Abstract
Background
Eosinophilic oesophagitis (EoE) is a chronic, inflammatory condition of the oesophagus, characterised by intermittent dysphagia, food bolus obstruction (FBO) and histologically proven, eosinophil-mediated inflammation. EoE is identified in up to 50% of FBO presentations.
Objective
To evaluate the management of patients presenting with FBO to our centre against current clinical guidelines.
Design
A retrospective analysis of acute FBO was performed between January 2008 and August 2014. Patients were identified using the ICD 10 code T18.1, ‘foreign body in oesophagus’ in their electronic discharge document. Data were collected on admitting specialty, previous FBO, endoscopy findings, biopsy sites and findings, eosinophil count and diagnosis of EoE.
Results
310 acute episodes of FBO were included in the final study cohort. 202 (65.2%) flexible oesophagogastroduodenoscopies (OGDs) were performed, with 50 (34.5%) of those occurring in those admitted under ENT (n=145), versus 28 (93.3%) and 124 (91.9%) in general medicine (n=30) and surgery (n=135), respectively. 80 (39.6%) had oesophageal biopsies taken, and 21 novel diagnoses of EoE were made (26.3% biopsy-proven rate). Five (23.8%) of the novel diagnoses had a formal eosinophil count included in the histopathology report, and eight (38.1%) had up to three previous OGDs that had not diagnosed their condition of EoE.
Conclusion
Our study highlights wide variation in adherence to the guidelines for the management of FBO depending on admitting specialty. We advocate an FBO protocol involving single specialty management, flexible OGD, ≥6 biopsies from the upper and lower oesophagus, and standardisation of oesophageal biopsy reports with a formal eosinophil count.
Keywords: food bolus, eosinophilic oesophagitis, dysphagia
Introduction
Food bolus obstruction (FBO) has an estimated annual incidence rate of 13 episodes per 100 000, with increased prevalence reported over the last 15 years.1 2 With the exclusion of paediatric and intentional adult foreign body ingestion, the aetiology of FBO includes complications of gastro-oesophageal reflux disease (eg, peptic stricture), oesophageal strictures (eg, Schatzki’s ring), oesophageal motility disorders such as achalasia, and malignancy, but the single most common cause is eosinophilic oesophagitis (EoE).1 3
There is increasing recognition that EoE is a common and preventable cause of dysphagia and FBO.4 Studies have shown overall incidence rates of EoE have increased to a current figure of 7.2 new patients per 100 000 inhabitants yearly.5 EoE prevalence ranges from 0.4% to 0.7%, making it more common than inflammatory bowel disease (0.3%) and achalasia (0.01%).6–8 The only reliable diagnostic test for EoE is endoscopic evaluation of the oesophagus with flexible oesophagogastroduodenoscopy (OGD) and oesophageal biopsies.9 A minimum of six oesophageal biopsies should be taken from the upper and lower oesophagus, with a particular focus on areas with mucosal abnormalities at endoscopy.9 An eosinophil count ≥15 eosinophils per high-power field (eos/hpf) is diagnostic of EoE.9 With regards to managing FBO, guidelines recommend routine therapeutic OGD and gently pushing the bolus into the stomach or retrieval if this fails, as well as the procurement of oesophageal biopsies to determine the underlying cause.10
The aim of our study was to investigate the management of FBO in our institution, and to determine whether we conform to international consensus guidelines for the management of FBO and diagnosis of EoE.9 10 Our primary objectives were to identify the proportion of patients who underwent flexible OGD with biopsies taken as inpatient or outpatient, those presenting with recurrent FBO and those subsequently diagnosed with EoE.
Methods
We performed a retrospective analysis of all adult patients admitted with ICD 10 code T18.1 ‘foreign body in oesophagus’ to our trust between 1 January 2008 and 31 August 2014. Patients were excluded if there was a lack of clinical data, if their admitting specialty was paediatrics or if they had ingested a non-food bolus foreign body. Research ethics committee approval was not required for this study, as confirmed by the decision-making tool on the online National Research Ethics Service.11 Patients were identified using their Community Health Index number. Hospital databases were accessed for admission and investigative data, admitting specialty, previous FBO, OGD findings, biopsy sites and findings, eosinophil count and diagnosis (established or novel) of EoE. This was entered on to an encrypted database with a unique study ID allocated to each subject as per Caldicott principles.
