Abstract
BTBR T+ Itpr3tf/J (BTBR) mice are an Autism Spectrum Disorder (ASD)-like model that exhibit behavioral and physiological deficits similar to those observed in patients with ASD. While behavioral therapy is a first line of treatment in ASD patients, comparable non-pharmacological treatments are less explored in murine models. Here, we administer a bio-behavioral intervention for BTBR mice by way of environmental enrichment (EE) — an experimental housing paradigm previously shown to improve systemic metabolism, learning/memory, anxious behavior, neurogenesis, locomotion, and immunocompetence in C57BL/6 mice. Juvenile BTBR mice were randomized to standard or EE housing and were subjected to metabolic and behavioral assessments up to 17 weeks. Following EE exposure, we report an EE-induced metabolic and behavioral phenotype. Male BTBR mice responded metabolically to EE, displaying reduced adiposity, increased lean mass, improved glycemic control, and decreased circulating leptin. The gene expressions of brain-derived neurotrophic factor (Bdnf) and its receptor (Ntrk2/TrkB) were upregulated in several brain areas in EE-BTBR males. EE-BTBR females showed modest reduction of adiposity and no changes in glycemic control, circulating leptin, or Bdnf/Ntrk2 gene expression. With regard to behavior, EE resulted in decreased anxiety, and increased social affiliation. Together, these results suggest that EE improves metabolic and behavioral health in BTBR mice.
Keywords: BTBR mice, autism spectrum disorder, environmental enrichment, sociability, metabolism, BDNF, TrkB
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social interaction and repetitive behavior. The former is characterized by: (1) deficits in social-emotional reciprocity, (2) deficits in nonverbal communicative behaviors, and (3) deficits in developing, maintaining, and understanding relationships. The latter is characterized by: (1) stereotyped or repetitive motor movements, speech, or use of objects, (2) insistence on sameness, (3) highly restricted or fixated interests, and (4) hyper- or hypo-reactivity to sensory inputs (American Psychiatric Association, 2013). Epidemiological studies performed by the Centers for Disease Control and Prevention (CDC) conclude ASD presents with a frequency of 1 in every 59 children, with males four times more likely than females to be diagnosed with ASD (Baio et al., 2018). The etiology of the disorder remains a topic of debate—epigenetic, genetic and environmental factors are all thought to play a role (Meyza and Blanchard, 2017).
Some intervention treatments, administered both in-person and online, have shown the ability to ameliorate symptoms in ASD patients. In one experiment, personalized online therapy led to improvements in learning, memory, anxiety, attention span, motor skills, eating, sleeping, sensory processing, self-awareness, communication, social skills, and mood (Aronoff et al., 2016). Early intensive behavioral intervention (EIBI) administered to autistic children at a young age has been shown to improve social behavior (Waters et al., 2018). Another recent meta-analysis suggests that cognitive behavioral therapy (CBT) may be useful for reduction of anxiety in some ASD presentations (Perihan et al., 2019). These examples of human intervention successes underscore the potential benefit of developing analogous treatments in animal models to elucidate mechanisms underlying ASD treatments.
Our previous work has shown environmental enrichment (EE) leads to anti-obesity, anticancer, and anxiolytic phenotypes (Cao et al., 2011; Cao and During, 2012; Cao et al., 2010; McMurphy, 2018). EE mice reside in larger-than-standard cages, and are provided with abundant bedding, running wheels, mazes, toys, and shelters that are rearranged on a weekly basis. Our mechanistic studies have identified a specific brain-fat axis underlying the EE-induced anticancer and anti-obesity response—the hypothalamic-sympathoneural-adipocyte (HSA) axis (Cao et al., 2011; Cao et al., 2010). EE provides physical, social, sensory, and cognitive stimuli that result in upregulation of brain-derived neurotrophic factor (BDNF) within the hypothalamus, the upstream mediator in the brain, leading to an increase in sympathetic tone to the adipose tissue (Cao et al., 2011). The downstream mediators at the fat level are multiple: a reduced level of leptin contributing to the anticancer effect, an increased level of vascular endothelial growth factor (VEGF) contributing to beige cell induction and elevated energy expenditure resulting in leanness, and improved glycemic control and resistance to obesity (Cao et al., 2011; Cao et al., 2010; During et al., 2015; McMurphy, 2018). Moreover, we have recently demonstrated that EE modulates immune functions in models of cancer and autoimmune disease via hypothalamic BDNF (Cao et al., 2010; Xiao et al., 2019; Xiao et al., 2016). This enhanced immunocompetence and antitumor phenotype has been confirmed in tumor models of pancreatic, melanoma, colon, and breast cancer models (Li et al., 2015; Nachat-Kappes et al., 2012). While the anti-obesity and anticancer effects of EE have been well characterized in C57BL/6 mice, the potential for metabolic and behavioral benefits in EE are not well explored in one ASD-like murine model: the BTBR T+ Itpr3tf/J (BTBR) mouse.
BTBR mice exhibit behavioral and physiological deficits similar to those observed in patients with ASD. The BTBR model has disturbed endocrine function and was originally bred for studies on abdominal obesity, insulin resistance, diabetes-induced nephropathy, and phenylketonuria (Clee et al., 2005; Meyza and Blanchard, 2017). Over the past decade, studies have characterized the model as a consistent manifestation of ASD-like behavior as well (Meyza and Blanchard, 2017). BTBR mice display reduced social approach, low reciprocal social interactions, impaired juvenile play, repetitive behaviors, and communication deficits (McFarlane et al., 2008). This strain is also thought to be a model of generalized anxiety, and can be used to study psychiatric comorbidities present in ASD (Chao et al., 2018; Miller et al., 2010; Wang et al., 2018b). Administration of a high-fat diet has been shown to exacerbate cognitive rigidity and social deficiency in BTBR mice, suggesting a link between the aforementioned metabolic deficiencies and behavior (Zilkha et al., 2017).
Here, using behavioral and metabolic assays, we examine the efficacy of like-peer environment enrichment as a neurobehavioral intervention treatment for obesity, anxiety, low sociability, and repetitive behaviors in an ASD-like murine model: BTBR mice.
METHODS
To investigate whether our like-peer EE could alleviate the metabolic and behavioral deficits of BTBR mice, young (4–5 week old) male and female BTBR mice were randomized to live in SE (standard environment) or EE. Body weight and food intake were monitored weekly for 17 weeks. Mice were subject to behavioral and metabolic assessments throughout the 17-week housing period (Fig. 1A). Mice were subjected to housing conditions at a juvenile age (4–5 weeks old) to mimic early-age intervention treatments. Housing was continued for 17 weeks to carry intervention into adulthood. No more than one test was performed each week in an effort to minimize experimental stress of tests. Previous work by our lab (Cao et al., 2011) suggests that four weeks of housing is sufficient to elicit metabolic changes in C57BL/6 mice. No testing occurred in the first four weeks while mice acclimated to their environment.
