Abstract
Purpose
To evaluate the prevalence of diabetic nephropathy and different categories of estimated glomerular filtration rate (eGFR) as calculated by the CKD-EPI equation among Chinese patients with type 2 diabetes in primary care in Hong Kong. The associated factors of diabetic nephropathy were also analyzed.
Methods
A cross-sectional study was conducted in 35,109 Chinese patients with type 2 diabetes followed up in all General Outpatient Clinics in a Hospital Authority cluster and had undergone comprehensive diabetic complication assessment from April 2013 to March 2016. The GFR was estimated by the CKD-EPI equation. Logistic regression was used to analyze the associated factors of diabetic nephropathy.
Results
The prevalence of diabetic nephropathy (with either or both albuminuria and impaired eGFR), impaired eGFR (with or without albuminuria) and albuminuria (with or without impaired eGFR) was 31.6%, 16.9% and 22.0% respectively. The prevalence of eGFR categories 1, 2, 3, 4 and 5 was 36.0%, 47.1%, 15.7%, 1.1% and 0.1% respectively. The comorbidity with hypertension or presence of other diabetic microvascular or macrovascular complications including diabetic retinopathy, peripheral neuropathy, peripheral vascular disease, history of stroke and history of ischemic heart disease had strong association with diabetic nephropathy. Obesity, smoking, suboptimal control of blood pressure, hemoglobin A1c and non-high density lipoprotein cholesterol were also significantly associated with diabetic nephropathy.
Conclusions
Diabetic nephropathy was common among Chinese patients with type 2 diabetes in primary care in Hong Kong. Early identification and control of the modifiable risk factors are of upmost importance in preventing the complication.
Keywords: Type 2 diabetes, Diabetic nephropathy, Glomerular filtration rate, Primary care
Introduction
Diabetes mellitus is a major public health problem. The World Health Organization reported that the global prevalence of diabetes was around 9% among adults aged above 18 years in 2014 [1]. According to the International Diabetes Federation, the number of people with diabetes is expected to increase above 500 million by 2030 [2]. Diabetes occurs in about 10% of Hong Kong population and is one of the most common chronic diseases encountered in primary care. About 2% of people aged less than 35 years and more than 20% of those older than 65 years are affected by the disease [3].
Diabetes is the leading cause of chronic kidney disease (CKD) worldwide [4]. Diabetic nephropathy is defined as diabetes with the presence of albuminuria, impaired glomerular filtration rate (GFR), or both [5]. The analyses of a national survey in 2011 estimated that among the US adults diagnosed with diabetes, the prevalence of diabetic nephropathy, impaired GFR (with or without albuminuria) and albuminuria (with or without impaired GFR) was 34.5%, 17.7% and 23.7% respectively [6]. A study in China reported similar prevalence of diabetic nephropathy (31.0%) and albuminuria (28.9%) [7]. According to the Hong Kong Renal Registry Report 2012, diabetes was the most common cause (45%) of end-stage renal failure (ESRF) [8]. Given the projected increase in the number of people diagnosed with diabetes, the number of people with ESRF due to diabetic nephropathy may increase significantly over the next few decades and impose heavy burden on our healthcare system.
