Abstract
Background:
Chronic opiate use leads to a sensitized behavioral response to acute pain, which in turn, leads to escalating doses of opiates. This study was designed to test the hypothesis that chronic opiate usage is also associated with a sensitized neurobiological response to acute pain in individuals that have used prescription opiates for 6 or more months.
Methods:
Fourteen patients with non-alcoholic chronic pancreatitis that have been taking prescription opiates for 6 or more months and 14 gender matched, non-opiate using controls were enrolled. Functional neuroimaging data was acquired while participants received blocks of thermal stimulation to their wrist (individually-tailored to their pain threshold).
Results:
Self-reported pain was significantly greater in opiate using patients (3.4±3.4) than controls (0.2±0.8: Brief Pain Inventory p<0.005), however no significant difference between groups was observed in the individually-tailored pain thresholds. Opiate using patients evidenced a significantly greater response to pain than controls in two established nodes of the “Pain Matrix”: somatosensory cortex (pFWE≤0.001) and anterior cingulate cortex (p≤0.01). This response was positively correlated with prescribed morphine equivalent dosages (average: 133.5±94.8mg/day).
Conclusion:
The findings suggest that in chronic pancreatitis patients, a dose of opiates that normalizes their behavioral response to acute pain is associated with an amplified neural response to acute pain. Further longitudinal studies are needed to determine if this neural sensitization hastens a behavioral tolerance to opiates or the development of an opioid use disorder.
Keywords: Pain, fMRI, Prescription Opiates, Opioids, Hyperalgesia
1. Introduction
In 2014, there were 245 million prescriptions for opiates written in the United States (Volkow and McLellan, 2016). Unfortunately, the widespread availability of these powerful analgesic drugs has led to a public health crisis and increases in mortality and morbidity associated with chronic prescribing. In 2015, 33,000 individuals had fatal overdoses caused by licit and illicit opioids (Rudd et al., 2016). Despite recent success in reducing the overall number of prescriptions, prescribing rates have remained high (Guy et al., 2017). The increased availability of opiates places many individuals at risk of conversion to opiate use disorder (Volkow et al., 2018), and with chronic use individuals are susceptible to a paradoxical increased sensitivity to pain known as opioid-induced hyperalgesia (Lee et al., 2011; Nusrat et al., 2012).
Decades of preclinical work has elucidated several mechanisms by which opiate usage can lead to states of hyperalgesia (Angst and Clark, 2006; Ossipov et al., 2005; Roeckel et al., 2016; Simonnet and Rivat, 2003). One commonly uncovered mechanism operating at the peripheral and spinal levels is NMDA-dependent, long-term potentiation (LTP) (Drdla et al., 2009; Zhou et al., 2010) at nociceptive afferents. These findings have been translated to clinical practice, with meta-analyses supporting the effectiveness of ketamine, an NMDA antagonist, in reducing post-surgical pain (Wu et al., 2015). Preclinical work has also uncovered alterations at the supraspinal level, with evidence for facilitory, pronociceptive activity within the rostral ventromedial medulla and periaqueductal gray (Rivat et al., 2009; Vanderah et al., 2001), as well as increases in protein kinase activity across the cortex (Sanna et al., 2014). In humans, the supraspinal mechanisms through which chronic prescription opiate usage alters brain reactivity to pain are not well understood, though neuroimaging is uncovering the regions involve in pain processing. Functional magnetic resonance imaging (fMRI) studies of acute pain in healthy individuals demonstrate that there is a reliable network of brain regions (the “Pain Matrix”) which are engaged by an acutely painful stimulus (Apkarian et al., 2005; Cauda et al., 2014; Tanasescu et al., 2016; Wager et al., 2013). These brain regions include: (1) the anterior cingulate cortex (ACC) and insula, which are primary nodes in the “Salience Network” (Seeley et al., 2007); (2) the somatosensory cortex and thalamus, which are primary sensory processing areas and their subcortical afferent; (3) as well as prefrontal regions and brainstem nuclei (Melzack, 2001; Petrovic et al., 2004). Positron emission tomography (PET) studies demonstrate that several of these areas have high endogenous opiate receptor levels, including the ACC (Vogt et al., 1995), insula (Baumgartner et al., 2006), and thalamus. Additionally, acute experimental pain evoked with the application of a thermal stimulus leads to an increase in opiate receptor binding specifically in the ACC and insula among healthy individuals (Sprenger et al., 2006). A recent meta-analysis demonstrated that the brain response to acute pain in chronic pain patients is similar to healthy controls (Tanasescu et al., 2016). Notably, however, none of these studies examined how opiate usage affects the pain response, with many studies excluding patients who use opiates. Given that the brain regions involved in processing acute pain contain high levels of opiate receptors, it is possible that chronic opiate use in individuals with chronic pain may lead to homeostatic dysregulation in this system.
