Abstract
Objectives
This study investigated the anti-hyperglycemic effects of concentrated hot water infusion of Phragmanthra incana leaves as well as its ameliorative effect on indices related to diabetic complications in a type 2 diabetes model of rats.
Methods
Type 2 diabetes was induced by feeding 10% fructose solution ad libitum for two weeks followed by an intraperitoneal injection of streptozotocin (40 mg/kg body weight (b.w.)). Concentrated plant infusion was administered orally at a dose of 150 and 300 mg/kg b.w. to two type 2 diabetes rat groups. Diabetic rats without treatment served as a negative control while the group administered with metformin was served as a positive control. The intervention lasted for 4 weeks when a single oral dose was given daily for 5 days a week. Body weight and blood glucose were determined every week. An oral glucose tolerance test was performed in the last week of treatment. The rats were sacrificed after 4 weeks of intervention, and the blood and organs were harvested for further analysis.
Results
Both dosages of the plant infusion significantly improved body weight, pancreatic β-cell function (HOMA-β), insulin secretion and reduced blood glucose, insulin resistance (HOMA-IR) with concomitant reduction in the elevated level of serum α-amylase activity, fructosamine, uric acid, urea, and liver function enzymes. The liver glycogen content was significantly improved while the activity of liver glucose-6-phosphatase was significantly reduced.
Conclusion
The results demonstrate the anti-hyperglycemic ability of P. incana and its ability to delay the onset of diabetic complications which can be exploited for the anti-diabetic drug discovery.
Keywords: Phragmanthra incana, Antihyperglycemic, Type 2 diabetes, Glucose homeostasis
Introduction
Over the years the life expectancy and the quality of life have been decreased gradually due to the unrelenting growth in numbers of people living with diabetes. The prevalence of diabetes has increased from 239 million in 2000 to 425 million in 2017 [1]. Consequently, diabetes has directly caused 1.6 million deaths in 2016 and is the major cause of kidney failure, blindness, heart failure and other complication that impact significantly on the quality of life [2].
Maintaining a normal blood glucose level is the underlying principle of the management and treatment of diabetes. Different classes of hypoglycemic agents used in the treatment of diabetes that utilize different mechanisms to achieve this goal. For example, biguanide suppresses hepatic glucose production, thus decreases blood glucose level [3]. Sulfonylureas work by stimulating insulin secretion from pancreatic β-cells [4], while α-glucosidase inhibitors decrease postprandial hyperglycemia and delay the breakdown of carbohydrates thereby decrease small intestinal glucose absorption [5]. However, none of these drugs are without side effects and relatively expensive, particularly for the people in developing world.
On the other hand, medicinal plants provide cheaper alternative therapy for the treatment of diabetes. Several medicinal plants have been investigated for their antihyperglycemic activity using different techniques in vitro, ex vivo and in vivo experimental models. They modulate blood sugar levels through a variety of mechanisms such as, increasing muscle glucose uptake [6], pancreatic β-cells regeneration, increasing insulin secretion [7, 8], and inhibiting carbohydrate digesting enzymes [9–11].
Phragmanthra incana or P. incana, commonly known as African mistletoe, is a parasitic plant species of the Loranthaceae family. It is used traditionally in the treatment of different disease conditions and ailments including hypertension, diabetes, inflammation, cancer, and insomnia [12]. However, scientific validations of its traditional use are scanty. The methanol and ethyl acetate extracts have been reported to demonstrate antibacterial activity [13]. The methanolic extract of the leaves showed antioxidant and inhibitory activities against Fe2+-induced lipid peroxidation [12]. The hepatoprotective effect of the methanol crude extract in alloxan-induced diabetes has also been reported [14]. Recently, the infusion of the leaf was shown to inhibit the carbohydrate digesting enzymes and promote glucose uptake in ex vivo model [15]. However, the antihyperglycemic effect of the leaves hot water infusion in diabetic rats has not been investigated.
Hence, this study was aimed at investigating the antihyperglycemic activities of P. incana in a fructose-fed streptozotocin-induced type 2 diabetes model of rats.
Materials and methods
Plant material
P. incana leaves were collected sometime in March 2016 from Owo community in Ondo State of Nigeria. The plant sample was authenticated at the Biological Science Department of Adekunle Ajasin University, Akungba, Nigeria when a voucher specimen number: PSB 178 was preserved at the same university.
Sample extraction
The leaves were washed with tap water and air-dried to a constant weight. The dried leaves were blended to fine powder using Thomas-Wiley Laboratory mill, Philadephia P.A, USA. One kilogram (1 kg) of the blended sample was infused in boiling water and allowed to stand for 20 min. The infusion was then filtered with Whatmann’s filter paper (No. 1) and concentrated in a water bath at 50 °C. It was then stored at 4 °C until further use.
Experimental animals
Forty-two male Sprague-Dawley (SD) rats of six week old (mean b.w. 193 ± 11.94 g) were procured from and housed at the Biomedical Resource Unit, University of Kwazulu-Natal, Durban, South Africa. The animals were maintained according to the guidelines of the Animal Research Ethics Committee (AREC) of the University of Kwazulu-Natal, Durban, South Africa (Protocol approval number: AREC/067/017D).
