Abstract
Objectives
The present study aimed to investigate the anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency.
Design
This study was a randomized, placebo-controlled, double-blinded clinical trial.
Trial registration code: IRCT20190204042621N1
Participants
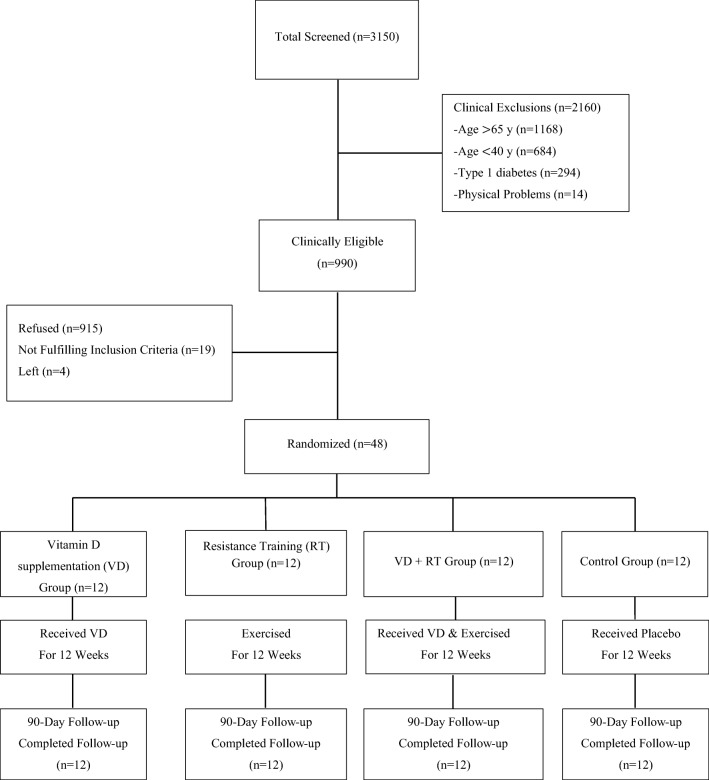
Forty-eight patients with type 2 diabetes aged 40–65 (from a total of 52 volunteers in Ardabil diabetes clinic) were randomly assigned to either the vitamin D supplementation with resistance training group (VD + RT: n = 12), the resistance training group (RT: n = 12), the vitamin D supplementation group (VD: n = 12), or the control group (CON: n = 12).
Intervention
The subjects in VD group took vitamin D supplements at 50000 IU per 2 weeks for 3 months; the subjects in RT group exercised 3 times per week for 12 weeks; and the subjects in VD + RT group participated in both treatments. Subjects in CON group were asked to maintain normal daily life pattern for the duration of the study.
Measurements
Serum Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α) and C-reactive protein (CRP) levels were determined at pre and post-test and the data were compared among the four groups and between two tests (4 × 2) using two-way ANOVA with repeated measures.
Results
IL-6 decreased significantly (P = 0.001) in all groups (VD + RT = % -71.73, RT = % -65.85, VD = % -61.70). TNF-α decreased significantly (P = 0.001) in VD + RT (% -44.90) and RT (% -40) groups. CRP showed no significant change in any group (P > 0.05).
Conclusion
Results demonstrated that vitamin D supplementation in addition to resistance training had positive effects on some inflammatory markers in T2D and vitamin D deficient men. Vitamin D supplementation was especially effective when it was complemented with exercise training.
Keywords: Inflammation, Resistance training, Type 2 diabetes mellitus, Vitamin D
Introduction
Type 2 diabetes mellitus (T2DM) as a metabolic disease may affect nearly every organ system in the body [1]. It is reaching epidemic proportions globally which remains a significant public health care concern and its related economic effect is seriously burdensome [2]. The World Health Organization estimated that T2DM was responsible for 1.6 million deaths worldwide each year, a figure making diabetes one of the world’s most common causes of preventable mortality [3]. Although genetic factors are important, it is necessary to take into account that diabetes is a multifactorial and heterogeneous disease [4].
Impaired glucose tolerance (IGT) leads to T2DM as a result of insulin resistance and β-cell malfunction, with following insulin deficiency which affects liver, adipose tissues and skeletal muscle [5]. Additionally, systemic inflammation is believed to play a significant role in the pathogenesis of T2DM and the development of insulin resistance [6]. Inflammatory cytokines downregulate insulin signaling cascades in tissues that are sensitive to insulin. This leads to the disruption of insulin sensitivity and the impairment of glucose homeostasis [7]. The association of elevated cytokines with T2DM has extensively been investigated but with conflicting results [8]. Recently, vitamin D has attracted the investigators, attention as an important factor that can improve insulin resistance [9], diabetes [1], inflammatory response and glycemic control [9, 10]. While, some studies indicated that vitamin D would not affect inflammatory and glycemic markers in diabetics [11].
