Abstract
The use of hydrogels in biomedical applications dates back multiple decades, and the engineering potential of these materials continues to grow with discoveries in chemistry and biology. The approaches have led to increasing complex hydrogels that incorporate both synthetic and natural polymers and functional domains for tunable release kinetics, mediated cell response, and ultimately use in clinical and research applications in biomedical practice. This review focuses on recent advances in hybrid hydrogels that incorporate nano/microstructures, their synthesis, and applications in biomedical research. Examples discussed include the implementation of click reactions, photopatterning, and 3D printing for the facile production of these hybrid hydrogels, the use of biological molecules and motifs to promote a desired cellular outcome, and the tailoring of kinetic and transport behavior through hybrid-hydrogel engineering to achieve desired biomedical outcomes. Recent progress in the field has established promising approaches for the development of biologically relevant hybrid hydrogel materials with potential applications in drug discovery, drug/gene delivery, and regenerative medicine.
Keywords: Biomedical application, drug/gene delivery, hybrid hydrogel, microstructure, nanostructure, regenerative medicine
Introduction
In their simplest form, hydrogels are hydrophilic polymer networks, that can absorb, swell, and retain large amounts of aqueous fluid [1]. Their high water content and permeability, as well as their tunable viscoelasticity and structural similarity to the extracellular matrix, make hydrogels inherently well suited for biological applications. These key properties make them attractive for biomedical use as pioneered by Wichterle and Lim in 1960, and continued today as platforms for drug delivery and tissue engineering [2]. Increased versatility and function of the materials has been enabled by chemical advances that allow incorporation of molecules that can direct cell activity and/or be released with temporal control, and also for inclusion of carriers such as microstructured domains and nanoparticles in the hydrogel network.
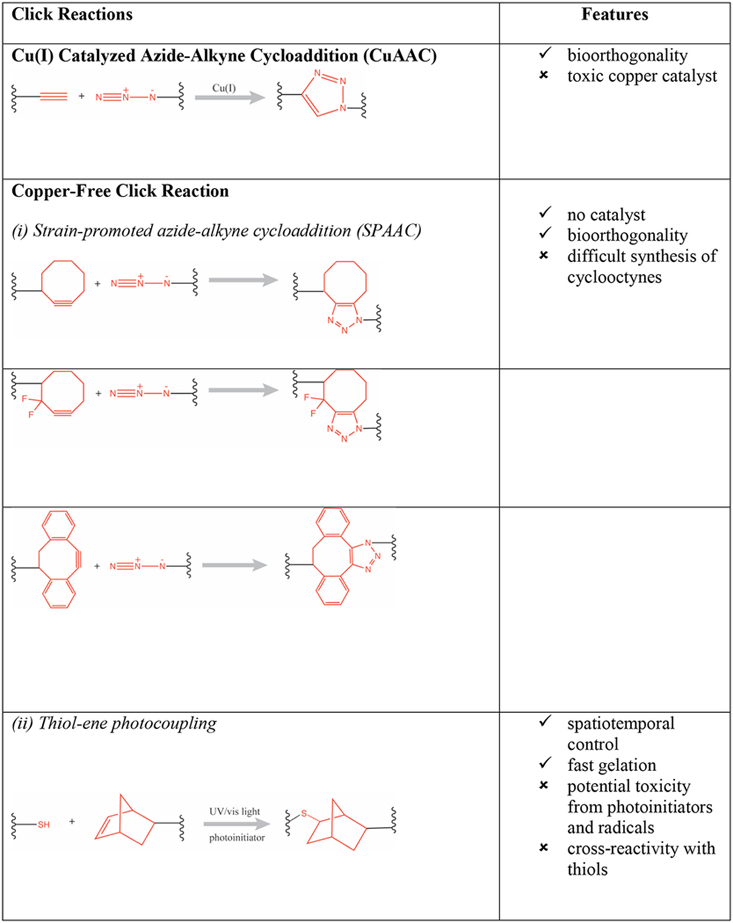
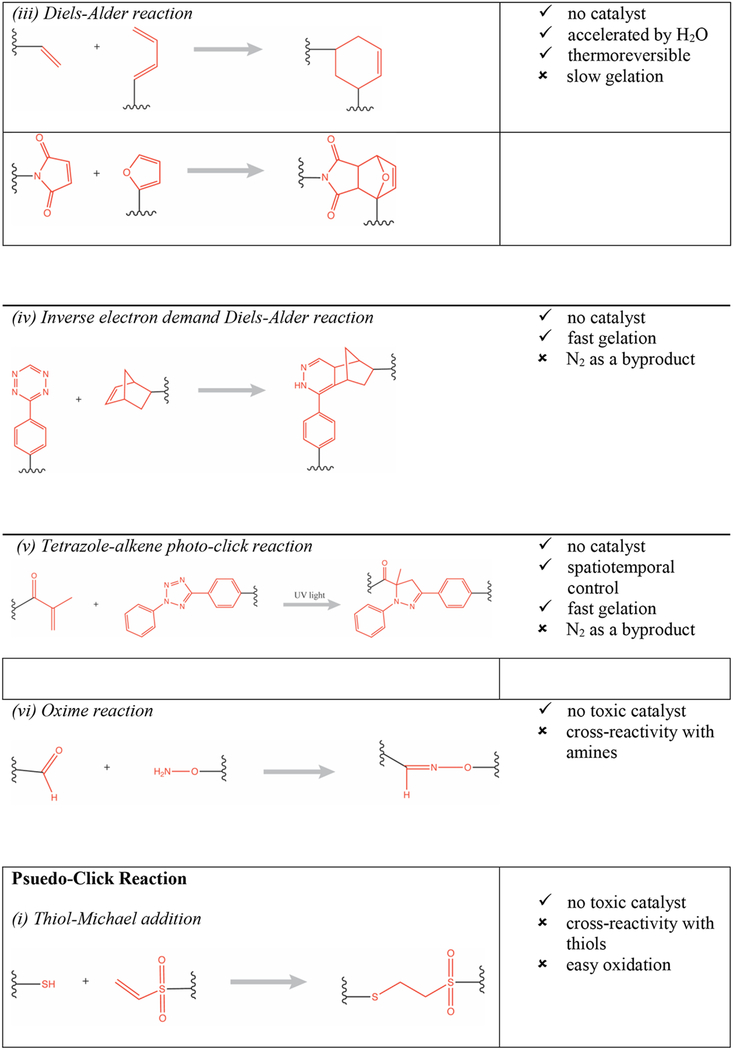
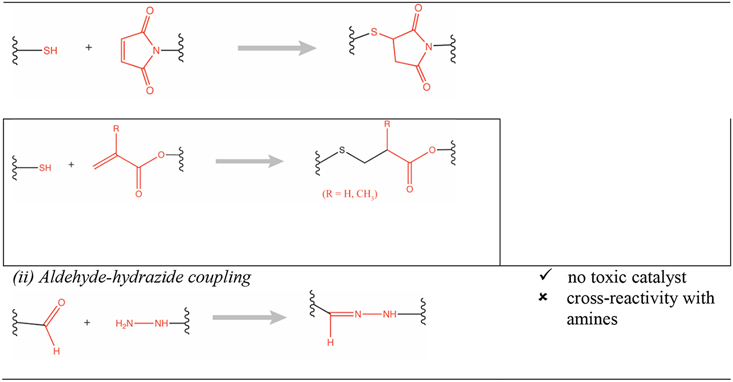
Various physical and chemical cross-linking strategies have been implemented for fabrication of hydrogels. Physical hydrogels are formed by using a variety of environmental triggers (temperature, pH, ionic strength) and physicochemical interactions (stereocomplexation, charge condensation, hydrophobic interactions or supramolecular chemistry) [3]. They can be formed under mild conditions but are usually weak and possess poor long-term stability [4]. Conversely, chemical hydrogels (e.g., photopolymerized [5] or enzymatically cross-linked [6]) are generally characterized by better stability and enhanced mechanical properties (e.g., enhanced hydrogel stiffness). Advances in modern polymer chemical transformations have enabled the design of sophisticated material systems with a broad range of applications. In particular, “click reactions” (e.g., thiol-maleimide Michael addition, thiol-norbornene click reaction) have been especially valuable because they are orthogonal to many naturally occurring chemical functionalities, have few byproducts, and the resulting thioether succinimide linkage can be tuned to show reversible and dynamic properties [7,8]. They have particular utility for creating hydrogels with high biocompatibility, tunable viscoelasticity, and most importantly the ability to carry, protect, and release cargo to the surrounding tissue in a responsive fashion (e.g., triggered by biological substrates like glutathione for glutathione-mediated cleavage of maleimide-thiol adducts, or triggered by a change in pH) [9]. For example, copper(I)-catalyzed azide-alkene cycloaddition (CuAAC) based hyaluronic acid hydrogels were used as cell scaffolds and drug reservoirs [10]. Furthermore, the potentially toxic catalysts used for copper-catalyzed reactions can be minimized with copper-free click chemistries such as those employed in radical mediated thiol-ene/yne chemistry [11], azide-alkyne cycloaddition [12], tetrazole-alkene photo-click chemistry [13], the Diels-Alder reaction [14], and the oxime reaction [15], as presented in Table 1 (adapted from Jiang et. al.) [16].