Animal grouping and induction of T2D
After the acclimatization of the animals for one week, they were randomly divided into six groups of seven animal in each group as shown below;
Normal control (NC)
Normal control +300 mg/kg b.w. of the infusion (NTPI)
Diabetic control group (DBC)
Diabetic + low dose (150 mg/kg b.w.) of the infusion (DPIL)
Diabetic + high dose (300 mg/kg b.w.) of the infusion (DPIH)
Diabetic + metformin (300 mg/kg b.w.) (DBM)
After an overnight fast, animals in diabetic groups (DBC, DPIL, DPIH and DBM) were given a 10% fructose solution ad libitum for two weeks to induce insulin resistance followed by a single injection of streptozotocin (STZ) (40 mg/kg b.w. dissolved in citrate buffer pH 4.5) intraperitonially to cause partial destruction of pancreatic β-cells [16]. The animals in normal groups (NC and NTAI) were given water ad libitum for two weeks and then injected with citrate buffer (40 mg/kg b.w). After one week of STZ injection, non-fasting blood glucose (NFBG) of all the animals was measured using a portable glucometer (Glucoplus Inc., Quebec, Canada). Animals with NFBG >18 mmol/L were considered diabetic while the animals with NFBG <18 mmol/L were excluded from the study.
Intervention period
After the confirmation of diabetes, the animals were given their respective treatment (as indicated in the grouping) for five days a week using a gastric gavage needle. While animals in the control groups were treated with a similar volume of the vehicle. During the intervention period, fluid and food intake were measured daily and body weight and NFGB were measured weekly in all the groups.
Oral glucose tolerance test (OGTT)
OGTT was performed in the last week of the intervention period. The animals were given an oral dose of glucose solution (2 g/kg b.w.) after an overnight (12 h) fast. Thereafter, the blood glucose levels were measured at 0 (just before glucose ingestion), 30, 90, and 120 min after the glucose ingestion using a portable glucometer. The area under the curve (AUC) was calculated according to the following formula:
Where Ci is the concentration of blood glucose at time ti.
Collection of blood and organs
At the end of the experimental period, the rats were sacrificed by euthanizing with halothane. Whole blood was collected via cardiac puncture from each animal into a plain tube and centrifuge at 3000 rpm for 15 min to obtain the serum which was preserved at -20 °C for subsequent analysis. The liver and pancreas of each animal were collected, washed with normal salaine, weighed and stored at -20 °C for further analysis. A small piece of the pancreatic tissue of each animal was cut and placed in a 10% formalin solution for histopathological examination.
Biochemical analysis
Serum insulin
Serum insulin concentration was measured using an ultrasensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Upsala, Sweden) according to the manufacturer’s manual. Serum fructosamine, urea, and creatinine concentrations as well as liver function enzymes; aspartate and alanine aminotransferases (AST and ALT) and alkaline phosphatase (ALP) were measured with an Automated Chemistry Analyzer (Labmax Plenno, Labtest Co. Ltd., Lagoa Santa, Brazil) with compatible commercial assay kits.
Serum amylase activity
This was determined using a colorimetric method with 3,5-dinitrosalicylic acid (DNS) reagent [17]. The assay is based on the conversion of starch to maltose by α – amylase and measured by the reduction of 3,5-dinitrosalicylic acid.
Liver glycogen estimation
Liver glycogen was estimated according to a previously described method by Lo et al. [18], and glycogen content was calculated from the glycogen standard curve using log-log graph and expressed as μg/mg of tissue.
Determination of glucose-6-phosphatase activity
The activity of glucose-6-phosphatase was measured by spectrophotometric determination of inorganic phosphate (Pi) production according to a method as described previously by Koide [19]. The amount of liberated Pi was extrapolated from a phosphorus standard curve. Glucose-6-phophatase activity was expressed as units/min/mg of protein in tissue.
Homeostatic model assessment
Homeostatic model assessment scores are used to determine the pancreatic β-cell function (HOMA- β) and insulin resistance (HOMA- IR) from fasting serum insulin and glucose concentrations [20]. These were calculated according to the following formula:
Histological examination of pancreatic tissue
Tissue sections were cut into a size of 4 μm and fixed on slides after necessary processing. They were embedded in paraffin and then subjected to hematoxylin and eosin staining. Slides were viewed using a Leica slide scanner (SCN 4000, Leica Biosystems, Germany).
Statistical analysis
Data are represented as means ± SD and analyzed with Windows SPSS statistical software package (version 26, IBM Corporation, New York, USA) using Tukey’s-HSD multiple range post hoc test. p< 0.05 was considered as significant.