We defined diagnostic conformity to international guidelines as follows9:
Endoscopy with oesophageal biopsy is the only reliable diagnostic test for EoE.
An eosinophil-predominant inflammation on oesophageal biopsy with a peak value of ≥15 eos/hpf.
At least six biopsies should be taken from the upper and lower oesophagus.
Statistical analysis was performed using SPSS V.22 software. Statistical significance was set at p value <0.05. Shapiro-Wilk test was used to assess normal distribution and subsequent non-parametric analysis using Mann-Whitney U test compared mean ages between groups. Pearson’s χ2 statistic was used to assess for the presence of a significant relationship between admitting specialty and frequency of OGDs performed.
Results
Patient demographics
A total of 354 episodes of acute FBO were identified. This number includes 24 subjects who presented twice (48 episodes) and 5 subjects who presented three times (15 episodes). Five subjects were excluded due to a lack of clinical data and 39 were excluded as they were admitted under the care of paediatrics and/or had ingested a non-food bolus foreign body. This left a final study cohort of 310 acute episodes of FBO (including recurrent episodes). A total of 211 (68.1%) subjects were men compared with 99 (31.9%) women. The mean age for men (55.3 years, 21.0 SD) was less than that of women (66.8 years, 19.1 SD) (p<0.05). Moreover, 145 (46.8%) admissions were managed by ENT, 135 (43.5%) by general surgery and 30 (9.7%) by general medicine.
Interventions
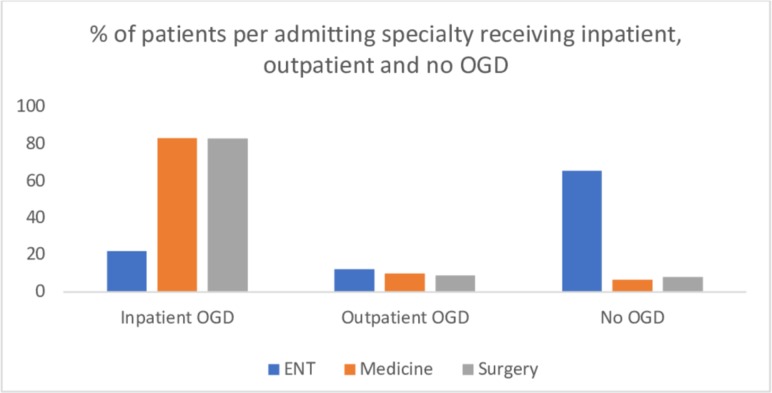
A total of 202 (65.2%) flexible OGDs were performed; 169 (83.7%) as an inpatient and 33 (16.3%) as an outpatient. In 108 (34.8%) episodes, no OGD was performed. The breakdown by admitting specialty is outlined in figure 1 and table 1; patients admitted under ENT were significantly more likely to have no OGD done during and after their admission (χ2=113.0, p<0.01). In the ENT cohort (n=145), 54 (37.2%) subjects received an inpatient rigid oesophagoscopy and 15 (10.3%) received a flexible laryngoscopy, with or without an inpatient or outpatient OGD.
Figure 1.
Graph illustrating the % breakdown of patients receiving an inpatient, outpatient or no oesophagogastroduodenoscopy (OGD) following their acute food bolus obstruction presentation.
Table 1.
Patients per specialty who received an inpatient, outpatient or no OGD following their presentation with acute FBO
| Inpatient OGD | Outpatient OGD | No OGD | ||||
| n | % total specialty cohort | n | % total specialty cohort | n | % total specialty cohort | |
| ENT | 32 | 22.1 | 18 | 12.4 | 95 | 65.5 |
| Medicine | 25 | 83.3 | 3 | 10 | 2 | 6.7 |
| Surgery | 112 | 83.0 | 12 | 8.9 | 11 | 8.1 |
| Total | 169 | 33 | 108 | |||
FBO, food bolus obstruction; OGD, oesophagogastroduodenoscopy.
