independently of p53 (Biggs et al., 1996; Datto et al., 1995; Macleod et al., 1995; Nakano et al., 1997). In our neonatal pituitary explants, onset of senescence was not dependent on p53 upregulation and was likely mediated solely by p21 induction. We observed that mature hormone expressing cells do not appear to be undergoing senescence based on the failure of hormone antibodies to co-stain with SA β-gal in genistein treated pituitary explants. However 43.7% of p21 expressing cells, induced by 36 μM genistein were also SOX9 positive and 13.1% of p21 cells also expressed PIT1. This supports the conclusion that 36 μM genistein-mediated senescence may be occurring in a population of pituitary progenitors, or in the case of PIT1, cells differentiating into somatotropes, lactotropes or thyrotropes. Importantly, the ability of genistein to induce senescence is somewhat specific because, in related experiments with the plant isoflavone ISL, we found that 200 μM ISL inhibited pituitary cell proliferation primarily though initiation of apoptosis, and not senescence (Weis and Raetzman, 2016).
The onset of cell senescence by 36 μM genistein could have some severe consequences in the immature pituitary gland. Senescent cells can activate the senescence associated secretory phenotype (SASP) whereby immune factors are secreted from affected cells with the goal of repairing cellular damage (Campisi, 2013; Coppé et al., 2010). While the SASP can be a protective response to counter genotoxic damage, SASP is a double edged sword which can lead to negative changes in the cellular chemoenvironment (Campisi, 2013; Coppé et al., 2010; Gonzalez-Meljem et al., 2017). In fact, recent studies in the pituitary gland have shown that the activation of SASP in senescent pituitary cells can actually lead to the development of a particularly devastating pediatric pituitary tumor, human adamantinomatous craniopharyngioma (ACP)(Gonzalez-Meljem et al., 2017; Gonzalez-Meljem and Martinez-Barbera, 2018). SASP develops slowly and can take 5 days or more to appear (Coppé et al., 2010). Due to the 48 hour duration of genistein treatment in our assays, we likely are not seeing SASP activation. However, the severity of the SASP has been shown to be increased by downregulation or inactivation of p53 and upregulation of RAS (Coppé et al., 2008), and we observed a significant downregulation of p53 mRNA by 36 μM genistein in our pituitary cultures. This suggests it is possible that genistein could elicit a SASP in the pituitary gland given more time.
Ultimately our study indicates that high levels of genistein exposure to the immature pituitary gland could have some detrimental developmental impacts, including limiting proliferation during a significant period of pituitary expansion and differentiation. Furthermore, the onset of senescence and the potential of the SASP could alter the chemical milieu in the pituitary gland, and in the worst case, possibly foster the formation of pituitary tumors such as ACP that are most prevalent in children. These results suggest the need for moderation in consumption of soy foods and especially soy infant formula during early childhood development. However, there are limitations to the study. These data only examine direct effects of genistein on the pituitary in vitro. In vivo, the regulation of pituitary proliferation may be masked or increased, depending on the contribution of other circulating factors. Examining the impact of neonatal genistein exposure in vivo would be a future direction. Additionally, the dose of genistein is an important consideration as there are most certainly distinct effects at different doses. Caution should be taken to avoid generalizing results of one dose to potential effects that might occur due to soy consumption.
Highlights.