The purpose of this pilot study was to evaluate the pattern and amplitude of neural activity associated with acute pain in a sample of chronic pancreatitis patients that have been using opiates daily for 6 or more months. Chronic pancreatitis is a particularly intransigent condition associated with visceral pain. Similar to other chronic pain conditions, pain originates from a specific location, but over time the etiology of this pain spreads. In part, this may be due to alterations in central processing, as chronic pancreatitis is associated with changes in brain structure in pain processing regions (Bouwense et al., 2013; Dimcevski et al., 2007a; Dimcevski et al., 2006; Dimcevski et al., 2007b), mimics neuropathies (Dimcevski et al., 2007a; Drewes et al., 2008; Staahl et al., 2007), and surgical intervention is not guaranteed to resolve pain symptoms (Cahen et al., 2007; Rosch et al., 2002). Given these difficulties, opiates are frequently prescribed to treat chronic pancreatitis (Goulden, 2013; Kleeff et al., 2017). Little is known, however, about the effects of chronic opiate use on the processing (behavioral and neurobiological) of acute pain in this population. Given the need to develop non-opiate based therapeutics for patients with chronic pain, evaluating the neural response to pain in these patients may elucidate potential treatment targets and inform future interventions.
2. Methods
2.1. Participants and Questionnaires
All procedures for this research were reviewed and approved by the Medical University of South Carolina’s (MUSC) Institutional Review Board. Individuals with chronic non-alcoholic pancreatitis (‘patients’, n=14, 10 female) currently using chronic opiates (>6 months) were recruited from the MUSC Pancreatitis Clinic. Non-opiate using control individuals (‘controls’, n=14, 10 female) were recruited from the local community. Following informed consent, participants completed a demographic questionnaire, the Brief Pain Inventory (BPI; (Cleeland and Ryan, 1994)), and the Current Opioid Misuse Measure (COMM; (Butler et al., 2007)). The Medoc Pathway System (Medoc Ltd, Ramat Yishai, Israel) was used to identify a hot temperature (°C) that each participant rated as a 7 out of 10, corresponding to an intense pain that could be tolerated without moving. This testing was done using a slightly adapted model of hot allodynia (Petersen and Rowbotham, 1999). Specifically, 0.1% capsaicin cream was applied to a 40 × 40 mm area of the skin 12 cm from the wrist on the left volar forearm. After 30 minutes the cream was removed, and 7 out of 10 testing was performed in the capsaicin sensitized region. Heat was delivered using a 30 × 30 mm ATS thermode.
2.2. MRI Data Acquisition
Each participant was positioned supine in a Siemens 3T TIM Trio, with their head positioned in a 12-channel head coil and secured by foam. Up to three runs of blood oxygen dependent signal (BOLD) data, reflecting functional brain activation, were acquired (TA: 13:12 36 slices, TR 2.2s, 35ms TE, FA: 90 degrees, 3×3×3mm). A high resolution T1-weighted, MPRAGE anatomical image was also collected (TE: 4.18ms, TR 1.75s, 1mm3 voxels).
2.3. Pain Task
2.3.1. Task Design.
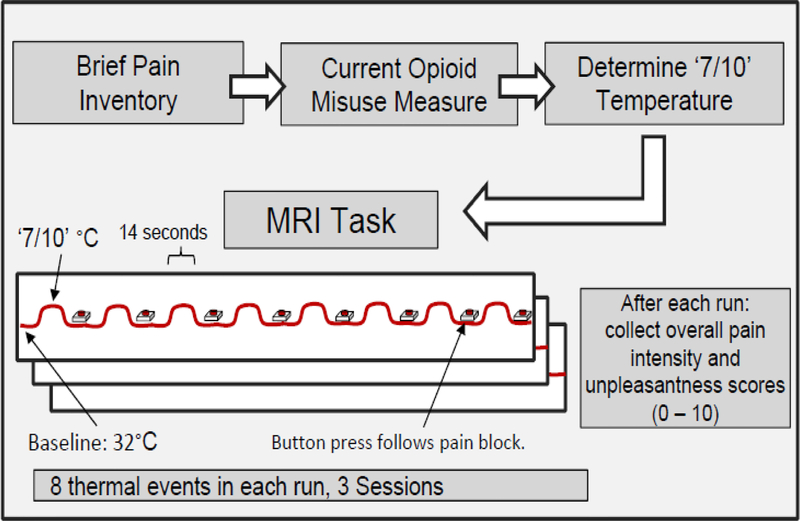
The experimental design is illustrated in Figure 1. During each experimental run, the thermode was placed on the capsaicin sensitized region of the left forearm and response buttons were positioned on both hands. During the first 5 minutes and 57 seconds of each run, the temperature alternated between a baseline of 32° (19 seconds), or each participant’s 7/10 temperature (14 seconds). Following each block of heat, participants performed an control task (button press) to ensure they were remaining awake, with their eyes opened. These tasks were repeated 8 times per run, after which participants self-reported overall pain intensity and pain unpleasantness. There were three sequential runs of the task. All participants completed all three runs with the exception of four patients and two controls (who completed two runs due to a delay in starting the fMRI acquisition).
Figure 1. Experimental Overview.