Table 1:
Summary of click chemistry strategies employed to form hydrogels (adapted from Jiang et. al. 2014) [16]. (Reproduced with permission from Elsevier, Copyright 2019.)
|
|
|
While hydrogels as a class of materials possess key properties that make them suitable for biomedical applications (e.g., drug delivery and tissue engineering) [17,18], their mechanical strength, release/degradation kinetics, and the bioactivities of incorporated therapeutic molecules must still be optimized. Traditional synthetic hydrogels exhibit significant heterogeneity and network defects (e.g., chain ends, entanglements, and phase-separated regions), which dramatically affect mechanical properties, and alter the diffusion rates and biological activity of active molecules [17]. Therefore, hybrid hydrogels have been developed to address and ameliorate the issues to improve existing formulations as well as to expand the range of applications from medicinal (e.g., spatially and temporally controlled drug release profiles [19,20]) to opto-electronics and magnetic materials (e.g., high-tech applications such as fabrication of organic electronic devices with excellent charge transport and related properties [21,22]). Hybrid hydrogels are composed of chemically, functionally, and morphologically distinct building blocks, which can include biologically active proteins, peptides, or nano/microstructures, interconnected via physical or chemical means. Proteins and peptides that are incorporated into networks are generally reacted with synthetic polymers via polymerization or conjugation (click chemistry) strategies to yield hybrid hydrogels compatible for in vitro (cell differentiation, proliferation and migration studies) and in vivo (drug delivery, tissue engineering, and wound healing) applications [23]. Nano/microstructures can be encapsulated within the hydrogels via physical or chemical encapsulation to yield improvement in mechanical reinforcement as well as for controlled cargo delivery [24] or for sequestration of growth factors from the surrounding milieu [25]. Furthermore, the heterogeneity of hybrid hydrogels can improve cell adhesion, organization, and cell-cell interactions to create tissue constructs with enhanced mechanical integrity, electroactivity, and improved cellular organization [26,27]. This review will focus on recent developments in the production of nano/microstructured hydrogels and their potential use in biomedical applications.
Hybrid hydrogels incorporating nanostructures
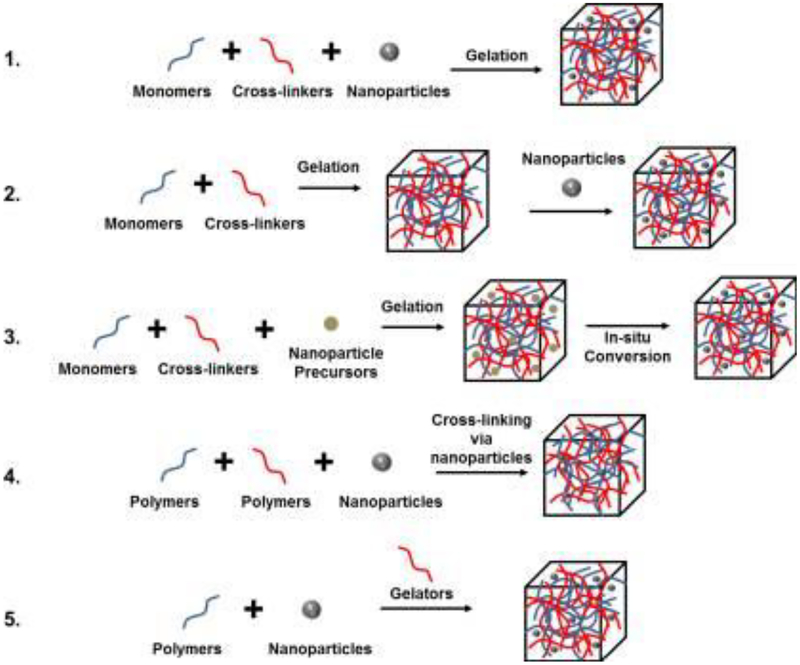
Recently, nanoparticles have gained increased attention for potential applications in biomedical fields owing to their small size, surface functionality, stability, and drug loading capacity. Different approaches can be implemented to develop nanoparticle-hydrogel composites with varying nanoparticle types and varying bulk hydrogel frameworks, either through noncovalent or covalent immobilization strategies [28]. The homogenous distribution of nanoparticles within hydrogels is achieved by five main approaches: (a) hydrogel formation directly in a suspension of nanoparticles; (b) physically embedding of the nanoparticles into a hydrogel matrix after gelation (typically achieved in three steps: a preformed hydrogel is placed in aprotic solvent to release water; the shrunken gel is placed in nanoparticle suspended aqueous solution; and the hydrogel is washed with water to remove weakly surface-adsorbed nanoparticles); (c) reactive nanoparticle formation within a preformed gel; (d) nanoparticle-mediated crosslinking to form hydrogels; and (e) gel formation by crosslinking a mixture of nanoparticles, polymers, and distinct gelator molecules. These various methods are presented in Figure 1 [28].
Figure 1.
Five main approaches that have been used to obtain hydrogel-nanoparticle conjugates with uniform distribution: (1) hydrogel formation directly in a suspension of nanoparticles; (2) physically embedding the nanoparticles into hydrogel matrix after gelation; (3) reactive nanoparticle formation within a preformed gel; (4) nanoparticle-mediated crosslinking to form hydrogels; and (5) gel formation by crosslinking a mixture of nanoparticles, polymers, and distinct gelator molecules. Adapted from Thoniyot et. al. [28]. (Reproduced with permission from John Wiley and Sons, Copyright 2015.)
Many types of nanoparticle-containing hydrogel networks have been aimed at enhancing the mechanical properties of the hydrogel, such as the incorporation of silica nanoparticles for improved mechanical stiffness, bioactivity of therapeutic molecules, and tissue stickiness as compared to unmodified hydrogels [29,30]. A 10-fold increase in stiffness of collagen-based hydrogels was realized following mixing with nitro-dopamine modified oleic acid coated iron oxide nanoparticles working as crosslinker epicenters that connect collagen chains on the surface of nanoparticles [31]. Incorporation of carbon nanotubes into methacrylated gelatin hydrogels led to a 3-fold increase in the hydrogel tensile modulus [26]. Addition of gold nanoparticles into poly(N-isopropylacrylamide) (PNIPAM) hydrogels enhanced shear modulus by 6-fold [32].
Conventional hydrogels have enabled controlled release of a variety of water-soluble therapeutic molecules; however, entrapment of hydrophobic therapeutic molecules has been limited [33]. These poorly soluble therapeutic agents have thus been encapsulated in nanoparticles such as micelles and liposomes to achieve controlled and sustained delivery. The direct loading of the hydrophobic drug dexamethasone acetate (DMSA) into poly-2-hydroxyethyl methacrylate (pHEMA)-based hydrogels for ocular drug delivery was reported [34]. Direct loading of DMSA in the hydrogel supported delivery for only three days, whereas DMSA-loaded poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) micelles, physically incorporated into the hydrogels, resulted in sustained release for up to 30 days–depending on the drug loading level. Erythromycin (a hydrophobic antibiotic drug) was encapsulated in Pluronic F-127 diacrylate macromer micelles, and hydrogels were developed by photopolymerization under a low-intensity UV light that sustained drug release for > 2 days [35]. A pH-responsive hydrogel containing poly(methyl methacrylate) (PMMA) nanoparticles, crosslinked with methacrylic acid grafted with poly(ethylene glycol) tethers, was developed for delivery of hydrophobic drugs [36]. A similar approach was used to develop a nanocomposite hydrogel for curcumin delivery that comprised gelatin and poly(3-hydroxybutyrate) (PHB) polymeric nanoparticles [37]. Uniform core-shell microparticles encapsulating cisplatin and paclitaxel were fabricated using coaxial electrohydrodynamic atomization technique and subsequently were embedded into an injectable hydrogel composed of a mixture of alginate-aldehyde and branched polyethyleneimine (PEI-25k). [38]. Controlled paclitaxel and cisplatin release for 45 days was evident with synergistic combination anticancer effects on MDA-MB-231 breast cancer cells.
