Abstract
The rapid adoption of nanocellulose-based engineered nanomaterials (CNM) by many industries generates environmental health and safety (EHS) concerns. This work presents the development of fluorescently tagged CNM which can be used to study their interactions with biological systems. Specifically, cellulose nano-fibrils and cellulose nano-crystals with covalently attached fluorescein isothiocyanate (FITC) molecules on their surface were synthesized. The fluorescence of the FITC-tagged materials was assessed along with potential FITC detachment under pH conditions encountered in the human gastrointestinal tract, in intracellular compartments, and in cell culture media. Finally, the potential cytotoxicity due to the presence of FITC molecules on the surface of CNM was assessed using a cellular gut epithelium model. The results showed that neither FITC-CNF nor FITC-CNC were cytotoxic and that they have a comparable bioactivity to their untagged counterparts, rendering them suitable for biological studies.
1-. Introduction
Cellulose is the most abundant and renewable polymer of natural origin. Nanocellulose-based engineered nanomaterials (CNM) are cellulosic extracts or processed materials with defined nano-scale structures. CNM include cellulose nano-crystals (CNC), cellulose nano-fibrils (CNF), and bacterial cellulose (BC). All of them consist of highly ordered bundles of cellulose chains aligned along the bundle axis, imparting them with high aspect ratios, useful mechanical properties as well as low thermal expansion and density. The surface-accessible hydroxyl groups on CNM allow for further modification of the surface chemistry, hence providing additional functionalities 1. CNM have been synthesized following various synthesis methods, including mechanical and chemical milling 2,3 which can result in particles with varied crystallinities, surface chemistries, and other intrinsic properties.
CNF and CNC are chemically similar, but they are considered to be in different categories because of vastly different particle morphologies resulted from the synthesis methods 1. CNC are rod-like crystalline cellulose nanoparticles typically derived from the acid hydrolysis of cellulose materials 4,5. On the other hand, CNF present a web-like fibrillar structure with both amorphous and crystalline parts. Some established approaches to synthesize CNF from cellulosic materials include purification and fibrillation of raw plant materials. In general, fibrillation is performed by different methods such as grinding 6–8, cryo-crushing 9, and high shear mechanical treatment combined with enzymatic or chemical pretreatments 10,11. Normally, it is desired to process CNF with narrowed fibril diameter distribution without compromising the crystallinity and degree of polymerization of the cellulose 12. CNF possess larger surface area than CNC potentially inducing higher mechanical properties to polymer matrices 12.
The unique properties of CNM (i.e., tailorable surface chemistry, interesting mechanical robustness, barrier properties 13–17) combined with the biocompatible and biodegradable nature of these materials 18 offer a wide range of opportunities for food and biomedical engineering applications. More recently, there has been a growing interest to use CNM in the field of food packaging 19,20, food additive or supplement 21,22, and drug controlled release 18,23. While US FDA classifies cellulose as a “generally recognized as safe” (GRAS) material for uses in food, very few nanotoxicology studies exist for the nanoscale cellulose materials 2,17. It is worth noting that currently, EPA Sustainable Futures classification categorizes CNM as “low concern level” 24. Yet, the human exposures of CNM is expected to increase due to emerging new technologies, especially in food and cosmetic applications. Therefore, it is necessary to ensure the safe large-scale manufacturing and use of CNM-enabled products 2,17 by assessing any potential EHS implications.
Owning to the variety of proposed applications for cellulose-based materials, toxicological studies of these materials are equally diverse. During the past decade, there has been extensive research on the use of CNF and CNC as scaffolds and hydrogels with improved mechanical properties and low cytotoxicity that can function as suitable substrates for cell growth 25,26. The overall biocompatibility of CNF- and CNC-based surfaces has been tested in these studies, among other endpoints. Along the years, concerns for pulmonary exposure also led to the testing of cellulose-based nanomaterials against lung cell lines using air-liquid interface, submersion cultures, and even in vivo studies 27–29. The immunogenic potential of several functionalized CNF and CNC has already been tested in vitro by some groups in order to elucidate if and how their surface chemistry can influence their biocompatibility 30,31. Recently, Ogonwski et al. used fluorescently tagged CNF for tracing their evacuation and systemic toxicity in Daphnia Magna 32. The conclusion from a decade of nanotoxicological studies is that cellulose-based materials are generally biocompatible and present a low toxic potential against cells, tissues, and organs.
Labeling of nanomaterials with fluorescent molecules has become a popular tool for in vitro monitoring of autophagy 33, nanotoxicological studies 34, and the development of therapeutic applications 35. Various fluorescent probes have been used, including semiconductor nano-crystals (quantum dots) 36–38, small organic fluorophores 39,40, and fluorescent proteins 41. Despite the benefits afforded by labeling probes, each fluorescence labelling technique has limitations: quantum dots are not biocompatible, small molecules have sub-optimal binding chemistry, and protein tags are relatively large. Therefore, it is imperative to consider the advantages and disadvantages of each technique and evaluate them for different applications in order to use them at their fullest capacity 42. Fluorescent labelling enables the visualization of engineered nanomaterials in cells and their quantification in biological environments 37. However, the use of fluorescent probes is contingent with certain drawbacks. For example, it is possible that some fluorescent molecules lose their ability to fluoresce over long periods of illumination, a phenomenon referred to as photobleaching37. Alternatively, misleading localization and quantification assessment may occur should the molecules detach from the surface of nanoparticles under the fluctuating pH conditions of biological systems. In addition, fluorescent molecules should be low in number relative to the surface of the material as to not change the chemical nature of the surface. In summary, fluorescent molecules have to be efficiently conjugated to the surface of engineered nanomaterials, be stable under the physicochemical conditions of the experiment and avoid rapid photobleaching 37,43.
Among various labels, fluorescein isothiocyanate (FITC) is a widely used probe in biology and medicine for labeling proteins, antibodies, and nanomaterials, mainly due to its biocompatibility and straightforward conjugation chemistry 43. FITC can be covalently linked to the cellulose surface using a three-step reaction 44: 1) cellulose hydroxyl groups are modified with epichlorohydrin to form an epoxy groups, 2) epoxy groups are converted to amine groups with ammonium hydroxide, and 3) the sulfur tail of FITC reacts with the amine group. FITC-labeled engineered nanomaterials provide desirable excitation and emission wavelengths as well as strong fluorescence intensity. FITC also displays pH-indicative properties owing to the hydroxyl groups in it, making it suitable for pH sensing measurements 45. Drawbacks of using FITC are the sensitivity to pH and its potential for photobleaching issues 46. As with many other fluorophores, it has been shown that FITC fluorescence is quenched by some metal based nanoparticles 47.
Fluorescent labeling of engineered nanomaterials may affect their toxicological profile. For example, the delignification and removal of hemicellulose in order to minimize potential interference with covalent linking of fluorescent probes may introduce considerable changes to their surface chemistry and may in turn alter their toxicological profile. Therefore, the physicochemical and toxicological characterization of CNM before and after their labeling as well as their behavior in biological media should be assessed in order to identify any changes in the toxicological properties of the modified material 48–51.
In our previous study, we presented an industrially relevant, highly reproducible technique for the synthesis of reference cellulose nano-crystals and nano-fibrils that can be used in nanotoxicology studies7. In the current work, these reference CNM were fluorescently labelled with FITC. Since cellulose-based materials are non-digestible by humans and cannot be efficiently hydrolyzed under physiologically acidic conditions, these fluorescently labelled CNM could have a potential use for biomedical applications and toxicological studies following ingestion. The conjugate stability in cell culture media and under pH values present in the gastrointestinal tract and in intracellular environment was assessed by measuring its fluorescent emission intensity and photobleaching. Finally, the cytotoxicity profile of both the FITC-tagged and unlabeled materials were assessed using a cellular model and compared to evaluate any changes in the toxicity to investigate whether FITC-tagged materials are suitable to be used in nanotoxicological studies.
