Abstract
Background
Osteosarcoma (OS) is one of the most frequent bone malignancies. Long noncoding RNAs (lncRNAs) have been revealed to participate in many cancers, including OS. This study aimed to explore the biological function of lncRNA homeobox A cluster antisense RNA2 (HOXA-AS2) and its potential mechanism in OS progression.
Methods
Twenty-seven OS patients were recruited for this study. U2OS and MG-63 cells were cultured for in vitro analyses. The levels of HOXA-AS2, microRNA-124-3p (miR-124-3p) and E2F transcription factor 3 (E2F3) were measured by quantitative real-time polymerase chain reaction or Western blot. OS progression was investigated by cell viability, migration and invasion using cell counting kit-8 or trans-well assay. The interaction among HOXA-AS2, miR-124-3p and E2F3 was explored by bioinformatics analysis, luciferase reporter assay, RNA immunoprecipitation and biotinylated RNA pull-down. Xenograft model was established by injecting U2OS cells into nude mice.
Results
HOXA-AS2 expression was increased in OS tissues and cells and associated with poor survival of patients. Knockdown of HOXA-AS2 inhibited cell viability, migration and invasion in OS cells. miR-124-3p could bind with HOXA-AS2 and its deficiency reversed the suppressive role of HOXA-AS2 knockdown. Moreover, E2F3 acted as a target of miR-124-3p and positively regulated by HOXA-AS2. Silence of E2F3 suppressed OS progression, which was abolished by miR-124-3p exhaustion. Interference of HOXA-AS2 attenuated U2OS xenograft tumor growth via upregulating miR-124-3p and downregulating E2F3.
Conclusion
HOXA-AS2 silence impeded OS progression possibly by functioning as a decoy of miR-124-3p to target E2F3, indicating novel evidence of HOXA-AS2 as a promising therapeutic target of OS.
Keywords: osteosarcoma, HOXA-AS2, miR-124-3p, E2F3
Introduction
Osteosarcoma (OS) is the most common bone tumor in children and adolescents with high mortality.1 Although much effort has been expended in decades, the overall survival of patients remains unsatisfactory.2 Hence, it is urgent to understand the pathogenesis of OS to ameliorate the outcomes of patients.
The emerging evidence suggests that noncoding RNAs, such as long noncoding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs, play important roles in regulating pathogenesis, diagnosis and prognosis of OS.3 LncRNAs could serve as essential biomarkers and therapeutic targets of OS by functioning as competitive endogenous RNAs (ceRNAs) for miRNAs to derepress mRNAs expression.4 Increasing evidences demonstrate that lncRNA homeobox A cluster antisense RNA2 (HOXA-AS2) acts as an oncogene to promote progression of cancers, including hepatocellular carcinoma, bladder cancer, papillary thyroid cancer, colorectal cancer and gallbladder carcinoma.5–9 However, the clinical value of HOXA-AS2 is rarely known in OS, except for the report of Wang et al.10 There is a need for better understanding the mechanism of HOXA-AS2 in OS progression.
miRNAs have been reported to be involved in OS pathogenesis by serving as oncogenes or tumor suppressors through multiple signaling.11 Previous studies reveal that miR-124-3p could play a suppressive role in bladder cancer, hepatocellular carcinoma and glioma.12–14 The available evidence indicates that miR-124 downregulated in serum plays as an important target for diagnosis and prognosis of OS.15 E2F transcription factor 3 (E2F3) is carcinogenic in human cancers and associated with cell proliferation, apoptosis and metastasis through miRNAs targeting.16 Bioinformatics analysis provided the potential binding sites of miR-124-3p and HOXA-AS2 or E2F3. Hence, we assumed HOXA-AS2 could regulate OS progression via miR-124-3p and E2F3. In this study, we measured the expression of HOXA-AS2 in OS tissues and cells. Moreover, we investigated the therapeutic effect of HOXA-AS2 on OS as well as the ceRNA regulatory network of HOXA-AS2/miR-124-3p/E2F3.
Materials and Methods
Patients and Specimens
A total of 27 OS patients were recruited from Shouguang People’s Hospital of Shandong Province and had signed informed consents. OS tissues and paratumor normal samples were harvested via surgical resection and stored at −80°C. A 5-year follow-up was performed for survival assay of all participants. This study was performed in accordance with the agreement of the Ethics Committee of Shouguang People’s Hospital of Shandong Province.
Cell Culture and Transfection
Normal human osteoblast cell line NHost and OS cell lines (U2OS and MG-63) were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in an incubator with 5% CO2 at 37°C. Cell culture medium was premixed Dulbecco’s Modified Eagle's Medium (Gibco, Carlsbad, CA, USA), 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA).