Meta-analyses have shown that impaired GFR and presence of albuminuria are independent risk factors for progressive CKD, ESRF, all-cause mortality and cardiovascular mortality in general population [9, 10]. It has been demonstrated that therapeutic interventions can be implemented to ameliorate the onset and course of diabetic nephropathy significantly if the condition is recognized at its initial stages [11–13]. Thus, early and accurate detection of impaired GFR and albuminuria in diabetic patients is of upmost importance for initiating and optimizing treatment to slow down the progression of kidney failure, assessing and modifying other comorbid conditions, and reviewing prescription of renally excreted or nephrotoxic drugs [14]. Although the Modification of Diet in Renal Disease (MDRD) Study equation had been recommended for estimating GFR for long time [5], it is known that it would underestimate the GFR in patients with normal or near normal kidney function as it was initially derived from data acquired from patients with CKD [15, 16]. It is also only valid if the age is from 18 to 85 years old [17]. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) had proposed a new equation in 2009 using the same four variables (age, sex, race, and serum creatinine level) as the MDRD Study equation [18]. The new equation is applicable in a wider age range including those people older than 85 years old [17]. The CKD-EPI equation has been shown to estimate GFR more precisely compared with the MDRD Study equation among various study populations including Chinese and different clinical conditions including patients with hypertension and diabetes [18–21]. There is also lower bias especially at higher estimated GFR when using the CKD-EPI equation and thus leads to fewer false positive diagnosis of CKD [18]. Moreover, studies have demonstrated that the CKD-EPI equation is more accurate in categorizing the clinical risk, including acute myocardial infarction, end-stage renal disease, stroke and all-cause mortality [22, 23]. Therefore, the CKD-EPI equation is more suitable for estimating kidney function than the MDRD Study equation in primary care in which more patients have normal or near normal renal function. The National Institute for Health and Care Excellence (NICE) 2014 guideline for CKD also recommended the use of CKD-EPI to estimate GFR [24]. Nevertheless; the CKD-EPI equation is not yet widely adopted in most Hospital Authority clinical settings in current practice.
Increased systolic blood pressure (BP), non-high density lipoprotein (non-HDL) cholesterol, poor glycemic control, long duration of diabetes, smoking history, older age, higher body mass index (BMI), previous retinopathy and previous sensory neuropathy have all been found to be risk factors of diabetic nephropathy [25–29]. Moderate alcohol consumption was reported to have association with reduced risk of diabetic nephropathy [30]. However, most of the evidence was derived from non-Chinese populations. The association of these risk factors in Chinese patients is yet to be explored.
To our knowledge, there was no published data regarding the prevalence of diabetic nephropathy and different categories of GFR among Chinese diabetic patients using the CKD-EPI equation in primary care in our locality.
This study therefore aimed to evaluate the prevalence of diabetic nephropathy and different categories of estimated GFR (eGFR) in Chinese diabetic patients in primary care in Hong Kong by using the new CKD-EPI equation. The associated risk factors would also be investigated. The results would provide us important information about the epidemiology of diabetic nephropathy in primary care in Hong Kong. This would be useful for devising the long-term management policy for our diabetic patients in primary care in order to reduce the incidence of ESRF caused by diabetic nephropathy.
Materials and methods
Study design
This was a cross-sectional study. The study was carried out in a local cluster of Hospital Authority, which covered two of the eighteen districts and around 15% of total population of Hong Kong. According to Hospital Authority statistics, the local cluster with eight General Out-patient Clinics (GOPCs) served about 42,000 diabetic patients in year 2016. All patients attending the GOPCs in the cluster who had undergone the comprehensive diabetic complication assessment program from 1st April 2013 to 31st March 2016 would be included. The inclusion and exclusion criteria were summarized as below:
Inclusion criteria:
Patients with diagnosis of type 2 diabetes who had undergone the diabetic complication assessment program within the study period
Exclusion criteria:
Non-Chinese patients
Patients with diabetes not confirmed
Patients with type 1 diabetes
Patients with renal transplant
Patients undergoing peritoneal dialysis or hemodialysis
Patients with missing serum creatinine measurement
Patients with missing urinary albumin creatinine ratio (ACR) measurement
A list of patients fulfilling the inclusion criteria was generated from the Hospital Authority Clinical Data Analysis and Reporting System for analysis. The sampling frame would be able to include all the eligible subjects being followed up in the participating clinics since each subject would attend the comprehensive diabetic complication assessment program at least once every 3 years and all patients newly diagnosed with diabetes mellitus would receive the comprehensive complication assessment within 6 months of diagnosis. The latest complication assessment results would be used for analysis in patients received more than one complication assessment within the study period.
Procedure
Collected variables included age, gender, blood pressure, BMI, current smoking and drinking status, duration of diabetes, co-morbidities including hypertension, ischemic heart disease and stroke, complications including retinopathy, peripheral neuropathy and peripheral vascular disease. Collected laboratory test results included urine ACR, serum creatinine, glycated hemoglobin A1c (HbA1c), total cholesterol and high density lipoprotein (HDL) cholesterol. The GFR was subsequently estimated by the CKD-EPI equation as below.