2-. Materials and methods
2–1. Synthesis of fluorescein isothiocyanate (FITC)-tagged cellulose nano-fibrils (CNF) and cellulose nano-crystals (CNC)
Step 1: Synthesis of cellulose nano-fibrils (CNF) and cellulose nano-crystals (CNC):
Before their tagging with FITC, CNF and CNC were synthesized following methods presented in a previous publication from our group 7. In brief, CNF were produced using kraft wood fibers (raw material) through an ultra-fine friction grinder, following a method similar to that described by Taniguchi and Okamura 8. Raw material was grinded into fibrillated cellulose by shearing and friction forces while a loop system collected and fed the processed material back to the grinder. To fabricate CNF with 50 nm fibrils in diameter, 30–40 passes were required through the grinder. The solid weight percentage of the suspensions was determined by drying 30 g of the as-processed raw material on a hot plate.
The synthesis of CNC was first described by Wang et al. 52 and Chen et al. 53 Our modifications on this method are presented in detail in a previous publication from our group 7. In brief, CNC were produced by acid hydrolysis at a ratio of 1: 10 (g/ml) of raw material to sulfuric acid 72% w/w. The reaction was quenched by diluting with deionized water (DI water) and neutralized by the slow addition of sodium hydroxide 4% w/w, while monitoring the pH. The CNC suspension was allowed to settle for approximately one hour, after which the top phase was decanted. The remaining part was further diluted with DI water, centrifuged at an acceleration of 10000✕ g relative centrifugal force (rcf) for ten minutes after which the supernatant was decanted. This washing/centrifuge procedure was repeated ten times.
Step 2: Fluorescein isothiocyanate (FITC) tagging of CNF and CNC:
CNF and CNC were tagged with FITC following the same, three-step functionalization process summarized in Figure 1. In the first step, 15 mL of 3.7% solids CNM suspension was reacted with 250 mL of epichlorohydrin in the presence of 50 mL of 1.34 molar sodium hydroxide for two hours at 60°C. During this time, CNM were stirred with a mechanical stirrer. The epoxy functionalized CNM were centrifuged at 17500 rcf for 5 minutes and then washed with DI water 3 times. The epoxy functionalized CNM after a 24-hour period, was brought to a pH of 12 with 1.34 molar sodium hydroxide and reacted with 2.8mL of ammonium hydroxide for 2 hours to amine functionalized at 60°C while stirring with a mechanical stirrer. The amine functionalized CNM were then centrifuged at 17500 rcf for 5 minutes and then washed with DI water 3 times. The amine functionalized CNM were placed into a sodium borate and sodium chloride buffer solution for half an hour. A water solution of FITC was prepared by adding 0.07 g of FITC (Sigma Aldrich) to 50 mL of DI water and stirred for half an hour. 1.05 g of sodium chloride and 2.3 g of sodium borate were added to the 1% solids CNM suspension and also stirred for half an hour. The FITC solution was then added to the CNM and stirred for 12 hours in the dark to prevent photobleaching of the FITC. Finally, the FITC-tagged materials were centrifuged at 17500 rcf for 5 minutes and washed with DI water 10 times. The final 4 wash waters were saved for UV-VIS analysis to determine how much FITC was coming off of the CNM; however, no detectable FITC was present in any of the wash waters, indicative of the covalent linking of the probe. The solid sample has a visible yellow color after tagging.
Figure 1.
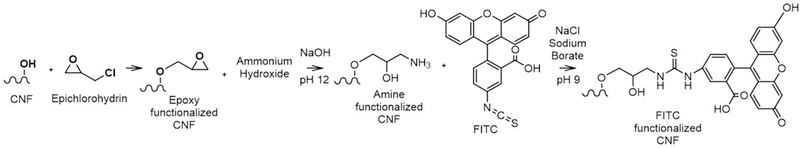
Three-step reaction to attach FITC to CNF (the functionalization process is the same for CNC).
2–2. Physicochemical properties of FITC-tagged CNF and FITC-tagged CNC
Measurement of FITC molecules covalently bound on CNF and CNC:
A small sample of FITC-tagged CNF (FITC-CNF) or FITC-tagged CNC (FITC-CNC) was degraded with a cellulase enzyme from Trichoderma reesei (Sigma Aldrich) to generate a clear solution for UV-VIS spectroscopy. The suspensions of FITC-tagged materials were lyophilized to remove the water. Sodium acetate buffer solution (pH 5) was added to the dry CNM to make a 1 wt% solution. 10 μL of cellulase enzyme was added for every mL of the solution and placed in a dry bath at 50 °C for at least 18 hours. Another 5 μL of the enzyme solution was added to the CNM solutions and placed back in the dry bath for 2 hours to complete the degradation of the cellulose. Using calibration curves of the FITC, the molar absorptivity of FITC in buffer solution is 226600 M−1cm−1. The degraded CNM is analyzed with UV-VIS spectroscopy.
The surface chemistry of FITC-CNF and FITC-CNC have been probed to using the following analytical methods:
Infrared spectroscopy (IR):
A portion of the undiluted suspensions of FITC-tagged materials was dried in a particle free environment. It was then cut into sections and was used for the FTIR spectroscopy.
X-ray photoelectron spectroscopy (XPS):
A portion of the undiluted suspensions of FITC-tagged materials was dried in a particle free environment. It was then cut into sections and was used for XPS measurements. XPS and elemental scans were done for all detected elements.
Transmission electron microscopy:
To determine an average fibril diameter, the CNF was diluted to 0.04 wt% using DI water and evaporated on copper grids using uranyl acetate as a negative dye. The grid sample was imaged by transmission electron microscopy (TEM) (CM10, Philips) to characterize the CNF in terms of average fiber diameter. Images were collected with the Gatan Microscopy software (v2.31) and analyzed using ImageJ software (v1.48). For each TEM image, a 4×4 grid was inserted using ImageJ and the diameter of every fiber that intersected the grid was determined.
Scanning electron microscopy:
Suspensions of FITC-tagged materials were deposited on a mica substrate (Ted Pella) precoated with poly-L-lysine, to enhance CNM adhesion. Scanning electron microscopy (SEM) images at a wide range of magnifications (15K✕ − 150K✕) were recorded using the in-lens detector of a Supra 55VPTM field-emission electron microscope by Carl Zeiss AG operated at an accelerating voltage of 5 keV. The obtained images were analyzed with ImageJ (NIH) software: fibril length was only measured when the two ends of each fibril could be clearly identified while their diameters were measured at a minimum five points across each fibril. One hundred (100) fibrils with distinct and defined outlines were measured.
2–3. Assessment of fluorescent properties of FITC-CNF and FITC-CNC
Identification of optimal absorbance wavelengths for FITC-CNF and FICT-CNC:
Suspensions of FITC-CNF and FITC-CNC at 7.5 mg/ml were prepared in DI water. Their UV-VIS spectra were then recorded between 400 and 700 nm in a disposable cuvette using a SpectraMax M5 spectrometer (Molecular Devices, LLC). Measurements were repeated twice by step size recording of 5 nm and 10 nm. The optimal excitation wavelength was considered the wavelength with the highest absorbance.