On the other hand, several studies related to diabetes have demonstrated that regular exercise improves glycemic control [12], regulates blood glucose, increases the physical strength in diabetics and prevents the progression from impaired glucose tolerance to T2DM [13]. For instance, resistance training specifically develops the total capacity of glucose uptake by enhancing muscle mass [14] and also improves systemic inflammation [15]. However, some studies have not reported the effects of exercise training on glucose metabolism [16] or systemic inflammation [17].
Studies verifying the effectiveness of the intake of vitamin D supplementation and resistance training as a complex treatment on inflammatory and glycemic markers in adult males with both diabetes and vitamin D deficiency remain insufficient. Thus, this study aimed to investigate the effects of the implementation of vitamin D intake for 12 weeks in addition to progressive resistance training on inflammatory markers in men with T2DM and vitamin D deficiency.
Materials and methods
Participants
Forty-eight inactive patients with type II diabetes aged 40–65 (from a total of 52 volunteers in Ardabil diabetes clinic-Ardabil-Iran) exhibiting symptoms of diabetes with fasting blood glucose higher than 126 mg/dl [18], glycated hemoglobin (HbA1c) <9% and vitamin D deficiency symptoms with 25-hydroxyvitamin D [25(OH)D] less than 20 ng/ml [19] were randomly assigned to either the vitamin D supplementation with resistance training group (VD + RT: n = 12), the resistance training group (RT: n = 12), the vitamin D supplementation group (VD: n = 12), or the control group (CON: n = 12). The sample size was calculated using the formula described by Whitley and Ball [20]. The purpose, procedures and risks of the study were explained to the subjects through an orientation session before conducting the pretest. Then, measures were taken to begin the study after receiving test consent forms from the subjects who wanted to voluntarily participate. In this study, inclusion criteria were: absence of hepatic, renal, bone and cardiovascular diseases, severe hypertension, diabetes type 1 and any history of using drugs such as insulin, anticonvulsants, calcium and vitamin D within 6 months prior to the study and HbA1c was <9%. All plans for this study were approved by ethics committee at the medical sciences faculty of Ardabil Azad University prior to the beginning of the study.
Study design
This study was conducted using a randomized, placebo controlled, double-blinded approach. According to the number of subjects and groups, a simple computer-generated program randomization list was used to put the subjects into 4 equal groups. The researchers, providers and patients were blinded to the method and process of randomization.
Trial registration code: IRCT20190204042621N1
Measurements
Body weight and height were measured while the subjects were in a standing position using scale (seca 220, Germany) and unstretched measuring tape. Body mass index (BMI) was calculated using the formula: weight (kg) divided by the square of the height (m) [weight (kg) / height 2 (meter)]. Percent body fat was estimated using Skinfold Caliper (Harpenden Model, Baty Company, England) and Jackson-Pollok 3-point (chest, abdominal and thigh) nomogram method [21] (Tables 1 and 3).
Table 1.
Changes in anthropometric characteristics in T2D and vitamin D deficient middle-aged men during 12 weeks interventions
| Group variables | VD + RT | RT | VD | CON |
|---|---|---|---|---|
| Height (cm) | 173.50 ± 6.33 | 169.16 ± 4.59 | 168.41 ± 3.75 | 169.83 ± 2.58 |
| Age (yr) | 53.75 ± 8.00 | 54.91 ± 5.86 | 53.83 ± 6.61 | 53.16 ± 8.12 |
VD+RT Vitamin D intake + Resistance Training Group, RT Resistance Training Group, VD Vitamin D intake Group, CON Control Group
Data are presented as Mean ± SD
Table 3.