Biopsies
Oesophageal biopsies were taken in 80 (39.6%) FBO episodes; 59 (34.9%) during inpatient OGDs and 21 (63.6%) during outpatient OGDs. Figure 2 illustrates the number of oesophageal biopsies taken. Five (6.3%) subjects had ≥6 biopsies taken. With regards to the procurement sites of the biopsies, 62 (77.5%) subjects had biopsies taken from one site, 5 (6.3%) from two sites, 4 (5.0%) from three sites and 9 (11.3%) had no procurement site specified.
Figure 2.
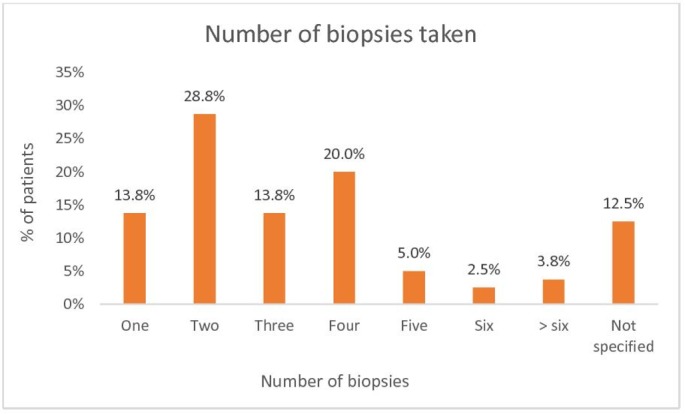
Number of biopsies taken on flexible oesophagogastroduodenoscopy (combined inpatient and outpatient).
Diagnosing EoE
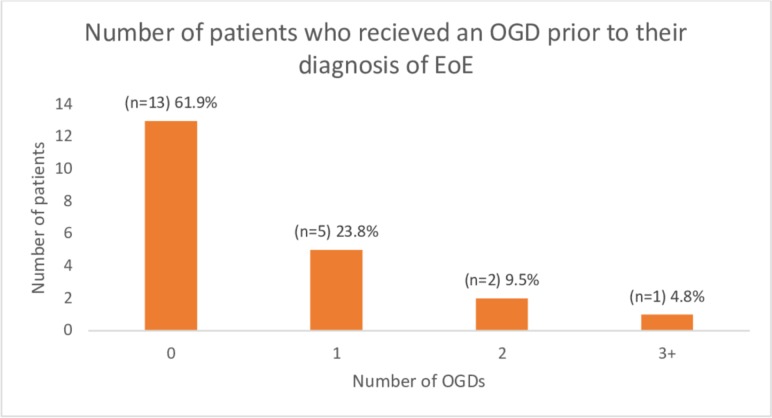
Excluding all repeat presentations, and considering all patients at first presentation, 276 individuals presented with FBO. Twenty-five (9.1%) were diagnosed with EoE; four (1.4%) had a known diagnosis of EoE, with a novel diagnosis of EoE made in 21 (7.6%) subjects. Twenty (80.0%) subjects were men and five (20.0%) were women. Five (23.8%) of the 21 novel diagnoses had a formal eosinophil count included in the histopathology report (all ≥15 eos/hpf). The remainder included phrases such as “features strongly suggestive of EoE” or “appearance entirely consistent with EoE” or included EoE in the list of differential diagnoses due to a subjectively described, large number of eosinophils in the biopsy specimen. Twenty-one novel diagnoses of EoE from 80 subjects biopsied gave a diagnostic rate of 26.3%, with 5 diagnoses made on inpatient OGD and 13 on outpatient follow-up OGDs. Three patients were diagnosed later at 7 months, 1 year and 3 years after experiencing recurrent FBO presentations. Eight (38.1%) subjects had previously undergone OGDs prior to their EoE diagnosis. A breakdown of OGDs prior to diagnosis is illustrated in figure 3.
Figure 3.
Number of oesophagogastroduodenoscopies (OGDs) received by patients prior to their diagnosis of eosinophilic oesophagitis (EoE).
