Abstract
Purpose
The association between selenium supplementation and glycemic indices seems to be a controversial issue. This systematic review and meta-analysis was conducted to evaluate the effect of selenium supplementation on glycemic indices.
Methods
We systematically searched PubMed/MEDLINE, ISI/WOS, and Scopus (from their commencements up to Jan 2016) for relevant studies examining the association between intake of selenium and glycemic indices. The data were extracted from relevant qualified studies and estimated using the random-effect or pooled model and standardized mean difference (SMD) with 95% confidence interval (CI).
Results
Twelve articles published between 2004 and 2016 were included. In all the studies, the participants were randomly assigned to an intervention group (n = 757) or a control group(n = 684). All the studies were double blind, placebo controlled trials. Selenium supplementation resulted in a significant decrease in homeostasis model of assessment-estimated β-cell function (HOMA-B) (SMD: -0.63; 95%CI: −0.89 to −0.38) and a significant increase in quantitative insulin sensitivity check index (QUICKI) (SMD: by 0.74; 95%CI: 0.49 to 0.1) as compared with the controls. There were no statistically significant improvements in glycemic indices, such as fasting plasma glucose (FPG), insulin, homeostasis model of assessment-estimated insulin resistance (HOMA-IR), Hemoglobin A1c (HbA1c) and adiponectin.
Conclusion
This meta-analysis indicated that selenium supplementation significantly decreased HOMA-B and increased QUICKI score. There was no statistically significant improvement in FPG, insulin, HOMA-IR, HbA1c and adiponectin indices following selenium supplementation.
Keywords: Selenium, Glycemic indices, FPG, Insulin, HbA1c
Introduction
Selenium is a beneficial trace mineral [1] that has a crucial role in maintaining immune-endocrine function, metabolism, cellular homeostasis, proper thyroid hormone function, cardiovascular system, neurodegeneration, cancer prevention, and glucose hemostasis. These effects are delivered mainly by the selenoproteins involved in processes, such as cellular proliferation, differentiation, and activation of the cells [2–5]. The selenoproteins have redox function and help the cells maintain membrane integrity, protect the production of prostacyclin and decrease the oxidative damage to lipids, lipoproteins, and DNA [6, 7].
A number of studies have reported that selenium plays a role in the metabolism of carbohydrate, as it can regulate the expression of genes responsible for the synthesis of enzymes that control the metabolism of carbohydrates and inflammation [8, 9]. Moreover, experimental studies on diabetic db/db mice suggested that selenium resulted in increased plasma insulin levels [10], which might be a function of gene expression stimulation in pancreatic β-cells and the enhancement of islet function by selenium [11]. Several studies revealed that selenium decreased the insulin resistance [12]; however, selenium is not clearly associated with blood sugar, insulin, and diabetes, and studies do not yield consistent results. A study found that the selenium supplement increased the risk of diabetes in patients with higher levels of selenium before therapy [13]. This finding was supported by another study, which indicated that selenium supplementation increased the risk of diabetes [14]. Moreover, studies on animals revealed that selenium supplementation increased the risk of glucose intolerance, hyperinsulinemia, and insulin resistance [15].
It seems that the correlation of selenium supplementation with glycemic status is a controversial issue, due to the lack of statistical power, small sample sizes, and the ethnic diversity of the population. Meta-analysis is considered as a statistical technique for combining the results of several studies. Therefore, we performed this meta-analysis to evaluate of the effect of selenium supplementation on glycemic indices, including insulin, FPG, HOMA-IR, HOMA-B, QUICKI, HbA1c, and adiponectin.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA-P), this systematic review was conducted to evaluate the probable effects of selenium supplementation on glycemic indices(such as fasting plasma glucose (FPG), insulin, homeostasis model of assessment-estimated insulin resistance (HOMA-IR), homeostasis model of assessment-estimated β-cell function (HOMA-B), quantitative insulin sensitivity check index (QUICKI), Hemoglobin A1c (HbA1c), and adiponectin) [16, 17]. The study protocol was published previously [18].
Search strategy
The most comprehensive international databases of PubMed/ MEDLINE, ISI/WOS, and Scopus searched for targeted papers aiming of the access to all the available relevant evidence. We entered search terms of “glycemic indices” and “Selenium supplementation”, without restriction of ages of participants and time of publication. For review papers, a list of references was assessed to find the related data. Grey literature and key journals were searched for additional data. Searches run on 1.1.2016.
Inclusion and exclusion criteria
Regarding the study design, randomized clinical trials (RCTs) and crossover trails were included if their control group received the placebo. There was no restriction for the study population. We included studies which applied single therapy or combination therapy of selenium. Duplicated publication and non-relevant publications were excluded. Titles, abstracts and the full texts of all included studies were refined by two independent investigators. Possible disagreements were resolved by third investigator.
Quality assessment and data extraction
The eligibility of studies was evaluated by two independent investigators. Data were extracted by using a data extraction form which included citation information, details of study design, year of publication, dose of supplementation, intervention group, control group, mean age of participant, outcome, intervention duration, follow up information, measurements and result and effect size.
Data synthesis and statistical analysis
The standardized mean difference (SMD)/Cohen’s d was used to evaluate the effect of selenium supplementation on glycemic indices. For the two groups (treatment and placebo), the SMD was calculated using the mean differences (endpoint from baseline) and standard deviations (SD) based on the following formula:
If the median and range of glycemic indices were reported by a study, we converted them to mean and SD by using the Hozo formula [19]. A random-effect model was applied if the level of significance of the Q-statistic for heterogeneity was set at 0.1 [20]. In other cases, the fixed-effect model was used [21]. The I2 statistic was used to quantify the degree of heterogeneity, estimating the total variation across studies because of heterogeneity [22]. I2 values were considered as 25%, 50%, and 75% for low, medium, and high heterogeneity, respectively. A random-effect meta-regression model was used to explore possible sources of heterogeneity (such as quality assessment score, duration of intervention, study subjects, mean age of participants, dose of selenium supplementation and female ration). Egger’s test was used to estimate publication bias which was deemed statistically significant at 0.1. All analyses were done using STATA version 10 [23]. P value≤0.05 was regarded as statistically significant.
Ethical considerations
The study protocol was approved by the ethical committee of Alborz University of Medical Sciences. All of included studies would be cited in future relevant reports and publications.