Following consent, subjects completed the Brief Pain Inventory (BPI), and Current Opioid Misuse Measure (COMM). Next, a temperature that corresponded to a 7/10 (‘intense, but tolerable pain’) on self-reported pain intensity was found for each participant. This temperature was used during the functional imaging session. The baseline for all subjects was identical: 32°C. Temperature events during functional imaging had a duration of 14 seconds. Each of the three sessions of thermal stimulation lasted 4 minutes, 28 seconds. There were 8 thermal events per session, for a total of 24 thermal events.
2.3.2. fMRI Data Processing.
The data were converted from DICOM format to NIfTI using dcm2niix. SPM12 running in Matlab 2017a (Mathworks) was used for rigid-body timeseries realignment. The mean images produced by realignment were used to perform normalization directly to MNI space using the EPI template provided with SPM, which may improve registration outcomes (Calhoun et al., 2017). Images were smoothed using an 8mm FWHM gaussian kernel and exported to the CONN functional connectivity toolbox version 17.f (Whitfield-Gabrieli and Nieto-Castanon, 2012). Consistent with prior work (Flodin et al., 2016; Kucyi et al., 2014; Zeidan et al., 2015a), we extracted the first 5 principle components (PCs) from eroded white matter and cerebral spinal fluid regions. These PCs were then regressed from the smoothed timeseries (i.e. CompCor; (Behzadi et al., 2007). Simultaneously high pass filtering (cutoff 100s) and the 6 realignment parameters with first order derivatives were also regressed.
The data were then used in subject-level general linear models to determine BOLD signal change due to (1) pain and (2) button pressing. Contrast maps produced by regression against a double gamma hemodynamic response function convolved with the task design were carried forward to a two-sample t-test. To examine other effects on pain response in the patient group, we performed a within-group model that included age, 7/10 temperature, current pain, morphine mg equivalents (MME) and total COMM score as covariates. Due to the slice prescription in some subjects, much of the occipital cortex and cerebellum were excluded. Statistical analyses followed typical fMRI methods, applying an initial threshold to limit the analyses to a subset of all the voxels (voxel threshold) and then determining significance by examining contiguous collections (‘clusters’) of voxels that survive that threshold. Within-group analyses used a voxel threshold of p<0.001, reporting clusters that were pFWE<0.05. Between-group analyses used a voxel threshold of p<0.01, reporting clusters >150 voxels. The covariate analyses used a voxel threshold of p<0.005, reporting clusters that were pFWE<0.05.
2.3.3. Self-Report Data.
The effect of the thermal stimulation on self-reported pain sensation (intensity, unpleasantness) was evaluated using a general linear model with group (patients vs. controls) as the between-subjects factor and fMRI run (1–3) and self-report type (“Intensity” or “Unpleasantness”) as within-subject factors (SPSS software Ver. 25, IBM).
3. Results
3.1. Demographics and Pain Characteristics
No group differences in gender were revealed (10 women and 4 men in both groups), however patients were older than controls (patients 48.8±8.2 years vs. controls 37.1±13.2 years, p<0.05). In comparison to the control group, the patient group had significantly higher scores on the BPI, including subscales for current (patients 3.4±3.4 vs. controls 0.2±0.8) and average pain (patients 5.1±2.3 vs. controls 0.8±1.2; p’s<.005; Supplementary Table S1 for details)1. No group differences in the individual-tailored pain threshold were revealed (patients 42.4±3.0 vs. controls 41.9±4.3). The patient group was prescribed 133.5±94.8mg morphine equivalents at the time of the study, with medication taken an average of 13.7±20.2 hours prior to scanning (self-reported range: 1–77 hours).
3.2. fMRI GLM Results
3.2.1. Within-Group fMRI Responses.
During pain processing, the control group and the patient group had elevated BOLD responses in several established nodes of the “Pain Matrix” including the left motor/sensory cortex, insula, prefrontal areas, and the ACC (voxel threshold p<0.001, cluster pFWE<0.05; see Figure 2). Detailed coordinates are included in Supplemental Table S22. During the button pressing task, both controls and patients had elevated activity in the primary motor and sensory cortices, anterior cingulate cortex, bilateral thalamus and insula (pFWE <0.05; Supplemental Figure S1)3
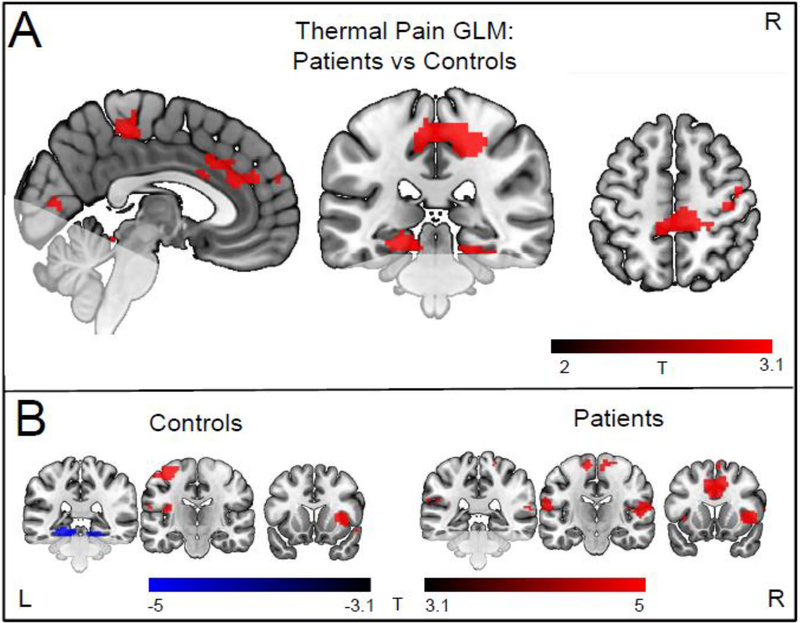
Figure 2. Between group difference in brain reactivity to heat pain.