Small interfering RNA (siRNA) targeting HOXA-AS2 (si-HOXA-AS2), targeting E2F3 (si-E2F3), negative control (si-NC), miR-124-3p mimic, miRNA negative control (miR-NC), miR-124-3p inhibitor (in-miR-124-3p) and inhibitor negative control (in-miR-NC) were synthesized by Genepharma (Shanghai, China). U2OS and MG-63 cells were seeded into 6-well plates and transfected with these oligonucleotides using Lipofectamine 3000 transfection reagent (Invitrogen) upon 70% confluence. After 48 hrs of the transfection, cells were harvested for following analyses.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
A protocol of Trizol-based RNA extraction was performed for total RNA isolation from tissues or cells. The samples were prepared in duplicate. All-in-One™ qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD, USA) was used for reverse transcribe and qRT-PCR. The primers for miRNA were purchased from GeneCopoeia and HOXA-AS2 or E2F3 were synthesized by Sangon Biotech (Shanghai, China). The specific primers were listed as follows: HOXA-AS2 (Forward, 5ʹ-CCCGTAGGAAGAACCGATGA-3ʹ; Reverse, 5ʹ-TTTAGGCCTTCGCAGACAGC-3ʹ); E2F3 (Forward, 5ʹ-AGAAAGCGGTCATCAGTACCT-3ʹ; Reverse, 5ʹ-TGGACTTCGTAGTGCAGCTCT-3ʹ); GAPDH (Forward, 5ʹ-ATTCCATGGCACCGTCAAGGCTGA-3ʹ; Reverse, 5ʹ-TTCTCCATGGTGGTGAAGACGCCA-3ʹ); miR-124-3p (Forward, 5ʹ-GCGTAAGGCACGCGGTGAATGCC-3ʹ; Reverse, the universal adaptor PCR primer provided by GeneCopoeia); U6 (Forward, 5ʹ-CTCGCTTCGGCAGCACATATACT-3ʹ; Reverse, the universal adaptor PCR primer). GAPDH or U6 was used as endogenous control. The relative expression levels of HOXA-AS2, miR-124-3p and E2F3 were analyzed via 2−ΔΔCt method.17
Cell Viability
After transfection, U2OS and MG-63 cells were seeded into 96-well plates at a density of 2500 cells per well. The samples were prepared in quadruplicate. At 0, 24, 48 or 72 hrs, cell viability was determined by the OD value at 450 nm using cell counting kit-8 (CCK-8) (Beyotime, Shanghai, China) and a microplate reader (Bio-Rad, Hercules, CA, USA).
Trans-Well Assay
Cell migration and invasion were investigated by using trans-well chamber (Corning, Corning, NY, USA). For invasion trans-well assay, the chamber was precoated with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). After transfection, cells were digested and washed with serum-free medium, followed by seeding into upper chambers (1×104 cells). After culture for 24 hrs at 37°C, the non-migrated or non-invasive cells were removed with cotton swabs and the migrated or invasive cells were stained with 0.5% crystal violet (Sigma, St. Louis, MO, USA). The stained cells were observed under a microscope (Olympus, Tokyo, Japan) with three random fields and counted.
Bioinformatics Analysis and Luciferase Reporter Assay
The putative binding sites of miR-124-3p and HOXA-AS2 or E2F3 were predicted by bioinformatics analysis using DIANA tools and TargetScan Release 7.2. The wild type (WT) or mutant (MUT) luciferase reporter constructs targeting HOXA-AS2 or E2F3 were generated using pGL3 vector (Promega, Madison, WI, USA). For luciferase reporter assay, U2OS and MG-63 cells were co-transfected with WT or MUT luciferase reporter vectors and miR-124-3p or miR-NC using Lipofectamine 3000 transfection reagent. After 48 hrs of post-transfection, a luciferase reporter assay kit (Promega) was used for luciferase activity analysis in each group.
RNA Immunoprecipitation (RIP) and Biotinylated RNA Pull-Down
For RIP assay, 1×107 U2OS and MG-63 cells with or without miR-124 overexpression were harvested and lysed in RIP buffer. RIP assay was conducted using a Magna RNA immunoprecipitation kit (Millipore, Billerica, MA, USA) and magnetic beads precoated with Ago2 or IgG antibody. The level of HOXA-AS2 in complex was measured by qRT-PCR after the treatment of Trizol reagent.
For biotinylated RNA pull down, the biotin-labeled HOXA-AS2 WT, SOXA-AS2 MUT or negative control (NC) probes were conjugated with streptavidin beads. Cell lysates were incubated with RNA-bound beads for 2 hrs at 4°C. The bound miR-124-3p abundance was analyzed by qRT-PCR.
Western Blot
Total protein was extracted from the treated cells or tissues by RIPA lysis buffer and centrifugation at 12,000 g for 10 min. The equal amounts of proteins (20 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Each sample was prepared in triplicate. After blocking the non-specific binding sites, the membranes were probed with antibodies against E2F3 (ab152126, 1:1000) or β-actin (ab8227, 1:3000) overnight at 4°C, along with horseradish peroxidase-labeled secondary antibody (ab6721, 1:10,000) for 1 hr at room temperature. The antibodies were purchased from Abcam (Cambridge, MA, USA). Following incubating with BeyoECL Plus (Beyotime), the blots were visualized using X-OMAT BT film (Carestream Health, Rochester, NY, USA) in the dark. β-actin was used as an endogenous control. The relative protein level was analyzed according to the gray value of bands using Quantity One software (Bio-Rad) and normalized to the control group.
Murine Xenograft Assay
The lentiviral vectors with sh-HOXA-AS2 or corresponding control (sh-NC) were constructed by GeneCopoeia. U2OS cells were infected with these lentiviral vectors for 6 hrs and stably transfected cells were selected by puromycin treatment. Four-week-old male BALB/c nude mice (Vital River Laboratory Animal Technology, Beijing, China) were randomly divided into sh-HOXA-AS2 or sh-NC group (n=7 per group). Stably transfected cells (5 × 106) were injected subcutaneously into nude mice. The experiment was permitted by the Animal Research Committee of Shouguang People’s Hospital of Shandong Province and performed in accordance with the guidelines of the use of laboratory animals. Tumor volume was monitored every week with the formula: volume (mm3) = width2 × length/2. After 28 days following the inoculation, the mice in all groups were killed and tumor tissues were weighed and then collected for following molecular analyses.