Genistein inhibits proliferation in the neonatal mouse pituitary
Genistein induces the cell cycle inhibitor CDKN1A in the neonatal pituitary
Genistein triggers cellular senescence in the neonatal pituitary
Acknowledgements
This work was supported by the National Institutes of Health P50 AT006268 (NCCIH) and R01 DK 076647 (NIDDK).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y, 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem 262, 5592–5. [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG, 2001. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 61, 5045–50. [PubMed] [Google Scholar]
- Asa SL, Ezzat S, 2002. The pathogenesis of pituitary tumours. Nat. Rev. Cancer 2, 836–849. doi: 10.1038/nrc926 [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJJ, Hakkak R, Rowlands JC, Korourian S, 2002. The Health Consequences of Early Soy Consumption. J. Nutr 132, 559S–565S. doi: 10.1093/jn/132.3.559S [DOI] [PubMed] [Google Scholar]
- Barlow J, Johnson JAP, Scofield L, 2007. Early Life Exposure to the Phytoestrogen Genistein and Breast Cancer Risk in Later Years. Fact Sheet on the Phytoestrogen Geinistein. [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou L-VF, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Ørntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG, 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637. doi: 10.1038/nature05268 [DOI] [PubMed] [Google Scholar]
- Biggs JR, Kudlow JE, Kraft AS, 1996. The role of the transcription factor Sp1 in regulating the expression of the WAF1/CIP1 gene in U937 leukemic cells. J. Biol. Chem 271, 901–6. [DOI] [PubMed] [Google Scholar]
- Bilodeau S, Roussel-Gervais A, Drouin J, 2009. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol. Cell. Biol 29, 1895–908. doi: 10.1128/MCB.01885-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT, 2012. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol. Reprod 87, 82. doi: 10.1095/biolreprod.112.100636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, 2013. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol 75, 685–705. doi: 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, 2005. Suppressing cancer: the importance of being senescent. Science 309, 886–7. doi: 10.1126/science.1116801 [DOI] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ, 2009. Isoflavones in urine, saliva and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J. Expo. Sci. Environ. Epidemiol 19, 223–234. doi: 10.1038/jes.2008.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti V, Fioravanti L, Miodini P, Di Fronzo G, 2000. Genistein blocks breast cancer cells in the G(2)M phase of the cell cycle. J. Cell. Biochem 79, 594–600. [PubMed] [Google Scholar]
- Carter MW, Smart WW, Matrone G, 1953. Estimation of estrogenic activity of genistein obtained from soybean meal. Proc. Soc. Exp. Biol. Med 84, 506–8. [DOI] [PubMed] [Google Scholar]
- Chang B-D, Swift ME, Shen M, Fang J, Broude EV, Roninson IB, 2002. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl. Acad. Sci. U. S. A 99, 389–94. doi: 10.1073/pnas.012602599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Charn TH, Park S-H, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS, 2008. Estrogen Receptors á and β as Determinants of Gene Expression: Influence of Ligand, Dose, and Chromatin Binding. Mol. Endocrinol 22, 1032–1043. doi: 10.1210/me.2007-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jackson PK, Kirschner MW, Dutta A, 1995. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374, 386–8. doi: 10.1038/374386a0 [DOI] [PubMed] [Google Scholar]
- Chen Jun, Duan Y, Zhang X, Ye Y, Ge B, Chen Jian, 2015. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 6, 995–1000. doi: 10.1039/C4FO01141D [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Zhou C, Ben-Shlomo A, Zonis S, Tani Y, Ren S-G, Melmed S, 2013. Growth hormone is a cellular senescence target in pituitary and nonpituitary cells. Proc. Natl. Acad. Sci. U. S. A 110, E3331–9. doi: 10.1073/pnas.1310589110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Ben-Shlomo A, Wawrowsky K, Melmed S, 2010. Molecular mechanisms of pituitary adenoma senescence. Front. Horm. Res 38, 7–14. doi: 10.1159/000318489 [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, Melmed S, 2008. p21(Cipl) restrains pituitary tumor growth. Proc. Natl. Acad. Sci. U. S. A 105, 17498–503. doi: 10.1073/pnas.0804810105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Rubinek T, Yu R, Ben-Shlomo A, Kovacs K, Wawrowsky K, Melmed S, 2007. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 67, 10564–72. doi: 10.1158/0008-5472.CAN-07-0974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X-X, Zhang T, Chu X-L, ZFIEN J-L, ZFIANG D-J, 2018. The regulatory effect of Genistein on granulosa cell in ovary of rat with PCOS through Bcl-2 and Bax signaling pathways. J. Vet. Med. Sci 80, 1348–1355. doi: 10.1292/jvms,17-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimafranca MA, Davila J, Ekman GC, Andrews RN, Neese SL, Peretz J, Woodling KA, Helferich WG, Sarkar J, Flaws JA, Schantz SL, Doerge DR, Cooke PS, 2010. Acute and Chronic Effects of Oral Genistein Administration in Neonatal Micel. Biol. Reprod 83, 114–121. doi: 10.1095/biolreprod.109.080549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M, 2005. Tumour biology: senescence in premalignant tumours. Nature 436, 642. doi: 10.1038/436642a [DOI] [PubMed] [Google Scholar]
- Constantinou A., Kamath N, Murley J., 1998. Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur. J. Cancer 34, 1927–1934. doi: 10.1016/S0959-8049(98)00198-l [DOI] [PubMed] [Google Scholar]
- Coppe J-P, Desprez P-Y, Krtolica A, Campisi J, 2010. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis 5, 99–118. doi: 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe J-P, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez P-Y, Campisi J, 2008. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 6, e301. doi: 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Yu Y, Wang XF, 1995. Functional analysis of the transforming growth factor beta responsive elements in the WAFl/Cipl/p21 promoter. J. Biol. Chem 270, 28623–8. [DOI] [PubMed] [Google Scholar]
- Davis SW, Keisler JL, Perez-Millan MI, Schade V, Camper SA, 2016. All Flormone-Producing Cell Types of the Pituitary Intermediate and Anterior Lobes Derive From Propl -Expressing Progenitors. Endocrinology 157, 1385–1396. doi: 10.1210/en.2015-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R, 2009. Oncogenic Braf Induces Melanocyte Senescence and Melanoma in Mice. Cancer Cell 15, 294–303. doi: 10.1016/j.ccr.2009.02.022 [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre’ M, Giovanni Nuciforo P, Bensimon A, Maestro R, Giuseppe Pelicci P, d’Adda di Fagagna F, 2006. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642. doi: 10.1038/nature05327 [DOI] [PubMed] [Google Scholar]
- Eckert RL, Katzenellenbogen BS, 1981. Human endometrial cells in primary tissue culture: modulation of the progesterone receptor level by natural and synthetic estrogens in vitro. J. Clin. Endocrinol. Metab 52, 699–708. doi: 10.1210/jcem-52-4-699 [DOI] [PubMed] [Google Scholar]
- Eckstrum KS, Edwards W, Banerjee A, Wang W, Flaws JA, Katzenellenbogen JA, Kim SH, Raetzman LT, 2018. Effects of Exposure to the Endocrine-Disrupting Chemical Bisphenol A During Critical Windows of Murine Pituitary Development. Endocrinology 159, 119–131. doi: 10.1210/en.2017-00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrum KS, Weis KE, Baur NG, Yoshihara Y, Raetzman LT, 2016. Icam5 Expression Exhibits Sex Differences in the Neonatal Pituitary and Is Regulated by Estradiol and Bisphenol A. Endocrinology 157, 1408–20. doi: 10.1210/en.2015-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards W, Raetzman LT, 2018. Complex integration of intrinsic and peripheral signaling is required for pituitary gland developmentt. Biol. Reprod 99, 504–513. doi: 10.1093/biolre/ioy081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguren M, Porlan E, Manchado E, Garcia-Higuera I, Cafiamero M, Farinas I, Malumbres M, 2013. The APC/C cofactor Cdhl prevents replicative stress and p53-dependent cell death in neural progenitors. Nat. Commun 4. doi: 10.1038/ncomms3880 [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson ICAF, 2008. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. U. S. A 105, 2907–12. doi: 10.1073/pnas.0707886105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez RA, Garcia-Palencia P, Sanchez MA, Gil-Gomez G, Sanchez B, Rollan E, Martin-Caballero J, Flores JM, 2011. Combined loss of p21(wafl/cipl) and p27(kipl) enhances tumorigenesis in mice. Lab. Invest 91, 1634–42. doi: 10.1038/labinvest.2011.133 [DOI] [PubMed] [Google Scholar]
