Abstract
Aims
There are no studies looking at the relationship between colonoscopy withdrawal time (CWT) and adenoma detection rate (ADR) in non-screening patients. Our aim is to explore the relationship between CWT and ADR, particularly in the proximal colon where colonoscopy is shown to be less protective for the development of cancers.
Methods
This is a retrospective study during November 2015 to December 2016 of non-screening colonoscopies done at a large teaching hospital. Incomplete and therapeutic procedures were excluded. The 39 endoscopists included were 15 gastroenterologists, 10 colorectal surgeons and 14 trainee colonoscopists. CWT was calculated by reviewing caecal intubation and rectal retroflexion images.
Results
783 colonoscopies were included, with mean patient age of 58.51 years (SD 15.5). The mean ADR was 21.45% in the study. The CWT could be calculated for 62.83% of the cases (n=492). 80% (393) of colonoscopies had CWT of ≥6 min. Mean CWT was 9.15 min (SD 4.4). The ADR positively correlated with longer CWT (r=0.31, p=0.0001). The ADR was significantly higher when CWT was ≥8 min compared with CWT <6 min or CWT of 6–8 min (p=0.0001). More polyps were detected in the proximal colon when CWT ≥8 min (p=0.078). Mean CWT of gastroenterologists was 9.8 min (SD 4.5), similar to the trainee group (10.3 min, SD 3.8), while mean CWT for colorectal surgeons was 5.7 min (SD 3.2). The ADR for gastroenterologists was 25.9% versus 17.5% for colorectal surgeons and 17.8% for trainees.
Conclusions
There is a moderately strong positive correlation between longer CWT and ADR in non-screening colonoscopies. CWT can differ between different endoscopists. Meticulous colonoscopy withdrawal may improve polyp detection in the proximal colon.
Keywords: colonic polyps, colonoscopy, colorectal adenomas, endoscopic polypectomy
Significance of this study.
What is already known on this topic
Withdrawal time in colonoscopy influences the adenoma detection in screening populations.
What this study adds
This is the first study done in an entirely non-screening population and this shows that withdrawal time in colonoscopy influences the adenoma detection in non-screening population.
How might it impact on clinical practice in the foreseeable future
Endoscopists who perform colonoscopies in non-screening patients are recommended to ensure that they spend the recommended 6 min during the withdrawal of the scope at colonoscopy. This, we anticipate, will increase adenoma detection.
Introduction
Colonoscopy is a common procedure used both in colorectal cancer screening and in investigating a wide range of colorectal pathologies. High-quality colonoscopy is considered the gold standard as it does allow direct visualisation of intraluminal lesions, tissue biopsy and excision of lesions.1–3 Colorectal cancer is the third most common cancer worldwide.4 In the UK, over 30 000 colorectal cancers were diagnosed in over 360 000 colonoscopies each year4 5 by individuals from different training backgrounds within the medical profession; for example, surgeons, gastroenterologists and clinical endoscopists (new term for nurse endoscopists in the National Health Service). As with many skills within medicine, there may be a variation in practice among clinicians. The most commonly used quality indicator for colonoscopy is the mean adenoma detection rate (ADR) of the endoscopist.6 7 ADR may be affected by a number of variables including quality of bowel preparation,6 8 9 caecal intubation rate7 10 and colonoscopy withdrawal time (CWT).11–13
A landmark study published in 2006 by Barclay et al 14 demonstrated a significant increase in ADR with longer CWT in a mixture of screening and non-screening colonoscopies. Subsequent screening studies, including a study of over 31 000 colonoscopies,11 have showed that the optimal CWT is between 6 and 10 min, with little effect gained beyond this point. A slower withdrawal may suggest that the clinician had time to adequately inspect the entire mucosa—any quicker may lead to some areas not adequately examined. Methods have been adopted to improve mucosal visualisation, such as repositioning of the patient, rectal retroflexion, adequate insufflation and suctioning were found to extend both the CWT and ADR simultaneously.11 15
The UK bowel cancer screening programme began in 2006 and, with the growing body of evidence, guidelines were developed in an attempt to standardise colonoscopies and minimise operator-dependent variation. In order to become accredited bowel cancer screening endoscopists and to prove their practice is of sufficient standards, clinicians must satisfy several criteria.16
Current guidelines in the UK state that it is mandatory to visualise the caecum at each colonoscopy and that endoscopists should maintain a caecal intubation rate of greater than 90%.1 10 17 Furthermore, the endoscopist should achieve an average CWT of greater than 6 min and retroflexion in the rectum images should be taken.18 These criteria are set to encourage complete and meticulous inspection of the entire colon. It is essential to document the entire procedure in detail. This has been achieved in bowel cancer screening programme with the intervention of specialist screening practitioners (SSPs).19 However, there is no equivalent to SSPs present during the non-screening colonoscopy.