Statistical Analysis
The data were analyzed using GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA) and expressed as the mean ± standard deviation (SD) based on three independent experiments. Survival curve was analyzed by Kaplan–Meier method and Log-rank test. The correlation among HOXA-AS2, miR-124-3p and E2F3 expression level in patients was analyzed via Spearman rank correlation analysis. Student’s t-test or ANOVA followed by Dunnett’s test was performed for comparisons between groups. P<0.05 was regarded significant.
Results
HOXA-AS2 Is Highly Expressed in OS
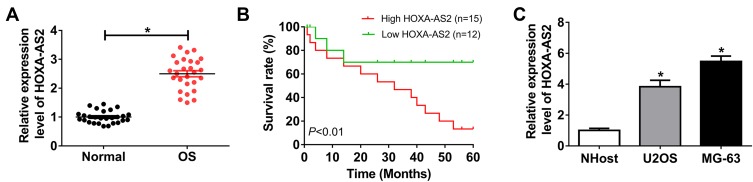
To explore the role of HOXA-AS2 in OS, its expression level was measured in OS tissues and cells. Compared with that in adjacent normal group, the abundance of HOXA-AS2 was significantly elevated in OS tissues (Figure 1A). Moreover, the patients were divided into two groups according to the mean level of HOXA-AS2 level in OS tissues, named as high (n=15) or low (n=12) HOXA-AS2 expression group. By a 5-year follow-up, data of survival rate showed that high HOXA-AS2 expression group displayed poor outcome of patients (Figure 1B). As demonstrated in Figure 1C, U2OS and MG-63 cells showed obviously higher expression level of HOXA-AS2 than NHost cells.
Figure 1.
HOXA-AS2 expression is increased in osteosarcoma. (A) The expression of HOXA-AS2 was measured in osteosarcoma tissues and normal samples by qRT-PCR, n=27. (B) The survival rate of patients was analyzed in high (n=15) or low (n=12) HOXA-AS2 expression group. (C) The level of HOXA-AS2 was detected in osteosarcoma cells by qRT-PCR. *P<0.05.
Knockdown of HOXA-AS2 Decreases Cell Viability, Migration and Invasion in OS Cells
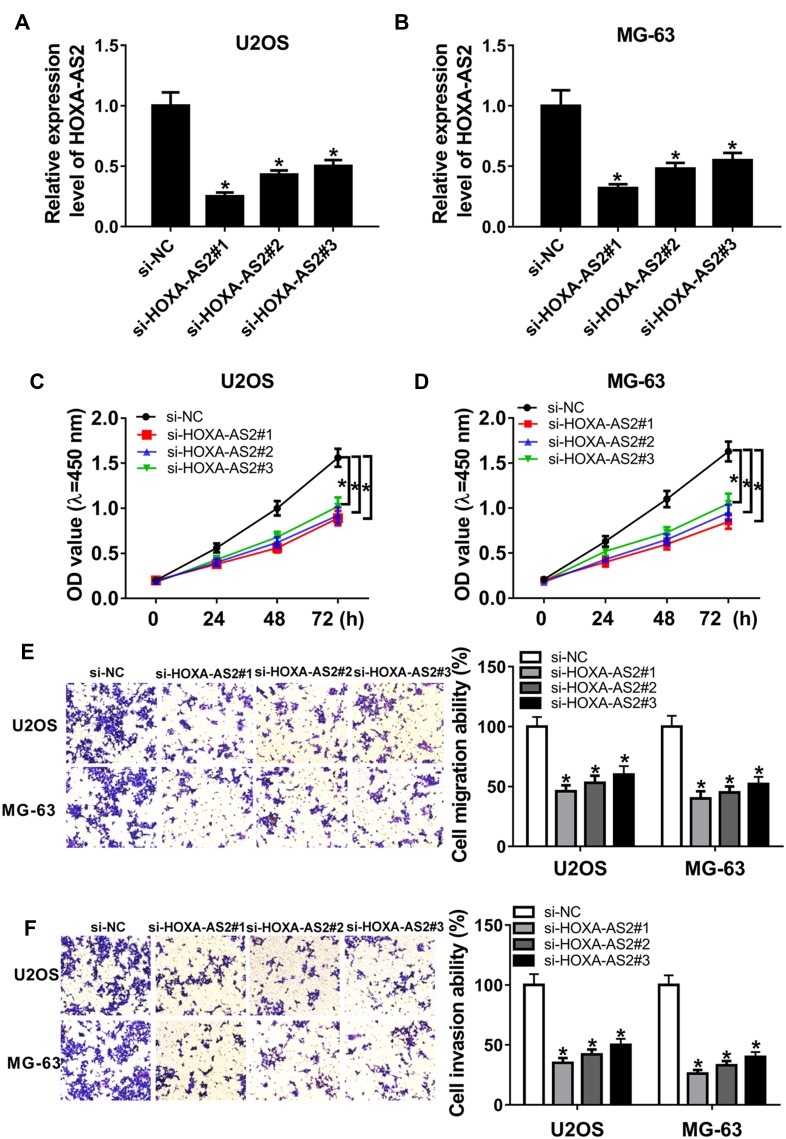
To explore the biological role of HOXA-AS2 in OS, U2OS and MG-63 cells were transfected with three siRNAs against HOXA-AS2 or si-NC. Transfection efficacy in U2OS and MG-63 cells was validated by qRT-PCR assay with the great reduction of HOXA-AS2 level in si-HOXA-AS2 transfected group (Figure 2A and B). As shown in Figure 2C and D, by CCK-8 assay of multiple points, knockdown of HOXA-AS2 evidently weakened the viability of U2OS and MG-63 cells at 72 hrs. In addition, analysis of trans-well revealed that interference of HOXA-AS2 led to obvious loss of migrated and invasive abilities in U2OS and MG-63 cells at 24 hrs (Figure 2E and F).