Results
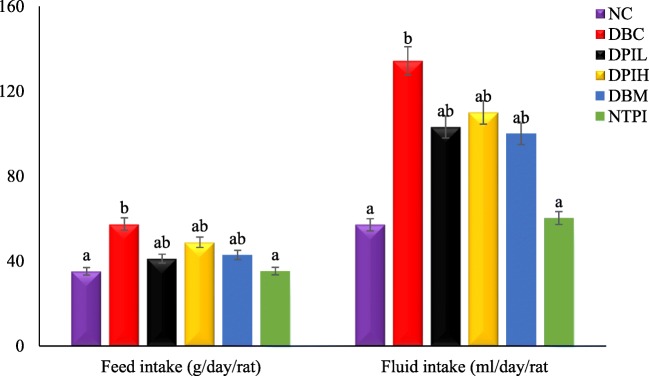
Treatment with P. incana infusion had a significant (p < 0.05) effect on both fluid and food intake as shown in Fig. 1. However, there was no significant difference in the fluid intake between the low and high doses of the treatment.
Fig. 1.
Food and fluid intake in different animal groups during the experimental period. Data are presented as the mean ± SD of 5-7 animals. a &b Different alphabets over the bars for a given animal group represent significance of difference (p < 0.05). NC, Normal Control; DBC, Diabetic Control; DPIL, Diabetic P. incana low dose; DPIH, Diabetic P. incana high dose; DBM, Diabetic Metformin; NTPI, Normal P. incana high dose
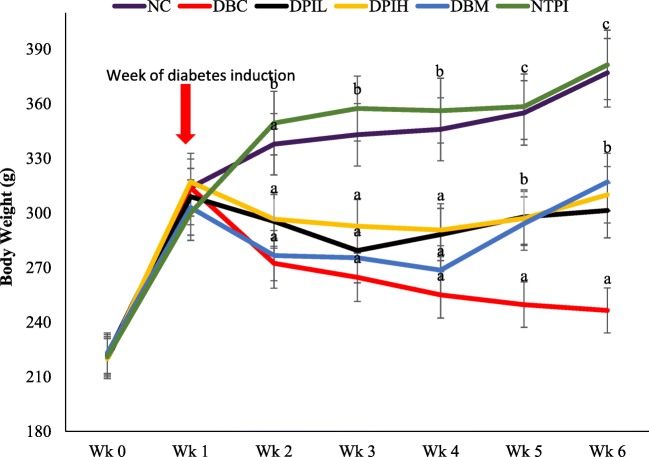
As indicated in Fig. 2, the body weight of diabetic animals was significantly (p < 0.05) reduced a week after the induction of diabetes. But along the intervention period, DAIL, DAIH, and DBM groups had significantly (p < 0.05) higher body weight gain compared to the DBC group.
Fig. 2.
Effect of oral treatment of hot water infusion of P. incana on body weight in different animal groups during the experimental period. Data are presented as the mean ± SD of 5-7 animals. a - c Different alphabets over the bars for a given animal group represent significance of difference (p < 0.05). NC, Normal Control; DBC, Diabetic Control; DPIL, Diabetic P. incana low dose; DPIH, Diabetic P. incana high dose; DBM, Diabetic Metformin; NTPI, Normal P. incana high dose
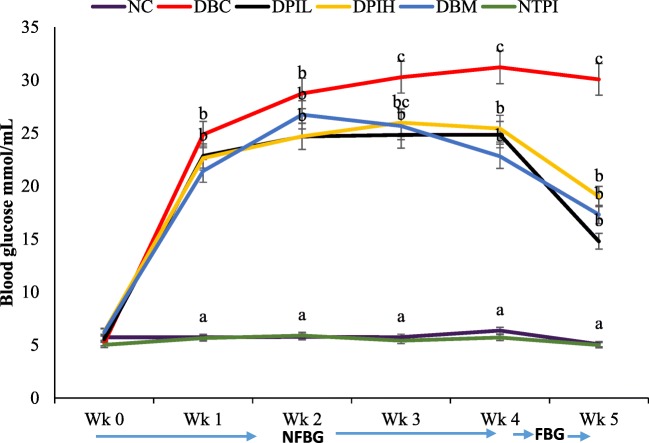
One week after the induction of diabetes, there was a significant (p < 0.05) increase in NFBG levels of diabetic groups (DBC, DPIL, DPIH and DBM) when compared to the normal control groups (NC and NTPI) as shown in Fig. 3. However, the administration of P. incana infusion significantly reduced the elevated blood glucose levels compared to the diabetic control group (DBC). Likewise, the calculated area under the curve (AUC) for DBC was significantly higher than DPIL, DPIH and DBM groups, while NC and NTAI groups were not significantly different from each other but were significantly lower than the diabetic groups (Table 1).
Fig. 3.
The effects of oral treatment of hot water infusion of P, incana leaves on weekly blood glucose concentrations in different animal groups during the intervention period. Data are presented as the mean ± SD of 5-7 animals. a-cValues with different letters for a given week are significantly different from one another (Tukey’s-HSD multiple range post hoc test, p < 0.05). NC, Normal Control; DBC, Diabetic Control; DPIL, Diabetic P. incana low dose; DPIH, Diabetic P. incana high dose; DBM, Diabetic Metformin; NTPI, Normal P. incana high dose; NFGB, non-fasting blood glucose; FGB, fasting blood glucose
Table 1.