Due to the rigorous documentation in screening colonoscopies and easily available data, most research has focused on bowel cancer screening colonoscopies only. Non-screening colonoscopies involve all diagnostic and therapeutic colonoscopies in symptomatic patients. Therefore, the primary goal of this study is to explore the relationship between the CWT and ADR, particularly in the proximal colon where colonoscopy is more likely to miss cancers20 21 by assessing the current practice of various endoscopists exclusively in non-screening colonoscopies.
Methods
This study was conducted at both sites of Sheffield Teaching Hospitals NHS Foundation Trust in the UK (Royal Hallamshire Hospital and Northern General Hospital). All diagnostic colonoscopies conducted during an 8-month period were retrospectively reviewed and included (from December 2015 to April 2016 and from September 2016 to November 2016). The following cases were excluded: screening colonoscopies, incomplete procedures, patients with colonic surgical anastomosis, colonoscopies with poor bowel preparation and all therapeutic procedures using diathermy or multiple cold polypectomies (≥3).
The population of this study consisted of the consecutive patients who had a routine or urgent diagnostic colonoscopy during the selected period. Our endoscopy department performs around 7000 colonoscopies (on average) per year across the two sites. The included colonoscopies were performed by 39 colonoscopists, who were divided into three groups according to their specialty and level of training (15 consultant gastroenterologists—group A, 10 colorectal surgeons—group B, 14 trainee colonoscopists—group C). The trainee colonoscopists group involved medical and surgical trainees and trainee nurse endoscopists. All the trainees conducted at least 100 colonoscopies prior to commencement of the study period. All trainees had a trainer in the room during the procedure unless they achieved full independence. Group A and B colonoscopists were all fully independent in performing colonoscopy.
The Olympus colonoscope (CF-H290L/I) was used for all the procedures. All the included colonoscopies were performed during the standard 30 min colonoscopy time slot. The standard bowel preparation used for our patients consisted of four sachets of Kleanprep.
All colonoscopies were performed either under conscious sedation or without sedation. All patients were offered Entonox as required during the procedure. The use of buscopan (hyoscine) was according to the colonoscopist preference (data not included).
Electronic Document and Records Management system (EDMS) and Infoflex V.5 software were used to retrieve the required data. The indication for the procedure, bowel preparation, patient tolerance (as assessed by endoscopy team), the time for caecal intubation and rectal retroflexion, presence of polyps±cold polypectomy and the site of the polyps were taken directly from the endoscopy report (available on both electronic systems) for the included colonoscopies. The polyps were classified according to the site into proximal and distal colonic polyps. We used the splenic flexure as a landmark. Therefore, any polyp reported to be in the splenic flexure or more distally was considered a distal colonic polyp; otherwise, the polyp was considered a proximal colonic polyp.
CWT was calculated by reviewing the time available on the caecal intubation and rectal retroflexion images on EDMS. CWT included time spent for manoeuvres such as biopsies and cold polypectomies.
Pearson correlation test was used to assess the relationship between the CWT and ADR. SPSS V.22.0 was used to conduct the statistical tests. Additionally, ANOVA with Bonferroni post hoc test was applied to compare the dependent variable CWT and the categorical independent variable (groups A, B and C). The assumptions for using ANOVA test were satisfied. We used χ2 test to examine the difference in ADR between colonoscopists with CWT that was less than 6 min, or those with CWT of 6–8 min and those with CWT of more than 8 min.
In an attempt to account for confounding, MANOVA test was used to investigate whether there is a difference in CWT between two groups of endoscopists with high or low ADR (high ADR ≥20% and low ADR <20%). The following confounding variables were explored: patient age, endoscopist specialty, indication for colonoscopy, patient tolerance, biopsy sampling, whether polypectomy was performed and its type. Results were considered significant if p value was<0.05.
Results
This retrospective study included 783 colonoscopies in total with a mean patient age of 58.51 years (SD 15.5).
The mean ADR was 21.45% (number of patients with at least one neoplastic polyp was 168 out of the total 783) across included colonoscopies. There were four sessile serrated lesions identified in the proximal colon. These were considered as adenomas in the analysis.