CKD-EPI equation [18]:
Scr is serum creatinine in mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and − 0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
The GFR categories (category 1: GFR ≥ 90 ml/min/1.73 m2; category 2: GFR 60 to 89 ml/min/1.73 m2; category 3: GFR 30 to 59 ml/min/1.73 m2; category 4: GFR 15 to 29 ml/min/1.73 m2; and category 5: GFR <15 ml/min/1.73 m2) were classified according to the NICE guideline [24].
Diabetic nephropathy was defined as diabetes with the presence of albuminuria, impaired GFR, or both [5]. Impaired GFR was defined as GFR less than 60 ml/min/1.73 m2 [5]. Albuminuria was defined as a spot urine ACR >2.5 mg/mmol for male and > 3.5 mg/mmol for female [31].
Non-HDL cholesterol was calculated by subtracting HDL-cholesterol from total cholesterol.
Diabetic retinopathy grading was performed with use of fundi photos by trained optometrists. The presence of diabetic peripheral neuropathy was defined as abnormal vibration threshold (more than 25 V) detected by a biothesiometer on the big toe or abnormal light touch perception tested with a 10-g monofilament at 4 plantar sites [32]. The presence of peripheral vascular disease was defined as abnormal pedal pulses detected by palpation and doppler ultrasound.
Outcomes
Primary outcomes were the prevalence of diabetic nephropathy based on the presence of albuminuria and/or impaired eGFR by using the CKD-EPI equation and the prevalence of different categories of eGFR among Chinese patients with type 2 diabetes in our GOPCs. Secondary outcome was the associated risk factors for development of diabetic nephropathy.
Statistical analysis
SPSS (Version 21.0) was used for statistical analysis. Continuous variables would be presented as means ± standard deviation (SD) and compared with Student’s t test. Skewed data would be presented in median and compared with Mann-Whitney test. Categorical variables would be presented as percentages and compared with Chi-square test. Logistic regression would be used to assess factors associated with diabetic nephropathy. A p value <0.05 would be considered statistically significant.
Results
Study population
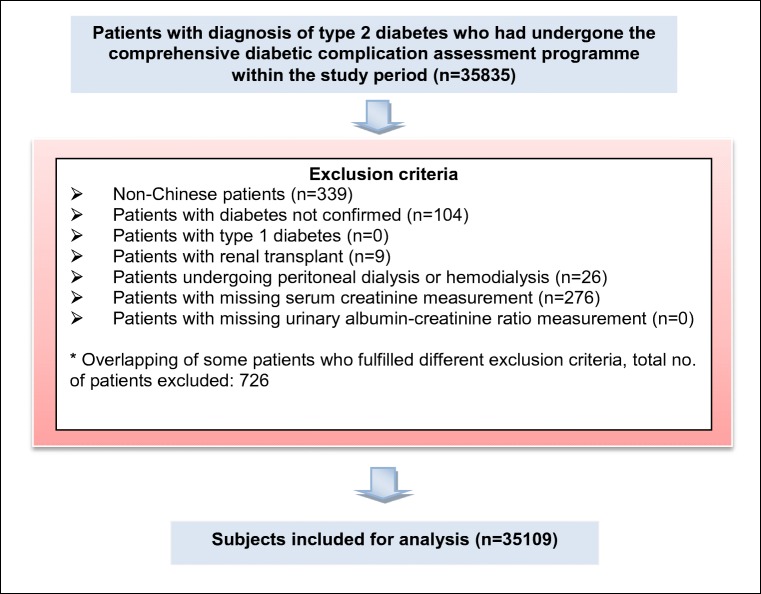
There were 35,835 patients underwent the comprehensive diabetic complication assessment program within the study period. 726 (2%) patients were excluded as shown in Fig. 1. A total of 35,109 individuals were included in the final analysis.
Fig. 1.
