Abstract
Acute liver failure (ALF) is a rare but life-threatening clinical syndrome with a broad range of causes. Significant improvements in outcome have occurred over the last 50 years, resulting not only from incremental improvements in specialist critical care and a step-change following the introduction of transplantation for this indication, but also better and more effective treatment started early at the site of first presentation.1 2 Emergency liver transplantation (LTx) remains an important intervention and the decision regarding the need for LTx remains key to management, though non-transplant therapies now appear effective for many causes of the condition. In this short review, we will outline issues in the recognition and management of ALF and ongoing challenges in its treatment.
Keywords: acute liver failure, liver transplantation, encephalopathy
Definition
‘Fulminant hepatic failure’ is a now historic term used first in 1970 to define a potentially reversible disorder resulting from a severe liver injury, in the absence of prior liver disease, with an onset of hepatic encephalopathy (HE) within 8 weeks of first symptom appearance.3 This definition has been refined over the years with central importance being given to the time interval between the development of jaundice and/or symptoms and onset of HE (figure 1). This interval provides clues in aetiology of the underlying disorder, likely complications and of prognosis without LTx.4–6 Using the O’Grady system,4 most frequently used in the UK, a ‘hyper-acute’ presentation where this interval is a week or less is typically secondary to paracetamol (acetaminophen or APAP) toxicity or viral hepatitis. This is generally associated with a good prognosis with medical management alone, in contrast to the poor non-transplanted survival seen in patients with an indolent ‘sub-acute’ presentation where this interval is between 1 and 3 months. This is typically seen in idiosyncratic drug-induced liver injury (DILI) or cases with indeterminate aetiology, which may be mistaken for chronic liver disease.7
Figure 1.
Subclassifications of acute liver failure.
Aetiologies
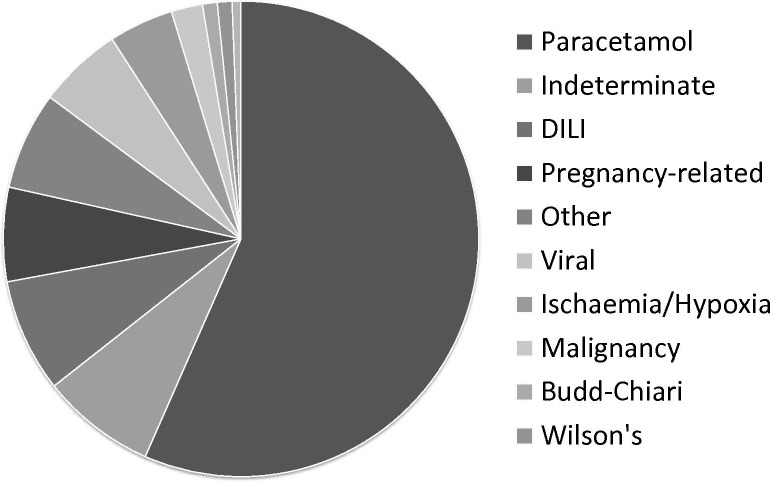
ALF is a condition most commonly affecting younger adults in previous good health and is more common in the developing than the developed world. Viral hepatitides are the most common causes of ALF in the former; their incidence in the developed world has fallen markedly by public health measures including improved sanitation and vaccination programme. Globally, the greatest number of cases probably results from acute hepatitis E infection, but in the UK by far the most common precipitant of ALF is severe paracetamol-induced hepatotoxicity, though a wide variety of other causes are seen (figure 2). Identification of aetiology at an early stage may make possible specific intervention to prevention of progression of the condition from liver injury without HE to frank liver failure.8 Further, identifying ‘poor prognosis’ aetiologies where there is little chance of native liver regeneration and recovery—particularly cases of non-paracetamol DILI or those of indeterminate aetiology—facilitates discussion with a transplantation centre at the earliest possible stage.
Figure 2.
Aetiology of acute liver disease admissions Kings College Hospital, 1999–2017, n=1502. DILI, non-paracetamol drug-induced liver injury.
General management
Initial management and when to discuss with a specialist centre
The European Association for the Study of Liver (EASL) disease guidelines for the management of ALF offer recommendations on the initial management of patients on presentation (box 1).9
Box 1. The European Association for the Study of Liver recommendations for measures at presentation of patients with acute liver failure.
In patients with severe acute liver injury, screen intensively for any signs of hepatic encephalopathy.
Exclude the presence of cirrhosis, alcohol-induced liver injury or malignant infiltration of the liver.
Consider whether the patient has contraindications for emergency liver transplantation: the finding of contraindications should not preclude transfer to a tertiary unit.