- Goldberg LB, Aujla PK, Raetzman LT, 2011. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev. Biol 358, 23–32. doi: 10.1016/j.ydbio.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Madak-Erdogan Z, Li J, Cheng J, Greenlief CM, Helferich W, Katzenellenbogen JA, Katzenellenbogen BS, 2014. Transcriptomic Analysis Identifies Gene Networks Regulated by Estrogen Receptor α (ERα) and ERβ that Control Distinct Effects of Different Botanical Estrogens. Nucl. Recept. Signal 12, nrs.12001. doi: 10.1621/nrs.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meljem JM, Haston S, Carreno G, Apps JR, Pozzi S, Stache C, Kaushal G, Virasami A, Panousopoulos L, Mousavy-Gharavy SN, Guerrero A, Rashid M, Jani N, Goding CR, Jacques TS, Adams DJ, Gil J, Andoniadou CL, Martinez-Barbera JP, 2017. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat. Commun 8, 1819. doi: 10.1038/s41467-017-01992-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meljem JM, Martinez-Barbera JP, 2018. Senescence drives non-cell autonomous tumorigenesis in the pituitary gland. Mol. Cell. Oncol 5, 00–00. doi: 10.1080/23723556.2018.1435180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O’Carroll A-M, Lolait SJ, 2009. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol 202, 223–36. doi: 10.1677/JOE-09-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikaus S, Winterhager E, Traub O, Grümmer R, 2002. Responsiveness of endometrial genes Connexin26, Connexin43, C3 and clusterin to primary estrogen, selective estrogen receptor modulators, phyto- and xenoestrogens. J. Mol. Endocrinol 29, 239–49. [DOI] [PubMed] [Google Scholar]
- Hernández LM, Lee PDK, Camacho-Hübner C, 2007. Isolated growth hormone deficiency. Pituitary 10, 351–357. doi: 10.1007/s11102-007-0073-3 [DOI] [PubMed] [Google Scholar]
- Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ, 1995. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell. Biol 15, 2672–81. doi: 10.1128/MCB.15.5.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopert AC, Beyer A, Frank K, Strunck E, Wünsche W, Vollmer G, 1998. Characterization of estrogenicity of phytoestrogens in an endometrial-derived experimental model. Environ. Health Perspect 106, 581–586. doi: 10.1289/ehp.98106581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y-C, Peng S-F, Lai K-C, Liao C-L, Huang Y-P, Lin C-C, Lin M-L, Liu K-C, Tsai C-C, Ma Y-S, Chung J-G, 2019. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol doi: 10.1002/tox.22698 [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Santell RC, Haslam SZ, Helferich WG, 1998. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 58, 3833–8. [PubMed] [Google Scholar]
- Jayakody SA, Andoniadou CL, Gaston-Massuet C, Signore M, Cariboni A, Bouloux PM, Le Tissier P, Pevny LH, Dattani MT, Martinez-Barbera JP, 2012. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J. Clin. Invest 122, 3635–3646. doi: 10.1172/JCI64311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR, 2007. Disruption of the developing female reproductive system by phytoestrogens: Genistein as an example. Mol. Nutr. Food Res 51, 832–844. doi: 10.1002/mnfr.200600258 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR, 2005. Adverse Effects on Female Development and Reproduction in CD-1 Mice Following Neonatal Exposure to the Phytoestrogen Genistein at Environmentally Relevant Doses. Biol. Reprod 73, 798–806. doi: 10.1095/biolreprod.105.041277 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Williams CJ, 2011. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod. Toxicol 31, 272–9. doi: 10.1016/j.reprotox.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, Khan I, Smillie TJ, Chittiboyina AG, Rotte SCK, Helferich WG, Katzenellenbogen JA, Katzenellenbogen BS, 2013. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 27, 4406–18. doi: 10.1096/fj.13-234617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG, 2001. Physiological Concentrations of Dietary Genistein Dose-Dependently Stimulate Growth of Estrogen-Dependent Human Breast Cancer (MCF-7) Tumors Implanted in Athymic Nude Mice. J. Nutr 131, 2957–2962. doi: 10.1093/jn/131.11.2957 [DOI] [PubMed] [Google Scholar]
- Jung SK, Lee M-H, Lim DY, Kim JE, Singh P, Lee S-Y, Jeong C-H, Lim T-G, Chen H, Chi Y-I, Kundu JK, Lee NH, Lee CC, Cho Y-Y, Bode AM, Lee KW, Dong Z, 2014. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem 289, 35839–48. doi: 10.1074/jbc.M114.585513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Iriti M, Wojtyczka RD, Buszman E, Stojko J, 2018. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 - a comparative study. Cell. Mol. Biol. (Noisy-le-grand) 64, 1–10. [PubMed] [Google Scholar]