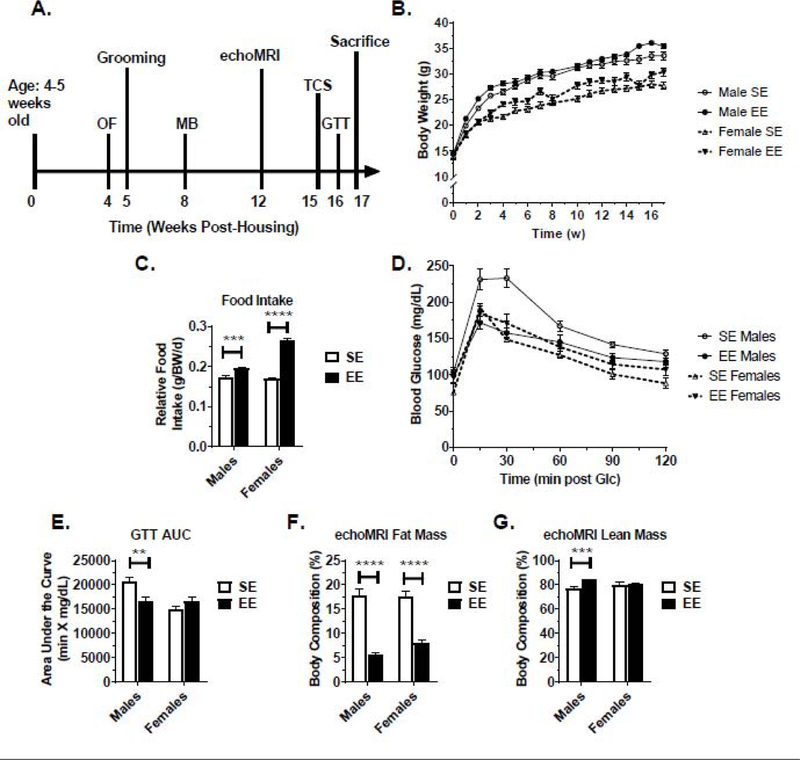
Figure 1.
EE-related metabolic changes in BTBR mice. (A) Experimental timeline. (B) Body weight. (C) Relative food intake. (D) Glucose tolerance test (GTT), performed 16 weeks post housing. (E) Area under the curve (AUC) of (D). (F) Percent fat mass, as measured echoMRI body composition assessment at 12 weeks post housing. (G) Percent lean mass, as measured by echoMRI. Data are means ± SEM. Males: n=8 SE, n=9 EE. Females: n=10 SE, n=9 EE. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
EE Protocol (Slater and Cao, 2015)
4–5 week old male and female BTBR T+ Itpr3tf/J (Jackson Laboratory #002282) were randomized to live in standard laboratory conditions (SE) or EE for 17 weeks. SE mice were group housed (3–5 mice) in standard laboratory environment cages (30.5 cm x 17 cm x 15 cm). EE mice were group housed (3–5 mice) in large cages (63 cm x 49 cm x 44 cm) supplemented with running wheels, igloos, toys, tunnels, a maze, and nesting material. All mice had ad libitium access to food (normal chow diet, 11% fat, caloric density 3.4 kcal/g, Teklad) and water. Mice were housed in temperature (22–23ºC) and humidity (30–70%) controlled rooms under a 12:12 light:dark cycle. All animal experiments were in accordance with the regulations of The Ohio State University Institutional Animal Care and Use Committee.
Open Field (OF) Test
At 4 weeks post-housing, mice were individually placed into the center of an open square arena (60cm x 60cm, enclosed by walls of 48 cm). Each mouse was allowed to explore the arena for 10 min, during which time and activity—in the center and the periphery of the OF—was recorded and analyzed via TopScan (Clever Sys, Inc.) software. Between each trial, the arena was cleaned with Opticide to remove odor cues.
Grooming Test (Kalueff et al., 2007)
At 5 weeks post-housing, mice were individually placed in a clear observation box (28 cm x 16 cm x 12 cm) and were video recorded for 10 min. The box itself functioned as a novelty stress to induce grooming, instead of misting the mouse with water, so that the behavior would reflect spontaneity. A blinded experimenter scored self-grooming as indicated by paw licking, nose/face/head wash, body grooming, leg licking, and/or tail/genital grooming in any order exceeding 1 second. The total number of grooming bouts and the average length of each bout for each mouse were measured.
Marble Burying (MB) Test
At 8 weeks post-housing, mice were individually placed into cages (30.5 cm x 17 cm x 15 cm) with evenly spaced glass marbles arranged in a three-by-four grid on the surface of clean aspen bedding (5 cm in depth). Cages were covered with plexiglass lids to prevent mouse escape. Mouse burying activity was video recorded for 30 min with an overhead camera. A blinded experimenter scored the latency to bury as well as the number of marbles buried. The marbles were washed with mild detergent and water between each trial.
Body Composition by EchoMRI
At 12 weeks post-housing, echoMRI was utilized to measure body composition of fat, lean, free water, and total water masses in live mice without anesthesia. Body composition analysis was performed with an echoMRI 3-in-1 Analyzer at the Small Animal Imaging Core of The Dorothy M. Davis Heart & Lung Research Institute, The Ohio State University.
Three-Chamber Sociability Test (Kaidanovich-Beilin et al., 2011)
At 15 weeks post-housing, mice were placed in an apparatus consisting of three connected plexiglass chambers (18 cm x 41 cm x 20 cm each) with removable dividers between each chamber. Each test subject was individually placed in the center plexiglass chamber for 5 minutes of habituation. In the first phase—testing social affiliation—another, unfamiliar mouse was placed in either the right or left chamber in a small wire cage, while another wire cage remained empty in the opposite chamber. The placement of the novel mouse in the right vs. left chamber was systematically alternated between each trial. The wire cage restricted social or aggressive interactions between the two mice beyond nose contact. Chamber dividers were lifted after the habituation period to allow the test subject to move freely about all three chambers for a 10-minute observation period. A second 10-minute test—assessing novel social engagement—was performed immediately afterward, using the conspecific from the first test (now denoted as a familiar mouse) and a novel unfamiliar mouse in the opposite chamber.
All trials were video recorded and a blinded experimenter analyzed for the time spent in each chamber and number of chamber entries. Between each trial, the arena was cleaned with Opticide to remove odor cues.
Glucose Tolerance Test (GTT)
At 16 weeks post-housing, mice were injected intraperitoneally with glucose solution (1.0 mg glucose per kg body weight) after a 16 h overnight fast. Blood was obtained from the tail at baseline, 15, 30, 60, 90, and 120 min after glucose injection. Blood glucose concentrations were measured with a portable glucose meter (Bayer Contour Next).
Tissue Collection
Following 17 weeks of housing conditions, mice were sacrificed. Mice were anesthetized with isoflurane and euthanized via decapitation or exsanguination cage-wise beginning at 0930 h. Tissues were flash frozen on dry ice and stored at −80°C until further analysis.
Quantitative Real-Time-PCR
Amygdala, hippocampus, and hypothalamus were dissected at sacrifice. Tissues were flash frozen on dry ice and stored at −80°C until further analysis. Following sonication, RNA was isolated using the QIAGEN RNeasy Mini kit with RNase-free DNase treatment. cDNA was reverse transcribed using Taqman Reverse Transcription Reagents (Applied Biosystems). qRT-PCR was completed on StepOnePlus Real-Time PCR System using Power SYBR Green (Applied Biosystems) PCR Master Mix. Primers are available upon request. We calibrated data to endogenous control Hprt1 and quantified the relative gene expression using the 2 −ΔΔCT method (Livak and Schmittgen, 2001).