Figure 2.
Knockdown of HOXA-AS2 suppresses osteosarcoma progression. U2OS and MG-63 cells were transfected with si-NC or si-HOXA-AS2. The expression of HOXA-AS2 (A and B), cell viability (C and D), migration and invasion (E and F) were detected in transfected cells by qRT-PCR, CCK-8 or trans-well assay. *P<0.05.
HOXA-AS2 Regulates Cell Viability, Migration and Invasion in OS Cells by Sponging miR-124-3p
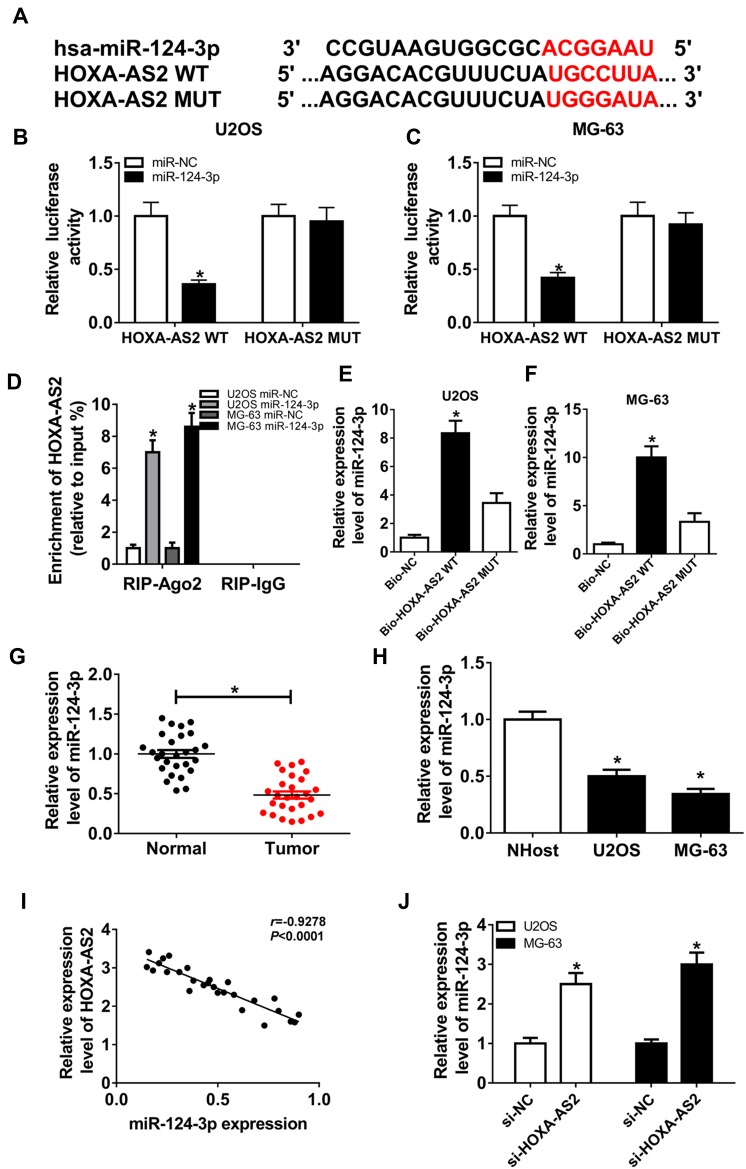
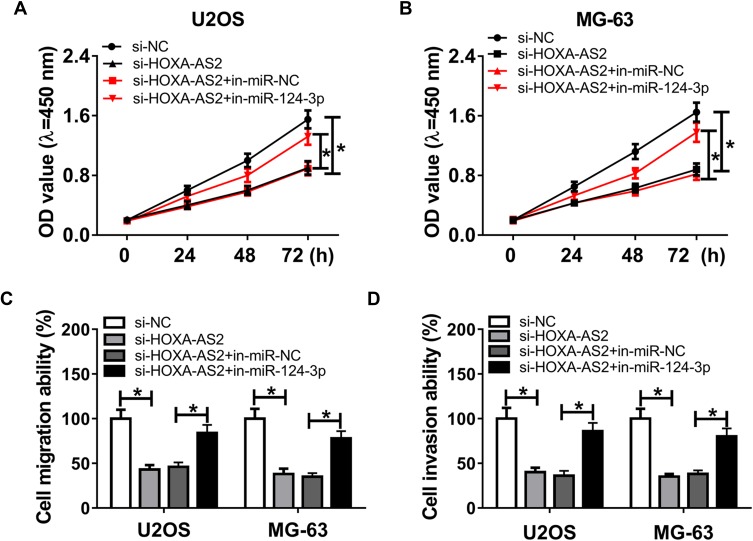
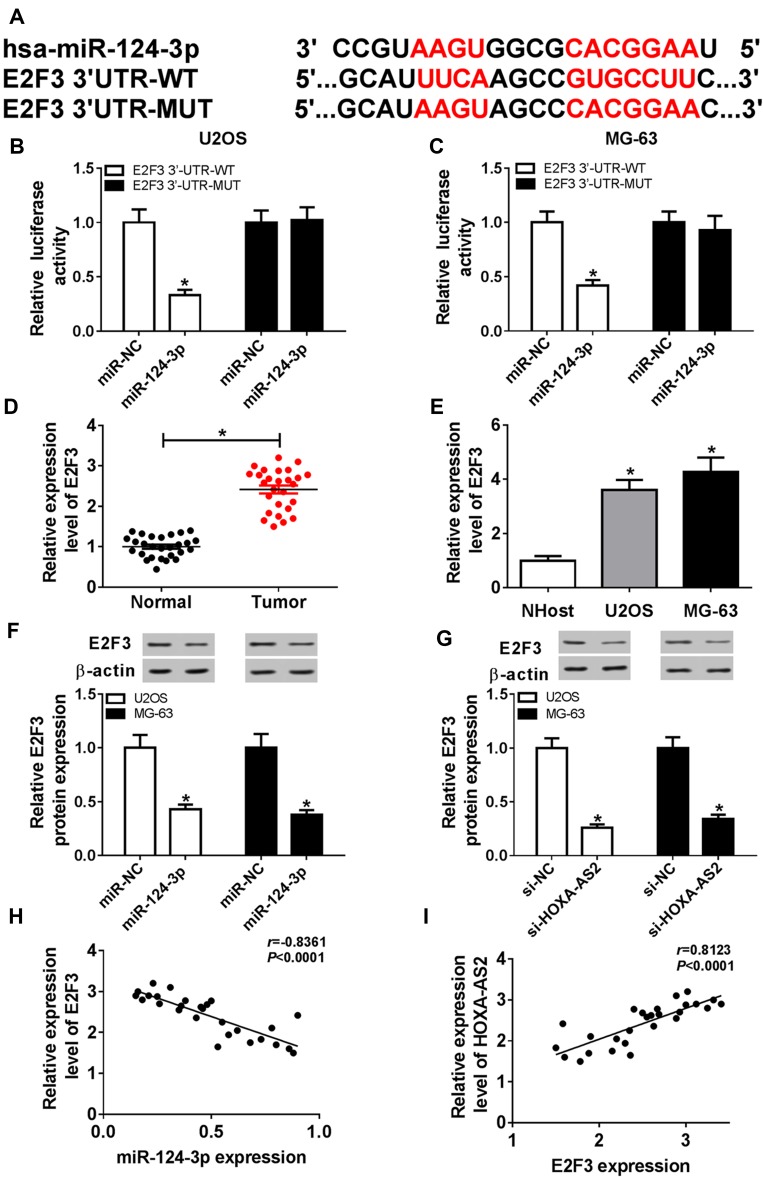
To elucidate the potential mechanism that allows HOXA-AS2 addressing OS progression, bioinformatics analysis was performed and predicted the binding sites of HOXA-AS2 and miR-124-3p using DIANA tools (Figure 3A). To identify this prediction, luciferase reporter assay was conducted by constructing luciferase reporter vectors HOXA-AS2 WT and HOXA-AS2 MUT. Results showed that transfection of miR-124-3p mimic resulted in a great reduction of luciferase activity compared with treatment of miR-NC in U2OS and MG-63 cells with WT reporter constructs, while its efficacy was lost by mutating the constructs (Figure 3B and C). Moreover, by a RIP assay based on Ago2, more abundance of HOXA-AS2 was enriched in U2OS and MG-63 cells with miR-124-3p overexpression (Figure 3D). Meanwhile, biotinylated RNA pull-down assay demonstrated in Figure 3E and F that the enrichment of miR-124-3p was specially increased in the two types of OS cells in WT biotinylated HOXA-AS2 group. Subsequently, the expression of miR-124-3p was measured in OS and qRT-PCR assay displayed lower expression level of miR-124-3p in OS tissues and cells compared with that in their corresponding controls, respectively (Figure 3G and H). As shown in Figure 