- Kang KH, Kim WH, Choi KH, 1999. p21 Promotes Ceramide-Induced Apoptosis and Antagonizes the Antideath Effect of Bcl-2 in Human Hepatocarcinoma Cells. Exp. Cell Res 253, 403–412. doi: 10.1006/EXCR.1999.4644 [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Koizumi H, Uchikoshi T, 1997. Expression of the G2-M checkpoint regulators cyclin B1 and cdc2 in nonmalignant and malignant human breast lesions: immunocytochemical and quantitative image analyses. Am. J. Pathol 150, 15–23. [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF, 1984. Corticosteroid Inhibition of ACTH Secretion*. Endocr. Rev 5, 1–24. doi: 10.1210/edrv-5-1-1 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Gieske MC, Hudgins S, Kim BG, Krust A, Chambon P, Ko C, 2007. Estrogen receptor alpha-induced cholecystokinin type A receptor expression in the female mouse pituitary. J. Endocrinol 195, 393–405. doi: 10.1677/JOE-07-0358 [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson J-Å, 1998. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 139, 4252–4263. doi: 10.1210/endo.139.10.6216 [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES, 2006. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5, 187–95. doi: 10.1111/j.1474-9726.2006.00199.x [DOI] [PubMed] [Google Scholar]
- Lee J, Ju J, Park S, Hong SJ, Yoon S, 2012. Inhibition of IGF-1 Signaling by Genistein: Modulation of E-Cadherin Expression and Downregulation of β-Catenin Signaling in Hormone Refractory PC-3 Prostate Cancer Cells. Nutr. Cancer 64, 153–162. doi: 10.1080/01635581.2012.630161 [DOI] [PubMed] [Google Scholar]
- Leffers H, Navarro VM, Nielsen JE, Mayen A, Pinilla L, Dalgaard M, Malagon MM, Castaño JP, Skakkebaek NE, Aguilar E, Tena-Sempere M, 2006. Increased expression of α- and β-globin mRNAs at the pituitary following exposure to estrogen during the critical period of neonatal sex differentiation in the rat. J. Steroid Biochem. Mol. Biol 99, 33–43. doi: 10.1016/J.JSBMB.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Leung LK, Wang TT, 2000. Bcl-2 Is Not Reduced in the Death of MCF-7 Cells at Low Genistein Concentration. J. Nutr 130, 2922–2926. doi: 10.1093/jn/130.12.2922 [DOI] [PubMed] [Google Scholar]
- Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO, 2013. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 12, 9. doi: 10.1186/1476-4598-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Upadhyay S, Bhuiyan M, Sarkar FH, 1999. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 18, 3166–3172. doi: 10.1038/sj.onc.1202650 [DOI] [PubMed] [Google Scholar]
- Liss MA, Schlicht M, Kahler A, Fitzgerald R, Thomassi T, Degueme A, Hessner M, Datta MW, 2010. Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics 7, 245–52. [PubMed] [Google Scholar]
- Lloyd HM, Meares JD, Jacobi J, 1975. Effects of oestrogen and bromocryptine on in vivo secretion and mitosis in prolactin cells. Nature 255, 497–8. [DOI] [PubMed] [Google Scholar]
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T, 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9, 935–944. doi: 10.1101/gad.9.8.935 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S, 2004. The G Protein-coupled Receptor GPR30 Mediates c- fos Up-regulation by 17β-Estradiol and Phytoestrogens in Breast Cancer Cells. J. Biol. Chem 279, 27008–27016. doi: 10.1074/jbc.M403588200 [DOI] [PubMed] [Google Scholar]
- Mahmoud AM, Yang W, Bosland MC, 2014. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol 140, 116–132. doi: 10.1016/J.JSBMB.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AM, Zhu T, Parray A, Siddique HR, Yang W, Saleem M, Bosland MC, 2013. Differential Effects of Genistein on Prostate Cancer Cells Depend on Mutational Status of the Androgen Receptor. PLoS One 8, e78479. doi: 10.1371/journal.pone.0078479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela S, Davis V, Tally W, Korkman J, Salo L, Vihko R, Santti R, Korach K, 1994. Dietary Estrogens Act through Estrogen Receptor-Mediated Processes and Show No Antiestrogenicity in Cultured Breast Cancer Cells. Environ. Health Perspect 102, 572–578. doi: 10.1289/ehp.94102572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Horwitz KB, Ryan Dale S, McGUIRE WL, 1978. Phytoestrogen Interaction with Estrogen Receptors in Human Breast Cancer Cells*. Endocrinology 103, 1860–1867. doi: 10.1210/endo-103-5-1860 [DOI] [PubMed] [Google Scholar]
- McCarver G, Bhatia J, Chambers C, Clarke R, Foster W, Hoyer P, Etzel R, Leeder SJ, Peters J, Rissman E, Rybak M, Sherman C, Toppari J, Turner K, 2010. NTP-CERHR MONOGRAPH ON SOY INFANT FORMULA Center for the Evaluation of Risks to Human Reproduction.