Measurement of fluorescence intensity as a function of concentration in environmental and in biological media:
Suspensions of FITC-CNF and FITC-CNC were prepared in PBS or RPMI-1640 cell culture medium supplemented with 10 % FBS without phenol red (hereafter referred to as “cell culture medium”) at a range of concentrations from 1 μg/ml to 2 mg/ml. Their photoluminescence spectra were recorded using the excitation wavelengths (λex) determined in the previous step (470 nm and 480 nm for CNF and CNC, respectively). Fluorescence emission spectra were collected in a range of 500–700 nm using a SpectraMax M5 spectrometer. Measurements were performed with top well illumination, using black/clear bottom 96-well micro-plates. Each suspension was measured in triplicate using 100 μl. The untagged CNM at a concentration of 0.5 mg/ml and the media alone were used as controls. Fluorescence of FITC-tagged materials was evaluated by subtracting the background fluorescence from the emission spectra of untagged CNM. The calculated fluorescence intensity was then described in relative fluorescence units (RFU) as a function of emission wavelength.
Measurement of fluorescence intensity at various pH values:
Suspensions of FITC-CNF and FITC-CNC at 0.5 mg/ml were prepared at 0.5 mg/ml in citrate-phosphate solution (pH 2), DI water (pH 5.5), PBS 1× (pH 7.4), and sodium borate (pH 9) in order to assess the material’s fluorescence intensity varying pH. Untagged materials suspended at the same concentration in the same solutions were used as controls. The photoluminescence spectra were recorded with a SpectraMax M5 spectrometer (Molecular Devices, LLC) according to the protocol described above.
2–4. Assessment of FITC detachment from FITC-CNF and FITC-CNC
Suspensions of FITC-CNF and FITC-CNC at 0.5 mg/ml were prepared in citrate-phosphate (pH 2), DI water (pH 5.5), PBS 1× (pH 7.4), and sodium borate (pH 9). In order to assess whether FITC detached from the surface of the materials, the as-prepared suspensions were centrifuged in a fixed-angle microcentrifuge at 12000 rcf for 2.5 h. After centrifugation, the supernatants were carefully collected while the pellets were washed three times with fresh PBS (pH 7.4). Untagged materials suspended at the same concentration in the same solutions were used as controls. The photoluminescence spectra of washed pellets, the collected supernatants, and the controls were recorded as described in Section 2–3.
2–5. Photobleaching assessment of FITC-CNF and FITC-CNC
Photobleaching impact of fluorometry assays:
Suspensions of FITC-CNF and FITC-CNC were prepared in PBS at 0.5 mg/ml and 100 μl were transferred in a black/clear bottom 96-well plate. The samples were irradiated at 470 nm using a SpectraMax M5. The real-speeds was 5 nm per step size and 6 lamp flashes per read. The light source was a Xenon flash lamp (50 Watts) with fluorescence intensity performance of 1 joule per flash. To quantify the fluorescence loss, the emission spectra were recorded in a range of 500–700 nm by step size 5nm up to 1 hour of exposure time. The fluorescence intensity obtained at 520 nm at different time points was plotted as a function of the illumination time. The gradually decline fluorescence intensity as a function of illumination time was considered as an effect of photobleaching.
Photobleaching impact of confocal laser scanning microscopy:
Suspensions of FITC-CNF and FITC-CNC were prepared in DI water at 50 μg/ml and a ~0.5 ml of each were transferred to 30 mm glass bottom dishes (WillCo-Dish®). The FITC-tagged materials were then observed under a Zeiss LSM 700 confocal microscope. Specifically, the samples were illuminated with a 10 mW 488 nm laser beam operating at 10% (FITC-tagged CNF) and 14% (FITC-tagged CNC). The materials were observed over a 60-minute period and their emitted fluorescence was periodically recorded. Photobleaching was defined as loss of pixel intensity of constantly illuminated particles.
2–6. Assessment of FITC detachment from and fluorescent intensity of FITC-CNF and FITC-CNC under simulated gastrointestinal conditions
A three-phase (mouth, stomach and small intestine) simulated gastrointestinal tract (GIT) digestion process developed by the authors was used in order to assess the detachment of FITC from FITC-CNF and FITC-CNC during digestion 48. In summary, samples were suspended in fasting media (phosphate buffer) at a starting concentration of 7.5 mg/ml. The suspensions of FITC-tagged materials were submitted to simulated mouth, stomach, and small intestinal digestion phases (Figure 2). More specifically, in the mouth phase of the GIT simulator, the FITC-CNF and FITC-CNC suspended in fasting media were mixed and incubated with a simulated saliva fluid composed of different salts and porcine gastric mucin type II for 2 min. The resulting mouth digesta (“bolus”) was then combined and incubated with a simulated gastric fluid comprising of sodium chloride and hydrochloric acid for 2 h which represented the stomach digestion phase. In the small intestinal phase, the stomach digesta (“chyme”) was combined with bile salts and proteins simulating the intestinal fluid and incubated for 2 h at a pH of 7.0, which was maintained constant by the use of a pH stat titration device. Samples were collected at the three different phases of the digestion, i.e. mouth, stomach, and small intestine, and stored at 4°C for fluorescence studies the following day. Clean DI water and/or untagged materials were also used as control samples. It is worth noting that the fasting digestion food model had a starting concentration of 7.5 mg/ml FITC-tagged materials, while the final cellulose concentration after small intestinal digestion was 0.62 mg/ml. The small intestinal digestas of FITC-tagged materials were evaluated in terms of their fluorescence intensity and FITC detachment following the protocols described in Section 2–4. The small intestinal phase digestas were also diluted in DMEM cell culture medium at a ratio of 1: 3 vol: vol, so that the final CNM concentration was ~0.2 mg/ml. The DMEM medium alone and digested untagged materials diluted in DMEM were used as control samples.
Figure 2.
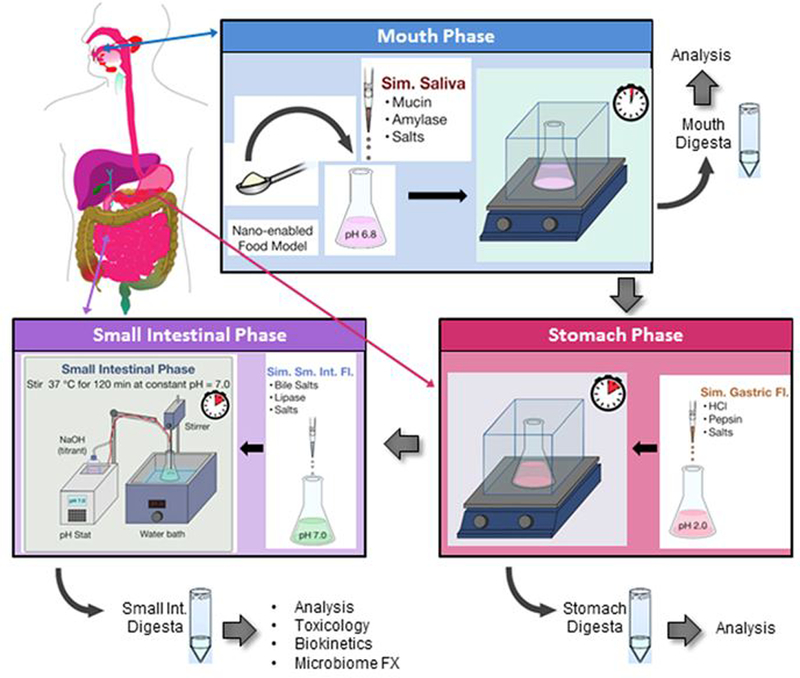
Simulated digestion of CNM present in food matrix in GIT representing mouth, stomach and small intestinal phases, (adapted from ref. 48)
2–7. Sterility/biological assessment of FITC-CNF and FITC-CNC
Endotoxin contamination:
The endotoxin content of the FITC-tagged materials was assessed using recombinant factor C (rFC) assay, EndoZyme® (Hyglos, U.S.) as described in a previous paper by the authors 7. In summary, samples were diluted in sterile endotoxin-free water at the concentration of 10 μg/ml and vortexed at high speed for 1 min. CNM were spun down from the concentrated suspensions at 14kx g for 5 min and the supernatant was collected and used for testing. To check for assay interferences, the initial suspension of the CNM samples was spiked with 0.5 EU/mL of Escherichia coli (E. coli) and underwent the same assessment described above.