3I, Spearman rank correlation analysis described a negative correlation between the expressions of HOXA-AS2 and miR-124-3p (r=−0.9278, P<0.0001). Besides, the results of qRT-PCR showed that miR-124-3p level was significantly enhanced in the two OS cell lines by silencing HOXA-AS2 (Figure 3J). To explore whether miR-124-3p was involved in HOXA-AS2-mediated development of OS, U2OS and MG-63 cells were transfected with si-NC, si-HOXA-AS2, si-HOXA-AS2 and in-miR-NC or in-miR-124-3p. Results showed that deficiency of miR-124-3p reversed the suppressive effect of HOXA-AS2 knockdown on viability, migration and invasion in U2OS and MG-63 cells (Figure 4A–D).
Figure 3.
miR-124-3p is bound to HOXA-AS2. (A) The potential binding sites of HOXA-AS2 and miR-124-3p. (B and C) Luciferase activity was measured in U2OS and MG-63 cells co-transfected with miR-124-3p or miR-NC and HOXA-AS2 WT or HOXA-AS2 MUT. (D) The enrichment of HOXA-AS2 was measured in the two cells transfected with miR-124-3p or miR-NC after Ago2 RIP. (E and F) The level of miR-124-3p was detected in U2OS and MG-63 cells after RNA pull-down. (G and H) The expression of miR-124-3p was detected in osteosarcoma tissues and cells by qRT-PCR. (I) The association between HOXA-AS2 and miR-124-3p levels was analyzed in osteosarcoma patients. (J) The level of miR-124-3p was measured in U2OS and MG-63 cells transfected with si-NC or si-HOXA-AS2 by qRT-PCR. *P<0.05.
Figure 4.
Deficiency of miR-124-3p reverses silencing HOXA-AS2-induced inhibition of osteosarcoma progression. U2OS and MG-63 cells were transfected with si-NC, si-HOXA-AS2, si-HOXA-AS2 and in-miR-NC or in-miR-124-3p. Cell viability (A and B), migration and invasion (C and D) were measured by CCK-8 or trans-well assay. *P<0.05.