A) areas in which patients had greater activity during thermal stimulation compared to controls (cluster forming threshold p<0.01). These areas include pre and postcentral regions (pFWE<0.005) and the anterior cingulate (puncorr =0.011). There were no areas in which patients had less activity. B) within-group responses to thermal stimulation (cluster forming threshold p <0.001). Both groups showed activation in canonical pain processing areas, such as the insula and anterior cingulate cortex. For coronal and axial slices, the right side of the image corresponds to the right side of the brain. Faded areas indicate regions that were not available for analysis.
3.2.2. Between-Group fMRI Responses.
Relative to controls, patients had significantly greater activity during the thermal stimulation blocks in 3 clusters: bilateral primary somatosensory cortices (cluster pFWE<0.01), left lingual gyrus and calcarine sulcus (cluster pFWE<0.05), and the bilateral anterior and middle cingulate (cluster puncorr=0.011) (voxel threshold p <0.01, see Figure 2A). There were no areas in which patients showed significantly less activation compared to controls. For the button press task there were no significant differences in brain activation or reaction time (Controls 727.4±163.8ms; Patients 759.6±132.2ms (p = 0.37)) between groups.
3.3. Self-Reported Pain Measures during the MRI task
The average pain intensity after each fMRI run was 6.9±1.6 in the controls and 6.7±1.3 in the patients. The average pain unpleasantness was 6.7±1.7 in the controls and 5.8±2.1 in the patients (see Supplemental Table S3 for all ratings)4. There was no interaction between group and fMRI run, nor a main effect of group or run. There was a significant main effect of self-report type (“Intensity” and “Unpleasantness”, p=0.043), as well as increasing self-report values over sessions (p = 0.047), however there were no group interactions.
3.4. Relationship between Pain and Opiate Dose on the Brain Response to Acute Pain
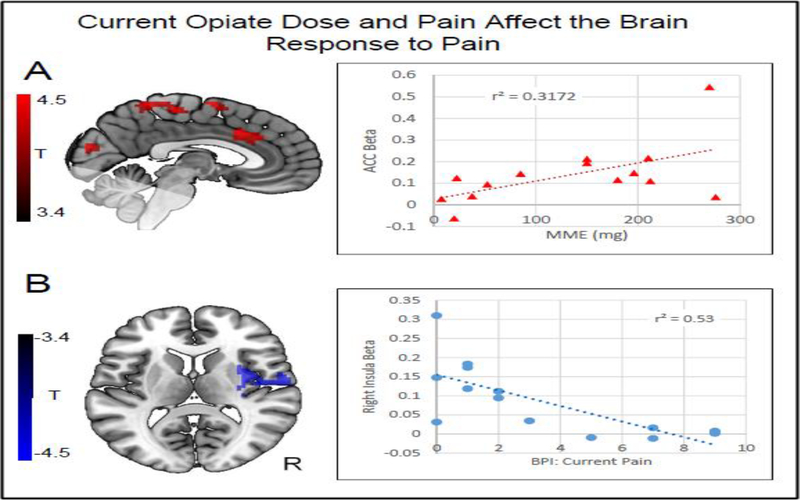
Morphine milligram equivalents (MME) were positively correlated with the response to pain in several clusters (pFWE<0.05). These included (1) the bilateral anterior and middle cingulate (a node in the “Pain Matrix” which was also different between controls and patients (Section 3.2, Figure 3A), (2) the right supramarginal/angular gyrus, (3) bilateral superior frontal cortex, (4) left primary motor/sensory cortex, (5) left supramarginal/angular gyrus, and (6) right primary motor/sensory cortex. In order to place the results in a more interpretable context, we determined the correlation between each participants MME and the average beta from the anterior cingulate cluster. MMEs explained 32% of the variance in the beta values, which reflect the magnitude of the brain response. With the most extreme MME values (>250) removed, 47% of the variance was explained.
Figure 3. Correlation between clinical variables and the brain response to pain.