Discussion
EoE was first described in the early 1990s and is now recognised as the leading cause of recurrent dysphagia and FBO in both children and young adults.12 13 There is now consensus agreement that EoE may be characterised clinically by symptoms that result in oesophageal dysfunction and histologically by an eosinophil-predominant inflammation.9
In our single-centre study, we report a biopsy-proven novel EoE diagnosis in 21 cases (26.3%). This is within the range (18.0%–54.8%) reported by other studies investigating FBO (see table 2).2 3 14–18 The variation in prevalence rates may be due to the different diagnostic criteria applied. All investigated the management of FBO, but some only included patients listed for endoscopy, indicating selection bias. In addition, some of these studies are likely to underestimate the true prevalence rates of EoE by setting the eos/hpf threshold too high or excluding patients who responded to proton pump inhibitor (PPI) therapy in their inclusion criteria.3 15 16 18 We now know that there is crossover with reflux disease and patients with EoE can respond to PPI therapy.9
Table 2.
OGD and biopsy findings from studies investigating EoE in adult patients presenting with FBO
| Study | Diagnosed with EoE (% cohort biopsied) | Number biopsied (% of OGD cohort) | OGD cohort | Diagnostic criteria |
| Desai et al 3 | 17 (54.8%) | 31 (100%) | 31 | Biopsies from upper and lower oesophagus, stomach and duodenum. ≥20 eos/hpf 13/17 pre-treated with PPI for 4–8 |
| Kerlin et al 14 | 14 (48.3%) | 29 (67.4%) | 43 | Biopsies from proximal and distal oesophagus, ≥15 eos/hpf |
| Sperry et al 15 | 45 (46%) | 98 (27%) | 363 | ≥15 eos/hpf, lack of response to PPI or normal pH monitoring. |
| Mahesh et al 2 | 73 (52%) | 141 (45%) | 315 | >15/eos/hpf+other descriptive histological findings, no specified oesophageal biopsy location |
| Heerasing et al 16 | 17 (33%) | 51 (60%) | 85 | Biopsies from proximal and distal oesophagus, ≥20 eos/hpf |
| Philpott et al 17 | 85 (30.6%) | 278 (24.6%) | 1132 | ≥15 eos/hpf, no specified oesophageal biopsy location |
| Truskaite and Dlugosz18 | 34 (18%) | 185 (77.7%) | 238 | Biopsies from proximal, mid, distal oesophagus, antrum and duodenum. ≥15 eos/hpf after PPI treatment |
| Our study | 21 (26.3%) | 80 (39.6%) | 202 | ≥15 eos/hpf, or diagnosis of EoE suggested on histopathology report |
EoE, eosinophilic oesophagitis; FBO, food bolus obstruction; OGD, oesophagogastroduodenoscopy; PPI, proton pump inhibitor; eos/hpf, eosinophils per high-power field.
In our study, 108 (34.8%) presentations were managed without an OGD. One hundred twenty-two (60.4%) of those who underwent OGD did not have oesophageal biopsies taken. This biopsy procurement rate in the investigation of FBO is not unusual, as shown in a recent study by Hiremath et al surveying the practices of 428 adult and paediatric gastroenterologists from three major USA gastroenterology societies.19 Only 34% of respondents reported always obtaining oesophageal biopsies irrespective of endoscopic findings, and 51% reported procuring biopsies only if the oesophagus appeared abnormal. The study goes on to estimate that approximately 10 000 patients who present to the emergency department in the USA with FBO are being missed each year as a result of inadequate investigation on index presentation and lack of appropriate follow-up.19
Within our single-centre study, using a 26.3% biopsy-proven rate, a further 60 patients may have undiagnosed EoE. Diagnostic sensitivity increases with the number of biopsies to 84%, 97% and 100% when two, three and six biopsy specimens are taken.20 Guidelines recommend at least six biopsies from the upper and lower oesophagus with a diagnostic cut-off of ≥15 eos/hpf.9 In our cohort, 5 (6.3%) of the 80 biopsied subjects had ≥6 biopsies taken, 9 (11.3%) had biopsies taken from at least two sites in the oesophagus and 5 (23.8%) of the novel EoE cases had a formally reported eosinophil count (all ≥15 eos/hpf). We report a lack of objective assessment of eosinophil counts with phrases such as ‘abundant’, ‘massive’, ‘heavy infiltration’ or ‘liberal sprinkling of eosinophils’ in the histopathology reports in place of a formal eosinophil count. We recognise that such reporting is subjective, not easily comparable and is a limitation on the objectivity of what truly defines EoE in our study. Therefore, caution should be exercised in using our study to report prevalence rates of EoE within a cohort presenting with FBO. Instead, we highlight the multiple problems within our system and likely others that exist in managing this group of patients. Our findings are additionally limited by the retrospective and single-centred nature of this study.