Serum Harvest and Analysis
Trunk blood was collected at euthanasia and allowed to clot on ice for at least 30 min before centrifugation at 10,000 rpm for 10 min at 4°C. The serum component was collected and stored at −20°C until further analysis. DuoSet ELISA kits were used to assay serum leptin (R&D Systems #DY498) and adiponectin (R&D Systems #DY1119). Triglycerides were measured using a colorimetric assay kit (Cayman Chemical #10010303).
Statistical Analysis
Data are expressed as mean ± SEM. GraphPad Prism 7 software (GraphPad, La Jolla, CA) and SPSS Statistics v25.0.0.0 (IBM, Armonk, NY) were used to analyze our data. Student’s t-tests were performed for qPCR data. Two-way ANOVAs with repeated measures were used for timepoint measures (body weight and GTT) and the Three-Chamber Sociability test as previously published (Silverman et al., 2015), following the guideline that the center chamber be presented in the figures as a technical control, and is not included for the data analysis. Two-way ANOVAs were used to analyze all other data; the main and interaction effects are listed in Supplemental Tables 1 and 2. Following ANOVA, the Holm-Bonferonni post hoc test was used to adjust multiple comparisons; these results are denoted in the figures. Normality was tested using the Shapiro-Wilk method.
RESULTS
EE Exerts Metabolic Improvement in BTBR Mice in a Sex-Dependent Manner
Over the 17-week experiment, body weights were measured (Fig. 1B). A housing F(1,32)=7.86, P=0.0084) and sex (F(1,32)=88.38, P<0.0001) main effect were observed in body weight measurements, with no interaction (Table S1). Contrary to our previous work in C57BL/6 mice, EE-BTBR mice were observed to weigh more than their SE counterparts. Relative food intake was increased in EE conditions for both male and female cohorts (Fig. 1C, A housing (F(1,582)=155.4, P<0.0001) and sex (F(1,582)=46.25, P<0.0001) main effect were observed for relative food intake (Table S1). For a glucose tolerance test (GTT), a trending housing main effect (F(1,32)=3.597, P=0.0670) and a significant sex main effect (F(1,32)=22.55, P<0.0001) were observed across time course measurements (Fig. 1D and Table S1). Male, but not female, mice living in EE displayed improved glycemic control as measured by an area under the curve (AUC) calculation (Fig. 1E). No housing main effect was observed for the GTT AUC, but a sex main effect (F(1,32)=11.24, P=0.0021) was observed (Table S1). Despite increased food intake, male and female EE mice displayed decreased fat mass when measured with an echoMRI in vivo imager (Fig. 1F). For fat mass measurements, a housing (F(1,30)=128.4, P<0.0001), but no sex main effect was observed (Table S1). Lean mass, as measured by echoMRI, was increased following EE in males but not females (Fig. 1G). For lean mass measurements, a housing (F(1,30)=8.554, P=0.0065), but no sex effect was observed (Table S1).
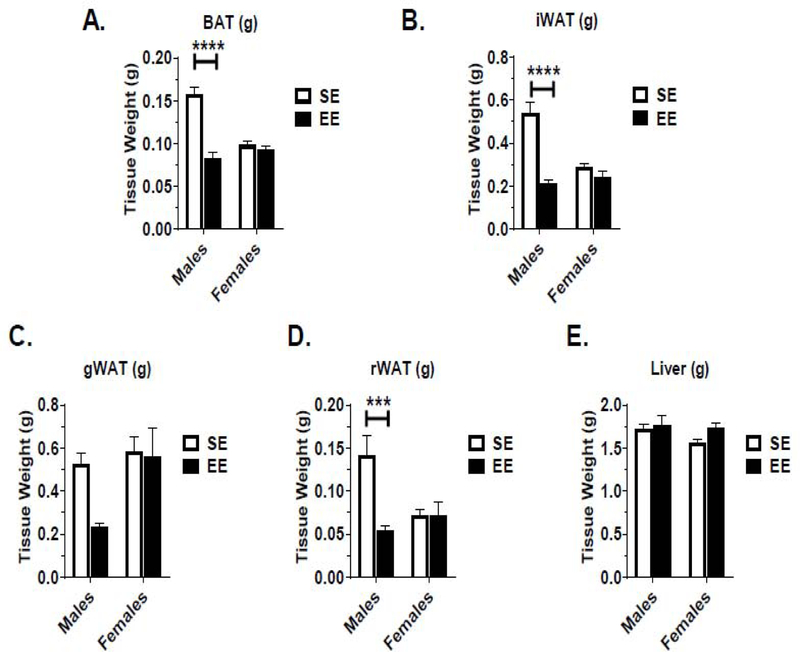
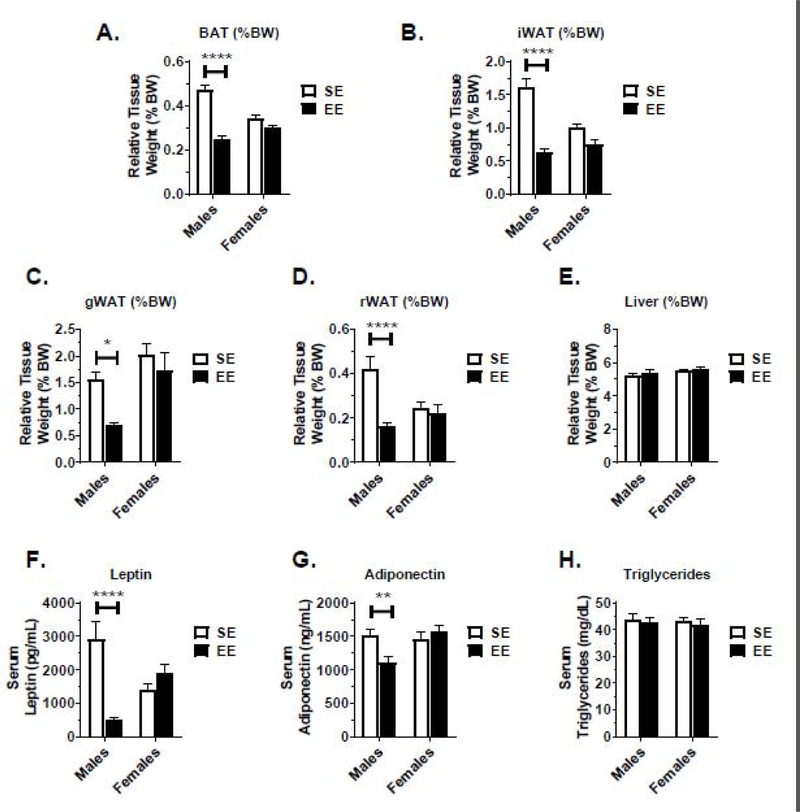
In vivo body composition observations were confirmed at sacrifice in gross tissue measurement (Fig. 2A–E) and when normalized (Fig. 3A–E) to body weight. Robust reductions in adiposity were observed in the EE male, but not female, brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), gonadal white adipose tissue (gWAT), and retroperitoneal adipose tissue (rWAT). No change in liver mass was observed in male or female EE mice.
Figure 2.