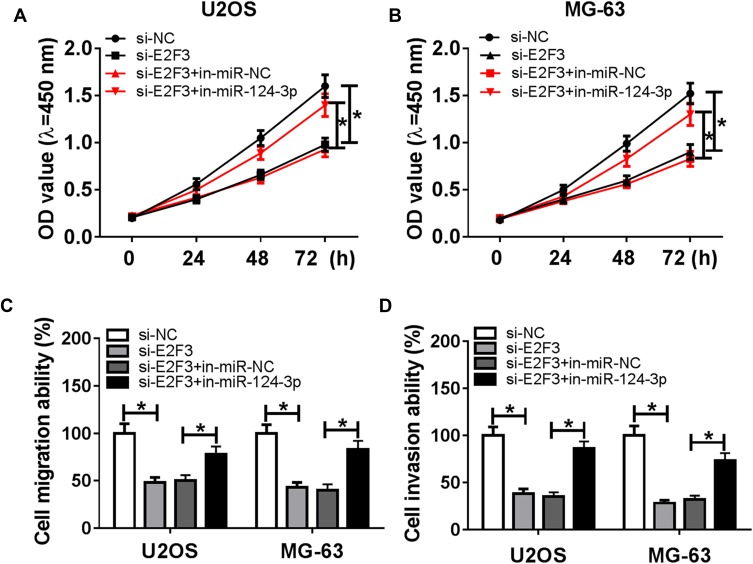
miR-124-3p Targets E2F3 to Mediate Cell Viability, Migration and Invasion in OS Cells
To further explore the underlying mechanism, the target of miR-124-3p was explored. Bioinformatics analysis showed the putative binding sites of miR-124-3p and E2F3 by TargetScan Release 7.2 (Figure 5A). Luciferase reporter assay by transfecting WT or MUT E2F3 luciferase reporter constructs displayed that luciferase activity was obviously inhibited in U2OS and MG-63 cells with WT constructs by overexpression of miR-124-3p, whereas it was not affected in MUT group (Figure 5B and C). Then, the expression of E2F3 was detected by qRT-PCR and results showed that the E2F3 mRNA level was aberrantly enhanced in OS tissues and cells (Figure 5D and E). Moreover, Western blot data revealed that overexpression of miR-124-3p or silence of HOXA-AS2 induced strong decrease of E2F3 protein level in U2OS and MG-63 cells (Figure 5F and G). Additionally, the expression level of E2F3 was negatively associated with miR-124-3p level (r=−0.8361, P<0.0001), but positively correlated with HOXA-AS2 abundance (r=0.8123, P<0.0001) (Figure 5H and I). To explore the role of E2F3 in OS development, U2OS and MG-63 cells were transfected with si-NC or si-E2F3. Results showed that interference of E2F3 impeded cell viability, migration and invasion in the two cell lines (Figure 6A–D). Besides, by co-transfecting inmiR-124-3p and si-E2F3, miR-124-3p exhaustion significantly attenuated the effect of E2F3 silence (Figure 6A–D).
Figure 5.
E2F3 is regulated by miR-124-3p and HOXA-AS2. (A) The binding sites of miR-124-3p and E2F3. (B and C) Luciferase activity was measured in osteosarcoma cells transfected with miR-124-3p or miR-NC and E2F3 WT or E2F3 MUT. (D and E) The expression of E2F3 mRNA was measured in osteosarcoma tissues and cells by qRT-PCR. (F and G) The level of E2F3 protein was detected in cells transfected with miR-NC, miR-124-3p, si-NC or si-HOXA-AS2 by Western blot. (H and I) The association between level of E2F3 mRNA in patients and miR-124-3p or HOXA-AS2 expression was investigated. *P<0.05.
Figure 6.
Knockdown of miR-124-3p reverses silencing E2F3-mediated inhibition of osteosarcoma progression. U2OS and MG-63 cells were transfected with si-NC, si-E2F3, si-E2F3 and in-miR-NC or in-miR-124-3p. Cell viability (A and B) and migration and invasion (C and D) were detected in the two transfected cells. *P<0.05.
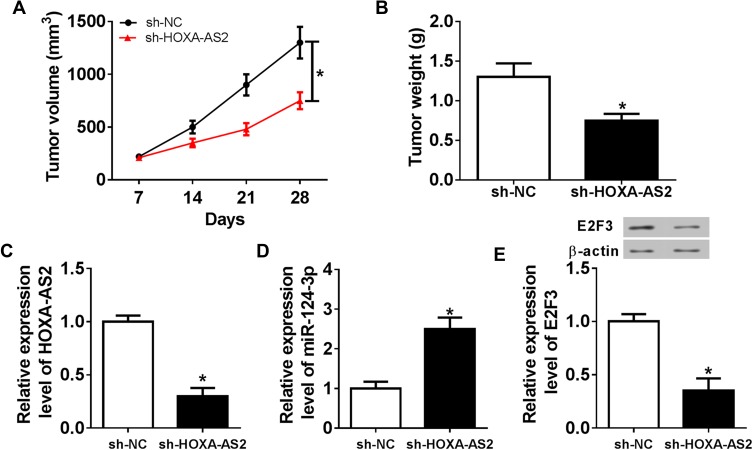
Knockdown of HOXA-AS2 Attenuates Xenograft Tumor Growth by Regulating miR-124-3p and E2F3
To better understand the mechanism in which HOXA-AS2 mediated OS progression, U2OS cells stably transfected with sh-HOXA-AS2 or sh-NC were injected into nude mice. As demonstrated in Figure 7A and B, the tumor volume and weight were significantly decreased in sh-HOXA-AS2 group compared with those in sh-NC group. Moreover, the tissues were harvested for further molecular analysis by qRT-PCR and Western blot. As displayed in Figure 7C–E, compared with control group, the expression levels of HOXA-AS2 and E2F3 protein were conspicuously reduced in sh-HOXA-AS2 group, while miR-124-3p abundance was remarkably upregulated.