Searching for an aetiology allows treatment to be instituted and facilitates prognostic stratification.
Transfer to a specialised unit early if the patient has an INR>1.5 and onset of hepatic encephalopathy or other poor prognostic features.
Early discussion with a transplant unit even if the patient does not need to transfer at that time point.
Source reference9.
Key messages are for early recognition of patients with features associated with ALF, rapid and effective intravenous volume resuscitation, metabolic stabilisation with correction of hypoglycaemia and acidosis, and early contact with a specialist liver unit. Early critical care review is often required, particularly if encephalopathy of any grade is present as there is potential for abrupt deterioration in conscious level and endotracheal intubation may be required. Other key interventions in the early stage after presentation include the administration of N-acetylcysteine (NAC) and antibiotics and are discussed below.
A low threshold for discussion with a liver centre should be maintained, guided by confirmed or suspected aetiology, the severity of liver injury, presence of extrahepatic organ failure or significant metabolic disarray. Discussion is mandatory in all patients with encephalopathy or a suspected aetiology that is associated with a poor prognosis before the onset of encephalopathy. After stabilisation, most patients with ALF will require transfer to a centre experienced in their care and where LTx may be available if required.9
Only a basic assessment of aetiology is usually performed at the presenting centre as transfer should not be delayed and detailed investigation of aetiology is best undertaken after this has occurred. In non-paracetamol-related disease, investigations often include cross-sectional imaging to exclude chronic liver disease, malignancy or alternate diagnoses. Liver biopsy is rarely indicated but can be useful in cases of diagnostic uncertainty.
Ideally, identification, discussion and transfer should occur before the onset of encephalopathy—but this is often not possible as alteration of consciousness may be the initial presenting feature.
NAC administration
The administration of intravenous NAC is well established as effective in the management of paracetamol intoxication after overdose, and has been shown to improve survival in patients who develop liver injury—when used both early and late after presentation.10 11 The mechanisms by which it mediates this are unclear but additional to its well-characterised antidotal effects, it has systemic antioxidant and anti-inflammatory attributes and vasodilatory effects that may improve microcirculatory flow.11 12 The use of NAC in non-paracetamol aetiologies is less well characterised but data suggest a survival benefit when administered at an early stage of illness.13 Given its good safety profile and that in the UK most cases of ALF or severe acute liver injury result from paracetamol poisoning, it is a frequent co-factor in liver injury of other causes and its efficacy falls rapidly over time after drug ingestion, it should probably be given to all cases at presentation. The NAC administration is usually limited to 5 days after presentation as there is little to suggest benefit from prolonged administration and there is a potential risk of enhancing functional immunosuppression.14
Management of sepsis
ALF is associated with a markedly increased risk of sepsis, which, when present, may trigger or worsen encephalopathy, impair hepatic regeneration and act as a contraindication to LTX. Its prevention and/or treatment is, therefore, of major importance. Historically, bacterial infections are common in ALF and fungal sepsis reported but less commonly.
The place of prophylaxis of sepsis versus active surveillance remains a controversial area with practice varying between centres.15 Conventional laboratory markers of infection, such as C reactive protein and procalcitonin, measurements may be unhelpful16 17 and prophylactic antimicrobial therapy has not been demonstrated to affect survival in ALF.18 The approach recommended by EASL is for active surveillance with repeat tissue and blood culturing and monitoring of fungal biomarkers and a low threshold for antimicrobial use. The presence of any degree of encephalopathy, clinical signs of infection or features of Systemic Inflammatory Response Syndrom (SIRS) should trigger administration of broad-spectrum antimicrobial therapy.
The management of coagulopathy
Coagulopathy is a universal feature of ALF and changes in laboratory measures including prothrombin time and INR are key to prognostic assessment. Changes in procoagulant and anticoagulant proteins, thrombocytopenia, and fibrin formation and breakdown have all been described. However, the functional consequences are complex and there is not a greatly increased risk of bleeding and some patients may be hypercoagulable.8 19 20 The risk of bleeding with procedures, such as central venous catheter placement and even transjugular liver biopsy, is relatively small.21 Correction of coagulopathy complicates prognostic evaluation by changing the laboratory measures utilised and should be reserved for active bleeding or more invasive procedures, such as surgery or insertion of intracranial pressure (ICP) monitoring devices, though severe thrombocytopenia or hypofibrinogenaemia may be addressed prophylactically.9 The role of pharmacological venous thromboembolism prophylaxis is yet to be defined.