We have identified the scope for education of the multidisciplinary team involved with the management of FBO in our centre. Management between specialties varies widely; it seems that those managed by ENT are the least likely to have management that conforms to international guidelines. Twenty-eight (93.3%) of 30 and 124 (91.9%) of 135 patients presenting to medical or surgical specialties respectively underwent an OGD. Only 50 (34.5%) of 145 patients managed by ENT underwent flexible OGD. ENT surgeons were more likely to perform rigid oesophagoscopy (37.2%) or flexible laryngoscopy (10.3%).
We have no protocol for the management of FBO. Currently, patients are admitted to one of three specialties: general medicine, general surgery and ENT. Management of FBO should be protocol driven and managed by a single specialty. Given that, in our centre, the majority of OGDs are performed by gastroenterology colleagues, admission under the care of general medicine seems to be the obvious choice. FBO protocols should include mandatory flexible OGD with a standardised approach to food bolus removal. Moreover, ≥6 biopsies should be taken from the upper and lower oesophagus, focusing on mucosal abnormalities. Patients should also be offered appropriate follow-up (eg, clinic appointment or follow-up endoscopy) to further investigate the underlying cause.10 21 Failing to investigate and follow up patients presenting with FBO delays commencement of treatment and results in repeat presentations and unnecessary intervention.17 In our study, 24 subjects presented twice and 5 subjects presented three times. Eight (38.1%) of our 21 novel EoE cases had received up to three OGDs prior to their EoE diagnosis.
Conclusion
Our study highlights a sporadic and unpredictable adherence to the guidelines for the management of FBO, and the investigation and diagnosis of EoE. There is a wide variation in practice among admitting specialties. We would advocate a formal FBO protocol that involves management by a single specialty, flexible OGD, multiple oesophageal biopsies, routine outpatient follow-up, standardisation of oesophageal biopsy reports and a formal eosinophil count. This will ensure patients presenting with FBO are managed according to current clinical guidelines. Too many opportunities to diagnose EoE are missed with a delay in the commencement of effective treatment.
Significance of this study.
What is already known about this subject?
It has become increasingly recognised that the most common underlying aetiology of patients presenting with food bolus obstruction (FBO) is eosinophilic oesophagitis (EoE).
Clear guidelines on the investigation and management of such patients exist.
What are the new findings?
Our single-centre study highlights wide variation in adherence to these guidelines depending on which specialty patients are admitted to.
We also highlight areas for improvement in histopathological assessment and reporting of oesophageal biopsy samples.
How might it impact on clinical practice in the foreseeable future?
This research has prompted the creation of an FBO protocol to avoid the wide disparities observed in clinical care.
This should improve the rate of missed diagnoses and ensure patients are managed in accordance with current guidelines.
Sharing this research will also prompt other centres to interrogate and improve their own clinical pathways for patients presenting with FBO who may have EoE.