The CWT could be calculated for 62.83% of the cases (492/783). This study showed that 80% (394) of colonoscopies had CWT of 6 min or more. The total mean CWT was 9.15 min (SD 4.4). CWT was longer when the endoscopist performed cold polypectomy during the diagnostic colonoscopy. The mean CWT was 12.9 min in cases where cold snare was performed, compared with 11.8 min when cold biopsy was used. In cases where no polyps were detected, the mean CWT was 8.3 min versus 8.9 min when polyps were found and left in situ.
Pearson χ2 test demonstrated a significant difference in ADR with longer CWT. The higher ADR positively correlated with longer CWT (r=0.31, p=0.0001). The ADR was significantly higher when CWT was 8 min or more compared with CWT of less than 6 min or CWT of 6–8 min (p=0.0001) (figure 1). In addition, the number of adenomatous polyps detected in the proximal colon was highest when CWT was 8 min or more, but this was not statistically significant (p=0.078) (figure 2). Colonoscopies with less than 6 min withdrawal time were only successful in identifying six polyps in total. All of them were situated on the distal colon.
Figure 1.
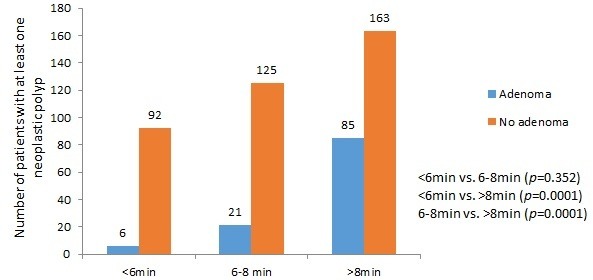
Relationship between colonoscopy withdrawal time groups (<6 min, 6–8 min and >8 min) and the number of patients with at least one neoplastic polyp detected.
Figure 2.

Relationship between colonoscopy withdrawal time groups (<6 min, 6–8 min and >8 min) with the site of the neoplastic polyps.
The CWT tends to be longer in well-tolerated colonoscopies. This was statistically significant (p=0.044). The mean CWT across all groups in relation to patient tolerance is as follows: mean CWT in poorly tolerated colonoscopies is 6.1 min (SD 3.1), acceptable tolerance is 8 min (SD 3.3) and good tolerance is 9.3 min (SD 4.4).
There was a statistically significant difference at the p level of <0.05 in CWT for the three groups (p=0.0001). Post hoc comparison using Bonferroni test indicated that the mean CWT for group A (mean=9.8 min, SD 4.5) and for group C (mean=10.3 min, SD 3.8) were significantly different from group B (mean=5.7 min, SD 3.2) (p=0.0001). Group A CWT did not differ significantly from group C (p=0.707). Table 1 compares mean CWT with the baseline and the study ADR for each endoscopist.
Table 1.
Comparison of the mean CWT with the baseline ADR and study ADR for each endoscopist
| Group A (gastroenterologists) |
Group B (colorectal surgeons) |
Group C (trainee endoscopists) |
|||||||||
| Code | Mean CWT (min) | Baseline ADR (%) | Study ADR (%) | Code | Mean CWT (min) | Baseline ADR (%) | Study ADR (%) | Code | Mean CWT (min) | Baseline ADR (%) | Study ADR (%) |
| 11 | 10.4 | 31.6 | 20.5 | 1 | 5.0 | 26.7 | 15.8 | 26 | 9.4 | 19.9 | 9.7 |
| 12 | 9.0 | 47.1 | 20.6 | 2 | 5.7 | 37.0 | 15.4 | 27 | 11.2 | 22.2 | 10.0 |
| 13 | 8.8 | 32.1 | 43.5 | 3 | 5.6 | 21.6 | 6.3 | 28 | 10.0 | 37.7 | 34.8 |
| 14 | 5.0 | 32.0 | 33.4 | 4 | 6.8 | 23.0 | 18.1 | 29 | 20.0 | NA | 25.0 |
| 15 | 6.8 | 19.5 | 16.7 | 5 | 6.1 | 23.0 | 18.4 | 30 | 9.0 | 31.9 | 25.0 |
| 16 | NA | 34.2 | 45.0 | 6 | 3.5 | 22.4 | 22.8 | 31 | 6.3 | 24.0 | 20.0 |
| 17 | 8.5 | 14.8 | 22.3 | 7 | 5.4 | 19.6 | 21.7 | 32 | 9.7 | 31.8 | 11.1 |
| 18 | 7.6 | 27.9 | 18.2 | 8 | 3.0 | 25.8 | 22.2 | 33 | 10.5 | 24.7 | 25.0 |
| 19 | 9.6 | 25.0 | 20.0 | 9 | 4.0 | 17.2 | 11.1 | 34 | 10.0 | 21.0 | 20.0 |
| 20 | 9.6 | 44.6 | 27.3 | 10 | 7.0 | 41.7 | 33.3 | 35 | 11.5 | 16.7 | 21.0 |
| 21 | 11.8 | 45.0 | 20.2 | 36 | 10.3 | 41.0 | 45.0 | ||||
| 22 | 10.7 | 36.2 | 21.7 | 37 | 8.4 | 14.5 | 45.0 | ||||
| 23 | 7.5 | 33.5 | 20.5 | 38 | 9.5 | 45.0 | 25.0 | ||||
| 24 | 4.0 | 22.0 | 33.3 | 39 | 15.0 | 40.0 | 25.0 | ||||
| 25 | 10.6 | 22.7 | 39.5 | ||||||||
ADR, adenoma detection rate; CWT, colonoscopy withdrawal time;NA, not available.