Changes in blood lipids, insulin resistance indices, inflammatory markers in T2D and vitamin D deficient middle-aged men during 12 weeks interventions
| Variables | Groups | Pre M ± SD | Post M ± SD | Δ% (Pre & Post) | P | Sig. |
|---|---|---|---|---|---|---|
| Weight (kg) | VD + RT | 87.52 ± 17.65 | *85.92 ± 17.53 | −1.82 |
Test Group Test × Group |
***0.001 0.37 ***0.001 |
| RT | 78.68 ± 14.02 | 77.75 ± 13.30 | −1.18 | |||
| VD | 79.35 ± 12.03 | 79.11 ± 12.04 | −0.37 | |||
| CON | 79.76 ± 6.32 | 79.92 ± 6.33 | 0.33 | |||
| BMI (kg/m−2) | VD + RT | 29.00 ± 4.65 | *28.30 ± 4.67 | −2.86 |
Test Group Test × Group |
***0.001 0.86 ***0.001 |
| RT | 27.94 ± 4.43 | *27.35 ± 4.23 | −2.56 | |||
| VD | 27.70 ± 3.55 | 27.54 ± 3.46 | −0.6 | |||
| CON | 27.79 ± 1.85 | 27.90 ± 1.45 | −1.17 | |||
| HbA1c (%) | VD + RT | 7.49 ± 1.27 | *6.55 ± 1.08 | −12.54 |
Test Group Test × Group |
***0.001 0.08 *0.018 |
| RT | 7.58 ± 1.26 | 6.99 ± 1.11 | −7.45 | |||
| VD | 8.17 ± 0.89 | *7.20 ± 0.86 | −11.82 | |||
| CON | 8.03 ± 1.01 | 8.51 ± 1.01 | 6.6 | |||
| 25(OH)D (ng/ml) | VD + RT | 14.60 ± 4.10 | 19.20 ± 6.30 | 31.50 |
Test Group Test × Group |
***0.001 0.20 ***0.001 |
| RT | 14.10 ± 3.70 | 13.40 ± 3.60 | −5 | |||
| VD | 12.50 ± 4.80 | *20.40 ± 6.50 | 63.2 | |||
| CON | 17.80 ± 5.70 | 17.70 ± 5.60 | −5.61 | |||
| BFP (%) | VD + RT | 26.20 ± 5.97 | 24.16.5.23 | −7.54 |
Test Group Test × Group |
**0.007 0.76 0.08 |
| RT | 24.37 ± 6.70 | 23.41 ± 6.14 | −3.67 | |||
| VD | 24.20 ± 3.68 | 23.91 ± 3.77 | 1.34 | |||
| CON | 25.62 ± 2.54 | 26.58 ± 2.15 | 1.87 | |||
| LDL (mg/dl) | VD + RT | 82.41 ± 23.89 | 71.58 ± 33.04 | −13.43 |
Test Group Test × Group |
**0.01 0.90 0.06 |
| RT | 92.58 ± 33.76 | *69.75 ± 24.33 | −24.60 | |||
| VD | 84.08 ± 22.14 | 68.75 ± 37.06 | −18.28 | |||
| CON | 83.08 ± 29.31 | 83.96 ± 28.55 | 1.19 | |||
| HDL (mg/dl) | VD + RT | 32.50 ± 4.98 | 34.66 ± 4.75 | 6.60 |
Test Group Test × Group |
*0.02 0.48 0.55 |
| RT | 34.33 ± 2.67 | 37.33 ± 6.56 | 8.45 | |||
| VD | 35.83 ± 5.33 | 37.58 ± 8.62 | 4.88 | |||
| CON | 34.83 ± 5.52 | 34.17 ± 5.50 | 1.5 | |||
| TG (mg/dl) | VD + RT | 212.66 ± 58.10 | 185.41 ± 39.12 | −12.74 |
Test Group Test × Group |
***0.001 0.71 *0.02 |
| RT | 228.75 ± 71.39 | *170.25 ± 63.67 | −25.86 | |||
| VD | 231.75 ± 81.81 | 184.66 ± 51.14 | −20.99 | |||
| CON | 189.50 ± 70.56 | 180.50 ± 70.55 | −3.32 | |||
| CL (mg/dl) | VD + RT | 158.33 ± 26.90 | *141.75 ± 30.58 | −10.50 |
Test Group Test × Group |
***0.001 0.87 **0.007 |
| RT | 176.00 ± 36.33 | *143.50 ± 22.27 | −18.41 | |||
| VD | 164.16 ± 33.87 | 14.66 ± 37.99 | −14.48 | |||
| CON | 153.83 ± 30.15 | 163.83 ± 30.16 | 7.72 | |||
| FBG (mg/dl) | VD + RT | 179.16 ± 42.81 | *127.58 ± 18.63 | −28.78 |
Test Group Test × Group |
***0.001 0.08 **0.01 |
| RT | 144.91 ± 51.01 | 129.16 ± 45.65 | −10.86 | |||
| VD | 181.00 ± 44.20 | 155.50 ± 27.82 | −14.08 | |||
| CON | 184.08 ± 64.80 | 170.08 ± 60.23 | −7.60 | |||
| FI ((μIU/ml | VD + RT | 3.40 ± 1.10 | *2.50 ± 1.0 | −26.47 |
Test Group Test × Group |
***0.001 0.10 *0.03 |
| RT | 3.30 ± 1.20 | 2.90 ± 1.30 | −12.12 | |||
| VD | 4.50 ± 2.10 | *3.50 ± 1.30 | −22.22 | |||
| CON | 4.60 ± 2.70 | 4.70 ± 2.80 | 2.17 | |||
| HOMA_IR | VD + RT | 1.50 ± 1.17 | *0.78 ± 0.61 | −48 |
Test Group Test × Group |
***0.001 *0.03 **0.004 |
| RT | 1.18 ± 1.54 | 0.92 ± 0.83 | −22.03 | |||
| VD | 2.01 ± 1.32 | *1.34 ± 1.18 | −35.05 | |||
| CON | 2.09 ± 1.89 | 1.97 ± 1.36 | −1.98 | |||
| IL-6 (pg/ml) | VD + RT | 46.09 ± 12.14 | *13.88 ± 3.25 | −71.73 |
Test Group Test × Group |
***0.001 ***0.001 ***0.001 |
| RT | 41.80 ± 6.21 | *14.65 ± 4.15 | −65.85 | |||
| VD | 47.30 ± 9.93 | 18.83 ± 4.15 | −61/70 | |||
| CON | 50.12 ± 10.94 | 51.10 ± 10.11 | 3 | |||
| TNF- α (pg/ml) | VD + RT | 20.45 ± 5.47 | *11.94 ± 2.91 | −44/90 |
Test Group Test × Group |
***0.001 0.73 ***0.001 |
| RT | 20.47 ± 4.92 | *12.67 ± 2.58 | −40 | |||
| VD | 18.73 ± 3.65 | 15.83 ± 1.16 | −16/66 | |||