The management of hepatic encephalopathy
The development of HE is a hallmark of ALF and of major prognostic significance.4 Its clinical course is fluctuant and ranges from changes in cognition and altered consciousness with additional manifestations of headache, vomiting, asterixis, agitation, hyperreflexia and clonus.22 Progression to grade 3 HE may occur rapidly and usually triggers intubation and mechanical ventilation to reduce the risk of aspiration and control oxygenation and ventilation.23 Intracranial hypertension (ICH) from cerebral oedema leading to cerebral herniation is a potentially catastrophic consequence of advanced HE. Its prevention requires clinical evaluation for risk stratification and the implementation of a package of neuroprotective supportive care to reduce the likelihood of development of cerebral oedema.1 Ammonia is thought to be the principal neurotoxin responsible for this and in contrast to chronic liver disease, arterial ammonia levels correlate closely with the severity of HE in ALF. Risk of ICH increases markedly with levels of >200 μmol/L, particularly if sustained.24 Arterial ammonia measurement is key to cerebral risk stratification in ALF, and its modulation an important therapeutic target. Continuous renal replacement therapy (RRT) has been demonstrated to have clinically meaningful ammonia clearance and can be used to lower circulating levels.25 26
The use of invasive ICP monitoring devices is controversial given the association of intracranial bleeding and data suggesting that it does not improve patient outcomes.27 28 Its role has been challenged in recent years and it is now seldom used by most UK centres. Non-invasive techniques including transcranial Doppler ultrasound and jugular venous oximetry may be useful in identifying evolving cerebral oedema and serves as a further tool for risk stratification.29
The package of care applied to patients with HE in ALF has evolved from practice in neurosurgical critical care. Currently utilised neuroprotective interventions include elevation of the head at a 30-degree upright angle, avoiding fever, hypoglycaemia and hyperglycaemia and maintaining serum sodium between 140 and 145 mmol/L by hypertonic saline infusion. RRT is introduced at an early stage to control hyperammonaemia. Ventilation maintains normocapnoea and high-level sedation is administered, often utilising propofol.9 Induced hypothermia does not appear helpful as a prophylactic measure and use of L-ornithine L-aspartate is ineffective.30 31 However, the overall approach now undertaken seems to have been successful with a remarkable fall in the incidence of ICH noted in recent years.1
Nutritional support
Patients with ALF have increased energy expenditure and protein catabolism and often require nutritional support; practice does not differ significantly from other critical illness.32 33 Oral or enteral support should be utilised in patients with acute liver injury without HE and in those with HE, should be accompanied by monitoring of arterial ammonia. A temporary reduction of protein load for 12–24 hours only may be used in patients with worsening hyperammonaemia or those assessed as at high risk for ICH.9 Other features associated with ICH include young age, a hyperacute presentation, systemic inflammation and a requirement for vasopressors or RRT. Acute pancreatitis and ileus are not infrequent complications in ALF and if encountered nutritional support approaches must also account for these pathologies.9 34 There is little to support the use of specific enteral or parental feed formulations in ALF.
Prognostic assessment and use of LTx
Prognostic assessment is performed at the liver transplant centre when the patient has been stabilised and disease aetiology and illness severity determined. A number of prognostic models have been used to predict outcome in ALF and identify those patients likely to benefit from emergency LTx.3 5 7 8 35 Variables assessed by the systems in most common use include patient age, the presence of encephalopathy and laboratory measures of liver injury severity, such as INR or blood lactate and bilirubin concentrations.
In the UK, current criteria for wait-listing patients with ALF for LTx are derived from the original Kings College Criteria (KCC), first developed in the late 1980s. These have separate criteria for paracetamol and non-paracetamol aetiologies, reflecting the differences in potential for native liver regeneration, response to medical therapy alone and, thus, in prognosis between these aetiological groups (box 2).
Box 2. Current UK acute liver failure indications for super-urgent liver transplantation registration.
Paracetamol
Arterial pH<7.25 more than 24 hours after overdose and after fluid resuscitation.
Co-existing INR>6.5 (PT>100S), HE grade≥3 and creatinine>300µmol/L.
-
Liver injury, coagulopathy and HE with
Arterial lactate>5 mmol/L on admission.
Arterial lactate>4 mmol/L >24 hours after admission.
Exclusion of other causes of elevated lactate.
Two criteria from Paracetamol Category 2 with clinical evidence of deterioration.
Non-paracetamol
-
Favourable aetiologies (ecstasy/hepatitis A) with HE
INR>6.5 (PT>100S) or
Three of: INR>3.5 (PT>50S), age>40 or <10 years, bilirubin>300 µmol/L and J-E>7 days.