A) Red clusters indicate areas in which the BOLD response to pain was positively correlated (pFWE<0.05) with morphine mg equivalence (MME) values (cluster forming threshold p<0.005). Color maps indicate T values. These areas include somatosensory and cingulate regions. A plot is shown indicating the relationship between the effect size (average beta from indicated cluster) and MME. MMEs explained nearly 32% of the variance in the brain response within the cingulate. The correlation remains significant following the removal of the most extreme values, with an R2 of 0.47. B) The blue cluster shows an area in the right insula in which the response to pain was negatively correlated (pFWE <0.05) with the Current Pain score, as derived from the Brief Pain Inventory. Color maps indicate T values. A plot is shown indicating the relationship between the effect size (average beta from cluster) and the Current Pain score. Current pain explained 53% of the variance in the brain response. The correlation remains significant following the removal of the first extreme value, with an R2 of 0.58. No other correlations were significant (Age, COMM score, 7/10 Temp). For the axial slice, the right side of the image corresponds to the right side of the brain. Faded areas indicate regions that were not available for analyses.
Current scores on the BPI were negatively correlated with the response to pain in one cluster: the right insula (also a node in the “Pain Matrix”, pFWE=0.011, Figure 3B). We examined the correlation between the Current Pain measure of the BPI and the average beta in the right insula cluster. The Current Pain scores on the BPI explained 53% of the variance in the brain activity in that location. With the most extreme value removed (BPI 0, Beta > 0.3) the correlation remains significant, explaining 58% of the variance. There were no significant correlations between the brain response to acute pain and age, individually calibrated pain threshold, or COMM score.
4. Discussion
4.1. Summary
While acute opiate usage is associated with acute pain relief, chronic opiate usage leads to a sensitized behavioral response to pain. Acute pain leads to elevated activity in a network of neural regions (e.g., the ACC, insula, and thalamus) that also have high opiate receptor concentrations. Very little is known, however, about the effects of chronic opiate usage on the brain response to acute pain. This study is the first to demonstrate that a dose of opiates that normalizes the behavioral response to acute pain in chronic pancreatitis patients is associated with an amplified neural response to acute pain. As expected, and consistent with prior reports, acute pain evoked a similar pattern of neural activity in the “Pain Matrix,” with particularly high levels in the somatosensory cortex, but the amplitude of this response was elevated in opiate using patients. Furthermore, the higher levels of activity in the ACC and insula associated with acute pain were positively correlated with morphine equivalent dose. That is, the higher the prescribed dose of opiates, the larger the pain response. The findings suggest that, while these patients are taking a dose of opiates that normalizes their behavioral response to pain (e.g., they did not report feeling more intense pain than controls), there is a sensitized brain response to pain in several key neural nodes which are not only key elements of pain processing circuitry but also locations of high opiate receptor binding. The findings provide a foundation for future longitudinal investigations which may seek to investigate if this homeostatic dysregulation may, in turn, contributes to the dangerous process of behavioral tolerance and opioid dose escalation.
4.2. Increased Responses in Patients using Chronic Opiates
In the present study, both patients and controls showed typical responses to pain, with activation in somatosensory areas, the insula and the ACC. However, the patient group had amplified brain responses compared to the control group. One cluster encompassed somatosensory regions, which provide information about the location and intensity of pain (Apkarian et al., 2005; Lee and Tracey, 2010). These areas, including the secondary somatosensory cortex (SII), also showed a positive relationship between opiate dose and pain-related activity. The SII has previously been targeted with an inhibitory form of non-invasive brain stimulation which led to reduced pain scores compare to a sham stimulation (Fregni et al., 2011). These overlapping findings may reflect changes in sensory processing from continued use of opiates. Future longitudinal work will be needed to determine if these somatosensory responses are indicative of an increased risk of hyperalgesia development. The cluster showing elevated activity in posterior regions of the brain suggests that there may also be changes in other aspects of sensory systems, including the representations within the cerebellum or visual processing areas, but interpretation of these findings is made difficult by the limited field of view.
The third cluster differentiating patients from controls encompasses the middle and anterior cingulate cortex (ACC). Activity in these regions is associated with motivational-affective processing of pain and tracks the unpleasantness of the stimulus (Apkarian et al., 2005; Price, 2000; Rainville et al., 1997). The affective dimension of pain may be a key component of visceral pain. For example, an EEG study in pancreatitis patients found decreased latencies of pain-related event potentials in the ACC (Dimcevski et al., 2007a), and research in irritable bowel syndrome, found increased ACC blood perfusion in response to painful stimuli (Mayer et al., 2005).