Endotoxin concentration was extrapolated from a standard curve prepared using E. coli endotoxin standards which were provided with the commercial kit. Fluorescence intensity at time zero (t0) and after incubation at 37°C for 90 mins (t90) at the excitation wavelength of 380/20 and emission wavelength of 440/40 was measured using a SpectraMax M5 plate reader. The fluorescence intensity recorded at t0 was subtracted from that at t90 and the unknown endotoxin concentration in the CNM was determined using the standard curve equation.
Microbiological contamination analysis:
The sterility of FITC-tagged materials was also tested according to a modified US Pharmacopeia protocol for sterility testing of pharmaceutical formulations 7. The samples were diluted at a concentration of 1 mg/mL using sterile endotoxin-free water. One (1) ml of the sample was added to 9 ml of Fluid Thioglycolate Media (FTM) in a 50-ml centrifuge tube and incubated inside a stationary incubator at 35°C. The samples were checked for turbidity and visual changes indicative of microbial growth. If any visual change were observed, the samples were further analyzed using two methods. 1) Sample was streaked onto a plate of Brain Heart Infusion Agar (BHIA) plate. The plates were then incubated overnight inside 37°C inside a stationary incubator and growth was checked after 18–24 hours of incubation. 2) One (1) ml of sample was added to plate count agar (PCA) as well as potato dextrose agar (PDA) and the pour plate method was employed. The plates were stored at room temperature (PDA) or 37°C (PCA). The results were analyzed after 48–72 hours of incubation.
2–8. Toxicological analyses of FITC-CNF and FITC-CNC
In the present study, we submitted FITC-tagged CNF and CNC to simulated gastrointestinal tract (GIT) transformations using a method that had been previously optimized in our group and reported by DeLoid et al 48. Figure 2 displays the simulated digestion of in a clockwise route starting from the top. Our goal was first to investigate the attachment and fluorescence intensity of FITC-tagged materials while they pass through simulated mouth, stomach, and small intestinal phases; second, we intended to assess the toxicological effect of the digested materials using in-vitro cellular studies. Five (5) mM of phosphate buffer was used as a fasting food model which was nano-enabled with the addition of cellulose-based nanomaterials. For their toxicological analysis, FITC-CNF and FITC-CNC were vortexed in fasting medium at 0.75% w/w (7.5 mg/ml) and 1.5% w/w (15 mg/ml) for 20 seconds. Untagged CNF and CNC and biological grade DI water were used as the control food models. All suspensions were passed through the GIT simulator as described above and the final small intestinal digestas were used to assess cytotoxicity using a gut epithelium triculture cellular model previously developed in detail by DeLoid et al.48
Triculture cellular model of the human gut epithelium:
In summary, Caco-2, HT29-MTX and Raji B cell lines were purchased from Sigma-Aldrich, MO. Caco-2 and HT29-MTX cells were grown in high-glucose DMEM supplemented with 10% heat-inactivated foetal bovine serum (FBS), 10 mM HEPES buffer, 100 IU/ml Penicillin, 100μg/ml Streptomycin and 1% non-essential amino acids. Raji B cells were cultured in RPMI-1640 media supplemented with 10% FBS, 10 mM HEPES buffer, 100 IU/ml Penicillin and 100 μg/ml Streptomycin.
The inserts of a 6-transwell plate system with 0.4 μm pores (Corning) were pre-coated with 10 μg/cm2 type I rat tail collagen solution (Sigma-Aldrich, MO). Caco-2 and HT-29-MTX cells were trypsinized using 5 mL TrypLE™ and detached from the bottom of the flask. 10 mL of complete DMEM media was then added to the flasks and mixed with the detached cells. The re-suspended cells were then centrifuged at 100xg and the pellet was re-suspended in fresh complete DMEM media at a concentration of 3 ×105cells/cm3. The Caco-2 and HT-29-MTX cells were combined at a ratio of 3: 1. The resulting cell mixture (1.5 ml) was deposited on the inserts (the “apical chamber”) of the transwells, and 2.5 ml of complete DMEM media was added to the space below the apical chamber (the “basolateral compartment”). The first change of media for the cells was done four days after seeding and was repeated every other day for the next 6 days. From culture days 10 to 15, the media was changed every day. When the cells had reached 15 days of age, the media in the basolateral compartment was substituted by 2.5ml of a Raji B cell suspension at a concentration of 1×106 cells/ml in 1: 1 DMEM: RPMI complete media. This procedure was repeated the next day, completing a total of seventeen days of culture for the trans-well plates, at which point cells were ready to be used for toxicity or biokinetics experiments 48.
Measurement of transepithelial electrical resistance (TEER):
Transwell plates were allowed to equilibrate to room temperature prior to measuring TEER using an EVOM2 Epithelial Volt/Ohm meter with a chopstick electrode set (World Precision Instruments). Electrodes were sterilized by rinsing in 70% ethanol and allowed to air dry prior to each use. For each transwell, measurements were made at three different insert locations and averaged. Measurements were corrected by subtracting resistance measured in a blank transwell (with media in basolateral and apical compartments, but no cells), and reported as the product of measured resistance and transwell insert membrane area (i.e. Ω.cm2). TEER measurements were taken every 3rd day till ingested nanoparticle exposure, immediately before exposing the cells to the digesta of FITC-tagged materials and after completion of exposure time period.
Cell viability and cytotoxicity analyses:
Following exposure of the gut triculture cellular model to the small intestinal phase digesta of FITC-tagged materials, cell viability and cytotoxicity were measured using lactate dehydrogenase (LDH) assay. At the end of the 24-hour exposure period, cell culture media containing FITC-tagged materials were removed and stored for determining cytotoxicity. For measuring the LDH in the stored cell culture media, we used the Pierce LDH cytotoxicity assay kit (ThermoFisher Scientific, Rockford, IL) and followed the protocol mentioned by the company. Absorbance was measured using SpectraMax M5 at 490 nm and 680 nm. The levels of LDH released from cells exposed to FITC-tagged materials were compared against those from cells exposed to untagged CNM.
Statistical analyses:
Cytotoxicity experiments were performed three times and the statistical significance of the results was tested using One-way ANOVA with Bonferroni correction post-hoc test using SigmaPlot® software.
3-. Results and discussion
3-1. Synthesis and physicochemical properties of FITC-CNF and FITC-CNC
The detailed morphological and structural characterization of the synthesized CNF and CNC prior to their fluorescent tagging has been presented in a previous work from our group 7.