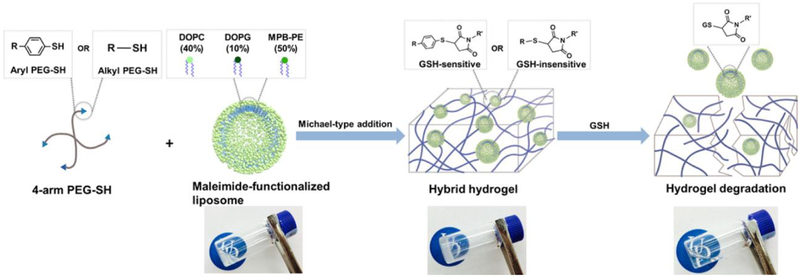
Stimuli-responsive hybrid hydrogels, such as those desired for chemotherapeutic applications [39], have motivated the development of chemical and biochemical approaches for hydrogel degradation and nanoparticle/drug release in response to specific stimuli. Liposome cross-linked hybrid hydrogels were developed by using the Michael-type addition of thiolated PEG-polymers with maleimide-linked liposomes, creating a hydrogel matrix that was capable of degradation in response to thiol-containing environments (i.e., GSH) (Figure 3) [40]. Liposomes encapsulating doxorubicin were prepared using maleimide-functionalized DSPE-PEG that cross-linked with alkyl- or aryl-thiol-functionalized four-arm PEG to form hybrid hydrogels. Controlled doxorubicin (DOX) release in PBS was observed with 25% drug release in 6 days, whereas addition of 10 mM GSH increased the cumulative release to 70% for the arylthiol-containing PEG-SH hybrid hydrogel. Furthermore, cytochrome c (CytC) also was encapsulated in the polymer network, enabling simultaneous release of DOX and CytC with differential release profiles driven by degradation-mediated release and Fickian diffusion, respectively. A bioactive nanocomposite hydrogel composed of hyaluronic acid and self-assembled pamidronate-magnesium nanoparticles (which acted as crosslinker for hyaluronic acid resulting in hybrid hydrogel formation within 30 sec) was developed for the localized elution and on-demand simultaneous release of bioactive ions (Mg2+) and a small drug (dexamethasone phosphate) following application in bone defects [41]. Osteogenic differentiation of the hydrogel-encapsulated human mesenchymal stem cells (hMSCs) and subsequent activation of alkaline phosphatase was promoted by the released magnesium ions. The activated alkaline phosphatase then catalyzed dephosphorylation (activation) of dexamethasone phosphate (a prodrug of dexamethasone) and expedited the drug release for the promotion of osteogenesis to enhance bone regeneration in the hydrogel implantation site. Biodegradable polycarbonate-based ABA triblock copolymers (synthesized via organocatalyzed ring-opening polymerization) were formulated into chemically cross-linked hydrogels by strain-promoted alkyne-azide cycloaddition [42]. Doxorubicin loaded catechol-functionalized polycarbonate micelles were encapsulated in the hydrogels to sustain the release of doxorubicin for one week (the cumulative drug release after 6 h in pH 7.4 media was 16±1 % and 79±3 % for micelles in hydrogels and free micelles, respectively). A pH-dependent doxorubicin release profile (i.e., higher release at pH 5.0 than at pH 7.4 due to doxorubicin’s higher solubility at lower pH) was evident with a corresponding reduction in MDA-MB-231 cell viability (< 25%). The significant alteration in MDA-MB-231 viability was attributable to accelerated drug release in the endolysosomes of cancer cells, ultimately enabling increased doxorubicin activity. These stimulus responsive behavior suggests the potential for such chemically degradable hybrid hydrogels as a potential platform for controlled and/or targeted delivery of multiple cargoes [41,43,44].
Figure 3:
A) Cumulative BSA release from 2% M-CHT spheres (blue circles) and spheroids (62% deformation) (red squares), up to 216 hours, with a zoomed-in view for the initial 2 hours (n = 3). Values plotted are the mean +/− error for 3 samples. Both experimental and curve fits using the Korsmeyer-Peppas equation are presented in the figure (lines with the same color of the corresponding symbols). Statistical significance of p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) are represented for time-points when applicable. (B) Numerical modelling studies for the release of a compound from spherical versus spheroidal particles with varying particle height. Variation in height of spheroidal particles (¾, ½, and ¼) is presented relative to spherical particles, corresponding to 1. Adapted from Bjørge et.al [64]. (Reproduced with permission from the Royal Society of Chemistry, Copyright 2018.)
Hydrogels incorporating nanoscale structures also have been studied for tuning cell behavior and responses through optimization of the mechanical properties or an enhancement in the stability of hydrogels [45]. Although the mechanical properties of hydrogel can be controlled by the density and chemistry of cross-links, highly cross-linked 3D hydrogels may limit cell proliferation, migration and morphogenesis. Therefore, nanoparticles including carbon-based nanostructures, dendrimers, and ceramic nanoparticles in hydrogels can be used for the development of biomimetic 3D environments enabling reconstruction of the complexity of native tissue for potential regenerative medicine and drug discovery applications [46,47]. Carbon-based nanostructures can be used to reinforce the mechanical properties of hydrogels [48]. Furthermore, the hybrid hydrogels can enhance cell proliferation [49,50] and differentiation [48] mediated by an increase in scaffold mechanical properties to match the properties of biological tissues such as bone, muscle and brain. Carbon-based nanocomposite hydrogels can also be used to engineer electrically conductive tissues such as muscles and cardiac tissues [26,48]. A 0.5 mg/ml carbon nanotube-gelatin methacrylate (CNT-GelMA) hydrogel improved compressive modulus by 3-fold when compared to 5% GelMA while facilitating cell survival and spreading [48]. Furthermore, the CNT-GelMA’s higher biocompatibility and modulation of cell morphology for hMSCs is expected to promote hMSC differentiation to skeletal muscular, osteoblastic, and neural lineages dependent on their stiffness [48]. An enhancement in cell proliferation was achieved by using primary amine-terminated polyamidoamine (PAMAM) dendrimer crosslinked collagen scaffolds [50]. The stability of naturally-derived proteins like collagen also plays a vital role in cell proliferation. A significantly higher cell proliferation rate was achieved for the dendrimer-collagen scaffolds (225%) as compared to the collagen scaffold alone (150%), attributable to the increased biostability of collagen nanofibers with dendrimers. Table 2 summarizes some of the examples of these hybrid hydrogels.
Table 2.
Hybrid hydrogels encapsulating nanoparticles for tuning cell behavior and responses.
Hybrid hydrogels incorporating microstructures
The addition of micron-sized particles or domains in a hydrogel network allows for better structural integrity and ultimately can serve as a method for directing cell behavior. It is well known that cells are sensitive to material properties such as elasticity, modulus, and pore size; therefore, taking advantage of these cell-modulating material properties is of great interest to develop materials that promote cell growth, proliferation, signaling, expression, and migration [56,57].
Despite the many outstanding properties of hydrogels for drug release and cell culture, they are typically isotropic materials that can only undergo uniform volumetric expansion and contraction in response to stimuli [58], in distinct contrast to the properties of ECM and various human tissues, which incorporate a vast array of microstructures such as spheres, tubules, and fibrils. Inspired by microscale structural motifs that occur naturally such as those mentioned, hybrid hydrogels that incorporate micron-sized domains have been developed to sustain cellular growth, promote microstructure-mediated cell migration, and release bioactive agents in a controlled fashion.
Much like nanoparticles, micron-sized particles can be covalently or non-covalently incorporated in a hydrogel network to yield increased mechanical strength, release bioactive molecules, and promote a variety of intended cellular responses. The mechanical properties of a hydrogel upon addition of graphene derivatives (GDs) in a matrix of peptide nanofibers was investigated for a set of hydrogels made from three different gelling β-sheet-forming, self-assembling peptides and five GDs with different surface chemistries to investigate the molecular interaction between the matrix and the filler [59]. By varying the physicochemical properties of the peptides and the surface chemistry of the GDs, the effect of coupled electrostatic and hydrophobic interactions and the subsequent outcome of those interactions on the modulus was determined [59]. When both the long-range electrostatic interactions and hydrophobic interactions are attractive, there is an increase in G’, and when the interactions are in competition it is the relative strength of each contribution that determines the result on G’ [59].