Results
Search results and characteristics of included studies
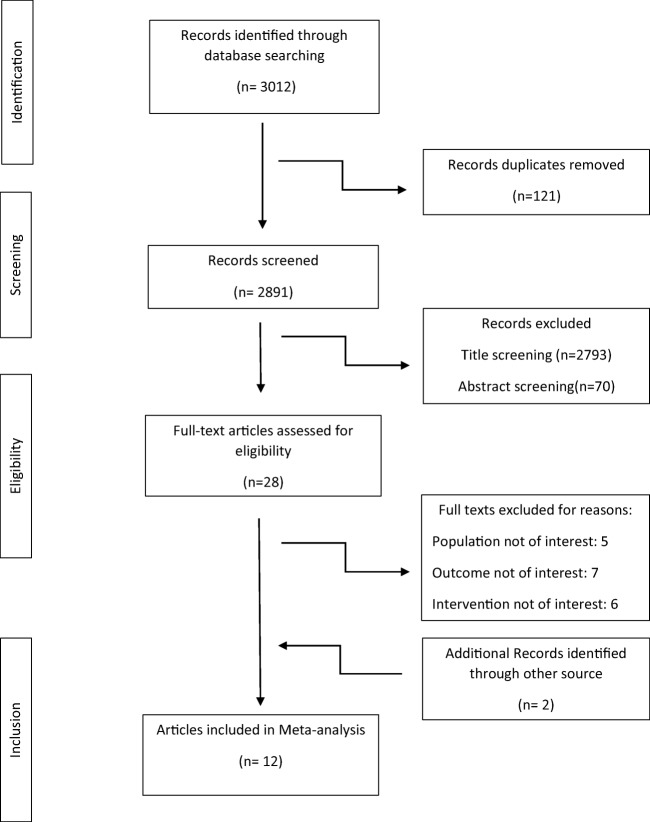
Figure 1 shows a flowchart of the study selection process. In total, twelve articles were included according to the study inclusion/exclusion criteria. Characteristics of included articles are shown in Table 1.The meta-analysis included twelve studies [24–35] published between 2004 and 2016. Randomized control trials (RCTs) were analyzed, but quasi-experimental studies were excluded. In all the studies, the participants were randomly assigned to an intervention group (n = 757) or a control group(n = 684). The age of the participants ranged from 10 years to 85 years. Seven RCTs recruited both men and women, but only female subjects were enrolled in the other five studies [24, 25, 30, 31, 35]. Nine trials used selenium alone as an oral supplement, and three trials used selenium combined with other vitamins or minerals [24, 32, 34]. The daily dose of selenium was different in various trials. Seven RCTs used a dose of 200 μg of daily selenium supplementation [24–28, 30, 35], one RCT used 100 μg daily dose [25], one RCT used 50 μg dose per day [24], and two studies used 60 and 90 μg daily doses, respectively [29, 31, 32]. In one study, three different groups were given three different doses (100,200,300 μg daily) so that they were considered as separate studies [33]. All the supplementations were administered orally; all the trials were placebo-controlled double-blinded. The intervention periods ranged from 42 days [25] to 180 days [33]. Women with gestational diabetes mellitus (GDM) [25], subjects with type 2 diabetes (T2D) [27–29], women with polycystic ovary syndrome [30, 35], patients with chronic heart disease [28], people with at least two cardiovascular risk factors [34], premenopausal women with central obesity [24], obese children and adolescents [32], and patients with diabetic nephropathy [26] were enrolled in the selected studies.
Fig. 1.
Flowchart of study selection
Table 1.
Characteristics of the included studies in the systematic review
| Author, y | Country | Study subject | Sample size | Dose | Intervention group | Mean age ± SD (year) | Outcome | Intervention duration | Result (means ± SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Mean change | Significance | SMD | ||||||||||
| 1 | Asemi et al. [25] | Iran | GDM Women | I = 35 | 200 mg | MT | 28.6 ± 4.6 | FPG | 6 weeks | I | −10.5 ± 11.9 | Yes | −0.51 |
| C = 35 | P | 4.5 ± 12.9 | |||||||||||
| 1 | Asemi et al. [25] | Iran | GDM Women | I = 35 | 200 μg | MT | 28.6 ± 4.6 | Insulin | 6 weeks | I | −1.98 ± 11.25 | Yes | −0.33 |
| C = 35 | P | 5.26 ± 9.33 | |||||||||||
| 1 | Asemi et al. [25] | Iran | GDM Women | I = 35 | 200 μg | MT | 28.6 ± 4.6 | HOMA-IR | 6 weeks | I | - 0.84 ± 2.76 | Yes | −0.40 |
| C = 35 | P | 1.47 ± 2.46 | |||||||||||
| 1 | Asemi et al. [25] | Iran | GDM Women | I = 35 | 200 μg | MT | 28.6 ± 4.6 | HOMA-B | 6 weeks | I | −1.71 ± 43.62 | No | −0.21 |
| C = 35 | P | 16.30 ± 36.69 | |||||||||||
| 1 | Asemi et al. [25] | Iran | GDM Women | I = 35 | 200 μg | MT | 28.6 ± 4.6 | QUICKI | 6 weeks | I | 0.008 ± 0.03 | No | 0.37 |
| C = 35 | P | −0.01 ± 0.01 | |||||||||||
| 2 | Shargorodsky et al. [34] | Israel | Patients with CVDRF | I = 36 | 100 μg | CT | 62.17 ± 6.21 | FPG | 6 months | I | 2 ± 60 | No | −0.02 |