FITC naturally adsorbs to cellulose-based materials through aromatic-carbohydrate type interactions where it can then covalently bond with their epoxy-functionalized surface. To ensure that FITC is not simply adsorbed on the surface of CNM, we have tested the FITC-tagged materials under pH values other than neutral (see results section 3-3). Although these tests did not allow for a direct quantification of the amount of covalently bonded FITC, the absence of FITC leaching strongly suggests that a considerable amount of the fluorophore is indeed covalently bonded to the surface of CNM.
The obtained XPS and FTIR spectra of FITC-CNF and FITC-CNC and those of the raw cellulose material confirmed that the tagging process does not significantly change the bulk chemical properties of the CNMs, as shown in Supporting Figure (SF) 1. Figure 3 shows typical SEM images of FITC-CNF and FITC-CNC with ~50 nm and ~25 nm in diameter, respectively. The fibrils in CNF samples appear as a web-like structure, connected at various node points. In contrast, CNC develop as long, rigid, and uniform structures with a narrow range of diameters and lengths 7,51. Image analysis revealed that FITC-CNF and FITC-CNC had an average length of 6.8 ± 5 μm and 270 ± 80 nm, respectively (SEM); their average diameters were 52 ± 20 nm and 23 ± 10 nm (TEM), respectively.
Figure 3.
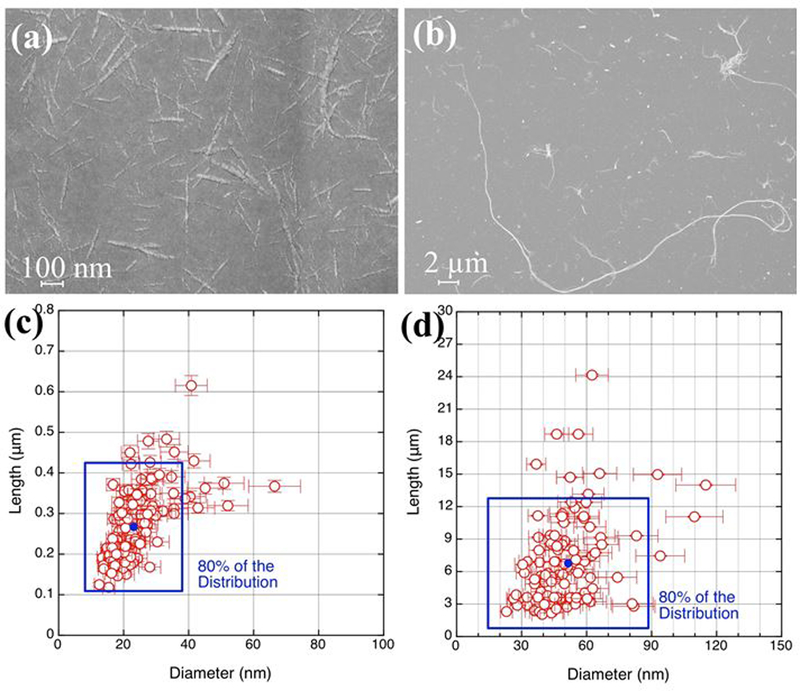
SEM analysis of the FITC-tagged materials: (a) and (b) a representative SEM image of CNC and CNF, respectively. (c) and (d) the data analysis of CNC and CNF, respectively.
3-2. Assessment of fluorescent properties of FITC-CNF and FITC-CNC
Identification of optimal absorbance wavelengths for FITC-CNF and FICT-CNC:
Figure 4 shows the absorbance spectra of CNF and CNC dispersed in DI water before and after their conjugation with FITC molecules. Figure 4 (a) presents the absorbance spectra of CNF and FITC-CNF. It is evident that FITC-CNF show higher UV-VIS absorbance than untagged CNF, attributed to the absorbance of FITC molecules. The inset presents the UV-VIS spectrum obtained when the signal from untagged CNF was subtracted from the those gained from FITC-CNF. Based on the UV-VIS absorbance, the excitation wavelength at 470 nm was chosen for the further fluorescent examination of FITC-CNF. Figure 4 (b) presents the absorbance spectra of CNC and FITC-CNC. It is evident that FITC-CNC show higher UV-VIS absorbance than untagged CNC, attributed to the absorbance of FITC molecules. The inset presents the UV-VIS spectrum obtained when the signal from untagged CNC was subtracted from the those gained from FITC-CNC. Based on the UV-VIS absorbance, the excitation of 480 nm was chosen for the further fluorescent examination of FITC-CNC. For both FICT-CNF and FITC-CNC, the driven spectra are very similar to the UV-VIS spectrum obtained from FITC in DI water which can be seen in SF2. The narrow absorbance peak indicates that conjugating FITC molecules to the surface of CNF does not significantly alter the chemical environment of FITC.
Figure 4.
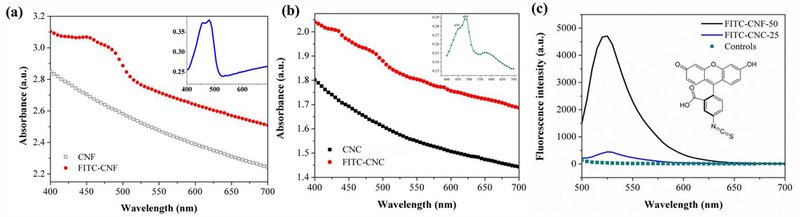
(a) UV-VIS absorption spectra of CNF and FITC-CNF. The inset presents the absorption spectrum of FITC-CNF without the contribution by the untagged CNF. (b) UV-VIS absorption spectra of CNC and FITC-CNC. The inset presents the absorption spectrum of FITC-CNC without the contribution by the untagged CNC. (c) Emission spectra of FITC-CNF and FITC-CNC upon excitation at 470 nm and 480 nm, respectively; the “Controls” are overlaid spectra of untagged CNF and untagged CNC. The molecular structure of fluorescein isothiocyanate (FITC) is shown as an inset. The concentration of FITC-CNF, FITC-CNC, untagged CNF, and untagged CNC were 0.5 mg/ml; cut-off wavelengths were set at 475 nm and 485 nm for FIC-CNF and FITC-CNC, respectively.
The recorded relative fluorescence intensity (RFU) spectra of FITC-CNF and FITC-CNC are presented in Figure 4 (c). Their fluorescence intensity was compared to that of untagged materials or PBS (grouped together as controls due to their absence of autofluorescence). As expected, the fluorescence intensity was markedly higher for FITC-CNF compared to their untagged counterparts. The lower UV absorbance of FITC-CNC manifests itself as lower fluorescence emission when compared to the FITC-CNF, but it is still markedly higher than the untagged CNC. This observed difference between FITC-tagged CNF and CNC could be attributed to fewer FITC molecules per glucose unit on the CNC. The exact cause of a lower degree of fluorescent tagging for CNC as compared to CNF is not clear. One of the major differences between CNF and CNC is that CNC has significantly fewer amorphous regions than CNF. Both the reaction and adsorption may be more efficient in the amorphous regions due to the greater availability of cellulose chains in the regions as compared to the crystalline domains that primarily make up CNCs.
Measurement of fluorescence intensity as a function of concentration in environmental and in biological media:
The fluorescence intensities of FITC-CNF and FITC-CNC were also evaluated in PBS and cell culture medium as shown in SF3 and SF4. Expectedly, fluorescence intensity was reduced at lower concentrations of the material. The minimum detectable concentration in PBS was measured to be 2 and 10 μg/ml for FITC-CNF and FITC-CNC, respectively; in cell culture medium, these values were 10 and 5 μg/ml for FITC-CNF and FITC-CNC, respectively.