Serum insulin and fructosamine concentrations as well as computed HOMA-IR and HOMA-β scores for different animal groups at the end of the experiment
| NC | DBC | DPIL | DPIH | DBM | NTPI | |
|---|---|---|---|---|---|---|
| Insulin (ρmol/L) | 72.60 ± 2.47c | 31.61 ± 3.20a | 70.62 ± 3.29bc | 42.72 ± 4.35ab | 59.23 ± 1.70b | 80.97 ± 0.72c |
| Fructosamine (μmol/L) | 119 ± 5.39a | 867 ± 19.38c | 387 ± 11.04b | 442 ± 12.58b | 633 ± 13.18b | 118 ± 2.88a |
| HOMA-IR | 1.85 ± 0.76a | 8.71 ± 1.54c | 3.95 ± 1.1ab | 4.46 ± 0.46b | 4.32 ± 0.97b | 2.2 ± 0.54a |
| HOMA-β | 64.34 ± 2.45d | 2.9 ± 0.32a | 32.01 ± 1.97c | 18.93 ± 3.67b | 13.59 ± 2.34b | 67.11 ± 1.23d |
Results are expressed as mean ± SD of 5-7 rats
NC Normal Control, DBC Diabetic Control, DPIL Diabetic P. incana low dose, DPIH Diabetic P. incana high dose, DBM Diabetic Metformin, NTPI Normal P. incana high dose, HOMA-IR Homeostatic model assessment - insulin resistant, HOMA-beta Homeostatic model assessment - beta cell function
a-dDifferent superscripts alphabets along a row indicate significant difference (Tukey’s-HSD multiple range post hoc test, p< 0.05)
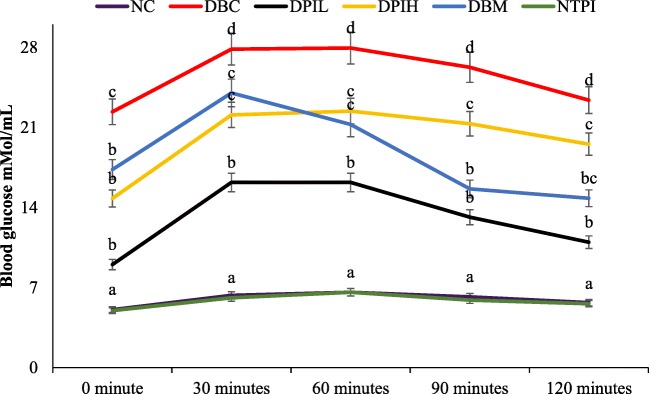
The result of OGTT are presented in Fig. 4. The glucose tolerance ability of the animals in normal control groups (NC and NTPI) was significantly (p < 0.05) better than the diabetic groups. However, diabetic groups treated with hot water infusion of P. incana were significantly ( p< 0.05) better than the diabetic control group. Besides, the calculated AUC for non-diabetic groups (NC and NTPI) were significantly lower than the diabetic groups (Table 1), while there was no significant difference between the DPIL, DPIH and DBM groups but were significantly (p < 0.05) lower than the DBC group.
Fig. 4.
Oral glucose tolerance test (OGTT) of different animal groups in the last week of experimental period. Data are presented as the mean ± SD of 5-7 animals. a - d Different alphabets over the lines for a given time represent significance of difference (p < 0.05). NC, Normal Control; DBC, Diabetic Control; DPIL, Diabetic P. incana low dose; DPIH, Diabetic P. incana high dose; DBM, Diabetic Metformin; NTPI, Normal P. incana high dose
The results of serum insulin concentration, fructosamine and homeostatic model assessment are presented in Table 2. The concentration of serum insulin and HOMA-β scores were significantly lower (p < 0.05) and the concentration of fructosamine and HOMA-IR scores were significantly higher (p < 0.05) in the DBC group when compared with other groups. Upon treatment with hot water infusion of P. incana, the serum insulin concentration and HOMA-β scores were significantly increased (p < 0.05) while fructosamine concentration and HOMA-IR scores were significantly (p < 0.05) decreased in DPIL, DPIH, and DBM groups when compared to the DBC group.
Table 2.
Liver glycogen concentrations and glucose-6-phosphatse as well as serum α-amylase activity in different animal groups at the end of the experimental period
| NC | DBC | DPIL | DPIH | DBM | NTPI | |
|---|---|---|---|---|---|---|
| Liver glycogen (mg/g tissue) | 5.36 ± 0.31d | 1.63 ± 0.88a | 3.58 ± 0.94c | 2.51 ± 0.58b | 3.58 ± 0.78c | 4.94 ± 0.88d |
| Serum α-amylase activity (U) | 58.40 ± 1.76a | 86.60 ± 1.73c | 62.7 ± 4.65ab | 64.3 ± 2.81b | 68.6 ± 2.10b | 60 ± 1.73a |
| Glucose-6-phosphatase (U) | 1.92 ± 0.03a | 6.21 ± 0.08d | 2.27 ± 0.22b | 3.24 ± 0.03c | 2.01 ± 0.03ab | 1.87 ± 0.04a |
Results are presented as mean ± SD of 5-7 animals
α-amylase activity 1 U = 1 μmoles maltose formed per min per ml
Glucose-6-phosphatase 1 U = 1 μmole phosphate liberated per min per mg of the liver tissue
NC Normal Control, DBC Diabetic Control, DPIL Diabetic P. incana low dose, DPIH Diabetic P. incana high dose, DBM Diabetic Metformin, NTPI Normal P. incana high dose
a-dValues with different alphabets along a row are significantly different from each other (Tukey’s-HSD multiple range post hoc test, p< 0.05)
The liver glycogen and, serum α-amylase and glucose-6-phosphatase activities are presented in Table 3. The liver glycogen content was significantly (p < 0.05) depleted in the DBC group but upon treatment with the hot water infusion of P. incana, the glycogen contents of the liver of DPIL and DPIH groups were significantly (p < 0.05) increased. Both the α-amylase and liver glucose-6-phosphatase activities were significantly increased in the DBC (p < 0.05) group, when the treatment with the infusion significantly reduced the activities of these enzymes.