Overall, the release of bioactive molecules from hybrid-hydrogels can occur through one or more of three mechanisms: (1) burst release associated with the dissolution of absorbed drug on the hydrogel surface; (2) release due to drug diffusion through the hydrogel matrix and/or through the micro-particle; and (3) release due to degradation of the hydrogel [60]. These mechanisms for release of bioactive molecules offer handles that can be modulated to influence transport; accordingly, polymer network and particle composition can be manipulated to control mesh size and rates of chemical degradation, and bio-responsive domains can be incorporated to enable cell-responsive behavior [61,62]. For example, upon inclusion of vancomycin hydrochloride HMPC (Van-HMPC) nanoparticles in a chitosan/glycerophosphate (Ch/Gp) hydrogel, there was a notable effect of extending the release of Van, attributed to a diffusional mechanism of transport [60]. In this case, the release of the drug is slowed by having to diffuse through both the microparticle and the hydrogel network. In another application, the release of an active drug species was tailored through generation of a carbohydrate based injectable hydrogel containing anionically-functionalized microgels (acrylic acid-functionalized poly(NIPAM)) [63]. In this system, the release of the drug bupivacaine was not only controlled through diffusive contributions, such as the cross-linking density of the hydrogel, but also through the partitioning affinity of the drug between the bulk and microgel phases, which allowed the initial burst release to be minimized.
However, the ability to tune the release from and into micro-domains is constrained due to geometry. With increased particle diameter, diffusion is limited as spheres possess a lower surface area to volume ratio [64]. In order to account for this shortcoming, particles of different geometries can be made to enable increased surface area and a correspondingly increased range in potential drug release rates. One such example is the potential of using spheroid particles generated by squeezing chitosan droplets between two superamphiphobic surfaces, followed by UV-crosslinking, to increase the release rate of encapsulated BSA. BSA was encapsulated in the particles and faster release was observed from spheroids than from spheres, shown in Figure 3 [64].
The benefits of anisotropic microparticles, such as spheroids, ellipsoids, rods, and disks, also have been shown to extend to hemodynamic settings, with both theoretical modeling and experimental studies indicating better margination, wall interaction, and adhesion [65]. The enhancement of both diffusion and cell viability (demonstrated by the incorporation of spheroid particles) [64], as well as the potential hemodynamic transport benefits arising from anisotropic particles, suggests the potential of employing more complex and anisotropic particles in hybrid hydrogels. Non-circular micropatterned regions in hybrid hydrogels have also been shown to increase the absorption of toxins, thus increasing the detoxification efficacy of these materials [66]. Through hemolytic assays, it was shown that star shaped channels in a poly(ethylene glycol) diacrylate (PEGDA)/red blood cell membrane-coated nanoparticles (RBC-NPs) hybrid hydrogel resulted in relatively higher detoxification efficacy compared to circular channels, potentially due to the increased surface perimeter, multiple surface planes, and planar area [66].
Rather than the fabrication and subsequent incorporation of microparticles in hydrogels, a variety of other approaches have been used to produce hydrogels that include micron-sized domains such as liquid-liquid phase separation, emulsion stabilization, and photopatterning [67]. While all of these methods can yield desirable microstructure with various levels of control, the exploitation of phase separation has particular benefits, owing to the one-step fabrication, tunability, and specificity of the behavior [62]. These methods have been employed to produce microstructured resilin-like polypeptide (RLP)-PEG hydrogels in which liquid-liquid phase separation (LLPS) of the acrylate-functionalized RLP and PEG, combined with UV-crosslinking, is used to generate RLP-rich hydrogel microdomains that are dispersed in a PEG hydrogel matrix [62]. The RLP-PEG hydrogels support high cell viability as well as direct cell localization around RLP-rich domains. The domain size can be easily tuned by selection of the timepoint at which the phase-separating solutions are crosslinked after mixing, and selection of LLPS conditions can afford bicontinuous networks when crosslinking occurs during the initial stages of spinodal decomposition. Figure 4 below [62] demonstrates the tunability of the domain size of the microsphere structures with time and acrylate functionality. While this method is easily employed in situ, and offers tunability in terms of the size of the domains, the range of structures is limited.
Figure 4:
A) Schematic of hydrogel formation and microstructure development. B) Autofluorescence images of photo-crosslinked 10wt% 50/50 RLP-6Ac/PEG-4Ac and 10 wt% 50/50 RLP-2Ac/PEG-4Ac hydrogels. Microscale RLP-rich domains grow in diameter when gel precursors were incubated at room temperature for 0, 5, or 10 min prior to photo-crosslinking. Adapted from Lau et. al. [62]. (Reproduced with permission from John Wiley and Sons, Copyright 2018)
In order to develop hydrogels with detailed and specific microstructures, methods such as photopatterning and 3D printing have been used. However, techniques such as these can be labor-intensive and resolution-limited, which restricts production efficiency and fabrication flexibility [68]. In order to address these shortcomings, more rapid and equally accurate techniques are being explored, such as laser-mediated, UV photo-patterning [69]. Such photopatterning of gelatin hydrogels with riboflavin-5’phosphate as a non-toxic UV-photosensitizer has permitted more rapid generation of micropatterned substrates with low variability and spatial resolution commensurate with that of traditional photolithographic and micromolding methods [68]. These approaches could be used in conjunction with casting and crosslinking of polymeric materials to generate hybrid-hydrogels with finely tuned domains. Furthermore, micropatterned gels could be stacked in order to generate multilayered heterogeneous materials with embedded high-resolution microchannels that allow for perfusion [70]. By use of silicon carbide as an adhesive to facilitate strong binding (0.39 ± 0.03kPa) between micropatterned alginate and collagen hybrid hydrogel films, the result is a hydrogel with hollow microchannels (150 μm - 1 mm) that allows for high cell viability (90.61 ± 3.28%) when tested over a 7 day period [70].
Biomedical applications of hybrid hydrogels
Hybrid hydrogels offer expanded opportunities in biomedical applications by enabling modulation of microscale hydrogel properties (suitable for cell adhesion, migration, and proliferation) as well as the inclusion and tailoring of drug or gene delivery features (suitable for microenvironment-sensitive and targeted therapy) (Figure 5). They have been employed as therapeutic interventions in a variety of conditions including wound healing [71,72], osteogenesis [51,73], cancers [39,74], myocardial infarction [75], Parkinson’s disease [76], and infections [77,78] along with the development of biodevices and biosensors or contact lenses [79].
Figure 5:
Examples of types of hybrid hydrogel compositions and select biomedical applications.
Of particular interest are hydrogels that incorporate biologically inspired molecules and delivery methods. A collagen mimetic peptide (CMP)-polyplex mediated delivery of platelet-derived growth factor-BB (PDGF-BB) genes from collagen gels improved PDGF-BB expression and promoted a diverse range of cellular processes associated with wound healing, including proliferation, extracellular matrix production, and chemotaxis [72]. An injectable form of heat shock protein 27 (HSP27, molecular chaperones that protect heart muscle from ischemic injury) fused with TAT peptide and contained in a hybrid hydrogel system composed of poly(lactic-co-glycolic acid) (PLGA) microsphere and alginate hydrogel sustained the release of HSP27 for two weeks in vitro based on the pore size [75]. Intramyocardial injection of this hybrid hydrogel into a murine myocardial infarction model substantially suppressed apoptosis (3-fold decrease) in the infarction site, and improved the ejection fraction (2-fold increase), end-systolic volume (3-fold decrease), and maximum pressure development (1.3-fold increase) in the heart compared to PBS treatment [75]. A hydrolytically degradable hydrogel composed of PEG-b-poly(lactide)-b-dimethacrylate (PEG-b-PLA-b-DM)-containing, siRNA nanoparticles (siRNA/NPs) targeting Wwp1 (i.e., WWdomain-containing E3 ubiquitin protein ligase 1) was tested for sustained and localized delivery to bone fractures. These structures showed accelerated healing and increased bone formation in a murine mid-diaphyseal femur fracture model [80]. In another example, a phenylacrylamide nanoparticle hybrid hydrogel was prepared and abiotic hydrogel nanoparticles were engineered to bind to and modulate the activity of a diverse array of phospholipase A2 (PLA2) and three-finger toxin (3FTX) isoforms found in Elapidae snake venoms, thus inhibiting the dermonecrotic activity of the venom in a dose-dependent manner [81], with potential to limit local tissue damage following a snake bite.