When the ADR was calculated for the various colonoscopist groups for non-screening procedures, ADRs were 25.9%, 17.5% and 17.8% in groups A, B and C respectively. Figure 3 gives the total number of the included colonoscopies performed by each group and the number of procedures in which adenomas were detected.
Figure 3.
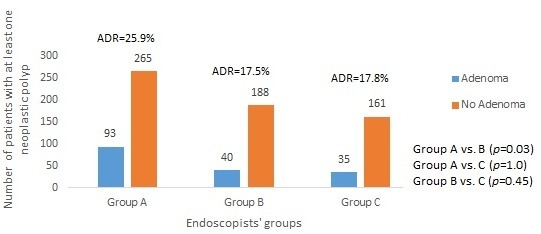
Total number of patients with at least one neoplastic polyp detected by endoscopists‘ specialty. ADR, adenoma detection rate.
The total number of patients who had biopsies done was 482. There was no statistical significant difference between CWT groups (<6 min, 6–8 min and >8 min) whether biopsies were taken or not (p=0.239).
Table 2 illustrates the multivariate analysis based on two endoscopist groups those with high ADR (≥20%) and low ADR (<20%). This analysis demonstrated that there was a statistically significant difference between high and low ADR groups in relation to the withdrawal time (p=0.001) while taking into account other variables: endoscopist specialty, biopsy sampling, patient tolerance and polypectomy procedure type.
Table 2.
Relationship between variables and high and low ADR
| Variables | Subgroups | Low ADR (<20%) n=147 | High ADR (≥20%) n=305 | P value (95% CI) |
| Mean age (SD) years | 58.7 (15.8) | 58.6 (15.6) | 0.941 | |
| Mean CWT (SD) min | 8.21 (4.08) | 9.63 (4.4) | 0.001 | |
| Endoscopist specialty | Trainee | 110 | 86 | 0.0001 |
| Gastroenterologist | 17 | 341 | ||
| Surgeon | 155 | 73 | ||
| Polypectomy | No polypectomy | 238 | 413 | 0.044 |
| Cold biopsy | 22 | 63 | ||
| Cold snare | 22 | 25 | ||
| Tolerance | Poor | 8 | 13 | 0.044 |
| Acceptable | 5 | 29 | ||
| Good | 232 | 423 | ||
| Biopsies | No biopsy | 80 | 221 | 0.0001 |
| Biopsies | 202 | 280 | ||
| Indications | Change bowel habit | 125 | 242 | 0.436 |
| Anaemia | 42 | 91 | ||
| Melaena/PR bleed | 31 | 55 | ||
| Polyp surveillance | 37 | 38 | ||
| Abdominal pain | 12 | 25 | ||
| Colitis surveillance | 13 | 22 | ||
| Abnormal radiology | 7 | 11 | ||
| Diarrhoea | 6 | 8 | ||
| Weight loss | 3 | 1 | ||
| Family Hx of colorectal cancer | 1 | 2 | ||
| Constipation | 1 | 1 | ||
| Incontinence | 0 | 1 | ||
| Missing data | 4 | 4 |
ADR, adenoma detection rate; CWT, colonoscopy withdrawal time; Hx, history; PR, per rectal.