Footnotes
Presented at: A poster for this work was presented at the 2017 Res Medica RMS conference and an abstract for this work published in 2017 Res Medica RMS Conference abstracts. Res Medica. 24(1);107–117. DOI: https://doi.org/10.2218/resmedica.v24i1.2510
Contributors: YN and IB collected and analysed the data as well as wrote the manuscript. MW provided a supervisory role, reviewed and amended subsequent draft copies of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Research Ethics Committee approval was not required for this study, as confirmed by the decision-making tool on the online National Research Ethics Service. The study was registered with and approved by the local Caldicott guardian.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1. Longstreth GF, Longstreth KJ, Yao JF. Esophageal food impaction: epidemiology and therapy. A retrospective, observational study. Gastrointest Endosc 2001;53:193–8. 10.1067/mge.2001.112709 [DOI] [PubMed] [Google Scholar]
- 2. Mahesh VN, Holloway RH, Nguyen NQ. Changing epidemiology of food bolus impaction: is eosinophilic esophagitis to blame? J Gastroenterol Hepatol 2013;28:963–6. 10.1111/jgh.12135 [DOI] [PubMed] [Google Scholar]
- 3. Desai TK, Stecevic V, Chang C-H, et al. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointestinal Endoscopy 2005;61:795–801. 10.1016/S0016-5107(05)00313-5 [DOI] [PubMed] [Google Scholar]
- 4. Kanakala V, Lamb CA, Haigh C, et al. The diagnosis of primary eosinophilic oesophagitis in adults: missed or misinterpreted? Eur J Gastroenterol Hepatol 2010;22:848–55. 10.1097/MEG.0b013e32832c7709 [DOI] [PubMed] [Google Scholar]
- 5. Arias Á., Pérez-Martínez I, Tenías JM, et al. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016;43:3–15. 10.1111/apt.13441 [DOI] [PubMed] [Google Scholar]
- 6. Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007;56:615–20. 10.1136/gut.2006.107714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. SC N, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 8. O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. WJG 2013;19:5806–12. 10.3748/wjg.v19.i35.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spergel JM, Dellon ES, Liacouras CA, et al. Summary of the updated international consensus diagnostic criteria for eosinophilic esophagitis. Ann Allergy Asthma Immunol 2018;121:281–4. 10.1016/j.anai.2018.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birk M, Bauerfeind P, Deprez P, et al. Removal of foreign bodies in the upper gastrointestinal tract in adults: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:489–96. 10.1055/s-0042-100456 [DOI] [PubMed] [Google Scholar]
- 11. NHS Health Research Authority NHS health research authority decision making tool. Available: http://www.hra-decisiontools.org.uk/research/
- 12. Attwood SE, Smyrk TC, Demeester TR, et al. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38:109–16. [DOI] [PubMed] [Google Scholar]
- 13. Straumann A, Spichtin HP, Bernoulli R, et al. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr 1994;124:1419–29. [PubMed] [Google Scholar]
- 14. Kerlin P, Jones D, Remedios M, et al. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol 2007;41:356–61. 10.1097/01.mcg.0000225590.08825.77 [DOI] [PubMed] [Google Scholar]
- 15. Sperry SLW, Crockett SD, Miller CB, et al. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc 2011;74:985–91. 10.1016/j.gie.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heerasing N, Lee SY, Alexander S. Prevalence of eosinophilic oesophagitis in adults presenting with oesophageal food bolus obstruction. WJGPT 2015;6:244–7. 10.4292/wjgpt.v6.i4.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Philpott HL, Nandurkar S, Thien F, et al. Seasonal recurrence of food bolus obstruction in eosinophilic esophagitis. Intern Med J 2015;45:939–43. 10.1111/imj.12790 [DOI] [PubMed] [Google Scholar]
- 18. Truskaite K, Dlugosz A. Prevalence of eosinophilic esophagitis and lymphocytic esophagitis in adults with esophageal food bolus impaction. Gastroenterol Res Pract 2016;2016:1–6. 10.1155/2016/9303858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiremath G, Vaezi MF, Gupta SK, et al. Management of esophageal food impaction varies among gastroenterologists and affects identification of eosinophilic esophagitis. Dig Dis Sci 2018;63:1428–37. 10.1007/s10620-018-4972-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah A, Kagalwalla AF, Gonsalves N, et al. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol 2009;104:716–21. 10.1038/ajg.2008.117 [DOI] [PubMed] [Google Scholar]
- 21. WT W, Chiu CT, Kuo CJ, et al. Endoscopic management of suspected esophageal foreign body in adults. Dis Esophagus 2011;24:131–7. [DOI] [PubMed] [Google Scholar]