| CON | 17.27 ± 1.26 | 17.00 ± 1.19 | −5.55 | |||
| CRP (mg/l) | VD + RT | 3.53 ± 1.08 | 3.44 ± 0.97 | −2.5 |
Test Group Test × Group |
**0.01 0.10 0.52 |
| RT | 3.68 ± 0.64 | 3.43 ± 0.54 | −6.70 | |||
| VD | 3.33 ± 0.73 | 3.22 ± 0.75 | −3.33 | |||
| CON | 4.10 ± 0.65 | 3.98 ± 0.68 | −2.90 |
BMI Body Mass Index, HbA1c Hemoglobin A1c, 25(OH)D 25-hydroxyvitamin D, BFP Body Fat Percent, LDL Low-density Lipoprotein, HDL High-density Lipoprotein, TG Triglyceride, CL Cholesterol, FBG Fasting Blood Glucose, FI Fasting Insulin, HOMA_IR Homeostatic Model Assessment for Insulin Resistance, IL-6 Interleukin 6, TNF-α Tumor Necrosis Factor Alpha, CRP C-Reactive Protein. VD+RT Vitamin D intake + Resistance Training Group, RT Resistance Training Group, VD Vitamin D intake Group, CON Control Group
*: P < 0.05; **: P < 0.01; ***: P < 0.001
Intervention
The program of exercise training conducted in the present study consisted of 10 kinds of exercise methods: chest press, leg extension, leg curl, arm curl, push-up with knees against the floor, seated row, overhead pull-down, overhead press, weighted sit-up and toe raise [22]. The training session consisted of three sets of 10- repetition maximum exercises, with a 90 s. rest between the sets and 30 s. rest between exercises. The exercise frequency was 3 times a week for 12 weeks. Each session began with a warm-up that included walking and light stretching for 10 min and ended with a cool-down that included stretching for 10 min. The main exercise lasted 50 min. The training intensity was 55% of one repetition maximum (1RM) in the first month, 65% of 1RM in the second month and 75% of 1RM in the third month. The workloads were adjusted to the condition of diabetic patients. So, maximum strength (1RM) of subjects was calculated again for each exercise at weeks 4 and 8. 1RM was calculated using the formula [23] as follows:
The resistance training program applied in this study is the same as shown in Table 2.
Table 2.
Resistance training program for T2D and vitamin D deficient middle-aged men during 12 weeks interventions
| Stage | Mode/set /rest | Duration | Weekly exercise frequency |
|---|---|---|---|
| Warm-up | Walking, Running, Stretching | 10 min | 3 times |
| Main exercise |
3 × 10 Rest between sets = 90 s. Rest between exercises = 30 s. |
3 times | |
| Cool-down | Stretching | 10 min | 3 times |
Intensity: 55% of 1RM, first month; 65% of 1RM, second month; 75% of 1RM, third month
Vitamin D was distributed using the double blind method to accurately examine the effect of vitamin D supplementation. The subjects of: 1) the vitamin D and resistance training group and 2) the vitamin D group took oral capsules containing 50,000 IU vitamin D (Zahravi Pharm Co,Iran) every 2 weeks [24] for 3 months of treatment. On the other hand, capsules made of paraffin oil (Zahravi Pharm Co,Iran) were provided to 3) the resistance training group, and 4) the placebo group (control group). The capsules designed as placebos were not externally distinguishable from the vitamin D capsules (Fig. 1).
Fig. 1.
Flowchart of study design and timeline
Biochemical analysis
To standardize dietary intake prior to blood collection, all participants were asked to consume the same foods and drinks during the 24 h prior to each testing day (baseline and 12 weeks). All subjects arrived at 8:00 am on the day of the test in the laboratory after a 12- h fasting state. After keeping the subjects in a stable state for 15 min, 10 ml of venous blood was obtained from the antecubital vein using an anticoagulant treated syringe. The blood samples were placed in tubes that were not treated for anticoagulation, and were then centrifuged at 3000 rpm using a centrifugal separator for 10 min. After separating from the cellular components, the serum was put in a storage tube and stored in the refrigerator at −80 °C until analysis.