- McConnell BB, Starborg M, Brookes S, Peters G, 1998. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr. Biol 8, 351–354. doi: 10.1016/S0960-9822(98)70137-X [DOI] [PubMed] [Google Scholar]
- Medigović I, Ristić N, Trifunović S, Manojlović-Stojanoski M, Milošević V, Žikić D, Nestorović N, 2012. Genistein affects ovarian folliculogenesis: A stereological study. Microsc. Res. Tech 75, 1691–1699. doi: 10.1002/jemt.22117 [DOI] [PubMed] [Google Scholar]
- Melmed S, 1990. Acromegaly. N. Engl. J. Med 322, 966–977. doi: 10.1056/NEJM199004053221405 [DOI] [PubMed] [Google Scholar]
- Milkovic S, Milkovic K, Paunovic J, 1973. The Initiation of Fetal Adrenocorticotrophic Activity in the Rat1. Endocrinology 92, 380–384. doi: 10.1210/endo-92-2-380 [DOI] [PubMed] [Google Scholar]
- Monahan P, Himes AD, Parfieniuk A, Raetzman LT, 2012. p21, an important mediator of quiescence during pituitary tumor formation, is dispensable for normal pituitary development during embryogenesis. Mech. Dev 128, 640–52. doi: 10.1016/j.mod.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan P, Rybak S, Raetzman LT, 2009. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology 150, 4386–94. doi: 10.1210/en.2009-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y, Tokino T, Yamagishi H, Oka T, Nomura H, Sakai T, 1997. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J. Biol. Chem 272, 22199–206. [DOI] [PubMed] [Google Scholar]
- Nantie LB, Himes AD, Getz DR, Raetzman LT, 2014. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol. Endocrinol 28, 731–44. doi: 10.1210/me.2013-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A, 2004. Notch Activation Induces Endothelial Cell Cycle Arrest and Participates in Contact Inhibition: Role of p21Cip1 Repression. Mol. Cell. Biol 24, 8813–8822. doi: 10.1128/MCB.24.20.8813-8822.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteboom WD, Gorski J, 1963. Estrogenic Effect of Genistein and Coumestrol Diacetate. Endocrinology 73, 736–739. doi: 10.1210/endo-73-6-736 [DOI] [PubMed] [Google Scholar]
- Novakova Z, Hubackova S, Kosar M, Janderova-Rossmeislova L, Dobrovolna J, Vasicova P, Vancurova M, Horejsi Z, Hozak P, Bartek J, Hodny Z, 2010. Cytokine expression and signaling in drug-induced cellular senescence. Oncogene 29, 273–284. doi: 10.1038/onc.2009.318 [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Spritzer P, Poy M, Coronel AX, Dahlem N, Moraes JT, Barbosa-Coutinho LM, 1993. Progestin effects on prolactin secretion and on immunoreactive prolactin cells in estradiol-treated ovariectomized rats. Horm. Metab. Res. = Horm. und Stoffwechselforsch. = Horm. métabolisme 25, 600–2. doi: 10.1055/s-2007-1002187 [DOI] [PubMed] [Google Scholar]
- Oseni T, Patel R, Pyle J, Jordan V, 2008. Selective Estrogen Receptor Modulators and Phytoestrogens. Planta Med. 74, 1656–1665. doi: 10.1055/s-0028-1088304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hartman JA, Helferich WG, Flaws JA, 2017. Preconception exposure to dietary levels of genistein affects female reproductive outcomes. Reprod. Toxicol 74, 174–180. doi: 10.1016/j.reprotox.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Peretz J, Pan Y-X, Helferich WG, Flaws JA, 2016. Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol. Appl. Pharmacol 293, 53–62. doi: 10.1016/j.taap.2015.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G, Barnes S, 1991. Genistein inhibition of the growth of human breast cancer cells: Independence from estrogen receptors and the multi-drug resistance gene. Biochem. Biophys. Res. Commun 179, 661–667. doi: 10.1016/0006-291X(91)91423-A [DOI] [PubMed] [Google Scholar]