EE-related change in tissue weight, as measured at sacrifice. (A) Brown adipose tissue (BAT) weight. (B) Inguinal white adipose tissue (iWAT) weight. (C) Gonadal white adipose tissue (gWAT) weight. (D) Retroperitoneal white adipose tissue (rWAT) weight. (E) Liver tissue weight. Data are means ± SEM. Males: n=8 SE, n=9 EE. Females: n=10 SE, n=9 EE. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
Figure 3.
EE-related change in relative tissue weight, as measured at sacrifice, and serum profiling. (A) Brown adipose tissue (BAT) relative weight. (B) Inguinal white adipose tissue (iWAT) relative weight. (C) Gonadal white adipose tissue (gWAT) relative weight. (D) Retroperitoneal white adipose tissue (rWAT) relative weight. (E) Liver tissue relative weight. (H) Serum leptin at sacrifice. (I) Serum adiponectin at sacrifice. (J) Serum triglycerides at sacrifice. Data are means ± SEM. Males: n=8 SE, n=9 EE. Females: n=10 SE, n=9 EE. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
Serum profiling revealed sexually dimorphic adipokine profiles. Male EE mice displayed an 82.9% drop in serum leptin (Fig. 3F) and a 27.2% drop in adiponectin (Fig. 3G). Female EE mice showed no differences in serum leptin (Fig. 3F) and adiponectin (Fig. 3G). Neither sex displayed changes in serum triglycerides (Fig. 3H).
An interaction main effect between sex and housing main factors was observed for relative food intake (F(1,582)=54.22, P<0.0001), GTT (F(1,32)=21.48, P<0.0001), echoMRI lean mass (F(1,30)=5.045, P=0.0322), serum leptin (F(1,32)=24.47, P<0.0001), serum adiponectin (F(1,32)=6.996, P=0.0126), absolute and normalized BAT and WAT tissue weight. These data indicate the importance of both sex and housing in the metabolic EE-BTBR phenotype. Analysis of all main and interaction effects for metabolic measures are shown in Table S1.
In a shorter 8-week pilot experiment, similar results were found with regard to male and female body weight (Fig. S1A), male and female echoMRI (Fig. S1B and Fig S2C), and serum leptin (Fig. S1D). Of note, a housing effect was observed for echoMRI and leptin metrics. No housing effect was observed for body weight, which could be explained by the shorter time course of the pilot experiment. Full analysis of all main and interaction effects is shown in Table S2.
EE Reduces Anxiety in BTBR Mice
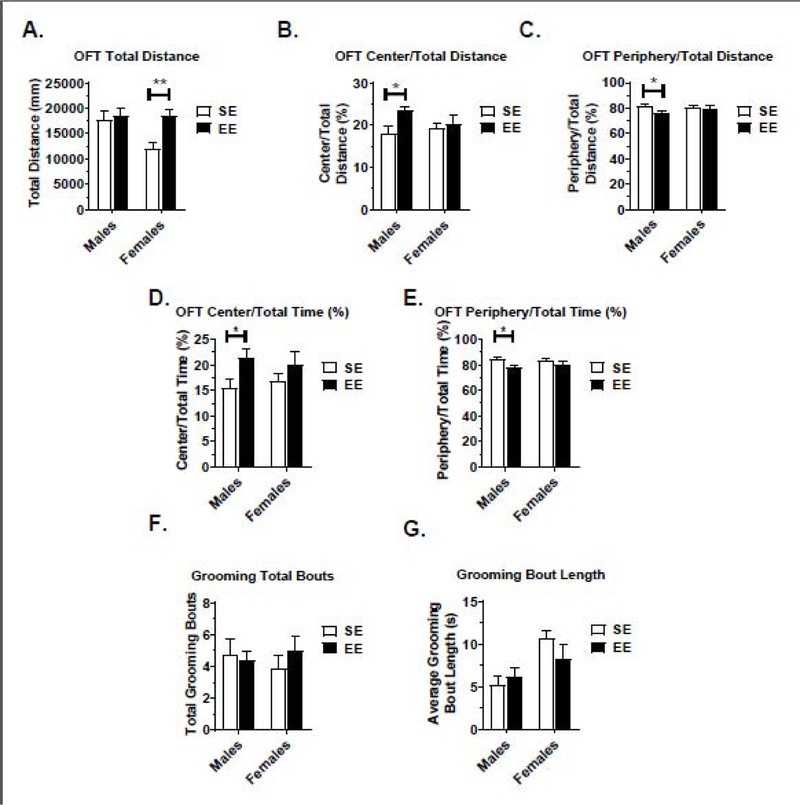
Our lab and others have shown that EE improves motor activity and reduces anxiety in C57BL/6 mice (Bailoo et al., 2018; McMurphy, 2018). After four weeks exposure to EE, BTBR mice were subjected to the open field (OF) test (Gould, 2009). By drawing on mouse prey tendencies and their desire to avoid open areas, the OF test is used to assess anxiety, among other measures. A reduced anxiety level is thought to be reflected via an increase of proportional time and/or distance traveled in the center portion of the OF.
A housing (F(1,32)=7.379, P=0.0106) main effect was observed for total distance traveled in the OF (Fig. 4A). Moreover, a housing main effect was observed for the total distance traveled in the center (Fig. 4B) and periphery (Fig. 4C) of the OF arena (both of these correlated measures had a result of F(1,32)=4.408, P=0.0437).
Figure 4.
Open Field (OF) and Grooming assessment results. (A) Total distance traveled during the OF test. (B) Relative distance traveled in the center of the OF test arena. (C) Relative distance traveled in the periphery of the OF test. (D) Relative time spent in the center of the OF test arena. (E) Relative time spent in the periphery of the OF test arena. (G) Total grooming bouts during the grooming test. (H) Average length of grooming bouts during the grooming test. Data are means ± SEM. Males: n=8 SE, n=9 EE. Females: n=10 SE, n=8–9 EE. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
Additionally, we assessed proportional time spent in the center (Fig. 4D) and proportional time spent in the periphery (Fig. 4E). A housing main effect was observed for these proportional time metrics (both of these correlated measures had a result of F(1,32)=6.509, P=0.016). Combined, these results indicate EE treatment ameliorated BTBR anxiety symptoms. Analysis of all main and interaction effects can be found in Table S1.
In a shorter 8-week pilot experiment, similar results were found with regard to EE-BTBR OF measures of anxiety (Fig. S1E–I). The main and interaction effect analyses of these data can be found in Table S2.
EE Does Not Affect Repetitive Behavior in BTBR Mice
Grooming behavior is common in rodents, representing 30–50% of their waking time (Kalueff et al., 2007). Highly repetitive self-grooming is thought to be a way to measure ASD-like repetitive behaviors in BTBR mice (Ellegood and Crawley, 2015). After 5 weeks in housing conditions, BTBR male and female mice were subjected to the Grooming Test. For male and female EE cohorts, no significant differences were observed in the number and average length of grooming bouts (Fig. 4F and 4G, Table S1).