4.3. Relationship Between Prescription, Pain and the Pain Response
The positive relationship observed between the amount of opiates prescribed and the neural response to pain highlights the potential risks associated with chronic opiate use. The cluster showing a positive correlation between dose and pain response in the ACC and middle cingulate directly overlaps with an area showing elevated activity in patients relative to controls. Although preliminary, these findings offer a functional correlate for previously found structural changes (Frokjaer et al., 2012). Collectively, the findings from this line of research support the idea that differences between groups in pain responses may be driven, in part, by increased opiate usage. This may reflect homeostatic dysregulation of the opiate system, such as the cingulate, as well as other areas such as the insula, thalamus and brain stem which are highly enriched with opiate receptors (Baumgartner et al., 2006; Corder et al., 2018; Vogt et al., 1995). Positive correlations were also found in motor and sensory cortices. As similar regions also differentiated the patient group from the controls, this may reflect the role that prescription opiates play in reducing the intensity of pain. Positive correlations in the supramarginal and angular gyrus may reflect alterations that opiates have on other aspects of pain processing. These regions are not typical included in the “Pain Matrix,” but nevertheless show relationships with pain, such as when expectations regarding pain are violated (Kokonyei et al., 2018; Zeidan et al., 2015b), or participants evaluate pain intensity (Kong et al., 2006). Overall, the distribution of the correlations throughout the brain is likely related to the widespread presence of pain processing activity (Atlas et al., 2014), and is similar in extent to changes seen in white matter structure in individuals using prescription opioids (Upadhyay et al., 2010).
One other measure, current pain, as indexed by the Brief Pain Inventory, also showed significant correlations with pain responses. Specifically, pain on the day of scanning was negatively correlated with a single cluster in the right posterior insula. This builds upon previous reports that chronic pancreatitis patient’s pain responses were shifted to a more posterior portion of the insula (Dimcevski et al., 2007a), and may relate to structural changes in that area (Frokjaer et al., 2012).
4.4. Implications for Opioid Use Disorder
The importance of these pilot data are underscored by the overlap in neural regions involved in processing acute pain as well as those associated with processing drug-cue reactivity (Becker et al., 2012; Elman and Borsook, 2016; Mitsi and Zachariou, 2016; Navratilova et al., 2015). Many individuals continue to use opiates due to concerns about pain (Barth et al., 2013). Previous work supported the idea that the presence of pain was protective against the development of an addiction phenotype (Colpaert et al., 1982; Colpaert et al., 2001; Lyness et al., 1989; Ozaki et al., 2004), however there is growing evidence that this is not the case (Ewan and Martin, 2013; Hou et al., 2015; Zhang et al., 2014) and epidemiological studies find evidence of misuse and addiction among individuals with chronic pain (Vowles et al., 2015). This study provides possible targets for treating the pain that is associated with chronic opiate usage. Building upon prior work that stimulated secondary somatosensory areas (Fregni et al., 2011), these findings support non-invasive brain stimulation targeting primary somatosensory regions or regions able to modulate the anterior cingulate cortex.
4.5. Relationship with Opioid Receptor Locations
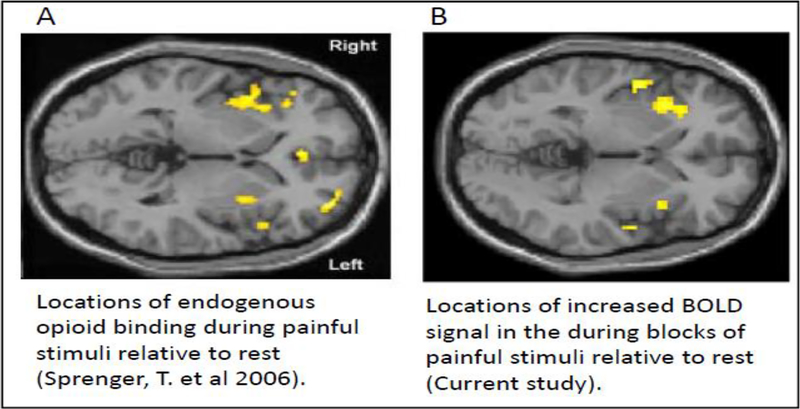
Opioid receptors are highly distributed throughout the brain (Corder et al., 2018), but the insula is particularly implicated in pain processing and is also enriched with opiate receptors (Baumgartner et al., 2006). To explore the relationship between the pain task used in the current study and opioid receptor binding we have compared the spatial extent of the BOLD response to pain (relative to rest) in healthy controls with previous work using positron emission tomography (PET). In Sprenger et al. (2006), [18F] DPN (a non-selective opioid ligand) was used as a tracer to measure changes in endogenous opioid binding during pain in 8 healthy controls, compared to 8 other control subjects at rest. They found that both the left and right insula showed reduced [18F] DPN ligand binding, suggesting endogenous opioid binding occurred at those locations (Figure 4A). These insula locations overlap with the increased BOLD response found in the current work Figure 4B), linking the current pain task BOLD response with locations of pain-sensitive opioidergic activity.
Figure 4. Comparing endogenous opioid binding with BOLD responses.
A) Adapted from Sprenger et al, (2006). Eight healthy controls were exposed to painful heat stimulus while opiate receptor binding was evaluated with a PET scan using [18F]DPN. These data were then compared to a separate group of healthy controls at rest. Endogenous opioid binding was found in both the left and right insula. B) In our study we evaluated the BOLD signal associated with pain blocks versus no pain (rest) blocks. Our findings within both the right and left insula correspond spatially to the locations of greater endogenous opiate binding found in Sprenger, T. et al 2006.