- Philipp-Staheli J, Kim K-H, Liggitt D, Gurley KE, Longton G, Kemp CJ, 2004. Distinct roles for p53, p27Kip1, and p21Cip1 during tumor development. Oncogene 23, 905–13. doi: 10.1038/sj.onc.1207220 [DOI] [PubMed] [Google Scholar]
- Pointis G, Mahoudeau JA, 1976. Release of immuno-reactive and biologically active LH from fetal mouse pituitary in response to synthetic gonadotropin releasing factor (LRF). Experientia 32, 1347–8. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M, 2011. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol 7, 715–26. doi: 10.1038/nrendo.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, McKinstry R, Gupta S, Gilfor D, Windle JJ, Hylemon PB, Grant S, Fisher PB, Dent P, 2002. Cyclin kinase inhibitor p21 potentiates bile acid-induced apoptosis in hepatocytes that is dependent on p53. Hepatology 36, 39–48. doi: 10.1053/JHEP.2002.33899 [DOI] [PubMed] [Google Scholar]
- Quereda V, Malumbres M, 2009. Cell cycle control of pituitary development and disease. J. Mol. Endocrinol 42, 75–86. doi: 10.1677/JME-08-0146 [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ, 2004. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev. Biol 265, 329–40. [DOI] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, Thomas JA, Umbach D, 2006. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res. Part B Dev. Reprod. Toxicol 77, 485–638. doi: 10.1002/bdrb.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino ME, Petiti JP, Sosa LDV, Pérez PA, Gutiérrez S, Leimgruber C, Latini A, Torres AI, De Paul AL, 2015. Evidence of cellular senescence during the development of estrogen-induced pituitary tumors. Endocr. Relat. Cancer 22, 299–317. doi: 10.1530/ERC-14-0333 [DOI] [PubMed] [Google Scholar]
- Sánchez-Criado JE, Martín De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A, 2004. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology 79, 247–58. doi: 10.1159/000079100 [DOI] [PubMed] [Google Scholar]
- Santell RC, Chang YC, Nair MG, Helferich WG, 1997. Dietary Genistein Exerts Estrogenic Effects upon the Uterus, Mammary Gland and the Hypothalamic/Pituitary Axis in Rats. J. Nutr 127, 263–269. doi: 10.1093/jn/127.2.263 [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE, 1998. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr 68, 1453S–1461S. doi: 10.1093/ajcn/68.6.1453S [DOI] [PubMed] [Google Scholar]
- Stahl S, Chun T-Y, Gray WG, 1998. Phytoestrogens Act as Estrogen Agonists in an Estrogen-Responsive Pituitary Cell Line. Toxicol. Appl. Pharmacol 152, 41–48. doi: 10.1006/TAAP.1998.8500 [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Lezmi S, Flaws JA, Schantz SL, Pan Y-X, Helferich WG, 2014. Genistein Exposure During the Early Postnatal Period Favors the Development of Obesity in Female, But Not Male Rats. Toxicol. Sci 138, 161–174. doi: 10.1093/toxsci/kft331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S-J, Chow N-H, Kung M-L, Hung T-C, Chang K-L, 2003. Effects of Soy Isoflavones on Apoptosis Induction and G2-M Arrest in Human Hepatoma Cells Involvement of Caspase-3 Activation, Bcl-2 and Bcl-XL Downregulation, and Cdc2 Kinase Activity. Nutr. Cancer 45, 113–123. doi: 10.1207/S15327914NC4501_13 [DOI] [PubMed] [Google Scholar]
- Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC, 2003. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol. Biomarkers Prev 12, 1213–21. [PubMed] [Google Scholar]