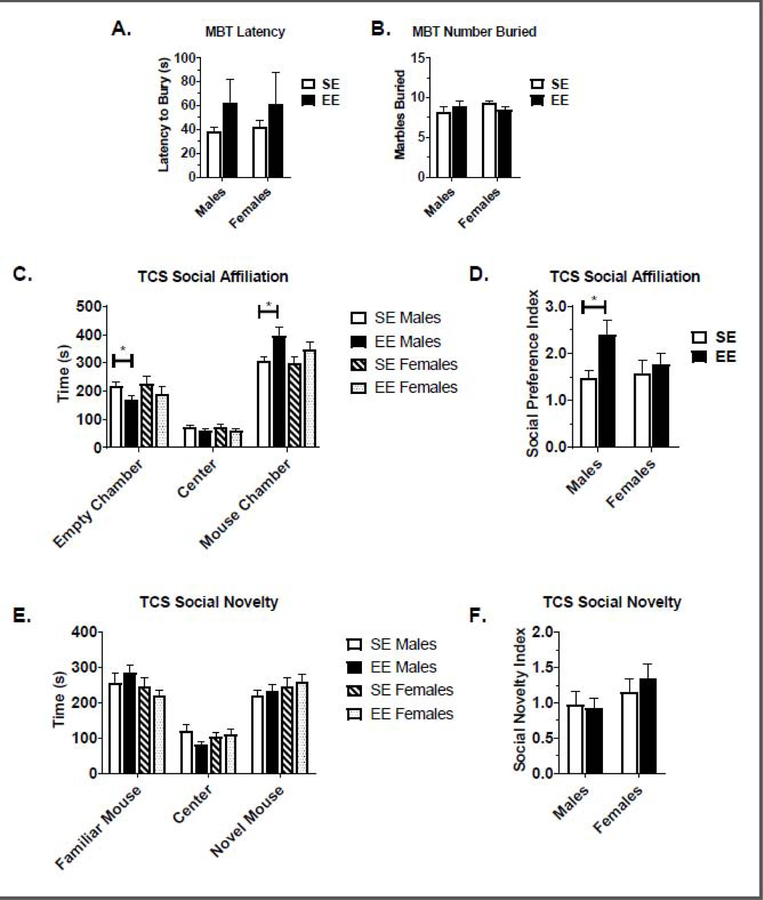
Spontaneous marble burying activity is thought to be representative of ASD-like repetitive behaviors (Angoa-Perez et al., 2013; Ellegood and Crawley, 2015). After 8 weeks in housing conditions, mice were subjected to the Marble Burying Test. For male and female EE cohorts, no significant differences were observed in the latency and the number of marbles buried (Fig. 5A and 5B). No housing, sex, or main effects were observed (Table S1). An additional pilot cohort corroborated these results from the Marble Burying test in males and females (Fig. S1J, Fig. S1K, and Table S2). In sum, these data suggest that EE did not ameliorate BTBR repetitive behaviors.
Figure 5.
Marble Burying (MB) and Three-Chamber Sociability (TCS) Test results. (A) Latency to bury marbles during the MB test. (B) Number of marbles buried during the MB test. (C) First phase of the TCS test – Social Affiliation. (D) Social preference index, measured during the first phase of the TCS test. (E) Second phase of the TCS test – Social Novelty Engagement. (F) Social Novelty index, measured during the second phase of the TCS test. Data are means ± SEM. Males: n=8 SE, n=9 EE. Females: n=10 SE, n=8–9 EE. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
EE Modulates Social Affiliation in BTBR Mice
BTBR mice display reduced social approach and low reciprocal social interactions (McFarlane et al., 2008). Researchers have used the Three-Chamber Sociability (TCS) test to study social affiliation and novel social engagement in mouse models. Mice are given the opportunity to wander three chambers while investigating social stimuli. In the first test phase—assessing social affiliation—mice are exposed to a novel confined peer and an opposing empty chamber. In the second test phase-assessing social novelty engagement—mice are exposed to both familiar and novel confined peers in opposing chambers (Kaidanovich-Beilin et al., 2011).
During the first test phase assessing social affiliation, a trending and significant housing main effect was observed for the time the test subject spent in the empty (F(1,30) =4.045, P=0.0530) and the mouse-filled (F(1,32)=7.814, P=0.0088) chambers, respectively. The social preference index is calculated by finding the ratio of time spent in the mouse chamber over the time spent in the empty chamber. For the social preference index, a housing main effect (F(1,30)=4.848, P=0.0355) was observed. Together, these data indicate that EE housing improves social affiliation.
During the second test phase assessing social novelty engagement, EE mice showed no significant improvements in social engagement, showing no preference for a novel social conspecific (Fig. 5E). No housing main effect was observed for the amount of time the test subject spent in the familiar and unfamiliar mouse chambers (Table S1). The social novelty index is calculated by finding the ratio of time spent in the novel mouse chamber over the familiar mouse chamber. No significant changes were observed in the social novelty index either (Fig. 5F), suggesting no EE-mediated improvements in social novelty engagement. Analysis of all main and interaction effects can be found in Table S1
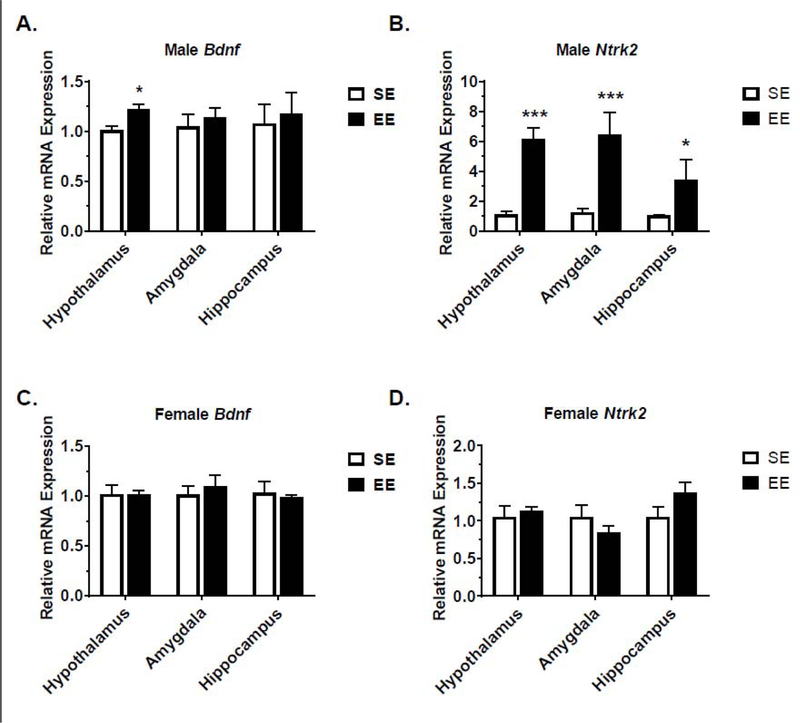
EE Regulates BDNF and Receptor Expression in the Brain in Male BTBR Mice
Metabolic data showed that male BTBR mice responded more readily to EE. Real-time quantitative RT-PCR was used to profile expression of Bdnf and Ntrk2 (encoding the BDNF protein’s receptor, TrkB) in the hypothalamus, amygdala, and hippocampus. Hypothalamic Bdnf was significantly upregulated in male EE mice, which is consistent with activation of the HSA axis (Fig. 6A). No changes in Bdnf expression were observed in the amygdala or hippocampus of male EE mice (Fig. 6A). Interestingly, Ntrk2 expression was upregulated significantly by 3–6 folds in the hypothalamus, amygdala, and hippocampus of male EE mice (Fig. 6B). No such changes in Bdnf (Fig. 6C) and Ntrk2 (Fig. 6D) were observed in the EE-BTBR female hypothalamus, amygdala, and hippocampus.