Flow chart showing the exclusion criteria
The demographic and clinical characteristics of all individuals included in the study were shown in Table 1. The mean age of our patients was 65.3 years and there were more female patients (53.4%). Most of the patients were non-smokers (69.7%) and non-drinkers or ex-drinkers (80.0%). The mean duration of diabetes was 9.1 years. 81.2% of patients had hypertension. 5.9% and 6.1% of patients had already developed ischemic heart disease and stroke respectively. The mean systolic and diastolic blood pressure was 130 and 73 mmHg and nearly half of the patients (46.7%) had blood pressure controlled to less than 130/80 mmHg. About three-quarters (74.6%) of patients were overweight or obesity. 61.7% of patients had their HbA1c levels controlled to less than 7% (53 mmol/mol) while 28.9% of patients had non-HDL cholesterol levels less than 2.5 mmol/L. Diabetic retinopathy, peripheral neuropathy and peripheral vascular disease was present in 36.4%, 9.7% and 0.1% of patients respectively.
Table 1.
Demographic data and clinical characteristics of patients (N = 35109)
| Mean (SD) | Number (%) | |
|---|---|---|
| Age (years) | 65.3 (11.4) | |
| < 40 | 428 (1.2) | |
| 40–49 | 2192 (6.3) | |
| 50–59 | 8512 (24.2) | |
| 60–69 | 11,419 (32.5) | |
| 70–79 | 8424 (24.0) | |
| ≥ 80 | 4134 (11.8) | |
| Sex | ||
| Male | 16,370 (46.6) | |
| Female | 18,739 (53.4) | |
| Smoking status | ||
| Non-smoker | 24,472 (69.7) | |
| Ex-smoker | 6978 (19.9) | |
| Smoker | 3641 (10.3) | |
| Unknown | 18 (0.1) | |
| Drinking status | ||
|
Non and ex-drinker Social-drinker Drinker |
28,099 (80.0) 6043 (17.2) 895 (2.6) |
|
| Unknown | 72 (0.2) | |
| Duration of diabetes (years) | 9.1 (6.5) | |
| < 5 | 10,022 (28.5) | |
| 5–10 | 12,910 (36.8) | |
| > 10 | 12,140 (34.6) | |
| Unknown | 37 (0.1) | |
| History of hypertension | ||
| Yes | 28,506 (81.2) | |
| No | 6603 (18.8) | |
| History of ischemic heart disease | ||
| Yes | 2091 (5.9) | |
| No | 32,785 (93.4) | |
| Unknown | 233 (0.7) | |
| Yes | 2140 (6.1) | |
| No | 32,791 (93.4) | |
| Unknown | 178 (0.5) | |
| Systolic blood pressure (mmHg) | 130.1 (15.2) | |
| < 130 | 18,354 (52.3) | |
| 130–139 | 8013 (22.8) | |
| ≥ 140 | 8287 (23.6) | |
| Unknown | 455 (1.3) | |
| Diastolic blood pressure (mmHg) | 73.0 (9.8) | |
| < 80 | 26,220 (74.7) | |
| 80–89 | 6726 (19.1) | |
| ≥ 90 | 1708 (4.9) | |
| Unknown | 455 (1.3) | |
| Control of BP | ||
| Below target (BP < 130/80 mmHg) | 16,398 (46.7) | |
| Above target (BP ≥ 130/80 mmHg) | 18,256 (52.0) | |
| Unknown | 455 (1.3) | |
| Body mass index (kg/m2) | 25.7 (4.0) | |
| < 23 | 8769 (25.0) | |
| 23–24.9 (overweight) | 7526 (21.4) | |
|
≥ 25 (obesity) Unknown |
18,679 (53.2) 135 (0.4) |
|
| HbA1c (% / mmol/mol) | 7.0 (1.1) | |
| < 6.5 / < 48 | 11,757 (33.5) | |
| 6.5–6.9 / 48–52 | 9896 (28.2) | |
| ≥ 7 / ≥ 53 | 13,259 (37.8) | |
| Unknown | 197 (0.5) | |
| Non-HDL cholesterol (mmol/L) | 3.0 (0.8) | |