Table 3.
Indices of hepatic and renal damages for different animal groups at the end of the experiment
| NC | DBC | DPIL | DPIH | DBM | NTPI | |
|---|---|---|---|---|---|---|
| Urea (mg/dL) | 54.75 ± 5.21a | 75.00 ± 3.12d | 60.00 ± 2.27bc | 65.33 ± 3.04c | 61.25 ± 1.25c | 56.33 ± 4.48ab |
| Uric acid (mg/dL) | 1.14 ± 0.17a | 4.80 ± 0.92c | 2.85 ± 0.54b | 3.03 ± 0.21b | 1.95 ± 0.14a | 2.11 ± 0.53ab |
| CK-MB (U/L) | 2.13 ± 0.03 | 2.81 ± 0.08 | 2.45 ± 0.22 | 2.75 ± 0.03 | 2.61 ± 0.03 | 2.21 ± 0.04 |
| AST (U/L) | 61.25 ± 1.87a | 121.67 ± 2.54c | 73.50 ± 4.67b | 88.67 ± 1.57b | 78.33 ± 3.12b | 64.00 ± 2.67a |
| ALT (U/L) | 59.50 ± 5.23b | 93.50 ± 6.47a | 86.23 ± 3.25b | 87.00 ± 4.26b | 64.00 ± 12.24b | 61.67 ± 10.21b |
| ALP (U/L) | 109.5 ± 21.14a | 513.00 ± 13.41d | 159.00 ± 27.21c | 269.50 ± 14.21b | 207.00 ± 11.42b | 102.00 ± 11.25a |
Results are presented as mean ± SD of 5-7 animals
NC Normal Control, DBC Diabetic Control, DPIL Diabetic P. incana low dose, DPIH Diabetic P. incana high dose, DBM Diabetic Metformin, NTPI Normal P. incana high dose
a-dValues with different alphabets along a row are significantly different from each other (Tukey’s-HSD multiple range post hoc test, p< 0.05)
The indices of hepatic and renal damages are presented in Table 3. The serum level of creatine was not affected in all the experimental groups, but serum urea, uric acid, AST, ALT and ALP levels were significantly elevated (p < 0.05) in the DBC group compared to the NC group. However, hot infusion treatments of P. incana significantly (p < 0.05) reduced their levels in diabetic rats compared to the untreated DBC group (Table 3). No significant differences were observed between the NC and NTPI groups for all these parameters.
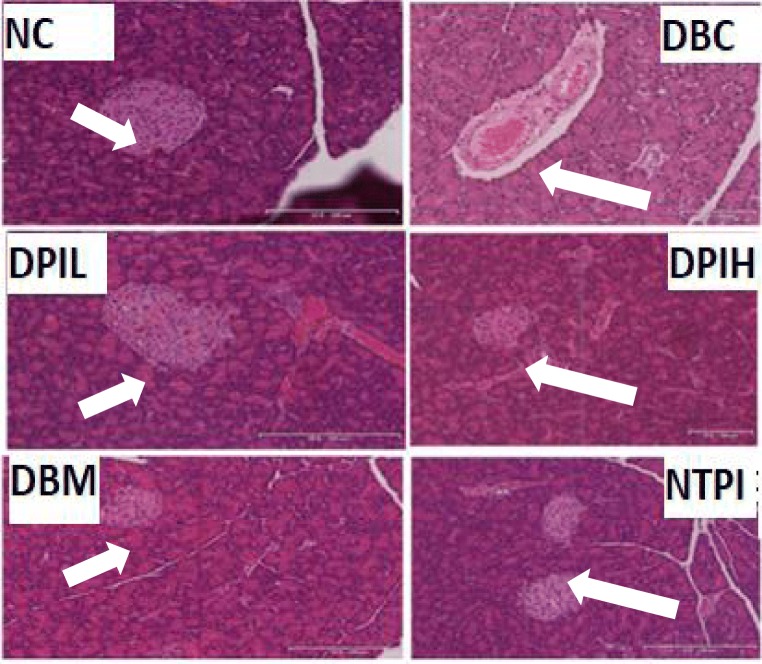
The results of the histopathological examination of pancreas are presented in Fig. 5. DBC group showed a reduction in the size of the pancreatic islet with a concomitant reduction in the number of β-cells when compared with NC group. However, no difference was observed in the number of β-cells in the islet of the pancreas in DPIL, DPIH and DBM groups. DBC group showed a lower number of β-cells in the islet of pancreas when compared with DPIL, DPIH and DBM groups.