Figure 6.
Gene expression profiling. (A) Relative mRNA expression of Bdnf in male mice. (B) Relative mRNA expression of full-length Ntrk2 in male mice. (C) Relative mRNA expression of Bdnf in female mice. (D) Relative mRNA expression of full-length Ntrk2 in female mice. Data are means ± SEM. n= 6–8 per group. * P<0.05, ** P<0.01, *** P<0.001.
DISCUSSION
Various methods of experimental manipulation—most commonly, pharmacological and transplant studies—have sought to ameliorate BTBR behavioral deficits. Researchers have found that many different drugs—including propofol, citalopram, metformin, and donepezil—can alleviate social deficits and repetitive behaviors in the BTBR strain, among other symptoms (Cai et al., 2019; Cai et al., 2017; Karvat and Kimchi, 2014; Wang et al., 2018a). Even a C57BL/6J bone marrow transplant was found to increase sociability in BTBR mice, suggesting a link between immunity and behavior within the BTBR strain (Schwartzer et al., 2017).
To date, studies combining BTBR mice and non-pharmacological treatments—like EE—are limited, and none report metabolic effects. For example, one group found that short-term EE reduced repetitive grooming behaviors in BTBR mice (Reynolds et al., 2013) and another found that EE improves novel object recognition in BTBR mice (MacPherson et al., 2008). In this study, we report metabolic and some behavioral improvements in BTBR mice following like-peer EE exposure.
Our previous findings in C57BL/6 mice indicate that activation of the HSA axis is a hallmark of EE treatment (Cao et al., 2011; Cao et al., 2010). In this investigation with a metabolically and socially deficient strain, we observed that EE-related stimuli resulted in upregulation of Bdnf within the male EE-BTBR hypothalamus, a central nervous system manifestation that is characteristic of HSA axis activation. Despite an increased total body weight, peripheral outcomes included reduced adiposity (in vivo and at sacrifice), increased lean mass, lower leptin level in circulation, and improved glucose tolerance in enriched male mice. These data suggest male BTBR mice experience metabolic improvement in response to EE. Female EE-BTBR mice, however, responded less readily than their male counterparts, displaying a more modest decrease in adiposity at sacrifice and no changes in female glycemic control, lean mass, or serum adipokines. Many of these metabolic panel results displayed an interaction main effect between housing and sex (Table S1), indicating the importance of these two variables in metabolic outcomes. These results suggest that EE-mediated HSA activation results in metabolic improvement in the BTBR strain—especially so in male mice.
Hallmark symptoms of ASD include impaired communication/social skills and repetitive behaviors (Meyza and Blanchard, 2017). Children with ASD are at an additional risk for developing anxiety and other psychiatric comorbidities (Chao et al., 2018; Perihan et al., 2019), creating a need for concurrent anxiolytic treatments. To assess EE as a bio-behavioral intervention for these symptoms, three ASD-like characteristics of BTBR mice were assessed: anxiety, repetitive behaviors, and inhibited sociability. The housing main effects of our OF test results suggest EE treatment reduces anxiety in BTBR mice. No changes in repetitive behaviors were observed in the Marble Burying and Grooming tests. In the Three-Chamber Sociability test, the significant housing main effects suggests that EE can improve social affiliation. Together, these results suggest an amelioration of some core behavioral symptoms in EE-BTBR mice.
The effects of enrichment have been explored in many other ASD murine models. In an induced ASD model using valproic acid, EE attenuated anxiety and social deficits (Yamaguchi et al., 2017). However, EE was shown to have minimal effects on rearing, self-grooming, or hole board exploration in the Shank3 model (Hulbert et al., 2018). The C58/J mouse model displays robust repetitive jumping behavior, behavioral inflexibility, and increased levels of self-grooming, which were reportedly dramatically reduced following EE treatment (Lewis et al., 2018). Lastly, the Mecp2− mouse model displays motor, cognitive, and emotional deficits. Two groups report EE-mediated Mecp2− improvements in motor coordination/learning, improved memory, and decreases in anxiety (Kerr et al., 2010; Lonetti et al., 2010).
Varying sex-specific differences at baseline have been identified for some of the most popular ASD models, including BTBR mice (Jeon et al., 2018). It is important to note that ASD behavioral presentations can also differ between male and female human patients (Aita et al., 2018; Milner et al., 2019; Ottosen et al., 2019), and males are four times more likely than females to be diagnosed with ASD (Baio et al., 2018). While there is no clear consensus, some studies have identified a difference in ASD treatment efficacy between the sexes (Kosaka et al., 2016; Tiura et al., 2017), a dichotomy that parallels several phenotypes observed in our EE-BTBR study. Researchers must consider sexual dimorphism when conducting and analyzing treatment-focused studies on BTBR mice, and future studies are needed to elucidate the mechanisms underlying these differences.
Many of the aforementioned studies on ASD models focus on assessment for repetitive behaviors following EE. Here, we report no EE associated changes in repetitive behaviors, as indicated by grooming and marble burying activity. Of note, our grooming results did not replicate previous work on enriched BTBR mice (Reynolds et al., 2013), despite testing at a similar timepoint post-housing and adherence to the same behavioral testing protocol (Kalueff et al., 2007). This discrepancy may result from differences in age at testing, EE housing protocol, optional measures (inducing spray vs. no spray), and manual scoring. When designing EE studies, it is important to be cognizant of reproducibility and replicability—inconsistencies between EE models in current literature may explain the discrepancy in some outcomes. Researchers may also consider using a social strain in tandem with ASD models. This can confirm if baseline deficits are apparent before intervention treatment, if symptom amelioration indeed occurs, and even if that amelioration is successful enough to approach wild-type scores. We did not observe a social strain concurrently, indicating one limitation of this study. However, EE’s metabolic and behavioral effects on the social C57BL/6 murine strain have been previously characterized within juvenile and aged mice following the same protocol.
Mixed-peer paradigms are reported in the literature—a pair of studies suggest that mixed-housing with social peers (C57BL/6J) can rescue BTBR social approach deficits (Yang et al., 2011) and correct memory deficits (Lipina and Roder, 2013) via co-learning. Our work shows that like-peer housing in EE was able to rescue BTBR social affiliation deficits decrease anxiety even without the benefit of social (C57BL/6J) role models. These results suggest that the non-social stimuli of enrichment may still facilitate social improvement. While we cannot make broader conclusions about like-peer and mixed-peer treatment methods for ASD mouse models and human patients, these considerations may be important for future intervention treatment research.