| C = 34 | P | 4.3 ± 51 | |||||||||||
| 2 | Shargorodsky et al. [34] | Israel | Patients with CVDRF | I = 36 | 100 μg | CT | 62.17 ± 6.21 | HbA1c % | 6 months | I | −0.75 ± 2.1 | Yes | −0.21 |
| C = 34 | P | 0.04 ± 1.5 | |||||||||||
| 3 | Rayman et al. [33] | USA | Elderly adults | I1 = 120 | 100 μg | MT | 67.5 | Adiponectin | 6 months | I1 | −0.02 ± 2.15 | No | 0.01 |
| I2 = 124 | 200 μg | I2 | −0.02 ± 1.8 | No | 0.02 | ||||||||
| I3 = 117 | 300 μg | I3 | 0.07 ± 1.85 | No | 0.04 | ||||||||
| C = 100 | P | −0.1 ± 1.94 | |||||||||||
| 4 | Alizadeh et al. [24] | Iran | Premenopausal women with central obesity | I = 17 | 200 μg/d | CT | 33.9 ± 8.5 | Insulin | 6 weeks | I | −3.4 ± 10.25 | No | −0.21 |
| C = 17 | P | 0.2 ± 5.33 | |||||||||||
| 4 | Alizadeh et al. [24] | Iran | Premenopausal women with central obesity | I = 17 | 200 μg/d | CT | 33.9 ± 8.5 | HOMA-IR | 6 weeks intervention period | I | −0.8 ± 2.05 | Yes | −0.2 |
| C = 17 | P | 0.1 ± 1.43 | 4 | ||||||||||
| 5 | Murer et al. [32] | Switzerland | Obese children and adolescents | I = 23 | 50 μg | CT | 12.7 ± 1.5 | Insulin | 4 months | I | 17.5 ± 178.56 | No | −0.04 |
| C = 21 | P | 33.26 ± 147.65 | |||||||||||
| 5 | Murer et al. [32] | Switzerland | Obese children and adolescents | I = 23 | 50 μg | CT | 12.7 ± 1.5 y | FPG | 4 months | I | −0.05 ± 0.57 | No | 0.05 |
| C = 21 | P | −0.11 ± 0.59 | |||||||||||
| 5 | Murer et al. [32] | Switzerland | Obese children and adolescents | I = 23 | 50 μg | CT | 12.7 ± 1.5 y | C – Peptide | 4 months | I | 52 ± 507.6 | No | −0.04 |
| C = 21 | P | 98.5 ± 570.02 | |||||||||||
| 6 | Mao [31] | UK | Primiparous women | I = 104 | 60 μg | MT | Adiponectin | From 12 weeks of gestation until delivery | I | −3.05 ± 5.45 | No | −0.01 | |
| C = 106 | P | −2.91 ± 5.1 | |||||||||||
| 7 | Jamilian et al. [30] | Iran. | Women with PCOS | I = 35 | 200 μg/d | MT | 25.4 ± 5.1 | FPG | 8 weeks | I | −0.23 ± 0.75 | No | −0.18 |
| C = 35 | P | −0.01 ± 0.33 | |||||||||||
| 7 | Jamilian et al. [30] | Iran. | Women with PCOS | I = 35 | 200 μg/d | MT | 25.4 ± 5.1 | Insulin | 8 weeks | I | −29.8 ± 47.29 | Yes | −0.29 |
| C = 35 | P | 9.07 ± 77.12 | |||||||||||
| 7 | Jamilian et al. [30] | Iran. | women with PCOS | I = 35 | 200 μg/d | MT | 25.4 ± 5.1 | HOMA-IR | 8 weeks | I | −1.15 ± 1.81 | Yes | −0.29 |
| C = 35 | P | 0.42 ± 3.09 | |||||||||||
| 7 | Jamilian et al. [30] | Iran. | Women with PCOS | I = 35 | 200 μg/d | MT | 25.4 ± 5.1 | QUICK 1 | 8 week | I | 0.03 ± 0.04 | Yes | 0.30 |
| C = 35 | P | 0.00 ± 0.05 | |||||||||||
| 7 | Jamilian et al. [30] | Iran. | women with PCOS | I = 35 | 200 μg/d | MT | 25.4 ± 5.1 | HOMA- B | 8 week | I | −19.0 ± 30.95 | Yes | −0.28 |
| C = 35 | P | 4.55 ± 47.99 | |||||||||||
| 8 | Faghihi et al. [27] | Iran | Patients with type 2 diabetes | I = 33 | 200 μg/d | MT | 53.54 ± (7.52) | HOMA-IR | 3 months | I | 0.13 ± 2.9 | No | 0.37 |
| C = 27 | P | −2.23 ± 2.95 | |||||||||||
| 8 | Faghihi et al. [27] | Iran | Patients with type 2 diabetes | I = 33 | 200 μg/d | MT | 53.54 ± (7.52) | FPG | 3 months | I | 17 ± 35.8 | Yes | 0.42 |
| C = 27 | P | −20.04 ± 43.5 | |||||||||||
| 8 | Faghihi et al. [27] | Iran | Patients with type 2 diabetes | I = 33 | 200 μg/d | MT | 53.54 ± (7.52) | HbA1c | 3 months | I | −0.38 ± 1.2 | Yes | 0.37 |