Discussion
Based on studies conducted in screening patient population, it is recommended to perform the colonoscopy withdrawal over a minimum period of 6 min22 23 with an the aspirational target of 10 min.22 To our knowledge, there are no studies examining the relationship between the CWT and ADR exclusively in non-screening colonoscopies. Our finding shows that procedures with longer CWT were associated with higher ADR.
Our analysis of the non-screening colonoscopies in a cohort of symptomatic patients revealed a significant variation in CWT and ADR among different groups of colonoscopists. The ADR was not significantly affected by patients’ age or the indication of the procedure. The ADR was directly linked to CWT which varied among the colonoscopists. There was a significant difference in ADR according to the endoscopist’s specialty.
We noticed that the rate of detecting polyps on the proximal colon was significantly higher when the CWT is equal or more than 8 min; however, this was not powerful statistically and could be attributed to a relatively small sample size.
The difference between the groups of colonoscopists was evident in terms of CWT and ADR. It could be argued that colonoscopists who had higher CWT were using techniques to improve mucosal visualisation like repeated irrigation, suctioning and position change.22
Multivariate analysis was conducted to compare the CWT in two groups (colonoscopists with high and low ADR) while taking into account a number of confounding variables. CWT remained significantly longer for colonoscopists with ADR equal or more that 20%.
This study has a number of limitations. First, we adopted a retrospective design, but it allowed us to eliminate the Hawthorne effect. Colonoscopists were not aware of study aims and were less likely to have altered their practice.12 24 25 In addition, there is a number of risk factors that may affect the ADR which were not considered such as gender and race,26–28 high body mass index,27 29 smoking30 31 and alcohol consumption.31–33 An important limitation is that while the CWT is longer, it could be translated as better performance and meticulousness during the inspection, and therefore the higher would be the probability to find adenomas. However, a longer inspection time can be attributed to the fact that endoscopists found adenomas and took time to remove them even with a cold snare technique. We attempted to account for this in the multivariate analysis. We additionally excluded procedures where polyps were removed using diathermy modalities like endoscopic mucosal resection. In these procedures, the CWT would increase significantly by the technicalities involved with the polyp removal via diathermy.
Conclusion
In non-screening colonoscopies, longer CWT is associated with higher ADR. CWT can differ between endoscopists. The detection of the polyps in the proximal colon might improve with thorough colonoscopy withdrawal. Studies of multicentric prospective design are recommended to increase the generalisability and applicability of the study findings.
Footnotes
Contributors: AA-R, ME-F, AH and MT planned and designed the study. AA-R, ME-F and IA-T collected data. MA and AA-R analysed the results. All authors revised the manuscript critically and approved the final version submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon request.
References
- 1. Rex DK, Cutler CS, Lemmel GT, et al. . Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- 2. Atia MA, Ramirez FC, Gurudu SR. Quality monitoring in colonoscopy: time to act. World J Gastrointest Endosc 2015;7:328–35. 10.4253/wjge.v7.i4.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaminski MF, Regula J, Kraszewska E, et al. . Quality indicators for colonoscopy and the risk of interval cancer. N Eng J Med 2010;362:1795–803. [DOI] [PubMed] [Google Scholar]
- 4. Atkin WS, Edwards R, Kralj-Hans I, et al. . Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. 10.1016/S0140-6736(10)60551-X [DOI] [PubMed] [Google Scholar]
- 5. National Cancer Intelligence Network Cancer Outcomes and Services Dataset 2015. Available: http://www.ncin.org.uk/collecting_and_using_data/data_collection/cosd
- 6. Aranda-Hernández J, Hwang J, Kandel G. Seeing better—evidence based recommendations on optimizing colonoscopy adenoma detection rate. World J Gastroenterol 2016;22:1767–78. 10.3748/wjg.v22.i5.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thoufeeq MH, Rembacken BJ. Meticulous cecal image documentation at colonoscopy is associated with improved polyp detection. Endosc Int Open 2015;3:E629–33. 10.1055/s-0034-1392783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong MCS, Ching JYL, Chan VCW, et al. . Determinants of bowel preparation quality and its association with adenoma detection: a prospective colonoscopy study. Medicine 2016;95:e2251 10.1097/MD.0000000000002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Widjaja D, Bhandari M, Loveday-Laghi V, et al. . Withdrawal time in excellent or very poor bowel preparation qualities. World J Gastrointest Endosc 2014;6:186–92. 10.4253/wjge.v6.i5.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pullens HJ, Siersema PD. Quality indicators for colonoscopy: current insights and caveats. World J Gastrointest Endosc 2014;6:571–83. 10.4253/wjge.v6.i12.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee TJW, Blanks RG, Rees CJ, et al. . Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy 2013;45:20–6. 10.1055/s-0032-1325803 [DOI] [PubMed] [Google Scholar]
- 12. Vavricka SR, Sulz MC, Degen L, et al. . Monitoring colonoscopy withdrawal time significantly improves the adenoma detection rate and the performance of endoscopists. Endoscopy 2016;48:256–62. 10.1055/s-0035-1569674 [DOI] [PubMed] [Google Scholar]
- 13. Xiang L, Zhan Q, Zhao X-H, et al. . Risk factors associated with missed colorectal flat adenoma: a multicenter retrospective tandem colonoscopy study. World J Gastroenterol 2014;20:10927–37. 10.3748/wjg.v20.i31.10927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barclay RL, Vicari JJ, Doughty AS, et al. . Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–41. 10.1056/NEJMoa055498 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt-Tänzer W, Eickhoff A. What influences the quality of prevention colonoscopy? Viszeralmedizin 2014;30:26–31. 10.1159/000358747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joint Advisory Group on GI Endoscopy Bowel cancer screening programmes, 2018. Available: https://www.saas.nhs.uk/Downloads.aspx [Accessed 23 Apr 2018].