Measurement of fluorescence intensity at various pH values:
It is well known that the fluorescence of FITC molecules is highly dependent on the pH environment due to ion dissociation. However, it is recovered when the pH is brought back to neutral values. Two hydroxyl groups in its molecular structure disassociate at low pH values resulting in a reduced molecular vibration and fluorescence intensity. Figures 5 (a) and 5 (b) illustrate the fluorescence intensity of 0.5 mg/ml of FITC-CNF and FITC-CNC obtained in different pH values. The emission spectra obtained from the controls (untagged CNM in various buffers) are also overlaid in the same figures. It is well known that the fluorescence of FITC molecules is highly dependent on the pH of its environment. At acidic pH, excess H+ from the environment lead to the protonation of the conjugated aromatic rings and thus lose their ability to emit light in the visible spectrum. At alkaline pH values, the fluorophore’s conjugation is also partially lost due the disassociation of its hydroxyl group and the creation of a dianion. A comprehensive description of these phenomena can be found in the work by Ma et al.54 and its potential applications are summarized by Obare et al.55
Figure 5.
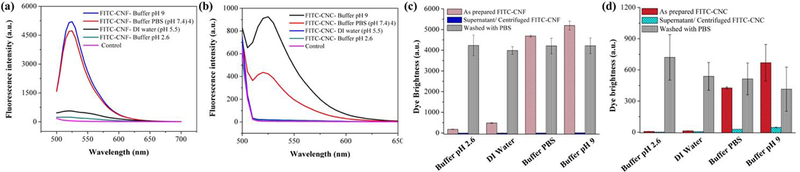
(a) Fluorescence emission spectra of FITC-CNF excited at 470 nm prepared at various pH values (pH 2.6 – 9). (b) Emission spectra of FITC-CNC excited at 480 nm prepared at various pH values (pH 2.6 – 9). For both (a) and (b), “Controls” are overlaid emission spectra of their untagged counterparts dispersed in the same buffers. Summary of fluorescence intensities at 520 nm obtained from FITC-tagged CNF (c) and FITC-tagged CNC (d) exposed to different pH values and compared against their respective supernatants following centrifugation. Dispersions of both FITC-tagged materials were further washed with PBS to evaluate the sustainability of fluorescence intensity at various pH values. The survey reflects the pH sensitivity of FITC fluorescence. At the same time, FITC is strongly attached to CNM and its fluorescence recovers in PBS. All suspensions are prepared at 0.5 mg/ml; standard deviations are obtained from 3 replicates (n=3).
3-3. Assessment of FITC detachment from FITC-CNF and FITC-CNC
FITC detachment was further evaluated as a role of pH change. The fluorescence of the as- prepared suspensions, their supernatants after centrifugation, and the control samples were recorded and given in SF5 and SF6. In both CNM, the fluorescence for the supernatant after centrifugation was decreased in all pH buffers and was comparable with the fluorescence of the control samples. In addition, dynamic laser scattering measurements were performed on the supernatants to ensure that no CNM was present. Results showed that the derived kilocounts per second values from the supernatants were comparable to that from a sample of deionized water (data not shown). This is additional evidence that the FITC molecules are strongly bound to the synthesized CNM and are not detaching as pH changes. Figures 5 (c) and 5 (d) summarize the probe fluorescence intensity and detachment in different buffers. The highest intensity was obtained at a buffer with a pH value 9. However, the intensity recovers when the pH of the buffer changes. To assess this, all solutions prepared in different pH values were washed three times with fresh PBS. The washed solutions retained the same amount of fluorescence intensities, comparable with the one observed in washed PBS. The slight difference in RFU of the as-prepared sample in PBS and after washing is attributed to the solid wt% change over the washing process. These results are in good agreement with the literature stating that the -COOH groups undergo H+ dissociation as a function of the pH 43,45,54.
3-4. Photobleaching assessment of FITC-CNF and FITC-CNC
Photobleaching impact of fluorometry assays:
The photobleaching of FITC-tagged materials was assessed by tracking the change in fluorescence intensity over 60-minute illumination. As shown in Figures 6 (a) and 6 (b), the fluorescence intensity of FITC-CNF and FITC-CNC was maintained when they were illuminated at 470 nm for the entire duration of the experiment.
Figure 6.
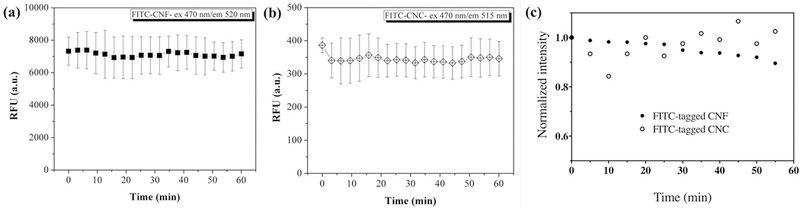
Fluorescence intensity of 0.5 mg/ml FITC-tagged materials: (a) FITC-CNF and (b) FITC-CNC. The samples are exposed to excitation of 470 nm for one hour. The standard deviations are obtained from 2 replicates (n=2). (c) Photobleaching impact of confocal laser scanning microscopy on FITC-tagged CNF (solid circles) and FITC-tagged CNC (open circles). Fluorescence intensities were recorded every 5 minutes and are normalized against the values at t = 0 min.
Photobleaching impact of confocal laser scanning microscopy:
Higher power of light illumination in confocal microscopy typically used in nanotoxicological studies might induce photobleaching of FITC molecules. By visual inspection alone, the fluorescence intensity of FITC-CNF and FITC-CNC did not change for the entire duration of the experiments. The recorded intensity of FITC-tagged CNF was still at ~85% of its original value after 60 minutes of illumination. The fluorescence intensity of FITC-tagged CNC appeared to fluctuate, but did not drop below ~80% of its initial value throughout the experiment.
3-5. Assessment of FITC detachment from and fluorescent intensity of FITC-CNF and FITC-CNC under simulated gastrointestinal conditions
Supporting Figure 7 (a) and SF7 (b) show the fluorescence emission spectra from digesta of FITC-tagged CNF and FITC-tagged CNC, respectively. Also plotted are the emission spectra from the supernatants of digested FITC-tagged materials as well as digestas of untagged materials. Results confirm the FITC stability on CNM through the GIT process, which render the tagged CNM suitable for simulated digestions in toxicological studies of ingested nanomaterials. As shown in SF7 (c) and SF7 (d), emission spectra were also collected from digesta of FITC-tagged materials after their dilution in DMEM in order to assess the probe’s utility in cellular toxicological studies. The fluorescence intensity of digested FITC-CNF, and FITC-CNC in DMEM were 5697 ± 169 arbitrary units (a.u.), and 524 ± 19.4 a.u., showing good fluorescence intensity indicative of their suitability for cellular biokinetic and cellular toxicological studies.
3-6. Sterility/biological assessment of FITC-CNF and FITC-CNC
Supporting Figure 8 presents the measured endotoxin levels in the utilized materials. No endotoxin contaminations were detected in the FITC-CNF or FITC-CNC using recombinant factor C (rFC) assay. The samples were also spiked with 0.5 EU/mL of lipopolysaccharide to determine any assay interference from the materials themselves, but the spiked endotoxin was fully recovered. Additionally, no biological contamination (bacteria or fungi) was detected in the samples.