| C = 27 | P | −1.26 ± 0.95 | |||||||||||
| 8 | Faghihi et al. [27] | Iran | Patients with type 2 diabetes | I = 33 | 200 μg/d | MT | 53.54 ± (7.52) | Insulin | 3 months | I | −1.19 ± 5.9 | No | 0.30 |
| C = 27 | P | −5.29 ± 6.85 | |||||||||||
| 9 | Faure et al. [29] | France | Diabetic patients | I = 27 | 960 μg/d | MT | 49 to 58 year | FPG | 3 months | I | 0.35 ± 1.75 | No | 0.03 |
| P = 21 | P | 0.21 ± 2.61 | |||||||||||
| 9 | Faure et al. [29] | France | Diabetic patients | I = 27 | 960 μg/d | MT | 49 to 58 year | HbA1c% | 3 months | I | −0.23 ± 1.85 | Yes | −0.02 |
| P = 21 | P | −0.14 ± 1.42 | |||||||||||
| 9 | Faure et al. [29] | France | Diabetic patients | I = 27 | 960 μg/d | MT | 49 to 58 year | Insulin | 3 months | I | 0.89 ± 5.1 | Yes | 0.09 |
| P = 21 | P | −0.04 ± 4.9 | |||||||||||
| 10 | Farrokhian et al. [28, 40] | Iran | Patients with type 2 diabetes & CHD | I = 30 | 200 μg/d | MT | 40–85 years | FPG | 8 weeks | I | - 2.2 ± 58.5 | No | 0.05 |
| C = 30 | P | - 7.3 ± 35.3 | |||||||||||
| 10 | Farrokhian et al. [28, 40] | Iran | Patients with type 2 diabetes & CHD | I = 30 | 200 μg/d | MT | 40–85 years | Insulin | 8 weeks | I | - 2.2 ± 4.6 | Yes | −0.39 |
| C = 30 | P | 3.6 ± 8.4 | |||||||||||
| 10 | Farrokhian et al. [28, 40] | Iran | Patients with type 2 diabetes & CHD | I = 30 | 200 μg/d | MT | 40–85 years | HOMA-IR | 8 weeks | I | - 0.7 ± 1.3 | Yes | −0.38 |
| C = 30 | P | 0.9 ± 2.4 | |||||||||||
| 10 | Farrokhian et al. [28, 40] | Iran | Patients with type 2 diabetes & CHD | I = 30 | 200 μg/d | MT | 40–85 years | HOMA-B | 8 weeks | I | - 7.5 ± 17.2 | Yes | −0.38 |
| C = 30 | P | 15.1 ± 34.5 | |||||||||||
| 10 | Farrokhian et al. [28, 40] | Iran | Patients with type 2 diabetes & CHD | I = 30 | 200 μg/d | MT | 40–85 years | QUICKI | 8 weeks | I | 0.01 ± 0.03 | Yes | 0.31 |
| C = 30 | P | - 0.01 ± 0.03 | |||||||||||
| 11 | Mohammad Hosseinzadeh et al. [35] | Iran | Patients with PCOS | I = 26 | 200 μg/d | MT | 18–42 years | FPG | 12 weeks | I | 5.38 ± 15.4 | No | 0.14 |
| C = 27 | P | 1.53 ± 10.97 | |||||||||||
| 11 | Mohammad Hosseinzadeh et al. [35] | Iran | Patients with PCOS | I = 26 | 200 μg/d | MT | 18–42 years | Insulin | 12 weeks | I | 0.74 ± 5.04 | No | 0.23 |
| C = 27 | P | −1.5 ± 4.31 | |||||||||||
| 11 | Mohammad Hosseinzadeh et al. [35] | Iran | PCOS patients | I = 26 | 200 μg/d | MT | 18–42 years | HOMA-IR | 12 weeks | I | 0.30 ± 1.27 | Yes | 0.27 |
| C = 27 | P | −0.37 ± 1.04 | |||||||||||
| 12 | Bahmani 2015 | Iran | diabetic nephropathy | I = 30 | 200 μg/d | MT | 45–85 years | FPG | 12 weeks | I | 0.7 ± 40.7 | No | −0.07 |
| C = 30 | P | 4.7 ± 45.1 | |||||||||||
| 12 | Bahmani 2015 | Iran | diabetic nephropathy | I = 30 | 200 μg/d | MT | 45–85 years | QUICKI | 12 weeks | I | 0.009 ± 0.01 | No | 0.39 |
| C = 30 | P | 0.00 ± 0.01 | |||||||||||
| 12 | Bahmani 2015 | Iran | diabetic nephropathy | I = 30 | 200 μg/d | MT | 45–85 years | Insulin | 12 weeks | I | −3.1 ± 4.6 | Yes | −0.31 |
| C = 30 | P | 0.5 ± 6.2 | |||||||||||
| 12 | Bahmani 2015 | Iran | diabetic nephropathy | I = 30 | 200 μg/d | MT | 45–85 years | HOMA-IR | 12 weeks | I | −0.9 ± 1.4 | Yes | −0.3 |
| C = 30 | P | 0.10 ± 1.7 | |||||||||||
| 12 | Bahmani 2015 | Iran | diabetic nephropathy | I = 30 | 200 μg/d | MT | 45–85 years | HOMA-B | 12 weeks | I | −11.3 ± 16.3 | Yes | −0.33 |