| < 2.5 | 10,138 (28.9) | |
| ≥ 2.5 | 24,838 (70.7) | |
| Unknown | 133 (0.4) | |
| Presence of diabetic retinopathy | ||
| Non-proliferative | 12,618 (35.9) | |
| Proliferative | 159 (0.5) | |
| No | 21,372 (60.9) | |
| Unknown | 960 (2.7) | |
| Presence of peripheral neuropathy | ||
| Yes | 3414 (9.7) | |
| No | 30,995 (88.3) | |
| Unknown | 700 (2.0) | |
| Presence peripheral vascular disease | ||
| Yes | 54 (0.1) | |
| No | 34,719 (98.9) | |
| Unknown | 336 (1.0) | |
BP blood pressure, HbA1c glycated hemoglobin A1c, Non-HDL non-high density lipoprotein
The prevalence of diabetic nephropathy
The prevalence of diabetic nephropathy, impaired eGFR (with or without albuminuria) and albuminuria (with or without impaired eGFR) was 31.6%, 16.9% and 22.0% respectively. More than four-fifths of patients had normal eGFR as estimated by the CKD-EPI equation (category 1: 36.0% and category 2: 47.1%). The prevalence of eGFR category 3 was 15.7%. A minority of patients had eGFR belonged to category 4 (1.1%) and category 5 (0.1%) (Table 2).
Table 2.
Prevalence of diabetic nephropathy, albuminuria and impaired eGFR
| No. of patients | |||
|---|---|---|---|
| eGFR Categories (ml/min/1.73 m2) | With albuminuria | Without albuminuria | Total no. of patients (%) |
| 1 (≥ 90) | 1833* | 10,797 | 12,630 (36.0%) |
| 2 (60–89) | 3327* | 13,226 | 16,553 (47.1%) |
| 3 (30–59) | 2277* | 3228* | 5505 (15.7%) |
| 4 (15–29) | 266* | 109* | 375 (1.1%) |
| 5 (< 15) | 31* | 15* | 46 (0.1%) |
| Total | 7734 (22.0%) | 27,375 (78.0%) | 35,109 (100.0%) |
No. of patient with eGFR <60 ml/min/1.73m2: 5926 (16.9%)
eGFR estimated glomerular filtration rate
*No of patients with diabetic nephropathy: 11086 (31.6%)
Factors associated with diabetic nephropathy
32,375 patients with no missing clinical parameters or laboratory data were included for analysis of factors associated with diabetic nephropathy in the logistic regression. The results revealed that the presence of other diabetic microvascular or macrovascular complications had strong association with diabetic nephropathy (proliferative diabetic retinopathy [Odd ratio (OR) 6.52, p < 0.001], peripheral vascular disease [OR 2.19, p = 0.023], stroke [OR 1.5, p < 0.001], peripheral neuropathy [OR 1.48, p < 0.001] and ischemic heart disease [OR 1.22, p < 0.001]). The presence of hypertension was also significantly associated with diabetic nephropathy [OR 1.99, p < 0.001]. Other demographic or clinical parameters like older age [OR 1.06, p < 0.001], male patients [OR 1.27, p < 0.001], smokers [OR 1.49, p < 0.001], longer duration of diabetes [OR 1.03, p < 0.001] and obesity [OR 1.51, p < 0.001] were also found to be associated with diabetic nephropathy. The risk of diabetic nephropathy in patients with suboptimal blood pressure, HbA1c and non-HDL cholesterol control was 1.26, 1.28 and 1.20 times respectively when compared with patients with those parameters controlled to target. (p < 0.001) (Table 3).
Table 3.