- Thorner M, Vance M, Laws E Jr, Horvath E, Kovacs K, 1998. The Anterio Pituitary, in: Wilson JD, Kronenberg HM, L.P.R. (Ed.), Williams Textbook of Endo-Crinology. W. B. Saunders, Philadelphia, PA, pp. 249–340. [Google Scholar]
- Tophkhane C, Yang S, Bales W, ARCHER L, OSUNKOYA A, THOR AD, YANG X, 2007. Bcl-2 overexpression sensitizes MCF-7 cells to genistein by multiple mechanisms. Int. J. Oncol 31, 867–874. [PubMed] [Google Scholar]
- Treier M, Rosenfeld MG, 1996. The hypothalamic-pituitary axis: co-development of two organs. Curr. Opin. Cell Biol 8, 833–43. [DOI] [PubMed] [Google Scholar]
- Varinska L, Gal P, Mojzisova G, Mirossay L, Mojzis J, 2015. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci 16, 11728–11749. doi: 10.3390/ijms160511728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM, 1996. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 17, 271–5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang N, Han S, Wang D, Mo S, Yu L, Huang H, Tsui K, Shen J, Chen J, 2013. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One 8, e68566. doi: 10.1371/journal.pone.0068566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis KE, Raetzman LT, 2016. Isoliquiritigenin exhibits anti-proliferative properties in the pituitary independent of estrogen receptor function. Toxicol. Appl. Pharmacol 313, 204–214. doi: 10.1016/j.taap.2016.09.027 [DOI] [PubMed] [Google Scholar]
- Wu G, Wei Q, Yu D, SHI F, 2018. Neonatal genistein exposure disrupts ovarian and uterine development in the mouse by inhibiting cellular proliferation. J. Reprod. Dev 2018–070. doi: 10.1262/jrd.2018-070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xue J, Yang Y, Zhu H, Chen F, Wang J, Lou G, Liu Y, Shi Y, Yu Y, Xia C, Hu Y, Chen Z, 2015. Isoliquiritigenin Inhibits Interferon-γ-Inducible Genes Expression in Hepatocytes through Down-Regulating Activation of JAK1/STAT1, IRF3/MyD88, ERK/MAPK, JNK/MAPK and PI3K/Akt Signaling Pathways. Cell. Physiol. Biochem 37, 501–14. doi: 10.1159/000430372 [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips III JA, Rosenfeld MG, 1998. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat. Genet 18, 147–149. doi: 10.1038/ng0298-147 [DOI] [PubMed] [Google Scholar]
- Wu YJ, Chen DW, Liu JL, Zhang JH, Luo HS, Cui S, 2008. Estradiol promotes pituitary cell proliferation and gonadotroph differentiation at different doses and with different mechanisms in chick embryo. Steroids 74, 441–8. doi: 10.1016/j.steroids.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D, 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704. doi: 10.1038/366701a0 [DOI] [PubMed] [Google Scholar]
- Yanagihara K, Ito A, Toge T, Numoto M, 1993. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 53, 5815–21. [PubMed] [Google Scholar]
- Yu X, Zhu J, Mi M, Chen W, Pan Q, Wei M, 2012. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med. Oncol 29, 349–357. doi: 10.1007/s12032-010-9770-2 [DOI] [PubMed] [Google Scholar]
- Yu Z, Li W, Liu F, 2004. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 215, 159–166. doi: 10.1016/J.CANLET.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Yu Z, Tang Y, Hu D, Li J, 2005. Inhibitory effect of genistein on mouse colon cancer MC-26 cells involved TGF-β1/Smad pathway. Biochem. Biophys. Res. Commun 333, 827–832. doi: 10.1016/j.bbrc.2005.05.177 [DOI] [PubMed] [Google Scholar]
- Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A, 2017. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 17, 813. doi: 10.1186/s12885-017-3829-9 [DOI] [PMC free article] [PubMed] [Google Scholar]