| C = 30 | P | 2.3 ± 22.0 | |||||||||||
RCT randomized controlled trial, CT Combination Therapy, MT Mono Therapy, I Intervention group, C Control group, FPG fasting plasma glucose, HOMA-IR homeostasis model of assessment-estimated insulin resistance, HOMA-B homeostasis model of assessment-estimated b cell function, QUICKI quantitative insulin sensitivity check index, HbA1c hemoglobin A1c, CVDRF cardiovascular diseases risk factors
* Quasi experimental study
Meta-analysis
Nine studies with 535 participants in selenium or placebo groups reported FPG and insulin as the outcomes at baseline and follow-up. Seven studies with 407 participants reported HOMA-IR, and four RCTs with 260 participants reported HOMA-B and QUICKI. In addition to these factors, three studies evaluated HbA1c levels as the outcome, and four studies with 871 participants reported adiponectin as the outcome at baseline and follow-up. These results are shown in Table 2.
Table 2.
Meta-analysis of effect of selenium supplementation on glycemic index
| Glycemic index | Group (Number)a | Number of Study | Pooled SMD (95% CI) | Model | Heterogeneity assessment | ||
|---|---|---|---|---|---|---|---|
| I2 | Q test | P value | |||||
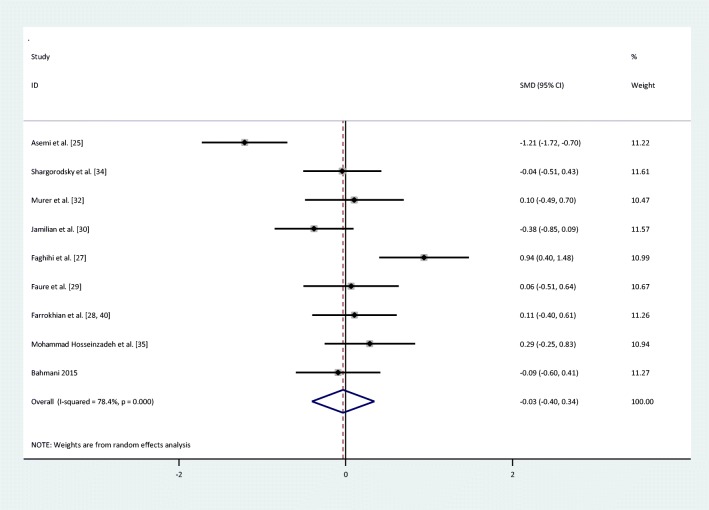
| FPG | I = 275 | 9 | −0.03(−0.4,0.34) | Random | 78.4% | 37.10 | <0.001 |
| P = 260 | |||||||
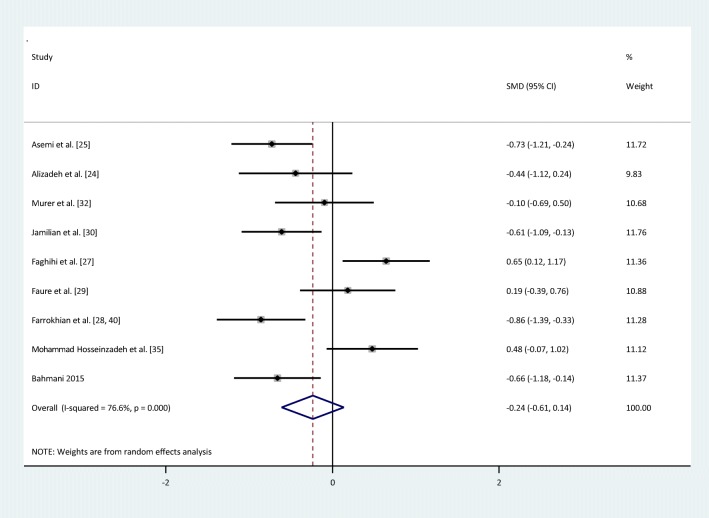
| Insulin | I = 256 | 9 | −0.23(−0.6,0.14) | Random | 76.6% | 34.22 | <0.001 |
| P = 243 | |||||||
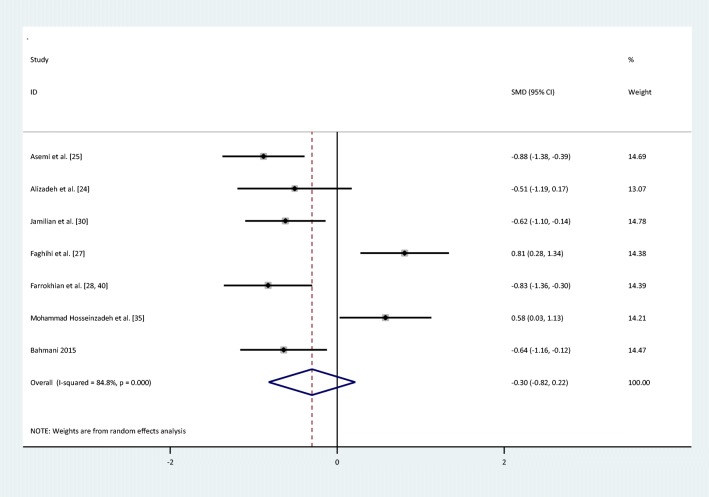
| HOMA-IR | I = 206 | 7 | −0.3(−0.82,0.22) | Random | 84.8% | 39.56 | <0.001 |
| P = 201 | |||||||
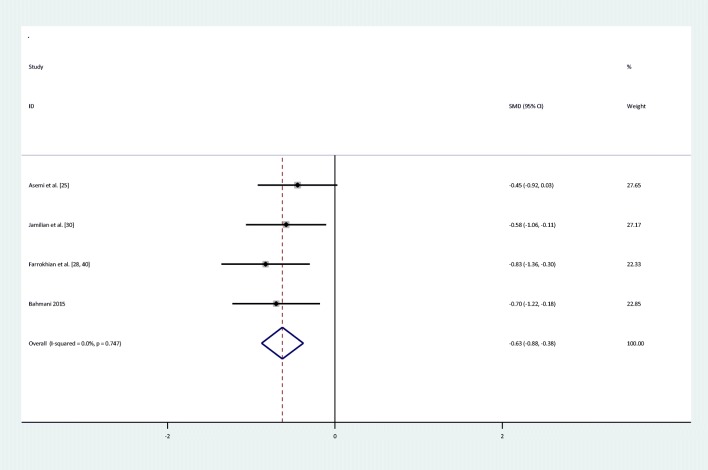
| HOMA-B | I = 130 | 4 | −0.63(−0.89,-0.38)* | Fixed | 0% | 1.23 | 0.75 |
| P = 130 | |||||||
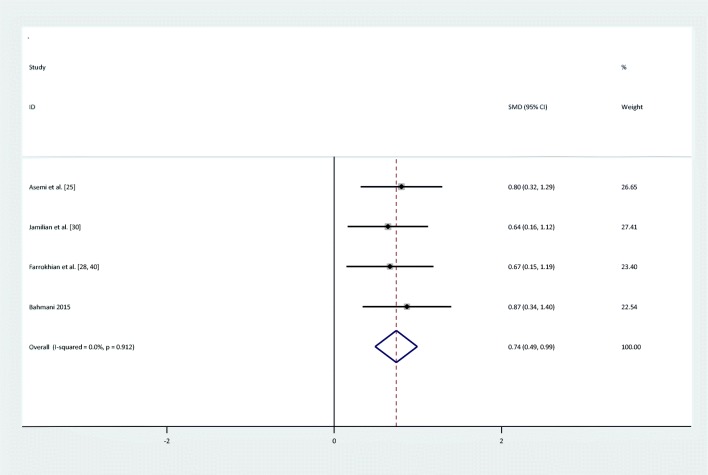
| QUIKI | I = 130 | 4 | 0.74(0.49,0.1)* | Fixed | 0% | 0.53 | 0.91 |
| P = 130 | |||||||
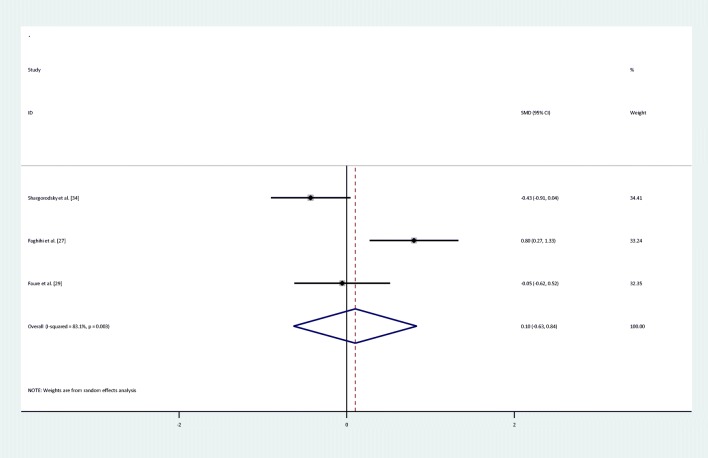
| HbA1c | I = 96 | 3 | 0.1(−0.63,0.84) | Random | 83.1% | 11.85 | 0.003 |
| P = 82 | |||||||
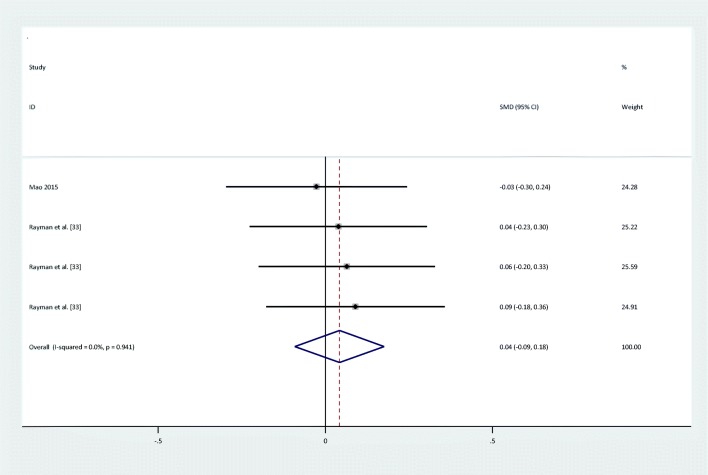
| Adiponectin | I = 465 | 4a | 0.04 (−0.09,0.17) | Fixed | 0% | 0.40 | 0.94 |
| P = 406 | |||||||
I Intervention group, C Control group, FPG fasting plasma glucose, HOMA-IR homeostasis model of assessment-estimated insulin resistance, HOMA-B homeostasis model of assessment-estimated b cell function, QUICKI quantitative insulin sensitivity check index, HbA1c hemoglobin A1c
a In one study, three different doses of adiponectin were used and we considered it as three studies
*Statistically significant
There were no statistically significant improvements in glycemic indices, such as FPG [pooled standardized mean difference (SMD): -0.03, 95%CI: (−0.4,0.34)] with obvious heterogeneity (Q = 37.10; P = 0,0; I2% = 78.4), insulin [(SMD): -0.23,95%CI: (−0.6,0.14)] with obvious heterogeneity (Q = 34.22; P = 0.0; I2% = 76.6), HOMA-IR [(SMD): -0.3, 95%CI: (−0.82,0.22)] with obvious heterogeneity (Q = 39.54; P = 0.0; I2% = 84.8), HbA1c levels [(SMD): 0.1,95%CI: (−0.63,0.84)] with obvious heterogeneity (Q = 11.83; P = 0.003; I2% = 83.1) and adiponectin[(SMD): 0.04,95%CI: (−0.09,0.17)] with non-significant heterogeneity (Q = 0.4; P = 0.94; I2% = 0) with selenium supplementation.