-
Unfavourable aetiologies (idiosyncratic drug-induced, indeterminate)
INR>6.5 (PT>100S).
In absence of HE: INR>3.5 and age>40 or <10 years.
In presence of HE: J-E>7 days and bilirubin>300 µmol/L.
-
Acute presentation of Wilson’s disease of Budd-Chiari syndrome
A combination of coagulopathy and any grade of HE.
HE, hepatic encephalopathy; J-E, jaundice to encephalopathy interval; PT, prothrombin time. Citation: NHS Blood and Transplant. Liver Transplantation: Selection Criteria and Recipient Registration. NHS Blood and Transplant. Accessed 29 March 2019 (online: http://odt.nhs.uk/pdf/liver_selection_policy.pdf).
Recent meta-analysis of the performance of the original KCC in predicting outcome in ALF demonstrated relatively high specificity but low sensitivity, and deterioration in test performance in recent case series.36 These changes likely reflect the improvements in non-transplanted survival with advances in medical care seen in some but not all aetiologies of ALF. A large body of research has sought to address these changes in prognosis though development of new predictive models and/or introduction of alternate or supplemental measures of prognosis, though to date none have been universally accepted. Prognostic scoring systems remain an adjunct to clinical assessment and are interpreted on an individual patient basis by experienced multidisciplinary teams.
Selection of LTx candidates is not based on fulfilling poor prognostic criteria alone, but also with consideration of relative or absolute contraindications to surgery. Making this assessment in critical patients with encephalopathy and rapidly progressive multiple organ failure is challenging and must assess not only the potential for recovery of native liver function, severity of acute illness, comorbidity and physiological reserve, but also whether a potential recipient has the capacity to cope with the long-term requirements of post-transplant life. Outcomes of such ‘super-urgent’ transplantation are good, with most recent reports from the UK showing a 1-year patient survival of >90%. The factor most consistently associated with recipient mortality is age, with markedly inferior outcomes seen in recipients older than 50 years, reflecting the extreme physiological stress associated with emergency transplantation.
Future therapies?
Recent data suggest that plasma exchange may have a useful role in some patients with ALF, with a randomised controlled trial suggesting a reduction in mortality.37 However, its place in clinical management and which patients will benefit from its use is yet to be fully established, and it is the subject of on-going research. Liver-assist devices, hepatocyte and stem cell transplants are attractive therapeutic interventions in patients with ALF; however, they have yet to show confirmed benefit and their use is currently restricted to clinical trials.9
Conclusions
ALF is a rare critical illness with high mortality that requires specialist management. Early recognition and prompt referral and transfer is key to successful management. Approaches to supportive care and the use of transplantation are now highly evolved and are associated with markedly improved outcomes.
Footnotes
Correction notice: This article has been corrected since it published Online First. The fifth heading under General Management has been corrected.
Contributors: ODT conceived the review and wrote the first draft. WB revised and amended prior to submission and acts as a guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol 2013;59:74–80. 10.1016/j.jhep.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 2. Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med 2016;164:724–32. 10.7326/M15-2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis 1970;3:282–98. [PubMed] [Google Scholar]
- 4. O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273–5. [DOI] [PubMed] [Google Scholar]
- 5. Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis 1986;6:97–106. 10.1055/s-2008-1040593 [DOI] [PubMed] [Google Scholar]
- 6. Mochida S, Nakayama N, Matsui A, et al. Re-evaluation of the Guideline published by the acute liver failure Study Group of Japan in 1996 to determine the indications of liver transplantation in patients with fulminant hepatitis. Hepatol Res 2008;38:970–9. 10.1111/j.1872-034X.2008.00368.x [DOI] [PubMed] [Google Scholar]
- 7. Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl 2008;14 Suppl 2:S67–S79. 10.1002/lt.21612 [DOI] [PubMed] [Google Scholar]
- 8. Koch DG, Speiser JL, Durkalski V, et al. The natural history of severe acute liver injury. Am J Gastroenterol 2017;112:1389–96. 10.1038/ajg.2017.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wendon, J, Cordoba J, Dhawan A, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047–81. 10.1016/j.jhep.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 10. Harrison PM, Keays R, Bray GP, et al. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 1990;335:1572–3. 10.1016/0140-6736(90)91388-Q [DOI] [PubMed] [Google Scholar]