4.6. Limitations
The findings are preliminary with a relatively small sample size, and did not capture pain responses in the brainstem and cerebellum. Future work should leverage advances in fMRI sequences to determine if these pain processing and modulating regions also show altered activity in patients using chronic opiates. It will also be important to incorporate other stimulus types (e.g., cold or mechanical pain) to confirm that the findings are broadly applicable and not specific to heat-induced pain.
5. Conclusion
Individuals using chronic prescription opiates for pain have elevated neural responses to a thermal pain stimulus relative to healthy controls. The amount of opiates used, as measured by morphine milligram equivalents, is positively associated with larger brain responses to pain. These findings need to be explored in a larger sample and over a longer period of time to determine whether and how chronic opiate usage may increase risk for conversion to nonmedical prescription opioid use, opioid use disorder, or opioid-induced hyperalgesia.
Supplementary Material
Highlights.
Individuals using prescription opiates have elevated brain responses to pain
Larger doses of prescription opiates are associated with larger brain responses
Higher current pain was associated with reduced responses to experimental pain
Acknowledgements
The authors would like to thank the Center for Biomedical Imaging for their support.
Role of Funding Source
This work was supported by F31DA043330 (Dowdle), R21DA044503 (Hanlon), R25DA020537 (Back and Brady), K02 DA039229 (Back), R01DA038971 (Borckardt).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflict declared.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: ...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
References
- Angst MS, Clark JD, 2006. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104, 570–587. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK, 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Lindquist MA, Bolger N, Wager TD, 2014. Brain mediators of the effects of noxious heat on pain. Pain 155, 1632–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth KS, Maria MM, Lawson K, Shaftman S, Brady KT, Back SE, 2013. Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence. Am. J. Addict. 22, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Hohnemann S, Piel M, Rosch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, Schreckenberger M, 2006. High opiate receptor binding potential in the human lateral pain system. Neuroimage 30, 692–699. [DOI] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P, 2012. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci. Lett. 520, 182–187. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwense SA, Ahmed Ali U, ten Broek RP, Issa Y, van Eijck CH, Wilder-Smith OH, van Goor H, 2013. Altered central pain processing after pancreatic surgery for chronic pancreatitis. Br. J. Surg. 100, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN, 2007. Development and validation of the Current Opioid Misuse Measure. Pain 130, 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Lameris JS, Dijkgraaf MG, Huibregtse K, Bruno MJ, 2007. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N. Engl. J. Med. 356, 676–684. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Wager TD, Krishnan A, Rosch KS, Seymour KE, Nebel MB, Mostofsky SH, Nyalakanai P, Kiehl K, 2017. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum. Brain Mapp. 38, 5331–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Costa T, Diano M, Sacco K, Duca S, Geminiani G, Torta DM, 2014. Massive modulation of brain areas after mechanical pain stimulation: A time-resolved fMRI study. Cereb. Cortex 24, 2991–3005. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM, 1994. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 23, 129–138. [PubMed] [Google Scholar]
- Colpaert FC, Meert T, De Witte P, Schmitt P, 1982. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 31, 67–75. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W, 2001. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain 91, 33–45. [DOI] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G, 2018. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 41, 453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, Drewes AM, 2007a. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology 132, 1546–1556. [DOI] [PubMed] [Google Scholar]
- Dimcevski G, Schipper KP, Tage-Jensen U, Funch-Jensen P, Krarup AL, Toft E, Thorsgaard N, Arendt-Nielsen L, Drewes AM, 2006. Hypoalgesia to experimental visceral and somatic stimulation in painful chronic pancreatitis. Eur. J. Gastroenterol. Hepatol. 18, 755–764. [DOI] [PubMed] [Google Scholar]
- Dimcevski G, Staahl C, Andersen SD, Thorsgaard N, Funch-Jensen P, Arendt-Nielsen L, Drewes AM, 2007b. Assessment of experimental pain from skin, muscle, and esophagus in patients with chronic pancreatitis. Pancreas 35, 22–29. [DOI] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J, 2009. Induction of synaptic long-term potentiation after opioid withdrawal. Science 325, 207–210. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Krarup AL, Detlefsen S, Malmstrom ML, Dimcevski G, Funch-Jensen P, 2008. Pain in chronic pancreatitis: The role of neuropathic pain mechanisms. Gut 57, 1616–1627. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2016. Common brain mechanisms of chronic pain and addiction. Neuron 89, 11–36. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2013. Analgesics as reinforcers with chronic pain: Evidence from operant studies. Neurosci. Lett. 557 Pt A, 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P, 2016. Intrinsic brain connectivity in chronic pain: A resting-state fMRI study in patients with rheumatoid arthritis. Front. Hum. Neurosci. 10, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Potvin K, Dasilva D, Wang X, Lenkinski RE, Freedman SD, Pascual-Leone A, 2011. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur. J. Pain 15, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer JB, Bouwense SA, Olesen SS, Lundager FH, Eskildsen SF, van Goor H, Wilder-Smith OH, Drewes AM, 2012. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 10, 434–438. [DOI] [PubMed] [Google Scholar]