Meta-analyses showed that compared to placebo, the intake of selenium significantly decreased HOMA-B levels [(SMD): -0.63,95%CI: (−0.89,-0.38)] and increased QUICKI score [(SMD): 0.74, 95%CI: (0.49,0.1)], and there was no significant heterogeneity between HOMA-B and QUICKI (Q = 1.23; P = 0.75; I2% = 0) & (Q = 0.53; P = 0.91; I2% = 0). The forest plots showing the effect of the selenium supplementation on glycemic indices are shown in Figs. 2, 3, 4, 5, 6, 7, 8.
Fig. 2.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of fasting plasma glucose
Fig. 3.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of insulin
Fig. 4.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of Homa-IR
Fig. 5.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of HOMA-B
Fig. 6.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of QUIKI
Fig. 7.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of HBA1C
Fig. 8.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of Adiponectin
Quality assessment
The quality of the included studies is shown in Table 3. Six studies were classified as high quality with CONSORT scores higher than 30 [24–26, 28, 30, 33], five studies as medium quality with CONSORT scores in the range of 25–30 [27, 31, 32, 34, 35], and one study as low quality with the CONSORT score lower than 25 [29]. Randomization, as a prerequisite for inclusion in this meta-analysis, was conducted in twelve studies. All the twelve RTCs were double-blind, but only two studies were classified as blind [28, 30].
Table 3.
Quality assessment of included studies according to the CONSORT checklist
| Item | Asemi et al. [25] | Shargorodsky et al. [34] | Alizadeh et al. [24] | Murer et al. [32] | Jamilian et al. [30] | Faghihi et al. [27] | Farrokhian et al. [28, 40] | Bahmani [26] | Faure et al. [29] | Mohammad Hosseinzadeh et al. [35] | Mao [35] | Rayman et al. [33] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| 1b | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3b | No | No | No | No | No | No | No | No | No | No | No | No |
| 4a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6b | No | No | No | No | No | No | No | No | No | No | No | No |
| 7a | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes |
| 7b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8a | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| 8b | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| 9 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes |
| 10 | Yes | No | No | No | Yes | No | Yes | Yes | No | Yes | No | Yes |
| 11a | No | No | No | No | Yes | No | Yes | Yes | No | No | No | Yes |
| 11b | No | No | No | No | No | No | No | No | No | No | No | No |
| 12a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14b | No | No | No | No | No | No | No | No | No | No | Yes | No |
| 15 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 17b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 19 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 20 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 23 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| 24 | No | No | No | No | No | No | No | No | No | No | No | Yes |
| 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 30 | 25 | 30 | 29 | 32 | 26 | 32 | 31 | 23 | 28 | 29 | 33 |
(1a) Identification as a randomized trial in the title; (1b) Structured summary of trial design, methods, results, and conclusions; (2a) Scientific background and explanation of rationale; (2b) Specific objectives or hypotheses; (3a) Description of trial design (such as parallel, factorial) including allocation ratio; (3b) Important changes to methods after trial commencement (such as eligibility criteria), with reasons; (4a) Eligibility criteria for participants; (4b) Settings and locations where the data were collected; [5] The interventions for each group with sufficient details to allow replication, including how and when they were actually administered; (6a) Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed; (6b) Any changes to trial outcomes after the trial commenced, with reasons; (7a) How sample size was determined; (7b) When applicable, explanation of any interim analyses and stopping guidelines; (8a) Method used to generate the random allocation sequence; (8b) Type of randomization; details of any restriction (such as blocking and block size); [9] Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned; [10] Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions; (11a) If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how; (11b) If relevant, description of the similarity of interventions; (12a) Statistical methods used to compare groups for primary and secondary outcomes; (12b) Methods for additional analyses, such as subgroup analyses and adjusted analyses; (13a) For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome; (13b) For each group, losses and exclusions after randomization, together with reasons; (14a) Dates defining the periods of recruitment and follow-up; (14b) Why the trial ended or was stopped; [15] A table showing baseline demographic and clinical characteristics for each group; [16] For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups; 17a) For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval); 17b) For binary outcomes, presentation of both absolute and relative effect sizes is recommended; [18] Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory; [19] All important harms or unintended effects in each group (for specific guidance see CONSORT for harms); [20] Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses; [21] Generalizability (external validity, applicability) of the trial findings; [22] Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence; [23] Registration number and name of trial registry; [24] Where the full trial protocol can be accessed, if available; [25] Sources of funding and other support (such as supply of drugs), role of funders
Meta-regression
The effect of influencing factors was analyzed using a random-effect meta-regression model. There was no effect of quality assessment score, duration of intervention, mean age of participants, dose of intervention, study subjects, and female ration on the heterogeneity of glycemic indices such as FPG, insulin, HOMA-IR, and HbA1c (P > 0.05).
Publication bias
The results of Egger’s test did not support the existence of publication bias through selenium supplementation on FPG (coefficient = 9.41, P = 0.37), insulin (coefficient = 5.12, P = 0.50), HOMA-IR (coefficient = 4.7, P = 0.67), HOMA-B (coefficient = −10.56, P = 0.17), QUICKI (coefficient = 3.71, P = 0.58), HbA1c (coefficient = 12.05, P = 0.7), and adiponectin (coefficient = −22.67, P = 0.3).