The IL-6 and TNF-a concentrations were measured from the serum sample using commercially available high-sensitivity ELISA kits (Diaclone, French). The CRP concentration was measured from the serum sample using commercially available quantitative kits (Roche Diagnostics Company, Swiss). Serum 25-hydroxy Vitamin D was assessed using commercially ELISA kit (Bioactiva Diagnostica, Germany). HbA1c was measured by chromatographic method using commercial kit (Biosystem, Spain). Fasting insulin concentration was measured using commercially ELISA kit (Monobind Inc., USA). Fasting plasma glucose was analyzed by enzymatic method using commercially available kit (glucoseoxidase, Pars Azmun, Iran). The triglyceride (TG), total cholesterol (TC), HDL-C, and LDL-C concentrations were measured from the serum sample using commercially available kits (Pars Azmun Kit, Iran) through a spectrophotometric method. In addition, HOMA-IR (homeostasis model for insulin resistance) was calculated using the following equation in order to evaluate insulin resistance [25]:
Statistical analysis
Descriptive data were presented as the mean values and standard deviation (SD). The Kolmogorov-Smirnov test was used to examine the normal distribution of variables. The homogeneity of variances was assessed using Leven’s test. In order to simultaneously analyze the average difference of the dependent variables between the four groups and between two tests, repeated two-way ANOVA was used. Tukey post hoc test was applied to determine the difference between groups. The results obtained in this study were analyzed using SPSS (version 21) for Windows. The significant level (α) of all statistical analysis was 0.05.
Results
Anthropometric changes
Percent body fat showed no significant changes in all groups (P = 0.76). The main effect of the test as well as the interaction between the group and the test on weight and BMI were significant (P = 0.001). Body weight and BMI improved in RT + VD and RT groups significantly (Table 3).
HbA1c and 25(OH) D changes
Although, significant differences between groups in HbA1c and 25(OH) D were not observed but the main effect of the test and the interaction between the group and the test in the HbA1c and 25(OH) D analyses were significant (P = 0.001). HbA1c decreased significantly in RT + VD and VD groups and 25(OH) D increased significantly in VD group (P < 0.005) (Table 3).
Changes in blood lipids
The main effect of the test and the interaction between the group and the test in the TG (P = 0.001), LDL (P = 0.01) and TC (P = 0.001) analyses were significant. TG and LDL in RT group as well as TC in RT + VD and RT groups significantly decreased (P < 0.05). Significant changes in HDL were not observed in any group (P > 0.05) (Table 3).
Changes in insulin resistance index
Compared to CON group, HOMA-IR decreased significantly in VD + RT and VD groups (P < 0.01) but the main effect of the test (P = 0.001), group (P = 0.03) and the interaction between the group and the test (P = 0.004) in the HOMA-IR analyses were statistically significant. In the fasting insulin and the fasting blood glucose analyses, the main effect of the test (P = 0.01) and the interaction between the group and the test (FI with P = 0.03 and FBG with P = 0.01) were statistically significant. Fasting insulin in RT + VD and RT groups as well as fasting blood glucose in RT + VD group, significantly decreased (P < 0.01) with tending toward improvement in other groups (Table 3).
Changes in inflammatory markers
IL-6 (P = 0.001) significantly changed in all groups (VD + RT with % -71.73, RT with % -65.85, VD with % -61.70). The main effect of the test (P = 0.001), group (P = 0.001) and the interaction between the group and the test (P = 0.001) in the IL-6 analyses were statistically significant. TNF-α (P = 0.001) significantly decreased in VD + RT (% -44.90) and RT (% -40) groups, whereas there were no significant changes in the variables in VD and CON groups. The main effect of the test (P = 0.001) and the interaction between the group and the test (P = 0.001) in the TNF-α analyses were statistically significant. Finally, CRP showed no significant changes in all groups (P > 0.05). In the CRP analyses, the main effect of the test (P = 0.01) was statistically significant, whereas the group (P = 0.10) and the interaction between the group and the test (P = 0.52) were not statistically significant (Table 3).
Discussion
According to our knowledge, the effectiveness of the intake of vitamin D supplementation and resistance training as a complex treatment on inflammatory and glycemic markers in patients with both T2DM and vitamin D deficiency have not been investigated in previous studies. Therefore, this is the first study that aimed to investigate the anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency. Furthermore, this is the first study in which male subjects with type 2 diabetes took oral capsules containing 50,000 IU vitamin D every 2 weeks for 3 months. Previously, just Yazdchi R, et al. (2016) had used this method for female subjects with gesture diabetes mellitus (GDM) in their study for 2 months.