- Goulden MR, 2013. The pain of chronic pancreatitis: a persistent clinical challenge. Br. J. Pain 7, 8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr., Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D, 2017. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb. Mortal. Wkly. Rep. 66, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YY, Cai YQ, Pan ZZ, 2015. Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of GluA1 in rat central amygdala. J. Neurosci. 35, 3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Munoz JED, Neoptolemos JP, 2017. Chronic pancreatitis. Nat. Rev. Dis. Primers 3, 17060. [DOI] [PubMed] [Google Scholar]
- Kokonyei G, Galambos A, Edes AE, Kocsel N, Szabo E, Pap D, Kozak LR, Bagdy G, Juhasz G, 2018. Anticipation and violated expectation of pain are influenced by trait rumination: An fMRI study. Cogn Affect Behav Neurosci. 19, 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL, 2006. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 27, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD, 2014. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 34, 3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L, 2011. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14, 145–161. [PubMed] [Google Scholar]
- Lee MC, Tracey I, 2010. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr. Pain Headache Rep. 14, 124–131. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, Garvin RD, 1989. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sci. 45, 2217–2224. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L, 2005. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115, 398–409. [DOI] [PubMed] [Google Scholar]
- Melzack R, 2001. Pain and the neuromatrix in the brain. J. Dent. Educ. 65, 1378–1382. [PubMed] [Google Scholar]
- Mitsi V, Zachariou V, 2016. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 338, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Atcherley CW, Porreca F, 2015. Brain circuits encoding reward from pain relief. Trends Neurosci. 38, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat S, Yadav D, Bielefeldt K, 2012. Pain and opioid use in chronic pancreatitis. Pancreas 41, 264–270. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F, 2005. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 80, 319–324. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T, 2004. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J. Neurochem. 88, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Rowbotham MC, 1999. A new human experimental pain model: The heat/capsaicin sensitization model. Neuroreport 10, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Hansson P, Ingvar M, 2004. Brainstem involvement in the initial response to pain. Neuroimage 22, 995–1005. [DOI] [PubMed] [Google Scholar]
- Price DD, 2000. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC, 1997. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- Rivat C, Vera-Portocarrero LP, Ibrahim MM, Mata HP, Stagg NJ, De Felice M, Porreca F, Malan TP, 2009. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur. J. Neurosci. 29, 727–737. [DOI] [PubMed] [Google Scholar]
- Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F, 2016. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 338, 160–182. [DOI] [PubMed] [Google Scholar]
- Rosch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R, Hintze R, Adler A, Neuhaus H, Zavoral M, Zavada F, Schusdziarra V, Soehendra N, European Society of Gastrointestinal Endoscopy Research, G., 2002. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy 34, 765–771. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Sanna MD, Ghelardini C, Galeotti N, 2014. Regionally selective activation of ERK and JNK in morphine paradoxical hyperalgesia: a step toward improving opioid pain therapy. Neuropharmacology 86, 67–77. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet G, Rivat C, 2003. Opioid-induced hyperalgesia: Abnormal or normal pain? Neuroreport 14, 1–7. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tolle TR, 2006. Opioidergic activation in the medial pain system after heat pain. Pain 122, 63–67. [DOI] [PubMed] [Google Scholar]
- Staahl C, Dimcevski G, Andersen SD, Thorsgaard N, Christrup LL, Arendt-Nielsen L, Drewes AM, 2007. Differential effect of opioids in patients with chronic pancreatitis: An experimental pain study. Scand. J. Gastroenterol. 42, 383–390. [DOI] [PubMed] [Google Scholar]
- Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP, 2016. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci. Biobehav. Rev. 68, 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D, 2010. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 133(Pt 7), 2098–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NMH, Ossipov MH, Malan TP, Lai J, Porreca F, 2001. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J. Neurosci. 21, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Watanabe H, Grootoonk S, Jones AK, 1995. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Hum. Brain Mapp. 3, 1–12. [Google Scholar]
- Volkow N, Benveniste H, McLellan AT, 2018. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 69, 451–465. [DOI] [PubMed] [Google Scholar]
- Volkow ND, McLellan AT, 2016. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N. Engl. J. Med. 374, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN, 2015. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156, 569–576. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E, 2013. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Wu L, Huang X, Sun L, 2015. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: a meta-analysis. J. Clin. Anesth. 27, 311–324. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC, 2015a. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J. Neurosci. 35, 15307–15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Lobanov OV, Kraft RA, Coghill RC, 2015b. Brain mechanisms supporting violated expectations of pain. Pain 156, 1772–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tao W, Hou YY, Wang W, Lu YG, Pan ZZ, 2014. Persistent pain facilitates response to morphine reward by downregulation of central amygdala GABAergic function. Neuropsychopharmacology 39, 2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL, 2010. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.