Discussion
Our meta-analysis revealed that selenium supplementation resulted in a significant decrease in HOMA-B and a significant decrease in QUICKI in comparison with the placebo group. There were no statistically significant improvements in glycemic indices, such as insulin, FPG, HOMA-IR, HbA1c, and adiponectin.
Results from previous studies conducted on effect of selenium supplementation on glycemic indices may seem to be contradictory. Although Algotar et al.’s study showed that selenium supplementation did not change serum glucose levels [36], Shargorodsky et al.’s study indicated that selenium supplementation combined with other vitamins significantly lowered HbA1c levels in subjects with cardiovascular risk factors [34]. The effect of long-term selenium supplementation on the incidence of T2D was first examined by Stranges et al. (2007) and the results indicated that selenium supplementation did not seem beneficial in the prevention of T2D, and it might increase risk for the disease [37]. Moreover, the results obtained from both non-experimental and experimental studies demonstrated that selenium supplementation may lead to increased risk of T2D. Pooled results from the non-experimental studies revealed a direct relationship between selenium exposure and risk of diabetes. Furthermore, a dose–response meta-analysis of the studies with direct evaluation of dietary selenium intake revealed a similar association in the non-experimental studies [38]. Kohler et al. (2018) conducted a systematic review and meta-analysis and the results showed that a summary odds ratio (OR) for T2D risk in cases consuming selenium was 2.03. However, RCTs of selenium did not indicate a higher risk of T2D in individuals receiving selenium as compared with placebo group. In this study, the associations between selenium and risk of T2D were observed in the observational studies which were not repeated in the RCT studies [39].
Our result is consistent with the findings of Farrokhian et al. who reported that selenium supplementation resulted in a significant decrease in HOMA-B, and a significant increase in QUICKI score. However, selenium supplementation did not change FPG levels [40]. An animal study showed that zin supplementation led to in a significant improvement in insulin sensitivity indices as it increased insulin levels and decreased HOMA-B values [41].
The results in our study were concordant with a recent meta-analysis conducted by Tabrizi et al. who reported that the selenium supplementation led to a significant increase in QUICKI score, but it had no beneficial effects on FPG and HOMA-IR [42]. Study population which selected to evaluate effect of selenium supplementation on glycemic indices is heterogeneous which may justify the heterogeneous findings.
It seems that effect of selenium supplementation on insulin resistance is a controversial issue. Some studies reported that selenium supplementation is not capable of improving the insulin resistance [42, 43], while other studies showed that selenium reduces insulin resistance [12].
The biological plausibility of selenium justifying effect of selenium supplementation on glycemic indices can be attributed to antioxidant properties of selenium. Selenium consists of selenoproteins as selenocysteine has antioxidant properties. Selenoproteins with antioxidant functions contain glutathione peroxidases, which decrease hydrogen peroxide, lipid, glucose and phospholipid hydroperoxides [28]. Recently a meta-analysis conducted on the effect of selenium supplementation on antioxidant markers and the results showed that selenium supplementation could reduce oxidative stress and had a positive effect on glycemic indices [44].
The current meta-analysis was the first study that performed a systematic and quantitative analysis of the relationship between selenium supplementation and glycemic indices, which is a strong point of our practice. Moreover, we recruited only randomized clinical trials, which are the most powerful clinical studies with reliable and valid outcomes. However, some limitations of the power of this analysis should be considered. The number of studies was relatively small (twelve studies); moreover, the duration of supplementation, study population and dosage of supplementation were widely variable in the included studies.
In conclusion, the results of this meta-analysis demonstrated that selenium supplementation led to a significant reduction in HOMA-B and a significant increase in QUICKI score. Moreover, the results of this meta-analysis showed that selenium supplementation had no statistically significant effect on FPG, insulin HOMA-IR, HbA1c, and adiponectin.
Acknowledgements
This study was funded by Alborz University of Medical Sciences. The authors are thankful of Emam Ali clinical research development unit for their assistance.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Mostafa Qorbani and Mehdi Noroozi contributed as corresponding authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Armita Mahdavi Gorabi, Email: armitamahdavi61@gmail.com.
Mostafa Qorbani, Email: mqorbani1379@yahoo.com.
Mehdi Noroozi, Email: noroozimehdi04@gmail.com.
References
- 1.Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48(3):756–775. doi: 10.1007/s12020-014-0477-6. [DOI] [PubMed] [Google Scholar]
- 2.Carlson BA, Yoo M-H, Shrimali RK, Irons R, Gladyshev VN, Hatfield DL, Park JM. Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc. 2010;69(3):300–310. doi: 10.1017/S002966511000176X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LH D. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 2009;41(06):443–447. doi: 10.1055/s-0029-1220724. [DOI] [PubMed] [Google Scholar]
- 4.Hatfield DL, Gladyshev VN. The outcome of selenium and vitamin E Cancer prevention trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9(1):18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann FKW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140(6):1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diplock AT. Antioxidants and disease prevention. Mol Asp Med. 1994;15(4):293–376. doi: 10.1016/0098-2997(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Nève J. Selenium as a risk factor for cardiovascular diseases. J Cardiovasc Risk. 1996;3(1):42–47. [PubMed] [Google Scholar]
- 8.Liu R, Xia L, Zhuoya Z, Min Z, Yue S, Dinglei S, Xuebing F, Xiang G, Songtao S, Wanjun C. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci Rep. 2015;5:12777. doi: 10.1038/srep12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano E, Serini S, Mondella N, Trombino S, Celleno L, Lanza P, Cittadini A, Calviello G. Antioxidant and anti-inflammatory effects of selected natural compounds contained in a dietary supplement on two human immortalized keratinocyte lines. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/327452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Yang S, Zhang N, Mu Y, Ren H, Wang Y, Li K. Long-term supranutritional supplementation with selenate decreases hyperglycemia and promotes fatty liver degeneration by inducing hyperinsulinemia in diabetic db/db mice. PLoS One. 2014;9(7):e101315. doi: 10.1371/journal.pone.0101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell SC, Aldibbiat A, Marriott CE, Landy C, Ali T, Ferris WF, Butler CS, Shaw JA, Macfarlane WM. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett. 2008;582(15):2333–2337. doi: 10.1016/j.febslet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Stranges S, Ana N-A, Rayman MP, Eliseo G. Selenium status and cardiometabolic health: state of the evidence. Nutr Metab Cardiovasc Dis. 2010;20(10):754–760. doi: 10.1016/j.numecd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Stranges S, Marshall James R, Raj N, Donahue Richard P, Maurizio T, Combs Gerald F, Cappuccio Francesco P, Antonio C, Reid Mary E. Effects of long-term selenium supplementation on the incidence of type 2 DiabetesA randomized TrialSelenium supplementation and risk for type 2 diabetes. Ann Intern Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 14.Lippman SM, Klein Eric A, Goodman Phyllis J, Scott LM, Thompson Ian M, Ford Leslie G, Parnes Howard L, Minasian Lori M, Michael GJ, Ann HJ. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E Cancer prevention trial (SELECT) Jama. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Huang K, Lei XG. Selenium and diabetes—evidence from animal studies. Free Radic Biol Med. 2013;65:1548–1556. doi: 10.1016/j.freeradbiomed.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikuei P, Nahid D, Iman T, Ali KA. Predictive value of miR-210 as a novel biomarker for pre-eclampsia: a systematic review protocol. BMJ Open. 2016;6(9):e011920. doi: 10.1136/bmjopen-2016-011920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosravi M, Djalalinia S, Hasani M, Saeedi Moghaddam S, Kazemzadeh Atoofi M, Asayesh H, et al. Effects of selenium supplementation on cardiometabolic risk factors, inflammatory factors and antioxidant factors : a systematic review and meta-analysis protocol. IJPM. 2017; (Submitted). [DOI] [PMC free article] [PubMed]
- 19.Hozo SP, Benjamin D, Iztok H. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10(11):1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intercooled Stata. 8.0 for windows. College Station, TX: Stata Corp; 2003. 2007.