3-7. Toxicological analyses of FITC-CNF and FITC-CNC
The tri-culture cell model comprising of the enterocytes, M cells, and goblet cells was exposed to simulated digesta of the FITC-CNF and FITC-CNC, digested at the starting concentrations of 0.75% and 1.5% w/w as described in detail in methods section. After 24h, they were found to be biologically inert and non-reactive, similar to their untagged counterparts. Figure 7 (a) shows that the released LDH from the GIT triculture cells exposed to the FITC-CNF and FITC-CNC digesta after 24 h exposure was comparable to the negative control, regardless their concentration. More specifically, the LDH levels from the exposed cells were only 2–3% higher than the negative (unexposed) controls. Moreover, Figure 7 (b) shows that TEER values of the of the GIT triculture cells did not decrease when exposed to the small intestinal phase digestas of FITC-CNF and FITC-CNC over 24 hours. This was an indication that the integrity of the modeled human gut epithelium was not compromised, either because of cell death or loss of tight junctions between cells.
Figure 7.
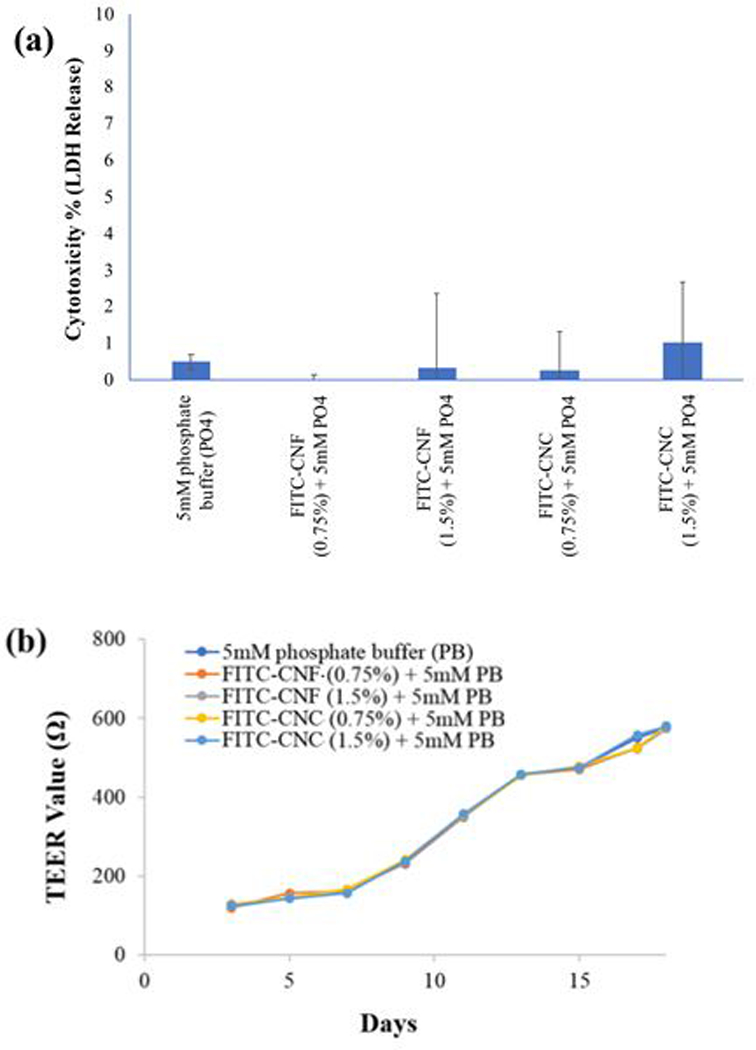
(a) Lactate dehydrogenase release (cytotoxicity percentage): LDH release analysis showed no membrane damage to the GIT tri-culture cells following 24 h exposure to the FITC-CNF and FITC-CNC digesta. (b) Transepithelial electrical resistivity (TEER) analysis: a gradual increase in TEER values indicated that the cell junctions of the cellular monolayer remained intact during 17 days of cell culture. On day 18, cells were exposed for 24 hrs to the FITC-CNF and FITC-CNC digesta and the TEER values across the transwell membranes did not decrease, thus indicating no loss of cell viability or loss of cellular monolayer integrity had occurred.
4-. Conclusions
In many of the potential medical and biomedical applications of CNM, thorough toxicity testing is essential in order to assess biological properties and potential toxicological implications of such emerging materials.
In this work, FITC-tagged CNM were developed and assessed in order to be used in nanotoxicology studies. The developed method for the FITC chemical bonding on CNM is robust and results in strongly bound molecules on the CNM. Further, the FITC remains attached to CNM at different pH conditions relevant to nano-bio interactions studies, including gastrointestinal digestion. Fluorescence intensity showed pH dependency and a steep trend as pH value increased around pH 5.5. However, it was observed that the fluorescence intensity was reversible and recovered whenever the pH was brought back to 7. This was also confirmed during the simulated GIT conditions where FITC did not detach from the tagged materials nor did it lose any of its fluorescence intensity, even when added to the tri-culture cellular model. It was also found that CNF and CNC preserve their non-toxic nature when their surface is covalently functionalized with FITC.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the HSPH Center for Nanotechnology and Nanotoxicology and National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number (NIH grant # U24ES026946) as part of the Nanotechnology Health Implications Research (NHIR) Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The engineered nanomaterials used in the research presented in this publication have been synthesized and characterized by the Engineered Nanomaterials Resource and Coordination Core of the NHIR consortium.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/C8EN01381K
References
- 1.Moon RJ, Schueneman GT and Simonsen J, JOM, 2016, 68, 2383–2394. [Google Scholar]
- 2.Ong KJ, Shatkin JA, Nelson K, Ede JD and Retsina T, NanoImpact, 2017, 6, 19–29. [Google Scholar]
- 3.Pyrgiotakis G, Vedantam P, Cirenza C, McDevitt J, Eleftheriadou M, Leonard SS and Demokritou P, Sci. Rep., 2016, 6, 21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Qin Z, Liang B, Liu N, Zhou Z and Chen L, J. Mater. Chem. A, 2013, 1, 3938. [Google Scholar]
- 5.† Stephanie Beck-Candanedo, ‡ and Maren Roman and † Derek G. Gray*, , DOI: 10.1021/BM049300P. [DOI]
- 6.Iwamoto S, Abe K and Yano H, Biomacromolecules, 2008, 9, 1022–1026. [DOI] [PubMed] [Google Scholar]
- 7.Pyrgiotakis G, Luu W, Zhang Z, Vaze N, DeLoid G, Rubio L, Graham WAC, Bell DC, Bousfield D and Demokritou P, Cellulose, 2018, 25, 2303–2319. [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi T and Okamura K, Polym. Int, 1998, 47, 291–294. [Google Scholar]
- 9.Chakraborty A, Sain M and Kortschot M, Holzforschung, 2006, 60, 53–58. [Google Scholar]
- 10.Zhang Y, Song P, Liu H, Li Q and Fu S, Compos. Sci. Technol, 2016, 125, 62–70. [Google Scholar]
- 11.Panthapulakkal S and Sain M, Int. J. Polym. Sci, 2012, 2012, 1–6. [Google Scholar]
- 12.Chakrabarty A, Teramoto Y, Chakrabarty A and Teramoto Y, Polymers (Basel)., 2018, 10, 517. [Google Scholar]
- 13.Abitbol T, Palermo A, Moran-Mirabal JM and Cranston ED, Biomacromolecules, 2013, 14, 3278–3284. [DOI] [PubMed] [Google Scholar]
- 14.Abitbol T, Rivkin A, Cao Y, Nevo Y, Abraham E, Ben-Shalom T, Lapidot S and Shoseyov O, Curr. Opin. Biotechnol, 2016, 39, 76–88. [DOI] [PubMed] [Google Scholar]
- 15.Dufresne A, Mater. Today, 2013, 16, 220–227. [Google Scholar]
- 16.Lin N and Dufresne A, Eur. Polym. J, 2014, 59, 302–325. [Google Scholar]
- 17.De France KJ, Hoare T and Cranston ED, Chem. Mater, 2017, 29, 4609–4631. [Google Scholar]
- 18.L. Sanga Pachuau, .