Glycated hemoglobin (HbA1c) is considered as an indicator of average blood glucose concentrations during the preceding 2–3 months and thus a long term marker of glucose homeostasis [26]. It has been mentioned that vitamin D supplementation has beneficial effect in reducing the progression from pre-diabetes to diabetes in high-risk groups and improves glycemic profile (HbA1c) in people with established diabetes [27]. In this regard, Yazdchi, et al. have reported that fasting blood glucose (FBG) and HbA1c in 76 women with GDM taking 50,000 IU of vitamin D (n = 38) or placebo (n = 38) every 2 weeks for 2 months, significantly decreased [24]. In the present study, Vitamin D levels were found to be negatively correlated with HbA1c levels that significantly improved in VD + RT and VD groups. This was in agreement with the studies in which HbA1c significantly decreased [28].
On the other hand, it has been demonstrated that regular physical activity is the most effective method for improving HbA1c and insulin resistance [22]. Consistent with that, Dixit, et al. have mentioned that 87 elderly patients with T2DM (control group = 47, study group = 40) participating in 8 weeks moderate-intensity (heart rate reserve 40–60%) exercise had a significant difference in the mean values of HbA1c at baseline and 8th week between two groups [12]. It seems that increased glucose uptake as a result of physical activity is the main factor to improve fasting glucose indices and HbA1c in the patients with T2DM. This is achieved through increased muscular blood flow, GLUT4 and glucose uptake capacity [15]. Based on the glycemic index results obtained in this study, it was mentioned that the results were not statistically significant. However, the group that combined vitamin D intake and resistance training showed the most positive changes. Therefore, it was determined that combining the two kinds of treatments rather than a single treatment involving only vitamin D intake or exercise training is more effective.
As previously mentioned, both vitamin D [4] and physical activity [29] are believed to affect systemic inflammation in diabetic people. It has been reported that increased serum 25(OH) D levels in diabetics improves inflammatory markers and glycemic control [28]. Consistent with that, Calton et al. [30] by conducting a meta-analysis of 23 research results which involved the impact of vitamin D levels on inflammatory status showed that vitamin D intake is effective in improving systemic inflammation. Briefly, taking higher dose of vitamin D may be required to affect diabetes risk and control high levels of inflammatory factors in patients with T2D and vitamin D deficiency [1]. Consistent with that, Miller, et al. [29] reported that in diabetic and overweight elderly patients participating in resistance training, 3 times a week for 12 weeks, some inflammatory markers significantly improved.
According to the results obtained in this study, significant changes were reported in IL-6 and to some extent in TNF-α, but no significant change was observed in CRP. This was in agreement with the studies in which, as a result of taking vitamin D and performing physical activity, some inflammatory markers significantly changed [15]. In the present study, the most significant reduction occurred in the group with vitamin D intake combined with resistance training. Therefore, it can be concluded that in situations where vitamin D intake is combined with exercise training, the improvements in some inflammatory markers are greater than situations with one single treatment. Similarly, Carillo et al. investigated the effects of vitamin D supplementation on inflammatory factors during resistance training in healthy overweight and obese people. In contrast to the present study, they concluded that vitamin D supplementation during resistance training has no influence on inflammatory biomarkers [17]. The conflicting results mentioned in these two studies may be as a result of different doses of vitamin D intake or subjects who participated in the studies.
Vitamin D may reduce inflammation through several mechanisms. Vitamin D has been shown to down-regulate the generation and action of cytokines, leading to increase in insulin sensitivity and β-cell survival [1]. It may also decrease in insulin resistance. Additionally, vitamin D has been demonstrated to up-regulate the expression of calbindin, a cytosolic calcium-binding protein which can protect against cytokine-induced apoptosis, in many tissues including β-cells and also down-regulate the activation of nuclear factor-κB (NF-κB) which is a major regulator of the genes related to pro-inflammatory cytokines, insulin resistance and stress responses [1]. It has been recognized that Vitamin D Receptor (VDR), the receptor of the steroid hormone 1,25(OH)2D3, is widely distributed in more than 38 tissues, where it clearly controls vital genes related to bone metabolism, oxidative damage, chronic diseases and inflammation [31]. VDR is constitutively expressed by macrophages and dendritic cells, which suggests that vitamin D plays an important role in the modulation of inflammatory response. The 1, 25(OH) 2D3 can be synthesized by both cell types, since they express the enzymes 25-hydroxylase and 1α-hydroxylase, which enables the production of 25OHD and 1, 25(OH) 2D3, respectively [32]. In macrophages, 1, 25(OH) 2D3 up-regulates the inhibitor protein of NF-κB (IκB-α) by increasing mRNA stability and decreasing IkB-α phosphorylation. The increase in IκB-α levels leads to a reduction in nuclear translocation of NF-κB, thereby causing a decline in activity and TNF-α production [33].
Additionally, with binding to VD receptors in monocytes, vitamin D can reduce pro-inflammatory cytokines such as TNF-α and IL-6, which have frequently been identified as key components in the development of insulin resistance and diabetes incidence [9]. The decrease in insulin sensitivity has been attributed to serine phosphorylation of insulin receptor substrate (IRS-1) and Akt substrate of 160 kDa protein (AS160) through the activation of the serine kinases C-Jun NH-terminal kinase (JNK) and adenosine monophosphate activated protein kinase (AMPK) [34].