The development of biohybrid drug carriers to deliver various cargo to targeted sites is an area of research that could overcome limitations of synthetic nanoparticles such as suboptimal distribution, innate toxicity, and limited transport through biological barriers [82]. A noncolvalent-bonded hybrid hydrogel was assembled by utilizing cucurbit[7]uril as a supramolecular linker to “stick” superparamagnetic γ-Fe2O3 nanoparticles onto the polymer backbone of catechol-functionalized chitosan [83]. The γ-Fe2O3 nanoparticles displayed vibrational movement (to promote chemotherapy release) and heat generation under an alternating magnetic field supporting both thermo- and chemotherapy modalities in vitro and in vivo in xenograft models bearing HeLa cells. An advanced hybrid hydrogel system comprised of RBC membrane wrapped PLGA polymeric nanoparticles loaded in acrylamide gel was developed for the antivirulence treatment of local bacterial infection [77]. In a methicillin resistant Staphylococcus aureus (MRSA) subcutaneous mouse model, treatment with the nanosponge-hybrid hydrogel markedly reduced MRSA skin lesion development, attributed to the retention of pore forming toxins within the hydrogels and their subsequent neutralization [77]. No involvement of antibiotics in this unique treatment broadens the treatment area to antibiotic resistant bacterial infections and also rules out the possibility of the development of any new bacterial resistance.
However, hybrid-hydrogels are not limited in their biomedical application to only clinical implementation. Hybrid-hydrogels could address the need for in vitro systems that more closely represent physiological microenvironments for applications such as drug-screening and disease models or disease detection [84,85]. A versatile hydrogel containing DNA-capped gold nanoparticles for simultaneous and sensitive imaging of intracellular multiplex miRNAs was developed using a toe-hold strand-displacement reactions and hairpin-locked, DNAzyme-assisted miRNA recycling for dual signal amplification [86]. A wide linear range, low limit of detection, and good selectivity for simultaneous detection of multiple miRNAs was exhibited by the hybrid hydrogels. Given the abundance of intracellular cancer-related miRNAs in different cancer types, these structures pose enormous potential in accurate and sensitive differentiation of cancer cells. In order to study the delivery of soluble molecules to target cells in their native environment, in vitro systems that utilize hydrogels with tunable chemical, physical, and mechanical properties that match native tissues can be used to determine cellular response [87]. In order to probe the nuanced relationship between the delivery of platelet derived growth factor-AA (PDGF-AA) and oligodendrocyte precursor cell (OPC) fate, a PEG-based hydrogel with encapsulated PLGA microparticles containing (PDGF-AA) was used to simulate seven different release schemes and study the effect of each on OPCs cultured in the hybrid hydrogel [87]. The results of these experiments support the hypothesis that burst release followed by withdrawal of PDGF-AA results in survival, proliferation, and differentiation of OCPs in the hybrid hydrogel and that OPC fate is dependent on release kinetics, rather than the amount of PDGF-AA delivered [87].
Table 3 summarizes studies involving a range of potential medical applications of hybrid hydrogels; these materials could also be used to as in vitro models of physiological microenvironments for applications such as drug screening [84,85].
Table 3:
Hybrid hydrogels and their reported biomedical applications
| Hybrid hydrogel components | Nano/microstructure incorporation strategy | Nano/microstructure or drug release mechanism | Targeted disease | Potential outcome | Reference |
|---|---|---|---|---|---|
| Sodium alginate/PLGA microspheres loaded with HSP27 (heat shock protein 27) fused to TAT peptide | Physical incorporation of microspheres followed by alginate crosslinking with calcium sulfate | Pore size regulated HSP27 release from microspheres | Myocardial infarction | Suppression of apoptosis and improved ejection fraction, end-systolic volume and maximum pressure developement in the heart | [75] |
| Sodium alginate/3D Ormocomp scaffold | Sodium alginate solution along with human adipose-derived stem cells (ASCs) were added to 3D scaffold and crosslinked using ionic cross-linking or RGD based crosslinking method | The gel containing scaffolds are stable for eight weeks and ASCs produced dopamine | Parkinson’s disease | Prevention of immune rejection of the non-autologous cells by the extremely small pore size of the alginate gel; higher dopamine secretion by hybrid hydrogel was evident as compared to conventional alginate hydrogel | [76] |
| PECA [poly(ε-caprolactone)-acryloyl chloride]/COS-GMA (glycidylmethacrylated chitooligosachharide)/NIPAm (N-isopropylacrylamide)/AAm (acrylamide)/Gold nanorods (GNR)/doxorubicin | Physical encapsulation of GNRs and doxorubicin in hydrogels developed by heat-initiated free radical polymerization using ammonium persulfate as a heat initiator | Temperature and pH dependent doxorubicin release profile; NIR laser irradiation of GNRs increased the temperature and thus improved doxorubicin release | Breast cancer | Significantly reduced postoperative tumor recurrence in in vivo mouse model | [74] |
| Acrylamide/RBC membrane coated PLGA nanoparticles (nanosponges) | Physical encapsulation of nanosponges before gelation | Pore forming α-toxin absorption by nanosponges | Methicillin resistant Staphyloco ccus aureus (MRSA) infection | Effective detoxification with marked reduction in MRSA skin lesion development in mouse models | [77] |
| Quaternized chitosan (HTCC)/silver nanoparticles/graphene oxide (GO)/voriconazole | Physical encapsulation of silver nanoparticles and GO in HTCC solution; electrostatic interactions between GO and HTCC resulted in hydrogel crosslinking | Voriconazole is loaded onto GO by Π-Π stacking interactions and released slowly | Fungal keratitis | Enhanced antibacterial properties in vitro and anti-fungal properties in vivo in fungal keratitis mouse model | [79] |
| Rapamycin-loaded unimolecular micelles/PLGA-PEG-PLGA | Physical dispersion in triblock gel | Diffusion of rapamycin from the unilamellar micelle dispersed triblock gel | Prevention of neointima-caused re(stenosis) after open surgery such as bypass surgery | Sustained rapamycin release for four months; inhibition of re(stenosis) by 80% even after three months as compared to no drug treatment | [88] |
| Carboxymethyl chitosan (CC)/Aldehyde hyaluronic acid (AHA)/VEGF loaded porous PLGA micro sphere/vancom ycin | Physical encapsulation of VEGF loaded porous PLGA microspheres in CC/AHA hydrogel | Vancomycin linked to injectable hydrogel via the reversible Schiffs base reaction is released by change in the pH from netural to acidic in infected wounds; VEGF release was dependent on the pore size of PLGA microspheres | Non-healing infected wounds | Inhibited bacterial growth; accelerated vein endothelial cell proliferation with reduced inflammation; promoted angiogenesis. | [78] |
| Chondriotin sulfate (CS) and poly(ethylene glycol) (PEG) | FXIIIa-mediated crosslinking of CS-Mal grafted with MMP-Lys and PEG-Gln | The degree of CS grafting with MMP-Lys and stoichiometry of the hydrogel components dictated hydrogel properties, and the gel was degradable by chondriotinase and MMP to promote cell proliferation, migration, and viability. | Osteogenesis | Tuned growth factor binding and release; generated a cell instructive matrix; promoted stem cell proliferation and osteogenic differentiation. | [73] |
Conclusions and future perspectives
The incorporation of nano/microstructures in hydrogel formulations allows for the generation of hybrid hydrogels that can achieve a multitude of functionalities for applications in biological systems. Incorporation of particles and fabrication of domains not only allows for targeted drug therapy, tuned cellular response, and stimuli-responsive material behavior, but also allows for improved mechanical and physical properties. As the development of hybrid-hydrogels continues, the design of new systems will continue to be inspired by biological structures and motifs that closely resemble the environment for which they are intended. Hybrid hydrogels that are easily synthesized by one-step methods to achieve this level of architecture will be influential to the field, as well as those that can be precisely tuned in a modular fashion for the desired application. The potential to generate materials through facile methods that mimic the native environment of cells for a wide range of biomedical applications is germane to the field. As innovation and research progresses in the creation of these hybrid hydrogels, advances in chemical, biomedical, and materials engineering as well as chemistry, biology, and medicine is inevitable.