- 24.Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A, Gargari BP. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. 2012;60(2):157–168. doi: 10.1159/000335470. [DOI] [PubMed] [Google Scholar]
- 25.Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: randomized, double-blind, placebo-controlled trial. Nutrition. 2015;31(10):1235–1242. doi: 10.1016/j.nut.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Bahmani F, Kia M, Soleimani A, Asemi Z, Esmaillzadeh A. Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol Trace Elem Res. 2016;172(2):282–289. doi: 10.1007/s12011-015-0600-4. [DOI] [PubMed] [Google Scholar]
- 27.Faghihi T, Radfar M, Barmal M, Amini P, Qorbani M, Abdollahi M, Larijani B. A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: effects on glucose homeostasis, oxidative stress, and lipid profile. Am J Ther. 2014;21(6):491–495. doi: 10.1097/MJT.0b013e318269175f. [DOI] [PubMed] [Google Scholar]
- 28.Farrokhian A, Bahmani F, Taghizadeh M, Mirhashemi SM, Aarabi MH, Raygan F, Aghadavod E, Asemi Z. Selenium supplementation affects insulin resistance and serum hs-CRP in patients with type 2 diabetes and coronary heart disease. Horm Metab Res. 2016;48(04):263–268. doi: 10.1055/s-0035-1569276. [DOI] [PubMed] [Google Scholar]
- 29.Faure P, Ramon O, Favier A, Halimi S. Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Investig. 2004;34(7):475–481. doi: 10.1111/j.1365-2362.2004.01362.x. [DOI] [PubMed] [Google Scholar]
- 30.Jamilian M, Razavi M, Fakhrie Kashan Z, Ghandi Y, Bagherian T, Asemi Z. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2015;82(6):885–891. doi: 10.1111/cen.12699. [DOI] [PubMed] [Google Scholar]
- 31.Mao J, Bath SC, Vanderlelie JJ, Perkins AV, Redman CWG, Rayman MP. No effect of modest selenium supplementation on insulin resistance in UK pregnant women, as assessed by plasma adiponectin concentration. Br J Nutr. 2016;115(1):32–38. doi: 10.1017/S0007114515004067. [DOI] [PubMed] [Google Scholar]
- 32.Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW, Taylor AW, Traber MG, Zimmermann MB. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144(2):193–201. doi: 10.3945/jn.113.185561. [DOI] [PubMed] [Google Scholar]
- 33.Rayman MP, Blundell-Pound G, Pastor-Barriuso R, Guallar E, Steinbrenner H, Stranges S. A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS One. 2012;7(9):e45269. doi: 10.1371/journal.pone.0045269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond) 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammad Hosseinzadeh F, Hosseinzadeh-Attar MJ, Yekaninejad MS, Rashidi B. Effects of selenium supplementation on glucose homeostasis and free androgen index in women with polycystic ovary syndrome: a randomized, double blinded, placebo controlled clinical trial. J Trace Elem Med Biol. 2016;34:56–61. doi: 10.1016/j.jtemb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Algotar AM, Stratton MS, Stratton SP, Hsu C-H, Ahmann FR. No effect of selenium supplementation on serum glucose levels in men with prostate cancer. Am J Med. 2010;123(8):765–768. doi: 10.1016/j.amjmed.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stranges S, Marshall James R, Raj N, Donahue Richard P, Maurizio T, Combs Gerald F, Cappuccio Francesco P, Antonio C, Reid Mary E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 38.Vinceti M, Filippini T, Rothman KJ. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Springer. 2018. [DOI] [PubMed]
- 39.Kohler L, Janet F, Connor K, Ana F, Colleen S, Chow HH, Paul H, Ken B, Nathan E, Kathylynn S. Selenium and Type 2 Diabetes: Systematic Review. Nutrients. 2018;10(12):1924. doi: 10.3390/nu10121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrokhian A, Bahmani F, Taghizadeh M, Mirhashemi SM, Aarabi MH, Raygan F, Aghadavod E, Asemi Z, Hormone J. Research metabolic. Selenium supplementation affects insulin resistance and serum hs-CRP in patients with type 2 diabetes and coronary. Heart Dis. 2016;48(04):263–268. doi: 10.1055/s-0035-1569276. [DOI] [PubMed] [Google Scholar]
- 41.Barman S, Krishnapura S. Zinc supplementation alleviates hyperglycemia and associated metabolic abnormalities in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2016;94(12):1356–1365. doi: 10.1139/cjpp-2016-0084. [DOI] [PubMed] [Google Scholar]
- 42.Tabrizi R, Maryam A, Mahmood M, Lankarani Kamran B, Taghi HS, Fariba K, Akbar MA, Azade S, Bita B, Mehri J. The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2017;49(11):826–830. doi: 10.1055/s-0043-119544. [DOI] [PubMed] [Google Scholar]
- 43.Katsuki A, Yasuhiro S, Gabazza Esteban C, Shuichi M, Masahiko F, Rika A-S, Yasuko H, Yutaka Y, Yukihiko A. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24(2):362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 44.W J, Li X, Li Z, Wu GR, Fu XF, Yang XM, Zhang XQ, Gao XB. The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. 2017;44:8–16. doi: 10.1016/j.jtemb.2017.04.009. [DOI] [PubMed] [Google Scholar]