Some studies showed a positive relationship between serum CRP and the incidence of T2DM [6]. A main mechanism by which CRP plays a crucial role in T2DM is primarily its effect on β-cells. CRP increases the rates of apoptotic cell death by preventing cell proliferation due to the CRP-mediated modulation of protein kinase B (PKB), a key factor for cell survival pathways and its ability to induce the production of TNF-α and IL-6 in a concentration-dependent situation [35].
The results related to the effects of physical activity on systemic inflammation are conflicting. Some studies have suggested that exercise reduces inflammatory cytokines. However, others showed no association between them [36]. To justify these conflicting results, some points are important to be considered. It has been reported that exercise training and supplementation intake can effectively impact inflammatory markers where baseline levels of these markers are high [37], meaning that when the values of inflammatory indices are lower at baseline, exercise training or supplementation intake cannot probably impact inflammatory markers effectively. Consequently, low baseline level of CRP in this study might be a factor for insignificant change in CRP.
On the other hand, it has been suggested that anti-inflammatory effects of exercise training may be due to the intensity and duration of exercise [38]. Moreover, age and sex differences are believed to affect the results of inflammatory markers [39]. Other factors such as differences in the times of blood sampling (interval between last training session and blood sampling), the methods of cytokines measures (ELISA or Flow Cytometric), the fitness level of subjects, the protocol and type of training can also be effective in the changes of inflammatory markers [40, 41]. In general, based on the results obtained in this study, it was demonstrated that the group which combined vitamin D intake and resistance training had the most positive changes, establishing that combining the two kinds of treatments rather than a single treatment involving only vitamin D intake or exercise training is more effective.
This study had some limitations including the small sample size. Furthermore, the sunlight exposure of each individual varies on a daily basis. Duration of the study was short. The strengths of the present study were its prospective, double-blind, controlled and randomized design with a placebo group, inclusion of only vitamin D deficient type II diabetes with insulin resistance. Future studies examining the benefits of vitamin D correction in type II diabetes are needed to confirm the true metabolic effects of vitamin D supplementation.
Conclusion
According to the results of the present study, it was concluded that the intake of vitamin D supplementation and progressive resistance training for 12 weeks, would have positive effects on the levels of inflammatory markers in men with T2DM and vitamin D deficiency. Particularly, the most important result obtained in this study was that the greater improvements in IL-6, TNF-α, HOMA-IR, HbA1c occurred in situations where vitamin D intake combined with resistance training than situations with one single treatment.
Acknowledgements
We would like to express gratitude to Dr. Mohammad Zaefizadeh, Dr. Ahad Jafari, Dr. Nasser Shokri, Ardabil Islamic Azad University for their excellent helps in conducting the present study and Sanandaj Islamic Azad University for Funding the study. This study was performed with the approval of the Faculty of Medical Sciences of Ardabil Azad University.
Funding/support
Sanandaj Branch, Islamic Azad University.
Author contributions
All authors equally contributed to the writing and revision of this paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seshadrj KG, Tamilselvan B, Rajendran A. Role of vitamin D in diabetes. J Clin Endocrinol Metab. 2011;12:47–56. [Google Scholar]
- 2.Marmett B, Nunes RB. Resistance and aerobic training in the treatment of type 2 diabetes mellitus. J Diabetes Metab Disord Control. 2017;4:00126. [Google Scholar]
- 3.World Health Organization (WHO).Global report on diabetes. Geneva, Switzerland 2018
- 4.Guadarrama-López Ana L., Valdés-Ramos Roxana, Martínez-Carrillo Beatríz E. Type 2 Diabetes, PUFAs, and Vitamin D: Their Relation to Inflammation. Journal of Immunology Research. 2014;2014:1–13. doi: 10.1155/2014/860703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumvoll M, Goldstein B, van Haeften T. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1134. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 6.Badawi A, Klip A, Haddad P, Cole DEC, Bailo BG, El Sohemy A, et al. Type 2 diabetes mellitus and inflammation: prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;26:173–186. doi: 10.2147/DMSO.S9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-a in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeggini E, Groves CJ, Parkinson JR, et al. Large-scale studies of the association between variation at the TNF/LTA locus and susceptibility to type 2 diabetes. Diabetologia. 2005;48:2013–2017. doi: 10.1007/s00125-005-1902-4. [DOI] [PubMed] [Google Scholar]