Figure 2.
Schematic representation of the formation and degradation of the liposome cross-linked hybrid hydrogels. Adapted from Liang and Kiick [40]. (Reproduced with permission from the American Chemical Society, Copyright 2016.)
Highlights.
Nano/microstructures can be incorporated into the hydrogels by various physical or chemical cross-linking methods including click reactions, liquid-liquid phase separation (LLPS), photopatterning, and 3D printing.
Nano/microstructures can enhance the mechanical properties of the hydrogel, control small molecule drug delivery, promote stimuli-responsive behavior, and tune cell behavior and responses.
Hybrid hydrogels have potential applications in drug discovery, drug/gene delivery, development of in vitro testing methods, and regenerative medicine.
Acknowledgments
Related work in the authors’ laboratories has been partially supported by grants from the National Institutes of Health (R01 AR067247A, RO1 DC011377A, and for instrument support: S10 OD016361, S10 RR027273, 1 P30 GM110758–01), the National Science Foundation (PFI-1700980, DMR 1609544), and the University of Delaware Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DECLARATION CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of oustanding interest
- [1].Peppas NA, Bures P, Leobandung W, Ichikawa H, Hydrogels in pharmaceutical formulations, Eur. J. Pharm. Biopharm 50 (2000) 27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- [2].Wichterle O, LÍM D, Hydrophilic gels for biological use, Nature 185 (1960) 117. doi: 10.1038/185117a0. [DOI] [Google Scholar]
- [3].Hoare TR, Kohane DS, Hydrogels in drug delivery: Progress and challenges, Polymer (Guildf) 49 (2008) 1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- [4].Caló E, Khutoryanskiy VV, Biomedical applications of hydrogels: A review of patents and commercial products, Eur. Polym. J 65 (2015) 252–267. doi: 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- [5].Censi R, Vermonden T, Deschout H, Braeckmans K, di Martino P, De Smedt SC, van Nostrum CF, Hennink WE, Photopolymerized thermosensitive poly(HPMAlactate)-PEG-based hydrogels: Effect of network design on mechanical properties, degradation, and release behavior, Biomacromolecules 11 (2010) 2143–2151. doi: 10.1021/bm100514p. [DOI] [PubMed] [Google Scholar]
- [6].Jin R, Hiemstra C, Zhong Z, Feijen J, Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates, Biomaterials 28 (2007) 2791–2800. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- [7].DeForest CA, Anseth KS, Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions, Nat. Chem 3 (2011) 925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koshy ST, Desai RM, Joly P, Li J, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ, Click-crosslinked injectable gelatin hydrogels, Adv. Healthc. Mater 5 (2016) 541–547. doi: 10.1002/adhm.201500757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baldwin AD, Kiick KL, Tunable degradation of maleimide–thiol adducts in reducing environments, Bioconjug. Chem 22 (2011) 1946–1953. doi: 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R, Novel hydrogels via click chemistry: Synthesis and potential biomedical applications, Biomacromolecules 8 (2007) 1844–1850. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- [11].Zhu W, Xiong L, Wang H, Zha G, Du H, Li X, Shen Z, Sustained drug release from an ultrathin hydrogel film, Polym. Chem 6 (2015) 7097–7099. doi: 10.1039/C5PY01204J. [DOI] [Google Scholar]
- [12].Jiang H, Qin S, Dong H, Lei Q, Su X, Zhuo R, Zhong Z, An injectable and fast-degradable poly(ethylene glycol) hydrogel fabricated via bioorthogonal strain-promoted azide–alkyne cycloaddition click chemistry, Soft Matter 11 (2015) 6029–6036. doi: 10.1039/C5SM00508F. [DOI] [PubMed] [Google Scholar]
- [13].Fan Y, Deng C, Cheng R, Meng F, Zhong Z, In situ forming hydrogels via catalyst-free and bioorthogonal “tetrazole–alkene” photo-click chemistry, Biomacromolecules 14 (2013) 2814–2821. doi: 10.1021/bm400637s. [DOI] [PubMed] [Google Scholar]
- [14].Fan M, Ma Y, Zhang Z, Mao J, Tan H, Hu X, Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering, Mater. Sci. Eng. C 56 (2015) 311–317. doi: 10.1016/j.msec.2015.04.004. [DOI] [PubMed] [Google Scholar]
- [15].Mukherjee S, Hill MR, Sumerlin BS, Self-healing hydrogels containing reversible oxime crosslinks, Soft Matter 11 (2015) 6152–6161. doi: 10.1039/C5SM00865D. [DOI] [PubMed] [Google Scholar]
- [16].Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z, Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering, Biomaterials 35 (2014) 4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [17].Jia X, Kiick KL, Hybrid multicomponent hydrogels for tissue engineering, Macromol. Biosci 9 (2009) 140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA, Hydrogels in regenerative medicine, Adv. Mater 21 (2009) 3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chivers PRA, Smith DK, Spatially-resolved soft materials for controlled release-hybrid hydrogels combining a robust photo-activated polymer gel with an interactive supramolecular gel, Chem. Sci 8 (2017) 7218–7227. doi: 10.1039/c7sc02210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, Zhang S, Benoit DSW, Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery, J. Control. Release 287 (2018) 58–66. doi: 10.1016/j.jconrel.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Babu SS, Praveen VK, Ajayaghosh A, Functional π-gelators and their applications, Chem. Rev 114 (2014) 1973–2129. doi: 10.1021/cr400195e. [DOI] [PubMed] [Google Scholar]
- [22].Amabilino DB, Smith DK, Steed JW, Supramolecular materials, Chem. Soc. Rev 46 (2017) 2404–2420. doi: 10.1039/c7cs00163k. [DOI] [PubMed] [Google Scholar]
- [23].Jonker AM, Löwik DWPM, van Hest JCM, Peptide- and protein-based hydrogels, Chem. Mater 24 (2012) 759–773. doi: 10.1021/cm202640w. [DOI] [Google Scholar]
- [24].Lau HK, Kiick KL, Opportunities for multicomponent hybrid hydrogels in biomedical applications, Biomacromolecules 16 (2015) 28–42. doi: 10.1021/bm501361c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maisani M, Pezzoli D, Chassande O, Mantovani D, Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment?, J. Tissue Eng 8 (2017) 204173141771207. doi: 10.1177/2041731417712073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan K-T, Palacios T, Dokmeci MR, Bae H, Tang XS, Khademhosseini A, Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators, ACS Nano 7 (2013) 2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK, Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach, ACS Nano 9 (2015) 3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- [28].Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ, Nanoparticle-hydrogel composites: Concept, design, and applications of these promising, multi-functional materials, Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger 2 (2015) 1400010. doi: 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo R, Du X, Zhang R, Deng L, Dong A, Zhang J, Bioadhesive film formed from a novel organic–inorganic hybrid gel for transdermal drug delivery system, Eur. J. Pharm. Biopharm 79 (2011) 574–583. doi: 10.1016/j.ejpb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Meng H, Konst S, Sarmiento R, Rajachar R, Lee BP, Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity, ACS Appl. Mater. Interfaces 6 (2014) 16982–16992. doi: 10.1021/am504566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jaiswal MK, Xavier JR, Carrow JK, Desai P, Alge D, Gaharwar AK, Mechanically stiff nanocomposite hydrogels at ultralow nanoparticle content, ACS Nano 10 (2016) 246–256. doi: 10.1021/acsnano.5b03918. [DOI] [PubMed] [Google Scholar]
- [32].Marcelo G, López-González M, Mendicuti F, Tarazona MP, Valiente M, Poly(N-isopropylacrylamide)/gold hybrid hydrogels prepared by catechol redox chemistry. Characterization and smart tunable catalytic activity, Macromolecules 47 (2014) 6028–6036. doi: 10.1021/ma501214k. [DOI] [Google Scholar]
- [33].McKenzie M, Betts D, Suh A, Bui K, Kim DL, Cho H, Hydrogel-based drug delivery systems for Poorly water-soluble drugs, Molecules 20 (2015). doi: 10.3390/molecules201119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu C, Yoganathan RB, Kociolek M, Allen C, Hydrogel containing silica shell cross-linked micelles for ocular drug delivery, J. Pharm. Sci 102 (2013) 627–637. doi: 10.1002/jps.23390. [DOI] [PubMed] [Google Scholar]
- [35].Liu T, Wu T, Liu H, Ke B, Huang H, Jiang Z, Xie M, Ultraviolet-crosslinked hydrogel sustained-release hydrophobic antibiotics with long-term antibacterial activity and limited cytotoxicity, J. Appl. Polym. Sci 131 (2014) 1–8. doi: 10.1002/app.40438. [DOI] [Google Scholar]
- [36].Schoener CA, Hutson HN, Peppas NA, pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the delivery of hydrophobic therapeutic agents, Polym. Int 61 (2012) 874–879. doi: 10.1002/pi.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bini RA, Silva MF, Varanda LC, da Silva MA, Dreiss CA, Soft nanocomposites of gelatin and poly(3-hydroxybutyrate) nanoparticles for dual drug release, Colloids Surfaces B Biointerfaces 157 (2017) 191–198. doi: 10.1016/j.colsurfb.2017.05.051. [DOI] [PubMed] [Google Scholar]
- [38].Davoodi P, Ng WC, Srinivasan MP, Wang CH, Codelivery of anti-cancer agents via double-walled polymeric microparticles/injectable hydrogel: A promising approach for treatment of triple negative breast cancer, Biotechnol. Bioeng 114 (2017) 2931–2946. doi: 10.1002/bit.26406.• • Uniform core-shell microparticles encapsulating cisplatin and paclitaxel were fabricated using coaxial electrohydrodynamic atomization technique and subsequently are embedded into an injectable hydrogel that provides a promising strategy to treat aggressive cancers and a modular platform for a broad range of localized multidrug therapies.