Recent work has sought to connect neuroanatomical and neuroplastic differences with BTBR metabolic and behavioral deficiencies (Stephenson et al., 2011). Transcriptomic and proteomic data suggest Bdnf is downregulated in the BTBR hippocampus and cortex (Daimon et al., 2015; Stephenson et al., 2011). Here, Bdnf was upregulated in the hypothalamus of male EE-BTBR mice. Our mechanistic studies in C57BL/6 mice have previously identified hypothalamic BDNF as the key brain mediator orchestrating the EE-induced metabolic, endocrine, and immune outcomes (Cao et al., 2011; Cao et al., 2010; Xiao et al., 2019; Xiao et al., 2016). Overexpressing BDNF in the hypothalamus reproduces the anxiolytic effect of EE in middle age female C57BL/6 mice (McMurphy, 2018; McMurphy et al., 2018). We are currently investigating whether hypothalamic BDNF overexpression could mimic the EE effects on metabolism, anxiety, and social affiliation observed in male BTBR mice. Another interesting finding of this study is the robust upregulation of the gene encoding TrkB (a receptor of BDNF) in the hypothalamus, amygdala, and hippocampus of male EE-BTBR mice. Further studies to examine TrkB signaling and its potential role in the EE-BTBR phenotype are underway in our lab. Of note, clinical studies report higher circulating BDNF in ASD patients (Armeanu et al., 2017; Meng et al., 2017), although it is not clear whether the peripheral BDNF level reflects the level in the brain or the BDNF signaling.
Future researchers may be interested in investigating the links between EE, BDNF, neurogenesis, and the BTBR murine model. BDNF is thought to be a regulator of neurogenesis and is upregulated within EE (Cao and During, 2012; Kowiański et al., 2018). Hypothalamic BDNF has been implicated in improved metabolism in young (Cao et al., 2011; Cao et al., 2010) and old C57BL/6 mice (McMurphy et al., 2018). Our recent work also suggests that hypothalamic BDNF promotes healthy aging in C57BL/6 mice (McMurphy et al., 2018). At baseline, BTBR mice have impaired neurogenesis and deficient BDNF signaling (Stephenson et al., 2011). One study found that anxiolytic improvements in EE are dependent upon neurogenesis (Schloesser et al., 2010). While we cannot conclude any causation or necessity with this study, exploring neurogenesis in the EE-BTBR model may be an interesting avenue for future research.
A large body of literature suggests that intervention treatments are valuable tools to ameliorate the symptoms of ASD in human patients (Aronoff et al., 2016; Fuller and Kaiser, 2019; Waters et al., 2018). The timing of intervention treatments is important for researchers and clinicians to consider. Because early ASD diagnosis and intervention are thought to be of clinical importance (Fernell et al., 2013), in this 17-week-long experiment, mice were placed in EE as juveniles (4–5 weeks old) and exposure to EE was maintained through adulthood. In a shorter pilot experiment, mice were placed in EE as young adults (6–10 weeks old). These results suggest that EE provides benefits when initiated at juvenile and young adult ages. Future experiments are needed to examine whether EE is effective for ASD-like symptoms when initiated at elderly stages, and moreover, to assess the optimal onset and duration of intervention to provide similar benefits.
Of interest, a pair of recent studies connects mouse and human EE treatments, bridging bench and bedside work. These studies observed that sensorimotor EE improved cognition and severity of autism scores in human patients (Woo et al., 2015; Woo and Leon, 2013). Considering the broader research base of EE literature may provide insight into treatment of conditions—including intellectual disability (ID), developmental coordination disorder (DCD), communication disorders affecting language, attention-deficit/hyperactivity disorder (ADHD), tic disorders, anxiety, and obesity (Curtin et al., 2010; Fernell et al., 2013)— that occur independently but are also frequently comorbid with ASD. Thus, the broad benefits demonstrated by EE—including improved learning/memory, neurogenesis, anxious behavior, sociability, locomotion, metabolism, and immunocompetence—might ameliorate symptoms within several relevant disorder models and warrant further investigation.
Supplementary Material
HIGHLIGHTS.
Environmental enrichment ameliorated metabolic/social deficits in an autism model
Male mice respond to an autism/metabolic treatment more readily than females
Researchers using BTBR mice must consider potential sex differences
ACKNOWLEDGEMENTS
We thank Dr. Zachary M. Weil, Julie Fitzgerald, and the Behavior Core at The Ohio State University for materials and consultation regarding the Three-Chamber Sociability Test. We also thank Jacqueline M. Anderson and Quais N. Hassan, II, for editorial assistance. This work was supported by NIH grants AG041250, CA166590, CA178227, CA163640 to L.C. Additional support provided by P30NS104177.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- Aita C, Mizoguchi Y, Yamamoto M, Seguch IY, Yatsuga C, Nishimura T, Sugimoto Y, Takahashi D, Nishihara R, Ueno T, Nakayama M, Kuroki T, Nabeta H, Imamura Y, Monji A, 2018. Oxytocin levels and sex differences in autism spectrum disorder with severe intellectual disabilities. Psychiatry research 273, 67–74. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Neurodevelopmental Disorders, Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association, Washington, DC. [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM, 2013. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp, 50978. [DOI] [PMC free article] [PubMed]
- Armeanu R, Mokkonen M, Crespi B, 2017. Meta-Analysis of BDNF Levels in Autism. Cell Mol Neurobiol 37, 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff E, Hillyer R, Leon M, 2016. Environmental Enrichment Therapy for Autism: Outcomes with Increased Access. Neural Plast 2016, 2734915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailoo JD, Murphy E, Boada-Sana M, Varholick JA, Hintze S, Baussiere C, Hahn KC, Gopfert C, Palme R, Voelkl B, Wurbel H, 2018. Effects of Cage Enrichment on Behavior, Welfare and Outcome Variability in Female Mice. Front Behav Neurosci 12, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF, 2018. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002) 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wang L, Nalvarte I, Xiao R, Li X, Fan X, 2019. Citalopram attenuates social behavior deficits in the BTBR T(+)Itpr3(tf)/J mouse model of autism. Brain research bulletin 150, 75–85. [DOI] [PubMed] [Google Scholar]
- Cai Y, Wang L, Xiao R, Li X, He X, Gao J, Xu H, Fan X, 2017. Autism-like behavior in the BTBR mouse model of autism is improved by propofol. Neuropharmacology 118, 175–187. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ, 2011. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 14, 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, During MJ, 2012. What is the brain-cancer connection? Annu Rev Neurosci 35, 331–345. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ, 2010. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, Yunger R, Yang YM, 2018. Behavioral assessments of BTBR T+Itpr3tf/J mice by tests of object attention and elevated open platform: Implications for an animal model of psychiatric comorbidity in autism. Behav Brain Res 347, 140–147. [DOI] [PubMed] [Google Scholar]
- Clee SM, Nadler ST, Attie AD, 2005. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. American journal of therapeutics 12, 491–498. [DOI] [PubMed] [Google Scholar]
- Daimon CM, Jasien JM, Wood WH 3rd, Zhang Y, Becker KG, Silverman JL, Crawley JN, Martin B, Maudsley S, 2015. Hippocampal Transcriptomic and Proteomic Alterations in the BTBR Mouse Model of Autism Spectrum Disorder. Front Physiol 6, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L, 2015. Adipose VEGF Links the White-to-Brown Fat Switch With Environmental, Genetic, and Pharmacological Stimuli in Male Mice. Endocrinology 156, 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Crawley JN, 2015. Behavioral and Neuroanatomical Phenotypes in Mouse Models of Autism. Neurotherapeutics 12, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernell E, Eriksson MA, Gillberg C, 2013. Early diagnosis of autism and impact on prognosis: a narrative review. Clinical epidemiology 5, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller EA, Kaiser AP, 2019. The Effects of Early Intervention on Social Communication Outcomes for Children with Autism Spectrum Disorder: A Meta-analysis. Journal of autism and developmental disorders. [DOI] [PMC free article] [PubMed]