Fig. 5.
Effect of oral treatments of hot water infusion of P. incana on histological examinations of the pancreas in different animal groups during the experimental period. The NC and NPAI group have the highest number of β-cells per islet. The islet of DBC group is distorted with fewest number of β-cells. DPIL, DPIH and DBM groups showed regeneration of the islet with more β-cells as compared to DBC. NC, Normal Control; DBC, Diabetic Control; DPIL, Diabetic P.incana low dose; DPIH, Diabetic P. incana high dose; DBM, Diabetic Metformin; NTPI, Normal P. incana high dose
Discussion
Medicinal plants have over the years played a crucial role in the health care delivery system all over the world [21]. However, the use of herbal medicine in the management of diseases has raised questions about the efficacy and safety [21, 22]. Our previous study revealed that the hot water infusion of the leaves of P. incana exhibited a potent α-glucosidase and α-amylase inhibitory activity and increased muscle glucose uptake [15]. This prompted us to further investigate its antihyperglycemic activity in a type 2 diabetes model of rats.
Obvious symptoms of diabetes mellitus include polydipsia and polyphagia with concomitant reduction of body weight [23], which were also observed in the diabetic groups of our experiment. Several studies have linked these parameters to prolong and stable diabetic conditions [16, 24, 25]. In the present study, treatment with the P. incana infusion although could not completely reverse the lost body weight (Fig. 2), polydipsia and polyphagia, its effect on theses parameters was better than the diabetic group without treatment. This may be as a result of the longer duration of the intervention period.
Elevated postprandial blood glucose is an indication of T2D. Therefore, maintaining glucose homeostasis is a major strategy for preventing complications associated with diabetes [25, 26]. Studies have attributed chronic hyperglycemia to insulin insufficiency followed by reduction of pancreatic β-cell mass and the insensitivity of peripheral tissues and cells to the action of insulin [27, 28]. This concurs with the elevated blood glucose (Fig. 3), low serum insulin (Table 1) and the disgruntled pancreatic morphology (Fig. 5) of the untreated diabetic rats in our study. The ability of the infusion to reduce blood glucose (Fig. 3) with concordant improvement of β-cell functions and increase in serum insulin (Table 1) depict antihyperglycemic effect of P. incana. Also, the regeneration of pancreatic β-cell and improved morphology (Fig. 5) further explain the antihyperglycemic potential of P. incana hot water infusion.
Apart from pancreatic β-cell dysfunction, the insensitivity of peripheral tissues and cells to insulin is the major characteristic of glucose intolerance in type 2 diabetes. Several studies have indicated the use of oral glucose tolerance test (OGTT) to evaluate glucose intolerance [29–31]. The glucose intolerance (Fig. 4) of the untreated diabetic rats further supports the pancreatic β-cell dysfunction and insulin insensitivity. The ability of the P. incana infusion to improve glucose tolerance and utilisation (Fig. 4) further portrays its antihyperglycemic ability. This is also evident by the low HOMA-IR scores (Table 1) of the P. incana treated groups of diabetic rats.
The liver plays a major role in overall glucose disposal and insulin sensitivity. In normal metabolic condition, elevated glucose is stored as glycogen in response to insulin action. In type 2 diabetes, the excess glucose disposal as glycogen is compromised which may further impact hepatic steatosis by diverting excess glucose to de novo fatty acid synthesis [32]. The depletion of liver glycogen in untreated diabetic rats (Table 2) indicates that both hepatosteatosis and insulin resistance have compromised glycogen synthesis [31]. The ability of the infusion to increase liver glycogen (Table 2) indicates improved glucose tolerance independent of insulin signalling [33] which further explain its antidiabetic potentials.
Furthermore, hepatic glucose production contributes significantly to the elevated postprandial hyperglycemia in diabetic subjects. It is well established that insulin inhibits hepatic glucose production [34–36]. This is evident in our study as the blood glucose level is higher in diabetic rats (Fig. 3) with concomitant reduction of serum insulin (Table 1). The processes of de novo glucose production (gluconeogenesis) involve in the activation of glucose-6-phosphatase which is one of the rate-limiting steps of gluconeogenesis [37]. The increased glucose-6-phosphatase as seen in the untreated diabetic rats (Table 2), indicates an activation of hepatic glucose production. The ability of the infusion to decrease the glucose-6-phosphatase activity (Table 2) indicates a suppressive effect of gluconeogenesis.