Factors associated with diabetic nephropathy (N = 32,375)
| Associated factors | Odd ratio (OR) |
95% confidence interval (CI) |
p value |
|---|---|---|---|
| Age | 1.06 | 1.06–1.06 | < 0.001 |
| Male | 1.27 | 1.19–1.35 | < 0.001 |
| Smoking | |||
| Ex-smoker | 1.20 | 1.11–1.29 | < 0.001 |
| Smoker | 1.49 | 1.35–1.64 | < 0.001 |
| Drinking | |||
| Social drinker | 0.89 | 0.82–0.96 | 0.002 |
| Drinker | 0.72 | 0.61–0.85 | < 0.001 |
| Duration of diabetes | 1.03 | 1.02–1.03 | < 0.001 |
| Hypertension | 1.99 | 1.84–2.16 | < 0.001 |
| History of ischemic heart disease | 1.22 | 1.11–1.35 | < 0.001 |
| History of stroke | 1.50 | 1.35–1.66 | < 0.001 |
| BP ≥ 130/80 mmHg | 1.26 | 1.20–1.33 | < 0.001 |
| Body mass index | |||
| Overweight | 1.08 | 1.00–1.17 | 0.043 |
| Obesity | 1.51 | 1.41–1.61 | < 0.001 |
| HbA1c ≥ 7% (53 mmol/mol) | 1.28 | 1.21–1.35 | < 0.001 |
| Non-HDL cholesterol ≥2.5 mmol/L | 1.20 | 1.13–1.27 | < 0.001 |
| Diabetic retinopathy | |||
| Non-proliferative | 1.47 | 1.40–1.55 | < 0.001 |
| Proliferative | 6.52 | 4.53–9.36 | < 0.001 |
| Peripheral neuropathy | 1.48 | 1.36–1.61 | < 0.001 |
| Peripheral vascular disease | 2.19 | 1.12–4.29 | 0.023 |
BP blood pressure; HbA1c glycated hemoglobin A1c, non-HDL non-high density lipoprotein
On the other hand, social drinking [OR 0.89, p = 0.002] and current drinking [OR 0.72, p < 0.001] were found to have significant negative association with diabetic nephropathy (Table 3).
Discussion
The overall prevalence of diabetic nephropathy in type 2 diabetes patients in our study was 31.6%. Albuminuria was present in 22.0% of patients while 16.9% of patients had impaired eGFR.
Similar prevalence of diabetic nephropathy, albuminuria and impaired eGFR were also found in other studies in China (diabetic nephropathy 31.0%, albuminuria 28.9%) [7] and US (diabetic nephropathy 34.5%, albuminuria 23.7%, impaired eGFR 17.7%) [6]. Two primary care studies in Spain and a Mediterranean area also showed comparable prevalence of diabetic nephropathy to our results (27.9% and 34.1%) although their prevalence of albuminuria was a bit lower (15.4% and 19.5%) [33, 34].
The results of the NEFRON study demonstrated a relatively high prevalence of diabetic nephropathy in patients with type 2 diabetes in the setting of Australian primary care. The rates of diabetic nephropathy, albuminuria and impaired eGFR were found to be 47.1%, 34.6% and 23.1% respectively [35]. The higher prevalence might be attributed to a higher mean HbA1c level (mean HbA1c 7.4% (57 mmol/mol) vs 7.0% (53 mmol/mol) in our study population), more patients (80%) were smokers or ex-smokers, different patients’ ethnicity and using MDRD study equation for estimating the GFR in the study.
A significant proportion of patients with type 2 diabetes was found to have diabetic nephropathy in our study. As discussed, both impaired GFR and albuminuria are independently associated with ESRF and all-cause and cardiovascular mortality [9, 10]. Although there was relatively small proportion of patients (1.2%) had severe chronic kidney disease with eGFR <30 ml/min/1.73 m2, as compared to 6.3% and 8.4% in the studies performed in Spain and a Mediterranean area [33, 34], it could still cause a significant increase in the burden to our health care system with the rising prevalence of diabetes. Therefore, early recognition of the condition and control of the modifiable factors are of upmost importance in primary care.
A substantial proportion of patients in our study had hypertension. Our study supported the well-known fact that hypertension and suboptimal BP control are independent risk factors for diabetic nephropathy [25–28]. Suboptimal glycemic control and longer duration of diabetes were also factors associated with diabetic nephropathy [25–28]. Tight blood glucose control targeting at HbA1c less than 7% (53 mmol/mol) is important to prevent the development of microalbuminuria and delay the progression to overt nephropathy [27]. Suboptimal control of non-HDL cholesterol was also associated with diabetic nephropathy as shown by our study and Toth [29]. This supported the need for achieving cholesterol goals as well in order to most optimally reduce the risk of diabetic nephropathy.