Figure 7.
Inhibition of HOXA-AS2 attenuates xenograft tumor growth by regulating miR-124-3p and E2F3. U2OS cells were infected with sh-NC or sh-HOXA-AS2, and then stably transfected cells were introduced into nude mice. (A) Tumor volume was measured every week. (B) Tumor weight was detected at end point. (C–E) The expressions of HOXA-AS2, miR-124-3p and E2F3 protein were measured in tumor tissues. *P<0.05.
Discussion
In the present study, we have displayed the therapeutic role of HOXA-AS2 knockdown in OS progression. Specially, the expression of HOXA-AS2 was enhanced in OS tissues and cells. Downregulation of HOXA-AS2 was associated with the decreased cell viability, migration and invasion in vitro. Moreover, we found that the mechanism allows HOXA-AS2 participating in OS development might be mediated by miR-124-3p and E2F3. This study revealed a novel potential mechanism of HOXA-AS2 in the development and therapeutics of OS.
The evidence proved the importance of ceRNA regulatory network of lncRNA/miRNA/mRNA in progression, recurrence and treatment of OS.18 HOXA-AS2 (a 1048-bp lncRNA) has been reported to promote tumorigenesis and malignancy in multiple cancers.5–9 However, little is known about the role and mechanism of HOXA-AS2 in OS progression. In this study, we exhibited that HOXA-AS2 increased in OS tissues and cells predicted poor outcomes of patients, suggesting that HOXA-AS2 might play as an oncogene in OS, which is also in agreement with a former work.10 Cell proliferation and metastasis are the main progresses of OS malignancy. This paper using loss-of-function experiments demonstrated that HOXA-AS2 knockdown inhibited OS cell viability, migration and invasion by CCK-8 and trans-well assays, indicating the therapeutic effect of HOXA-AS2 silence.
This study focused on the novel molecular pathway of HOXA-AS2 in OS progression. The ceRNA regulatory network is the main mechanism of lncRNA in human cancers. A number of investigators have reported HOXA-AS2 as ceRNA by sponging miR-125b or miR-520c-3p in many cancers.5–7 To investigate whether HOXA-AS2 could serve as a ceRNA in OS, the potential interaction with miRNA was explored by bioinformatics analysis. Here, we first validated HOXA-AS2 as a decoy of miR-124-3p in OS cells by luciferase reporter assay, RIP and RNA pull-down analyses. Former works suggested that miR-124, the precursor miRNA of miR-124-3p, could suppress proliferation, migration and invasion of OS by targeting receptor tyrosine kinase-like orphan receptor 2, B7 homolog 3, sphingosine kinase 1 or tumor necrosis factor receptor-associated factor 6.19–22 More particularly, miR-124-3p was reported as a tumor suppressor in OS by regulating snail family zinc finger 2 or Rho-associated kinase 1.23,24 In this study, qRT-PCR assay showed low expression of miR-124-3p in OS, uncovering that miR-124-3p might play a tumor-suppressive role. By the rescue experiments downregulating miR-124-3p on the basis of HOXA-AS2 inhibition, we found that miR-124-3p exhaustion counteracted the suppressive effect of HOXA-AS2 interference, suggesting that HOXA-AS2 mediated OS progression by sponging miR-124-3p. To further explore the ceRNA regulatory network, we confirmed E2F3 as a functional target of miR-124-3p by bioinformatics analysis and luciferase reporter assay. Here, we described E2F3 expression was upregulated in OS and its silence inhibited OS progression, revealing the carcinogenic role of E2F3, which is also consistent with former efforts.25,26 Besides, E2F3 expression level was negatively regulated by miR-124-3p but positively correlated with HOXA-AS2 in vitro and in vivo, indicating that HOXA-AS2 could act as a ceRNA for miR-124-3p to derepress E2F3 level in OS cells.
Cell viability or proliferation is usually mediated by cell cycle progress and apoptosis. Previous studies suggested that HOXA-AS2 knockdown could decrease cell proliferation by inducing G1 arrest and promoting apoptosis in gallbladder carcinoma, colorectal cancer, hepatocellular carcinoma and OS.5,8–10 Hence, we hypothesized the viability inhibition induced by HOXA-AS2 silence might also be caused by G1 arrest and apoptosis production, which should be explored in future.
Conclusion
In conclusion, our findings summarized the therapeutic effect of HOXA-AS2 knockdown on OS development, possibly by targeting miR-124-3p and E2F3. This study first provided the ceRNA regulatory network of HOXA-AS2/miR-124-3p/E2F3, indicating HOXA-AS2 as a novel target of OS treatment.
Disclosure
All authors declare that they have no conflicts of interest.