Delay in postprandial glucose absorption from the small intestine is one of the ways of managing diabetes [38]. Pancreatic α-amylase play an essential role in the breakdown of dietary carbohydrates to glucose for subsequent absorption by the intestine. The increased serum α-amylase activity in the untreated diabetic rats (Table 2) may contribute to their elevated blood glucose level (Fig. 3). This correlates with previous reports that increased serum amylase activity in diabetic ketoacidosis might be enhanced by the rate of α-amylase release [39]. The reduced activity of the enzyme in diabetic rats treated with the P. incana infusion connotes an inhibitory effect. This further corroborates our previous report on in vitro inhibitory potential of the plant on carbohydrate digestive enzymes [15].
Persistent hyperglycemia have been implicated in many of the diabetic complications such as retinopathy, nephropathy, atherosclerosis and neuropathy [40] which involve multiple cascade of reaction such as advanced glycation end product, hexosamine pathway, polyol pathway and activation of protein kinase C. Fructosamine is a product of glycated protein in an early stage which later form advanced glycation end product. It has been used as a glycemic biomarker for the detection and control of diabetes [41]. The increased level of fructosamine in the untreated diabetic rats corresponds with its increased blood glucose (Fig. 3), which indicates a glycation cascade reaction and the onset of diabetic complications (Table 1). The reduced level of fructosamine in the P. incana infusion treated rats, suggests the attenuation of the glycation cascade.
Likewise, elevated serum uric acid and urea have been linked with the development of diabetic nephropathy [42]. Their reduced levels in the treatment groups further supports the ability of the infusion to ameliorate the progression of diabetic complications in type 2 diabetes. Elevated liver function enzymes levels in hyperglycemia has been linked to lipid peroxidation and recruited inflammatory cells as a result of oxidative stress and increase proinflammatory cytokines, which may cause hepatocellular injury [43]. The depleted serum level of these enzymes in normal and diabetic rats treated with the P. incana infusion (Table 3) also indicates its safety on healthy tissues as well as on diabetics.
Conclusion
The results of this study indicate the antihyperglycemic potentials of concentrated hot water infusion of P. icana thus, give credence to its folkloric claims. Our future work will focus on the detailed studies on the molecular mechanism(s) and metabolic pathways that may be involved behind its antihyperglycemic activity.
Acknowledgements
This study was supported by a Competitive Research Grant from the Research Office of the University of KwaZulu-Nata, Durban; and Grant Support for Women and Young Researchers from the National Research Foundation (NRF), Pretoria, South Africa. First author received a Doctoral study scholarship from NRF as well.
Compliance with ethical standards
This study was conducted according to the rules and regulations of the Animal Research Ethics Committee of the University of KwaZulu-Natal, Durban, South Africa.
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.I.D.F. IDF diabetes atlas. Brussels: International Diabetes Federation. 2017; 8th edition Online: www.diabetesatlas.org.
- 2.Roglic Gojka. WHO Global report on diabetes: A summary. International Journal of Noncommunicable Diseases. 2016;1(1):3. [Google Scholar]
- 3.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Renström E, Barg S, Thévenod F, Rorsman P. Sulfonylurea-mediated stimulatio expert Opin Pharmacother. Of insulin exocytosis via an ATP-sensitive K+ channel–independent action. Diabetes. 2002;51(suppl 1):S33–SS6. doi: 10.2337/diabetes.51.2007.s33. [DOI] [PubMed] [Google Scholar]
- 5.Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015;16(13):1959–1981. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- 6.Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, Benhaddou-Andaloussi A, Nistor L, Afshar A, Arnason JT, Haddad PS. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res. 2010;54(7):991–1003. doi: 10.1002/mnfr.200900218. [DOI] [PubMed] [Google Scholar]
- 7.Abd El Latif A, El Bialy BES, Mahboub HD, Abd Eldaim MA. Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of β cells and reduction of pyruvate carboxylase expression. Biochem Cell Biol. 2014;92(5):413–419. doi: 10.1139/bcb-2014-0081. [DOI] [PubMed] [Google Scholar]
- 8.Patel D, Prasad S, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyebode OA, Erukainure OL, Chukwuma CI, Ibeji CU, Koorbanally NA, Islam S. Boerhaavia diffusa inhibits key enzymes linked to type 2 diabetes in vitro and in silico; and modulates abdominal glucose absorption and muscle glucose uptake ex vivo. Biomed Pharmacother. 2018;106:1116–1125. doi: 10.1016/j.biopha.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Oyebode OA, Erukainure OL, Koorbanally NA, ISLAM S. Acalypha wilkesiana ‘Java white’: identification of some bioactive compounds by GC-MS and their effects on key enzymes linked to type 2 diabetes. Acta Pharma. 2018;68(4):425–439. doi: 10.2478/acph-2018-0037. [DOI] [PubMed] [Google Scholar]
- 11.Sanni O, Erukainure OL, Chukwuma CI, Koorbanally NA, Ibeji CU, Islam MS. Azadirachta indica inhibits key enzyme linked to type 2 diabetes in vitro, abates oxidative hepatic injury and enhances muscle glucose uptake ex vivo. Biomed Pharmacother. 2019;109:734–743. doi: 10.1016/j.biopha.2018.10.171. [DOI] [PubMed] [Google Scholar]