In keeping with the results of the study by Ravid et al., male sex, obesity and smoking were all significantly associated with diabetic nephropathy [26]. The other diabetic complications including diabetic retinopathy, peripheral neuropathy, peripheral vascular disease and history of stroke and ischemic heart disease were also demonstrated to have association with diabetic nephropathy in our study. Thus, screening and managing those modifiable factors in patients with type 2 diabetes is important in primary care before the development of microvascular and macrovascular complications.
The ADVANCE trial reported patients with type 2 diabetes who had moderate alcohol drinking had less microvascular complications including nephropathy than non-drinkers [30]. Our study showed a compatible finding with negative association between social or current drinking and diabetic nephropathy. The possible beneficial association might be explained by an anti-atherosclerotic effect with moderate alcohol consumption through increased HDL cholesterol and improved insulin sensitivity [36].
Limitations
There were several limitations in this study. Firstly, the cross-sectional design of our study limited the establishment of causal relationship between diabetic nephropathy and the associated risk factors. To determine their causal relationship, future longitudinal studies are needed. Secondly, only a single reading of urine ACR and estimation of GFR were used to determine the presence of albuminuria and diabetic nephropathy. Thus, we could not differentiate individuals with persistent elevation of urine ACR or impairment of eGFR and those with transient abnormality caused by some reversible conditions such as urinary tract infection, febrile illness, nephrolithiasis or drugs. Ideally, repeating the measurements at 3 months to confirm the diagnosis would be recommended. Thirdly, we did not take into consideration the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) and their effects when evaluating the associated factors for diabetic nephropathy. As recommended by international guidelines, they are the first line anti-hypertensive medications for patients with diabetes and we expected that most of our patients were taking ACEI or ARB. Therefore, the prevalence of diabetic nephropathy might be underestimated with the microalbuminuria detected in some patients reversed by treatment with ACEI or ARB. Finally, a small number of patients who were managed in our primary care clinics for diabetes but did not undergo the diabetic complication assessment program during the study period were not included in our study. Patients with missing creatinine or urinary ACR measurement were also excluded. These patients might have poorer treatment compliance leading to less optimal control of blood pressure, diabetes and hyperlipidemia. As a result, there could be underestimation of the prevalence of diabetic nephropathy in our study. On the other hand, overestimation of the prevalence might be due to impaired eGFR or albuminuria caused by other diseases including congenital kidney diseases, obstructive nephropathy or glomerulonephritis.
Conclusion
Our study demonstrated that diabetic nephropathy was a highly prevalent complication among Chinese patients with type 2 diabetes in primary care in Hong Kong. Age, male sex, longer duration of diabetes, obesity, smoking, hypertension, suboptimal control of blood pressure, HbA1c and non-HDL cholesterol, presence of diabetic complications including diabetic retinopathy, peripheral neuropathy and peripheral vascular disease and history of stroke and ischemic heart disease were all shown to have significant association with diabetic nephropathy. Strategies to recognize diabetic nephropathy and the associated risk factors and control the modifiable factors at early stages are warranted to prevent or delay the progression of diabetic nephropathy.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global status report on noncommunicable diseases 2014 [article online]. 2014. Available from: http://www.who.int/nmh/publications/ncd-status-report-2014/en/. Accessed 13 Mar 2016.