- 11. Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 1991;303:1026–9. 10.1136/bmj.303.6809.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison PM, Wendon JA, Gimson AE, et al. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med 1991;324:1852–7. 10.1056/NEJM199106273242604 [DOI] [PubMed] [Google Scholar]
- 13. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856–64. 10.1053/j.gastro.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stravitz RT, Sanyal AJ, Reisch J, et al. Effects of N-acetylcysteine on cytokines in non-acetaminophen acute liver failure: potential mechanism of improvement in transplant-free survival. Liver Int 2013;33:1324–31. 10.1111/liv.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rabinowich L, Wendon J, Bernal W, et al. Clinical management of acute liver failure: results of an international multi-center survey. World J Gastroenterol 2016;22:7595–603. 10.3748/wjg.v22.i33.7595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rule JA, Hynan LS, Attar N, et al. Procalcitonin identifies cell injury, not bacterial infection, in acute liver failure. PLoS One 2015;10 10.1371/journal.pone.0138566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silvestre JPdaS, Coelho LMdaC, Póvoa PMSR. Impact of fulminant hepatic failure in C-reactive protein? J Crit Care 2010;25:657.e7–657.e12. 10.1016/j.jcrc.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 18. Karvellas CJ, Cavazos J, Battenhouse H, et al. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol 2014;12:1942–9. 10.1016/j.cgh.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habib M, Roberts LN, Patel RK, et al. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int 2014;34:672–8. 10.1111/liv.12369 [DOI] [PubMed] [Google Scholar]
- 20. Stravitz RT, Ellerbe C, Durkalski V, et al. Bleeding complications in acute liver failure. Hepatology 2018;67:1931–42. 10.1002/hep.29694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993;18:1370–6. 10.1002/hep.1840180614 [DOI] [PubMed] [Google Scholar]
- 22. Shawcross DL, Wendon JA. The neurological manifestations of acute liver failure. Neurochem Int 2012;60:662–71. 10.1016/j.neuint.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 23. Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet 2010;376:190–201. 10.1016/S0140-6736(10)60274-7 [DOI] [PubMed] [Google Scholar]
- 24. Bernal W, Hall C, Karvellas CJ, et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology 2007;46:1844–52. 10.1002/hep.21838 [DOI] [PubMed] [Google Scholar]
- 25. Slack AJ, Auzinger G, Willars C, et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int 2014;34:42–8. 10.1111/liv.12221 [DOI] [PubMed] [Google Scholar]
- 26. Cardoso FS, Gottfried M, Tujios S, et al. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 2018;67:711–20. 10.1002/hep.29488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vaquero J, Fontana RJ, Larson AM, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl 2005;11:1581–9. 10.1002/lt.20625 [DOI] [PubMed] [Google Scholar]
- 28. Karvellas CJ, Fix OK, Battenhouse H, et al. Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study. Crit Care Med 2014;42:1157–67. 10.1097/CCM.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajajee V, Williamson CA, Fontana RJ, et al. Noninvasive intracranial pressure assessment in acute liver failure. Neurocrit Care 2018;29:280–90. 10.1007/s12028-018-0540-x [DOI] [PubMed] [Google Scholar]
- 30. Bernal W, Murphy N, Brown S, et al. A multicentre randomized controlled trial of moderate hypothermia to prevent intracranial hypertension in acute liver failure. J Hepatol 2016;65:273–9. 10.1016/j.jhep.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 31. Acharya SK, Bhatia V, Sreenivas V, et al. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 2009;136:2159–68. 10.1053/j.gastro.2009.02.050 [DOI] [PubMed] [Google Scholar]
- 32. Schneeweiss B, Pammer J, Ratheiser K, et al. Energy metabolism in acute hepatic failure. Gastroenterology 1993;105:1515–21. 10.1016/0016-5085(93)90159-A [DOI] [PubMed] [Google Scholar]
- 33. Plauth M, Cabré E, Riggio O, et al. ESPEN guidelines on enteral nutrition: Liver disease. Clin Nutr 2006;25:285–94. 10.1016/j.clnu.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 34. Kuo PC, Plotkin JS, Johnson LB. Acute pancreatitis and fulminant hepatic failure. J Am Coll Surg 1998;187:522–8. 10.1016/S1072-7515(98)00222-1 [DOI] [PubMed] [Google Scholar]
- 35. Du W-B, Pan X-P, Li L-J. Prognostic models for acute liver failure. Hepatobiliary Pancreat Dis Int 2010;9:122–8. [PubMed] [Google Scholar]
- 36. McPhail MJW, Farne H, Senvar N, et al. Ability of King's College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients With Acute Liver Failure: A Meta-analysis. Clin Gastroenterol Hepatol 2016;14:516–25. 10.1016/j.cgh.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 37. Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. Journal of Hepatology 2016;64:69–78. 10.1016/j.jhep.2015.08.018 [DOI] [PubMed] [Google Scholar]