- 9.Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elnasr SM, Ibrahim IM, Alkady MM. Role of vitamin D on glycemic control and oxidative stress in type 2 diabetes mellitus. J Res Med Sci. 2017;22:22. doi: 10.4103/1735-1995.200278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk A, Fitz Patrick T, Roella LC. Effect of vitamin D3 supplementation on inflammatory markers and glycemic measures among overweight or obese adults: a systematic review of randomized controlled trials. PLoS One. 2016;11(4):e0154215. doi: 10.1371/journal.pone.0154215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixit S, Maiya A, Shastry BA. Effect of moderate-intensity aerobic exercise on glycosylated haemoglobin among elderly patients with type 2 diabetes & peripheral neuropathy. Indian J Med Res. 2017;145:129–132. doi: 10.4103/ijmr.IJMR_699_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Kang CK, Park H, Lee MG. Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J Exerc Nutr Biochem. 2014;18:249–257. doi: 10.5717/jenb.2014.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duclos M, Oppert JM, Verges B, Coliche V, Gautier JF, Guezennec Y, Reach G, Strauch G, SFD diabetes and physical activity working group Physical activity and type 2 diabetes. Recommandations of the SFD (francophone diabetes society) diabetes and physical activity working group. Diabetes Metab. 2013;39(3):205–216. doi: 10.1016/j.diabet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Hagstrom AD, Marshall PWM, Lonsdale C, Papalia S, Cheema BS, Toben C, Baune BT, Fiatarone Singh MA, Green S. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2016;155(3):471–482. doi: 10.1007/s10549-016-3688-0. [DOI] [PubMed] [Google Scholar]
- 16.Gray S, Baker G, Wright A, Fitzsimons C, Mutrie N, Nimmo M. The effect of a 12 weeks walking intervention on markers of insulin resistance and systemic inflammation. Prev Med. 2009;48(1):39–44. doi: 10.1016/j.ypmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, Teegarden D. Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur J Appl Physiol. 2012;112(8):3045–3052. doi: 10.1007/s00421-011-2279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association (ADA). Standards of medical Care in Diabetes, Diabetes Care Volume 41, Supplement 1, January 2018.
- 19.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752–758. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care. 2002;6:335–341. doi: 10.1186/cc1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sportsmed. 1985;13:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 22.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, PC HES, Kristin Castorino K, Tate DF. Physical activity/ exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brzycki M. Strength testing: predicting a one – rep max from repetitions-to-fatigue. JOPERD. 1993;64:88–90. [Google Scholar]
- 24.Yazdchi R, Pourghassem Gargari B, Asghari-Jafarabadi M, Shahaf F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: a randomized, double-blind, placebo-controlled clinical trial. Nutr Res Pract. 2016;10(3):328–335. doi: 10.4162/nrp.2016.10.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Woerle HJ, Pimenta WP, Meyer C, Gosmanov NR, Szoke E, Szombathy T, Mitrakou A, Gerich JE. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004;164:1627–1632. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- 27.Das G. Vitamin D and type 2 diabetes. Pract Diabetes. 2017;34(1):19–24. doi: 10.1002/pdi.2072. [DOI] [Google Scholar]
- 28.Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Kalayi A, Shariatzadeh N, Khalaji N, Gharavi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev. 2012;28:424–430. doi: 10.1002/dmrr.2290. [DOI] [PubMed] [Google Scholar]
- 29.Miller EG, Sethi P, Nowson CA, Dunstan DW, Daly RM. Effects of progressive resistance training and weight loss versus weight loss alone on inflammatory and endothelial biomarkers in older adults with type 2 diabetes. Eur J Appl Physiol. 2017;117(8):1669–1678. doi: 10.1007/s00421-017-3657-2. [DOI] [PubMed] [Google Scholar]
- 30.Calton EK, Kevin N, Keane KN, Newsholm P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10(11):e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haussler MR, Haussler CA, Bartik L, Whitfield CK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66:S98–S112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 32.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 33.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Zhao M, Li Q, Zhao H, Wang J, LI Y. Acetyl-l-carnitine inhibits TNF-alpha-induced insulin resistance via AMPK pathway in rat skeletal muscle cells. FEBS Lett. 2009;583(2):470–474. doi: 10.1016/j.febslet.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 35.Nabata A, Kuroki M, Ueba H, Hashimoto S, Umemoto T, Wada H, Yasu T, Saito M, Momomura SI, Kawakami M. C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: implications for the destabilization of atherosclerotic plaque. Atherosclerosis. 2008;196:129–135. doi: 10.1016/j.atherosclerosis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Salamat KM, Azarbayjani MA, Yusof A, Dehghan F. The response of pre-inflammatory cytokines factors to different exercises (endurance, resistance, concurrent) in overweight men. Alex J Med. 2016;52:367–370. doi: 10.1016/j.ajme.2015.12.007. [DOI] [Google Scholar]
- 37.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 38.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 39.Levinger I, Goodman C, Peake J, Garnham A, Hare DL, Jerums G, Selig S. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet UK Diabet Med. 2009;26(6):220–227. doi: 10.1111/j.1464-5491.2009.02679.x. [DOI] [PubMed] [Google Scholar]
- 40.Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011;48(3):183–189. doi: 10.1007/s00592-011-0278-9. [DOI] [PubMed] [Google Scholar]
- 41.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-weeks randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298(4):E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]