- [39].Li X, Su X, Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy, J. Mater. Chem. B 6 (2018) 4714–4730. doi: 10.1039/c8tb01078a. [DOI] [PubMed] [Google Scholar]
- [40].Liang Y, Kiick KL, Liposome-cross-linked hybrid hydrogels for glutathione-triggered delivery of multiple cargo molecules, Biomacromolecules 17 (2016) 601–614. doi: 10.1021/acs.biomac.5b01541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang K, Jia Z, Yang B, Feng Q, Xu X, Yuan W, Li X, Chen X, Duan L, Wang D, Bian L, Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration, Adv. Sci 1800875 (2018). doi: 10.1002/advs.201800875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ono RJ, Lee ALZ, Voo ZX, Venkataraman S, Koh BW, Yang YY, Hedrick JL, Biodegradable strain-promoted click hydrogels for encapsulation of drug-loaded nanoparticles and sustained release of therapeutics, Biomacromolecules 18 (2017) 2277–2285. doi: 10.1021/acs.biomac.7b00377.• Introduced a simple method for formulating hydrogels incorporating drug loaded micelles, using a strain-promoted click reaction, to achieve sustained drug release profiles.
- [43].Wang Q, Zhang H, Xu H, Zhao Y, Li Z, Li J, Wang H, Zhuge D, Guo X, Xu H, Jones S, Li X, Jia X, Xiao J, Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair, Theranostics 8 (2018) 4429–4446. doi: 10.7150/thno.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lyu D, Chen S, Guo W, Liposome crosslinked polyacrylamide/DNA hydrogel: A smart controlled-release system for small molecular payloads, Small 14 (2018) 1–8. doi: 10.1002/smll.201704039. [DOI] [PubMed] [Google Scholar]
- [45].Wu S, Duan B, Liu P, Zhang C, Qin X, Butcher JT, Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds, ACS Appl. Mater. Interfaces 8 (2016) 16950–16960. doi: 10.1021/acsami.6b05199. [DOI] [PubMed] [Google Scholar]
- [46].Lutolf MP, Gilbert PM, Blau HM, Designing materials to direct stem-cell fate, Nature 462 (2009) 433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kumachev A, Greener J, Tumarkin E, Eiser E, Zandstra PW, Kumacheva E, High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation, Biomaterials 32 (2011) 1477–1483. doi: 10.1016/j.biomaterials.2010.10.033. [DOI] [PubMed] [Google Scholar]
- [48].Shin SR, Bae H, Cha JM, Mun JY, Chen YC, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S, Khademhosseini A, Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation, ACS Nano 6 (2012) 362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Agarwal S, Zhou X, Ye F, He Q, Chen GCK, Soo J, Boey F, Zhang H, Chen P, Interfacing live cells with nanocarbon substrates, Langmuir 26 (2010) 2244–2247. doi: 10.1021/la9048743. [DOI] [PubMed] [Google Scholar]
- [50].Zhong S, Yung LYL, Enhanced biological stability of collagen with incorporation of PAMAM dendrimer, J. Biomed. Mater. Res. - Part A 91 (2009) 114–122. doi: 10.1002/jbm.a.32188. [DOI] [PubMed] [Google Scholar]
- [51].Heo DN, Ko W-K, Bae MS, Lee JB, Lee D-W, Byun W, Lee CH, Kim E-C, Jung B-Y, Kwon IK, Enhanced bone regeneration with a gold nanoparticle–hydrogel complex, J. Mater. Chem. B 2 (2014) 1584–1593. doi: 10.1039/C3TB21246G. [DOI] [PubMed] [Google Scholar]
- [52].Paul A, Hasan A, Al Kindi H, Gaharwar AK, Rao VTS, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A, Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair, ACS Nano 8 (2014) 8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].El-Fiqi A, Lee JH, Lee E-J, Kim H-W, Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering, Acta Biomater 9 (2013) 9508–9521. doi: 10.1016/j.actbio.2013.07.036. [DOI] [PubMed] [Google Scholar]
- [54].Song W, Markel DC, Jin X, Shi T, Ren W, Poly(vinyl alcohol)/collagen/hydroxyapatite hydrogel: Properties and in vitro cellular response, J. Biomed. Mater. Res. Part A 100A (2012) 3071–3079. doi: 10.1002/jbm.a.34240. [DOI] [PubMed] [Google Scholar]
- [55].Zhou J, Yang X, Liu W, Wang C, Shen Y, Zhang F, Zhu H, Sun H, Chen J, Lam J, Mikos AG, Wang C, Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct, Theranostics 8 (2018) 3317–3330. doi: 10.7150/thno.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vikingsson L, Claessens B, Gómez-Tejedor JA, Gallego Ferrer G., Gómez Ribelles J.L., Relationship between micro-porosity, water permeability and mechanical behavior in scaffolds for cartilage engineering, J. Mech. Behav. Biomed. Mater 48 (2015) 60–69. doi: 10.1016/j.jmbbm.2015.03.021. [DOI] [PubMed] [Google Scholar]
- [57].Bauer A, Gu L, Kwee B, Li WA, Dellacherie M, Celiz AD, Mooney DJ, Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts, Acta Biomater 62 (2017) 82–90. doi: 10.1016/j.actbio.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jeon SJ, Hauser AW, Hayward RC, Shape-morphing materials from stimuli-responsive hydrogel hybrids, Acc. Chem. Res 50 (2017) 161–169. doi: 10.1021/acs.accounts.6b00570. [DOI] [PubMed] [Google Scholar]
- [59].Wychowaniec JK, Iliut M, Zhou M, Moffat J, Elsawy MA, Pinheiro WA, Hoyland JA, Miller AF, Vijayaraghavan A, Saiani A, Designing peptide/graphene hybrid hydrogels through fine-tuning of molecular interactions, Biomacromolecules 19 (2018) 2731–2741. doi: 10.1021/acs.biomac.8b00333. [DOI] [PubMed] [Google Scholar]
- [60].Mahmoudian M, Ganji F, Vancomycin-loaded HPMC microparticles embedded within injectable thermosensitive chitosan hydrogels, Prog. Biomater 6 (2017) 49–56. doi: 10.1007/s40204-017-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rehmann MS, Skeens KM, Kharkar PM, Ford EM, Maverakis E, Lee KH, Kloxin AM, Tuning and predicting mesh size and protein release from step growth hydrogels, Biomacromolecules 18 (2017) 3131–3142. doi: 10.1021/acs.biomac.7b00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lau HK, Paul A, Sidhu I, Li L, Sabanayagam CR, Parekh SH, Kiick KL, Microstructured elastomer-PEG hydrogels via kinetic capture of aqueous liquid–liquid phase separation, Adv. Sci 5 (2018) 1–13. doi: 10.1002/advs.201701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sivakumaran D, Maitland D, Hoare T, Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery, Biomacromolecules 12 (2011) 4112–4120. doi: 10.1021/bm201170h. [DOI] [PubMed] [Google Scholar]
- [64].Bjørge IM, Costa AMS, Silva AS, Vidal JPO, Nóbrega JM, Mano JF, Tuneable spheroidal hydrogel particles for cell and drug encapsulation, Soft Matter 14 (2018) 5622–5627. doi: 10.1039/C8SM00921J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sen Gupta A, Role of particle size, shape, and stiffness in design of intravascular drug delivery systems: Insights from computations, experiments, and nature, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 8 (2016) 255–270. doi: 10.1002/wnan.1362. [DOI] [PubMed] [Google Scholar]
- [66].Chen MS, Zhang Y, Zhang L, Fabrication and characterization of a 3D bioprinted nanoparticle-hydrogel hybrid device for biomimetic detoxification, Nanoscale 9 (2017) 14506–14511. doi: 10.1039/c7nr05322c. [DOI] [PubMed] [Google Scholar]
- [67].Garcia Garcia C., Kiick KL, Methods for producing microstructured hydrogels for targeted applications in biology, Acta Biomater (2018). doi: 10.1016/j.actbio.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nawroth JC, Scudder LL, Halvorson RT, Tresback J, Ferrier JP, Sheehy SP, Cho A, Kannan S, Sunyovszki I, Goss JA, Campbell PH, Parker KK, Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications, Biofabrication 10 (2018). doi: 10.1088/1758-5090/aa96de.• Developed a method to rapidly generate patterned gelatin hydrogels through laser-mediated, UV photo-patterning with riboflavin-5’phosphate as a non-toxic UV-photosensitizer with low variability and spatial resolution commensurate with that of traditional photolithographic and micromolding methods.