- 19.Trifol J, Plackett D, Sillard C, Hassager O, Daugaard AE, Bras J and Szabo P, J. Appl. Polym. Sci, 2016, 133, n/a-n/a. [Google Scholar]
- 20.Seoane IT, Fortunati E, Puglia D, Cyras VP and Manfredi LB, Polym. Int, 2016, 65, 1046–1053. [Google Scholar]
- 21.C. Gómez H., Serpa A, Velásquez-Cock J, Gañán P, Castro C, Vélez Land Zuluaga R, Food Hydrocoll., 2016, 57, 178–186. [Google Scholar]
- 22.DeLoid GM, Sohal IS, Lorente LR, Molina RM, Pyrgiotakis G, Stevanovic A, Zhang R, McClements DJ, Geitner NK, Bousfield DW, Ng KW, Loo SCJ, Bell DC, Brain J and Demokritou P, ACS Nano, 2018, 12, 6469–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J and Li J, J. Bioresour. Bioprod, 2017, 2, 1–3. [Google Scholar]
- 24.GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELLING OF CHEMICALS (GHS) Fifth revised edition UNITED NATIONS New York and Geneva, 2013 Copyright@United Nations 2013. All rights reserved, .
- 25.Rashad A, Mustafa K, Heggset EB and Syverud K, Biomacromolecules, 2017, 18, 1238–1248. [DOI] [PubMed] [Google Scholar]
- 26.Hua K, Ålander E, Lindström T, Mihranyan A, Strømme M and Ferraz N, Biomacromolecules, 2015, 16, 2787–2795. [DOI] [PubMed] [Google Scholar]
- 27.Endes C, Mueller S, Kinnear C, Vanhecke D, Foster EJ, Petri-Fink A, Weder C, Clift MJD and Rothen-Rutishauser B, Biomacromolecules, 2015, 16, 1267–1275. [DOI] [PubMed] [Google Scholar]
- 28.Ilves M, Vilske S, Aimonen K, Lindberg HK, Pesonen S, Wedin I, Nuopponen M, Vanhala E, Højgaard C, Winther JR, Willemoës M, Vogel U, Wolff H, Norppa H, Savolainen K and Alenius H, Nanotoxicology, 2018, 12, 729–746. [DOI] [PubMed] [Google Scholar]
- 29.Park E-J, Khaliullin TO, Shurin MR, Kisin ER, Yanamala N, Fadeel B, Chang J and Shvedova AA, J. Immunotoxicol, 2018, 15, 12–23. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez AS, Jaramillo F, Hemraz UD, Boluk Y, Ckless K and Sunasee R, Nanotechnol. Sci. Appl, 2017, 10, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomić S, Ilić N, Kokol V, Gruden-Movsesijan A, Mihajlović D, Bekić M, Sofronić-Milosavljević L, Čolić M and Vučević D, Int. J. Nanomedicine, 2018, Volume 13, 6941–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogonowski M, Edlund U, Gorokhova E, Linde M, Ek K, Liewenborg B, Könnecke O, Navarro JRG and Breitholtz M, Nanotoxicology, 2018, 12, 509–521. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Enriquez S, Kim I, Currin RT and Lemasters JJ, Autophagy, 2006, 2, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan N, Chen C-S, Hsieh H-H, Wu Y-C and Chang H-C, Nano Lett, 2010, 10, 3692–3699. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz NF, Nanomedicine Nanotechnology, Biol. Med., 2010, 6, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasco M, Chaniotakis N, Frasco MF and Chaniotakis N, Sensors, 2009, 9, 7266–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray RA, Escobar A, Bastús NG, Andreozzi P, Puntes V and Moya SE, NanoImpact, 2018, 9, 102–113. [Google Scholar]
- 38.Parak WJ, Pellegrino T and Plank C, Nanotechnology, 2005, 16, R9–R25. [DOI] [PubMed] [Google Scholar]
- 39.Fokkema J, Fermie J, Liv N, van den Heuvel DJ, Konings TOM, Blab GA, Meijerink A, Klumperman J and Gerritsen HC, Sci. Rep, 2018, 8, 13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez Porras MA, Durfee PN, Gregory AM, Sieck GC, Brinker CJ and Mantilla CB, J. Neurosci. Methods, 2016, 273, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippincott-Schwartz J and Patterson GH, Science, 2003, 300, 87–91. [DOI] [PubMed] [Google Scholar]
- 42.Miyawaki A, Sawano A and Kogure T, Lighting up cells: labelling proteins with fluorophores, 2003. [PubMed] [Google Scholar]
- 43.Hakeem A, Zahid F, Duan R, Asif M, Zhang T, Zhang Z, Cheng Y, Lou X and Xia F, Nanoscale, 2016, 8, 5089–5097. [DOI] [PubMed] [Google Scholar]
- 44.S. Dong and M. Roman, , DOI: 10.1021/JA076196L. [DOI]
- 45.Lanz E, Gregor M, Slavík J and Kotyk A, J. Fluoresc, 1997, 7, 317–319. [Google Scholar]
- 46.Jacobson K, Derzko Z, Wu E-S, Hou Y and Poste G, J. Supramol. Struct, 1976, 5, 565–576. [DOI] [PubMed] [Google Scholar]
- 47.Cheng D and Xu Q-H, Chem. Commun, 2007, 0, 248–250. [DOI] [PubMed] [Google Scholar]
- 48.DeLoid GM, Wang Y, Kapronezai K, Lorente LR, Zhang R, Pyrgiotakis G, Konduru NV, Ericsson M, White JC, De La Torre-Roche R, Xiao H, McClements DJ and Demokritou P, Part. Fibre Toxicol, 2017, 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClements DJ, DeLoid G, Pyrgiotakis G, Shatkin JA, Xiao H and Demokritou P, NanoImpact, 2016, 3–4, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta A, Simmons W, Schueneman GT, Hylton D and Mintz EA, ACS Sustain. Chem. Eng, 2017, 5, 1711–1720. [Google Scholar]
- 51.Sofla MRK, Brown RJ, Tsuzuki T and Rainey TJ, Adv. Nat. Sci. Nanosci. Nanotechnol, 2016, 7, 35004. [Google Scholar]
- 52.Wang QQ, Zhu JY, Reiner RS, Verrill SP, Baxa U and McNeil SE, Cellulose, 2012, 19, 2033–2047. [Google Scholar]
- 53.Chen L, Wang Q, Hirth K, Baez C, Agarwal UP and Zhu JY, Cellulose, 2015, 22, 1753–1762. [Google Scholar]
- 54.Shi W, Li X and Ma H, Angew. Chemie Int. Ed, 2012, 51, 6432–6435. [DOI] [PubMed] [Google Scholar]
- 55.Ma LY, Wang HY, Xie H and Xu LX, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2004, 60, 1865–1872. [DOI] [PubMed] [Google Scholar]
- 56.Obare SO, De C, Guo W, Haywood TL, Samuels TA, Adams CP, Masika NO, Murray DH, Anderson GA, Campbell K and Fletcher K, Sensors, 2010, 10, 7018–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