- 17. Bourikas LA, Tsiamoulos ZP, Haycock A, et al. . How we can measure quality in colonoscopy? World J Gastrointest Endosc 2013;5:468–75. 10.4253/wjge.v5.i10.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rembacken B, Hassan C, Riemann JF, et al. . Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 2012;44:957–68. 10.1055/s-0032-1325686 [DOI] [PubMed] [Google Scholar]
- 19. Logan RFA, Patnick J, Nickerson C, et al. . Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439–46. 10.1136/gutjnl-2011-300843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bressler B, Paszat LF, Vinden C, et al. . Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology 2004;127:452–6. [DOI] [PubMed] [Google Scholar]
- 21. Clarke E, Woolson KL, Saunders M, et al. . PWE-100 missed rates of colorectal cancer—diagnostic limitations. Gut 2016;65(Suppl 1):A187.2–A188. 10.1136/gutjnl-2016-312388.345 [DOI] [Google Scholar]
- 22. Rees CJ, Gibson ST, Rutter MD, et al. . UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65:1784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. East JE, Atkin WS, Bateman AC, et al. . British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017;66:1181–96. 10.1136/gutjnl-2017-314005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rex DK. Looking over your shoulder during colonoscopy: potential roles for videorecording colonoscopy withdrawals. Gastrointest Endosc 2012;75:134–7. 10.1016/j.gie.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 25. Rex DK, Hewett DG, Raghavendra M, et al. . The impact of videorecording on the quality of colonoscopy performance: a pilot study. Am J Gastroenterol 2010;105:2312–7. 10.1038/ajg.2010.245 [DOI] [PubMed] [Google Scholar]
- 26. Robinson E, Mohilever J, Zidan J, et al. . Colorectal cancer: incidence, delay in diagnosis and stage of disease. Eur J Cancer Clin Oncol 1986;22:157–61. 10.1016/0277-5379(86)90025-8 [DOI] [PubMed] [Google Scholar]
- 27. Kim Y, Kim Y, Lee S. An association between colonic adenoma and abdominal obesity: a cross-sectional study. BMC Gastroenterol 2009;9:4 10.1186/1471-230X-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCashland TM, Brand R, Lyden E, et al. . Gender differences in colorectal polyps and tumors. Am J Gastroenterol 2001;96:882–6. 10.1111/j.1572-0241.2001.3638_a.x [DOI] [PubMed] [Google Scholar]
- 29. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47. 10.1158/1055-9965.EPI-07-0708 [DOI] [PubMed] [Google Scholar]
- 30. Botteri E, Iodice S, Bagnardi V, et al. . Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. 10.1001/jama.2008.839 [DOI] [PubMed] [Google Scholar]
- 31. Shrubsole MJ, Wu H, Ness RM, et al. . Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am J Epidemiol 2008;167:1050–8. 10.1093/aje/kwm400 [DOI] [PubMed] [Google Scholar]
- 32. Fedirko V, Tramacere I, Bagnardi V, et al. . Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958–72. 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 33. Cho E, Smith-Warner SA, Ritz J, et al. . Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med 2004;140:603–13. [DOI] [PubMed] [Google Scholar]