- Gould TD, 2009. Mood and anxiety related phenotypes in mice : characterization using behavioral tests. Humana Press, New York, NY. [Google Scholar]
- Hulbert SW, Bey AL, Jiang YH, 2018. Environmental enrichment has minimal effects on behavior in the Shank3 complete knockout model of autism spectrum disorder. Brain and behavior 8, e01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SJ, Gonzales EL, Mabunga DFN, Valencia ST, Kim DG, Kim Y, Adil KJL, Shin D, Park D, Shin CY, 2018. Sex-specific Behavioral Features of Rodent Models of Autism Spectrum Disorder. Exp Neurobiol 27, 321–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR, 2011. Assessment of social interaction behaviors. J Vis Exp. [DOI] [PMC free article] [PubMed]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P, 2007. Analyzing grooming microstructure in neurobehavioral experiments. Nature protocols 2, 2538–2544. [DOI] [PubMed] [Google Scholar]
- Karvat G, Kimchi T, 2014. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Silva PA, Walz K, Young JI, 2010. Unconventional transcriptional response to environmental enrichment in a mouse model of Rett syndrome. PLoS One 5, e11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H, Okamoto Y, Munesue T, Yamasue H, Inohara K, Fujioka T, Anme T, Orisaka M, Ishitobi M, Jung M, Fujisawa TX, Tanaka S, Arai S, Asano M, Saito DN, Sadato N, Tomoda A, Omori M, Sato M, Okazawa H, Higashida H, Wada Y, 2016. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: a 24-week randomized clinical trial. Translational Psychiatry 6, e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J, 2018. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cellular and molecular neurobiology 38, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Lindenmaier Z, Boswell K, Edington G, King MA, Muehlmann AM, 2018. Subthalamic nucleus pathology contributes to repetitive behavior expression and is reversed by environmental enrichment. Genes Brain Behav 17, e12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Gan Y, Fan Y, Wu Y, Lin H, Song Y, Cai X, Yu X, Pan W, Yao M, Gu J, Tu H, 2015. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci Rep 5, 7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Roder JC, 2013. Co-learning facilitates memory in mice: a new avenue in social neuroscience. Neuropharmacology 64, 283–293. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T, 2010. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry 67, 657–665. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV, 2008. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain research 1210, 179–188. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN, 2008. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 7, 152–163. [DOI] [PubMed] [Google Scholar]
- McMurphy T, Huang W, Liu X, Siu JJ, Queen NJ, Xiao R, Cao L, 2018. Hypothalamic gene transfer of BDNF promotes healthy aging in mice. Aging Cell, e12846. [DOI] [PMC free article] [PubMed]
- McMurphy T, Huang W, Liu X, Siu JJ, Queen NJ, Xiao R, & Cao L, 2018. Implementation of environmental enrichment after middle age promotes healthy aging. Aging 10, 1698–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng WD, Sun SJ, Yang J, Chu RX, Tu W, Liu Q, 2017. Elevated Serum Brain-Derived Neurotrophic Factor (BDNF) but not BDNF Gene Val66Met Polymorphism Is Associated with Autism Spectrum Disorders. Mol Neurobiol 54, 1167–1172. [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Blanchard DC, 2017. The BTBR mouse model of idiopathic autism - Current view on mechanisms. Neurosci Biobehav Rev 76, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT, 2010. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One 5, e14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner V, McIntosh H, Colvert E, Happe F, 2019. A Qualitative Exploration of the Female Experience of Autism Spectrum Disorder (ASD). Journal of autism and developmental disorders. [DOI] [PMC free article] [PubMed]
- Nachat-Kappes R, Pinel A, Combe K, Lamas B, Farges MC, Rossary A, Goncalves-Mendes N, Caldefie-Chezet F, Vasson MP, Basu S, 2012. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One 7, e51525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottosen C, Larsen JT, Faraone SV, Chen Q, Hartman C, Larsson H, Petersen L, Dalsgaard S, 2019. Sex Differences in Comorbidity Patterns of Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. [DOI] [PubMed]
- Perihan C, Burke M, Bowman-Perrott L, Bicer A, Gallup J, Thompson J, Sallese M, 2019. Effects of Cognitive Behavioral Therapy for Reducing Anxiety in Children with High Functioning ASD: A Systematic Review and Meta-Analysis. Journal of autism and developmental disorders. [DOI] [PubMed]
- Reynolds S, Urruela M, Devine DP, 2013. Effects of environmental enrichment on repetitive behaviors in the BTBR T+tf/J mouse model of autism. Autism Res 6, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M, 2010. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Molecular psychiatry 15, 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Onore CE, Rose D, Ashwood P, 2017. C57BL/6J bone marrow transplant increases sociability in BTBR T(+) Itpr3(tf)/J mice. Brain Behav Immun 59, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN, 2015. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology 40, 2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater AM, Cao L, 2015. A Protocol for Housing Mice in an Enriched Environment. Journal of visualized experiments : JoVE, e52874. [DOI] [PMC free article] [PubMed]
- Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D, 2011. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiura M, Kim J, Detmers D, Baldi H, 2017. Predictors of longitudinal ABA treatment outcomes for children with autism: A growth curve analysis. Research in Developmental Disabilities 70, 185–197. [DOI] [PubMed] [Google Scholar]
- Wang L, Cai Y, Fan X, 2018a. Metformin Administration During Early Postnatal Life Rescues Autistic-Like Behaviors in the BTBR T+ Itpr3tf/J Mouse Model of Autism. Front Behav Neurosci 12, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao S, Liu X, Zheng Y, Li L, Meng S, 2018b. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed Pharmacother 107, 262–269. [DOI] [PubMed] [Google Scholar]
- Waters CF, Amerine Dickens M, Thurston SW, Lu X, Smith T, 2018. Sustainability of Early Intensive Behavioral Intervention for Children With Autism Spectrum Disorder in a Community Setting. Behav Modif, 145445518786463. [DOI] [PubMed]
- Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M, 2015. Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behavioral Neuroscience 129, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Leon M, 2013. Environmental enrichment as an effective treatment for autism: A randomized controlled trial. Behavioral Neuroscience 127, 487–497. [DOI] [PubMed] [Google Scholar]
- Xiao R, Bergin SM, Huang W, Mansour AG, Liu X, Judd RT, Widstrom KJ, Queen NJ, Wilkins RK, Siu JJ, Ali S, Caligiuri MA, Cao L, 2019. Enriched environment regulates thymocyte development and alleviates experimental autoimmune encephalomyelitis in mice. Brain Behav Immun 75, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Bergin SM, Huang W, Slater AM, Liu X, Judd RT, Lin ED, Widstrom KJ, Scoville SD, Yu J, Caligiuri MA, Cao L, 2016. Environmental and Genetic Activation of Hypothalamic BDNF Modulates T-cell Immunity to Exert an Anticancer Phenotype. Cancer Immunol Res 4, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K, 2017. Environmental enrichment attenuates behavioral abnormalities in valproic acid-exposed autism model mice. Behav Brain Res 333, 67–73. [DOI] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN, 2011. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res 4, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilkha N, Kuperman Y, Kimchi T, 2017. High-fat diet exacerbates cognitive rigidity and social deficiency in the BTBR mouse model of autism. Neuroscience 345, 142–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.