References
- 1.Simpson E, Brown HL. Understanding osteosarcomas. JAAPA. 2018;31(8):15–19. doi: 10.1097/01.JAA.0000541477.24116.8d [DOI] [PubMed] [Google Scholar]
- 2.Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr. 2016;28(1):26–33. doi: 10.1097/MOP.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Jing J, Cheng L. Emerging roles of non-coding RNAs in the pathogenesis, diagnosis and prognosis of osteosarcoma. Invest New Drugs. 2018;36(6):1116–1132. doi: 10.1007/s10637-018-0624-7 [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Dou P, Liu T, He S. Application of long noncoding RNAs in osteosarcoma: biomarkers and therapeutic targets. Cell Physiol Biochem. 2017;42(4):1407–1419. doi: 10.1159/000479205 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Xu J, Zhang S, et al. HOXA-AS2 promotes proliferation and induces epithelial-mesenchymal transition via the miR-520c-3p/GPC3 axis in hepatocellular carcinoma. Cell Physiol Biochem. 2018;50(6):2124–2138. doi: 10.1159/000495056 [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Wu D, Chen J, et al. Long non-coding RNA HOXA-AS2 promotes the migration, invasion and stemness of bladder cancer via regulating miR-125b/Smad2 axis. Exp Cell Res. 2019;375(1):1–10. doi: 10.1016/j.yexcr.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 7.Xia F, Chen Y, Jiang B, et al. Long noncoding RNA HOXA-AS2 promotes papillary thyroid cancer progression by regulating miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. 2018;50(5):1659–1672. doi: 10.1159/000494786 [DOI] [PubMed] [Google Scholar]
- 8.Tong G, Wu X, Cheng B, et al. Knockdown of HOXA-AS2 suppresses proliferation and induces apoptosis in colorectal cancer. Am J Transl Res. 2017;9(10):4545–4552. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Cao P, Zhu X, et al. Upregulation of long non-coding RNA HOXA-AS2 promotes proliferation and induces epithelial-mesenchymal transition in gallbladder carcinoma. Oncotarget. 2017;8(20):33137–33143. doi: 10.18632/oncotarget.16561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang R, Cheng G, Xu R, Han X. Long non-coding RNA HOXA-AS2 promotes migration and invasion by acting as a ceRNA of miR-520c-3p in osteosarcoma cells. Cell Cycle. 2018;17(13):1637–1648. doi: 10.1080/15384101.2018.1489174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushlinskii NE, Fridman MV, Braga EA. Molecular mechanisms and microRNAs in osteosarcoma pathogenesis. Biochemistry (Mosc). 2016;81(4):315–328. doi: 10.1134/S0006297916040027 [DOI] [PubMed] [Google Scholar]
- 12.Wang JR, Liu B, Zhou L, Huang YX. MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer. Cancer Biomark. 2019;24(2):159–172. doi: 10.3233/CBM-182000 [DOI] [PubMed] [Google Scholar]
- 13.Long HD, Ma YS, Yang HQ, et al. Reduced hsa-miR-124-3p levels are associated with the poor survival of patients with hepatocellular carcinoma. Mol Biol Rep. 2018;45(6):2615–2623. doi: 10.1007/s13277-015-4527-3 [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Xu L, Wang C, Fan W, Yan H, Li Q. MicroRNA-124-3p represses cell growth and cell motility by targeting EphA2 in glioma. Biochem Biophys Res Commun. 2018;503(4):2436–2442. doi: 10.1016/j.bbrc.2018.06.173 [DOI] [PubMed] [Google Scholar]
- 15.Cong C, Wang W, Tian J, Gao T, Zheng W, Zhou C. Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark. 2018;21(2):449–454. doi: 10.3233/CBM-170672 [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Feng B, Lu L, et al. MiRNAs and E2F3: a complex network of reciprocal regulations in human cancers. Oncotarget. 2017;8(36):60624–60639. doi: 10.18632/oncotarget.17364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Ding L, Li X, Fan H. Identification of biomarkers associated with the recurrence of osteosarcoma using ceRNA regulatory network analysis. Int J Mol Med. 2019;43(4):1723–1733. doi: 10.3892/ijmm.2019.4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Hu Y, Wan J, He H. MicroRNA-124 suppresses the migration and invasion of osteosarcoma cells via targeting ROR2-mediated non-canonical Wnt signaling. Oncol Rep. 2015;34(4):2195–2201. doi: 10.3892/or.2015.4186 [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Kang FB, Sun N, et al. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol. 2016;37(11):14939–14947. doi: 10.1007/s13277-016-5386-2 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Han Y, Zhang Z, et al. MicroRNA-124 upregulation inhibits proliferation and invasion of osteosarcoma cells by targeting sphingosine kinase 1. Hum Cell. 2017;30(1):30–40. doi: 10.1007/s13577-016-0148-4 [DOI] [PubMed] [Google Scholar]
- 22.Meng Q, Zhang W, Xu X, et al. The effects of TRAF6 on proliferation, apoptosis and invasion in osteosarcoma are regulated by miR-124. Int J Mol Med. 2018;41(5):2968–2976. doi: 10.3892/ijmm.2018.3458 [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Liang Y, Xu M, Xiong J, Wang D, Ding Q. MicroRNA-124 acts as a tumor-suppressive miRNA by inhibiting the expression of Snail2 in osteosarcoma. Oncol Lett. 2018;15(4):4979–4987. doi: 10.3892/ol.2018.7994 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Cui M, Wang J, Li Q, Zhang J, Jia J, Zhan X. Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed Pharmacother. 2017;92:437–444. doi: 10.1016/j.biopha.2017.05.081 [DOI] [PubMed] [Google Scholar]
- 25.Dong D, Gong Y, Zhang D, Bao H, Gu. G. miR-874 suppresses the proliferation and metastasis of osteosarcoma by targeting E2F3. Tumour Biol. 2016;37(5):6447–6455. doi: 10.1007/s13277-015-4527-3 [DOI] [PubMed] [Google Scholar]
- 26.Ma C, Han J, Dong D, Wang N. MicroRNA-152 suppresses human osteosarcoma cell proliferation and invasion by targeting E2F transcription factor 3. Oncol Res. 2018;26(5):765–773. doi: 10.3727/096504017X15021536183535 [DOI] [PMC free article] [PubMed] [Google Scholar]