- 12.Ogunmefun O, Fasola T, Saba A, Akinyemi A. Inhibitory effect of Phragmanthera incana (Schum.) harvested from Cocoa (Theobroma cacao) and Kolanut (Cola nitida) trees on Fe2+ induced lipid oxidative stress in some rat tissues-in vitro. Int J Biomed Sci. 2015;11(1):16. [Google Scholar]
- 13.Ogunmefun O, Saba A, Fasola T, Akharaiyi F, Oridupa O. Phytochemistry and in-vitro Antimicrobial Evaluation of Phragmanthera incana (Schum.) Balle Extracts on Selected Clinical Microorganisms. 2016. [Google Scholar]
- 14.Ogunmefun O, Fasola T, Saba A, Oridupa O, Adarabioyo M. Haematology and serum biochemistry of alloxan-induced diabetic rats administered with extracts of Phragmanthera incana (Schum.) Balle. Afr J Pharm Pharmacol. 2017;11(43):545–553. [Google Scholar]
- 15.Sanni O, Erukainure OL, Oyebode OA, Koorbanally NA, Islam MS. Concentrated hot water-infusion of phragmanthera incana improves muscle glucose uptake, inhibits carbohydrate digesting enzymes and abates Fe2+−induced oxidative stress in hepatic tissues. Biomed Pharmacother. 2018;108:417–423. doi: 10.1016/j.biopha.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64(1):129–139. doi: 10.1016/s1734-1140(12)70739-9. [DOI] [PubMed] [Google Scholar]
- 17.Shai LJ, Masoko P, Mokgotho MP, Magano SR, Mogale AM, Boaduo N, Eloff JN. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S Afr J Bot. 2010;76(3):465–470. [Google Scholar]
- 18.Lo S, Russell JC, Taylor A. Determination of glycogen in small tissue samples. J Appl Physiol. 1970;28(2):234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 19.Koide H. Pathological occurrence of glucose-6-phosphatase in serum in liver disease. Clin Chim Acta. 1959;4:554–561. doi: 10.1016/0009-8981(59)90165-2. [DOI] [PubMed] [Google Scholar]
- 20.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14(11):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson TR, Akerele O. Medicinal plants: their role in health and biodiversity: University of Pennsylvania press. 2015. [Google Scholar]
- 22.Mahady GB. Global harmonization of herbal health claims. J Nutr. 2001;131(3):1120S–1123S. doi: 10.1093/jn/131.3.1120S. [DOI] [PubMed] [Google Scholar]
- 23.ADA Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 24.Islam MS. Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine. 2011;19(1):25–31. doi: 10.1016/j.phymed.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim MA, Islam MS. Effects of butanol fraction of Ziziphus mucronata root ethanol extract on glucose homeostasis, serum insulin and other diabetes-related parameters in a murine model for type 2 diabetes. Pharm Biol. 2017;55(1):416–422. doi: 10.1080/13880209.2016.1242632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin RR, Peyrot M. Psychological issues and treatments for people with diabetes. J Clin Psychol. 2001;57(4):457–478. doi: 10.1002/jclp.1041. [DOI] [PubMed] [Google Scholar]
- 27.Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4(2):287–302. doi: 10.1007/s13679-015-0155-x. [DOI] [PubMed] [Google Scholar]
- 28.S-i G, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, et al. Probucol preserves pancreatic β-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2002;57(1):1–10. doi: 10.1016/s0168-8227(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 29.Kjems L. L., Kirby B. M., Welsh E. M., Veldhuis J. D., Straume M., McIntyre S. S., Yang D., Lefebvre P., Butler P. C. Decrease in -Cell Mass Leads to Impaired Pulsatile Insulin Secretion, Reduced Postprandial Hepatic Insulin Clearance, and Relative Hyperglucagonemia in the Minipig. Diabetes. 2001;50(9):2001–2012. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 30.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 31.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 32.Samuel VT, Liu Z-X, Qu X, Elder BD, Bilz S, Befroy D. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci. 2007;104(31):12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, et al. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One. 2011;6(2):e16556. doi: 10.1371/journal.pone.0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205(1):97. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- 36.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Barrett EJ, Dalkin AC, Zwart AD, Chou JY. Effect of acute diabetes on rat hepatic glucose-6-phosphatase activity and its messenger RNA level. Biochem Biophys Res Commun. 1994;205(1):680–686. doi: 10.1006/bbrc.1994.2719. [DOI] [PubMed] [Google Scholar]
- 38.Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr. 2011;66(2):143–148. doi: 10.1007/s11130-011-0226-4. [DOI] [PubMed] [Google Scholar]
- 39.Krane EJ. Diabetic ketoacidosis: biochemistry, physiology, treatment, and prevention. Pediatr Clin N Am. 1987;34(4):935–960. doi: 10.1016/s0031-3955(16)36296-4. [DOI] [PubMed] [Google Scholar]
- 40.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122(4):333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 41.Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies–cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. doi: 10.1371/journal.pone.0111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovind P, Rossing P, Johnson RJ, Parving H-H. Serum uric acid as a new player in the development of diabetic nephropathy. J Ren Nutr. 2011;21(1):124–127. doi: 10.1053/j.jrn.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23(3):115–119. [Google Scholar]