- 2.Whiting D, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Wong K, Wang Z. Prevalence of type 2 diabetes mellitus of Chinese populations in mainland China, Hong Kong, and Taiwan. Diabetes Res Clin Pract. 2006;73(2):126–134. doi: 10.1016/j.diabres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Saunders WB. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.de Boer I, Rue T, Hall Y, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou Q-L, Ouyang X-J, Liu-Bao G, et al. Chronic kidney disease and associated cardiovascular risk factors in Chinese with type 2 diabetes. Diabetes Metab J. 2012;36(6):433–442. doi: 10.4093/dmj.2012.36.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho Y, Chau K, Choy B, et al. Hong Kong renal registry report 2012. Hong Kong J Nephrol. 2013;15(1):28–43. doi: 10.1016/j.hkjn.2013.03.005. [DOI] [Google Scholar]
- 9.Matsushita K, van der Velde M, Astor B, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gansevoort R, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Chronic Kidney Disease Prognosis Consortium Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goede P, Lund-Andersen H, Parving H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 12.Zoungas S, de Galan B, Ninomiya T, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med. 2008;Suppl 2:25–29. doi: 10.1111/j.1464-5491.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson C, Levey A, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part I. definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70(5):869–985. [PubMed] [Google Scholar]
- 15.Levey A, Bosch J, Lewis J, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Stevens L, Coresh J, Feldman H, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. Frequently asked questions about GFR estimates [article online]. 2014. Available from: https://www.kidney.org/sites/default/files/12-10-4004_FAQ-ABE.pdf. Accessed 23 Nov 2018.
- 18.Levey A, Stevens L, Schmid C, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inker L, Shaffi K, Levey A. Estimating glomerular filtration rate using the chronic kidney disease-epidemiology collaboration creatinine equation: better risk predictions. Circ Heart Fail. 2012;5(3):303–306. doi: 10.1161/CIRCHEARTFAILURE.112.968545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilbride H, Stevens P, Eaglestone G, et al. Accuracy of the MDRD (modification of diet in renal disease) study and CKD-EPI (CKD epidemiology collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61(1):57–66. doi: 10.1053/j.ajkd.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Liao Y, Liao W, Liu J, Xu G, Zeng R. Assessment of the CKD-EPI equation to estimate glomerular filtration rate in adults from a Chinese CKD population. J Int Med Res. 2011;39(6):2273–2280. doi: 10.1177/147323001103900624. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita K, Selvin E, Bash L, et al. Risk implications of the new CKD epidemiology collaboration (CKD-EPI) equation compared with the MDRD study equation for estimated GFR: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2010;55(4):648–659. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita K, Mahmoodi B, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management: clinical guideline 182 [article online]. 2014 [updated Jan 2015]. Available from: https://www.nice.org.uk/guidance/cg182. Accessed 15 Mar 2016. [PubMed]
- 25.Retnakaran R, Cull C, Thorne K, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 26.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158(9):998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 27.Gross J, de Azevedo M, Silveiro S, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Unnikrishnan R, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, Mohan V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai urban rural epidemiology study (CURES 45) Diabetes Care. 2007;30(8):2019–2024. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 29.Toth PP, Simko, et al. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012;11(1):109. doi: 10.1186/1475-2840-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomster JI, Zoungas S, Chalmers J, Li Q, Chow CK, Woodward M, Mancia G, Poulter N, Williams B, Harrap S, Neal B, Patel A, Hillis GS. The relationship between alcohol consumption and vascular complications and mortality in individuals with type 2 diabetes. Diabetes Care. 2014;37(5):1353–1359. doi: 10.2337/dc13-2727. [DOI] [PubMed] [Google Scholar]
- 31.UK Renal Association. Clinical Practice Guidelines for the Detection, Monitoring and Care of Patients with Chronic Kidney Disease. 5th Edition [article online]. 2009–2011. Available from: https://renal.org/detection-monitoring-care-of-patients-with-ckd-5th-edition-2/. Accessed 15 Mar 2016.
- 32.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Poncelas A, Garre-Olmo J, Franch-Nadal J, et al. Prevalence of chronic kidney disease in patients with type 2 diabetes in Spain: PERCEDIME2 study. BMC Nephrol. 2013;14:46. doi: 10.1186/1471-2369-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coll-de-Tuero G, Mata-Cases M, Rodriguez-Poncelas A, Pepió JMA, Roura P, Benito B, Franch-Nadal J, Saez M. Chronic kidney disease in the type 2 diabetic patients: prevalence and associated variables in a random sample of 2642 patients of a Mediterranean area. BMC Nehrol. 2012;13:87. doi: 10.1186/1471-2369-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas MC, Weekes AJ, Broadley OJ, et al. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Keefe J, Bybee K, Lavie C. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]