- [69].Duan B, Xu C, Das S, Chen JM, Butcher JT, Spatial regulation of valve interstitial cell phenotypes within three-dimensional micropatterned hydrogels, ACS Biomater. Sci. Eng (2019) 10.1021/acsbiomaterials.8b01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Attalla R, Ling CSN, Selvaganapathy PR, Silicon carbide nanoparticles as an effective bioadhesive to bond collagen containing composite gel layers for tissue engineering applications, Adv. Healthc. Mater 7 (2018) 1–9. doi: 10.1002/adhm.201701385.• Developed micropatterned alginate and collagen hybrid hydrogel films, with hollow microchannels (150 μm-1 mm) by using of silicon carbide as an adhesive to facilitate strong-binding (0.39 ± 0.03kPa) between stacked gels.
- [71].Ghavaminejad A, Park CH, Kim CS, In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application, Biomacromolecules 17 (2016) 1213–1223. doi: 10.1021/acs.biomac.6b00039. [DOI] [PubMed] [Google Scholar]
- [72].Urello MA, Kiick KL, Sullivan MO, Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides, Bioeng. Transl. Med 1 (2016) 207–219. doi: 10.1002/btm2.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Anjum F, Lienemann PS, Metzger S, Biernaskie J, Kallos MS, Ehrbar M, Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation, Biomaterials 87 (2016) 104–117. doi: 10.1016/j.biomaterials.2016.01.050. [DOI] [PubMed] [Google Scholar]
- [74].Qu Y, Chu BY, Peng JR, Liao JF, Qi TT, Shi K, Zhang XN, Wei YQ, Qian ZY, A biodegradable thermo-responsive hybrid hydrogel: therapeutic applications in preventing the post-operative recurrence of breast cancer, Npg Asia Mater 7 (2015) e207. doi:10.1038/am.2015.8310.1038/am.2015.83https://www.nature.com/articles/am201583#supplementary-informationhttps://www.nature.com/articles/am201583#supplementary-information . [Google Scholar]
- [75].Lee J, Cha M-J, Lim KS, Kim J-K, Lee S-K, Kim Y-H, Hwang K-C, Lee KY, Injectable microsphere/hydrogel hybrid system containing heat shock protein as therapy in a murine myocardial infarction model, J. Drug Target 21 (2013) 822–829. doi: 10.3109/1061186X.2013.829072. [DOI] [PubMed] [Google Scholar]
- [76].Kang KS, Lee S-I, Hong JM, Lee JW, Cho HY, Son JH, Paek SH, Cho D-W, Hybrid scaffold composed of hydrogel/3D-framework and its application as a dopamine delivery system, J. Control. Release 175 (2014) 10–16. doi: 10.1016/j.jconrel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- [77].Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang Q, Hu C-MJ, Fang RH, Copp JA, Pornpattananangkul D, Lu W, Zhang L, Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection, Adv. Mater 27 (2015) 3437–3443. doi: 10.1002/adma.201501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Huang J, Ren J, Chen G, Li Z, Liu Y, Wang G, Wu X, Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds, Mater. Sci. Eng. C 89 (2018) 213–222. doi: 10.1016/j.msec.2018.04.009. [DOI] [PubMed] [Google Scholar]
- [79].Huang J-F, Zhong J, Chen G-P, Lin Z-T, Deng Y, Liu Y-L, Cao P-Y, Wang B, Wei Y, Wu T, Yuan J, Jiang G-B, A hydrogel-based hybrid theranostic contact lens for fungal keratitis, ACS Nano 10 (2016) 6464–6473. doi: 10.1021/acsnano.6b00601. [DOI] [PubMed] [Google Scholar]
- [80].Wang Y, Malcolm DW, Benoit DSW, Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing, Biomaterials 139 (2017) 127–138. doi: 10.1016/j.biomaterials.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].O’Brien J, Lee SH, Gutiérrez JM, Shea KJ, Engineered nanoparticles bind elapid snake venom toxins and inhibit venom-induced dermonecrosis, PLoS Negl. Trop. Dis 12 (2018) e0006736. doi: 10.1371/journal.pntd.0006736.• Developed a unique method of controlling local tissue damage following snake bite using phenylacrylamide nanoparticle hybrid hydrogel.
- [82].Banskota S, Yousefpour P, Chilkoti A, Cell-based biohybrid drug delivery systems: The best of the synthetic and natural worlds, Macromol. Biosci 17 (2017) 1–16. doi: 10.1002/mabi.201600361. [DOI] [PubMed] [Google Scholar]
- [83].Qiao H, Jia J, Chen W, Di B, Scherman OA, Hu C, Magnetic regulation of Thermo-chemotherapy from a cucurbit[7]uril-crosslinked hybrid hydrogel, Adv. Healthc. Mater 1801458 (2018) 1801458. doi: 10.1002/adhm.201801458.• • Developed an iron oxide nanoparticles incorporated cucurbit[7]uril-crosslinked hybrid hydrogel for magnetic regulation of thermos-chemotherapy for potential cancer treatments.
- [84].Luni C, Serena E, Elvassore N, Human-on-chip for therapy development and fundamental science, Curr. Opin. Biotechnol 25 (2014) 45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- [85].Lee H, Cho DW, One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology, Lab Chip 16 (2016) 2618–2625. doi: 10.1039/c6lc00450d. [DOI] [PubMed] [Google Scholar]
- [86].Meng X, Zhang K, Dai W, Cao Y, Yang F, Dong H, Zhang X, Multiplex microRNA imaging in living cells using DNA-capped-Au assembled hydrogels, Chem. Sci 9 (2018) 7419–7425. doi: 10.1039/c8sc02858c.• • Explained a unique way of non-invasively imaging multiplex microRNAs in living cells using DNA-capped gold nanoparticles assembled hydrogels for potential application in cancer detection.
- [87].Pinezich MR, Russell LN, Murphy NP, Lampe KJ, Encapsulated oligodendrocyte precursor cell fate is dependent on PDGF-AA release kinetics in a 3D microparticle-hydrogel drug delivery system, J. Biomed. Mater. Res. - Part A 106 (2018) 2402–2411. doi: 10.1002/jbm.a.36432.• • Investigated the kinetics of delivering platelet derived growth factor using hydrogels with various release profiles and studied the subsequent effect on oligodendrocyte precursor cells.
- [88].Chen G, Shi X, Wang B, Xie R, Guo L-W, Gong S, Kent KC, Unimolecular micelle-based hybrid system for perivascular drug delivery produces long-term efficacy for neointima attenuation in rats, Biomacromolecules 18 (2017) 2205–2213. doi: 10.1021/acs.biomac.7b00617. [DOI] [PMC free article] [PubMed] [Google Scholar]