Abstract
Background
Lymph node status of clinical T1 (diameter ≤ 3 cm) lung cancer largely affects the treatment strategies in the clinic. In order to assess lymph node status before operation, we aim to develop a noninvasive predictive model using preoperative clinical information.
Methods
We retrospectively reviewed 924 patients (development group) and 380 patients (validation group) of clinical T1 lung cancer. Univariate analysis followed by polytomous logistic regression was performed to estimate different risk factors of lymph node metastasis between N1 and N2 diseases. A predictive model of N2 metastasis was established with dichotomous logistic regression, externally validated and compared with previous models.
Results
Consolidation size and clinical N stage based on CT were two common independent risk factors for both N1 and N2 metastases, with different odds ratios. For N2 metastasis, we identified five independent predictors by dichotomous logistic regression: peripheral location, larger consolidation size, lymph node enlargement on CT, no smoking history, and higher levels of serum CEA. The model showed good calibration and discrimination ability in the development data, with the reasonable Hosmer-Lemeshow test (p = 0.839) and the area under the ROC being 0.931 (95% CI: 0.906-0.955). When externally validated, the model showed a great negative predictive value of 97.6% and the AUC of our model was better than other models.
Conclusion
In this study, we analyzed risk factors for both N1 and N2 metastases and built a predictive model to evaluate possibilities of N2 metastasis of clinical T1 lung cancers before the surgery. Our model will help to select patients with low probability of N2 metastasis and assist in clinical decision to further management.
1. Introduction
Preoperative staging of patients with malignant lung cancer suggests the prognosis and the life quality afterwards. An accurate clinical staging can guide physicians to choose a proper treatment according to the authorized guideline and therefore standardizes the management procedure. Especially for those with positive mediastinal lymph nodes (N2 disease), preoperative chemotherapy is reported to reduce tumor size by 25% [1], downstage nearly half of the N2-positive patients [2–6], and increase the 5-year survival rate of 5-20% compared with surgery alone [7–11]. In that case, the accuracy of TNM staging before surgery is of paramount important.
The European Society of Thoracic Surgeons (ESTS) guidelines compared the diagnostic accuracy of different preoperative examinations for lymph node evaluation. Computed tomography is common and available in most countries, despite its low sensitivity (55%) and specificity (81%) [12, 13]. PET-CT scan is reported to be superior to CT in mediastinal lymph node staging and exhibits a high negative predictive value (NPV) for peripheral tumors. The sensitivity of PET-CT is 80-90%, and the specificity is 85-95% [12, 13]. However, PET-CT requires more expensive facilities and is not as popularized as CT. Besides, the negative predictive value of PET-CT decreases in patients with central tumors, tumors > 3 cm, and suspected N1 metastasis [12].
Reported data shows the prevalence of occult N2 disease in patients with clinical stage I NSCLC is about 5.0-6.5% [14, 15]. In order not to omit this part of patients, a predictive model in combination of assisted examination is needed and previous efforts have been made by researchers. In this study, we aim to analyze the clinical features of patients with lymph node metastasis and create a predicted formula of N2 metastasis for clinical T1 lung cancers.
2. Methods
2.1. Patients
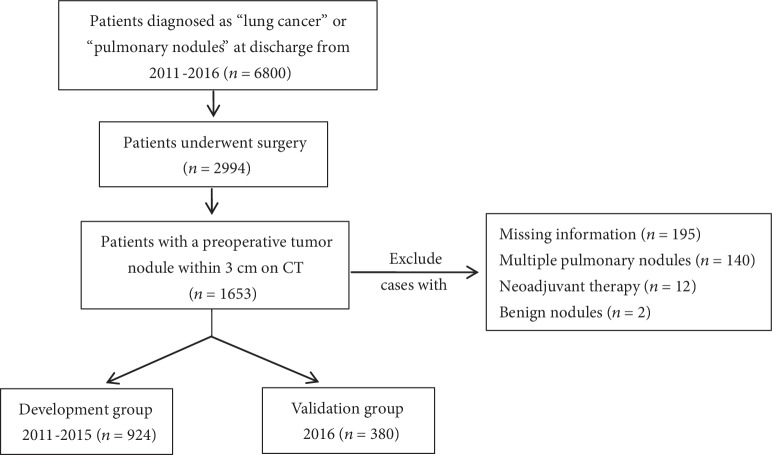
We retrospectively reviewed patients who were diagnosed with lung cancer and underwent radial surgical recession in Second Affiliated Hospital of Zhejiang University (SAHZU) during 2011-2016. Patients with a malignant nodule within 3 centimeters on CT (staged as cT1) were selected, all of which underwent lymph node evaluation via surgical operation. The exclusion criteria were as follows: (1) patients with multiple pulmonary cancers or metastatic pulmonary nodules, (2) patients with a history of preoperative therapy, and (3) patients without CT scan images before surgery. Patients from 2011 to 2015 were enrolled in the development group (n = 924), while patients from 2016 were included in the validation group (n = 380), as shown in Figure 1. This study was approved by the Institutional Ethics of Committee of SAHZU (2017-031).
Figure 1.
Flowchart of patient selection and exclusion.
2.2. Clinicopathological Variables
All the clinicopathological information was collected in the hospital information system (HIS). Information included gender, age, symptoms at presentation, smoking history, smoking index, chronic pulmonary diseases, cancer history, family history of cancer, levels of tumor markers within one month before surgery, histological type of lung cancer, pathological report of resected lymph nodes, tumor location (upper/middle/lower lobe, central/peripheral location), tumor size, consolidation size, C/T ratio (consolidation size/tumor size), and clinical N stage based on CT. Chronic pulmonary diseases included chronic bronchitis, emphysema, and chronic obstructive pulmonary disease (COPD). Tumor size was measured as the largest dimension on CT section in pulmonary window while consolidation size was measured in mediastinal window. Tumors were defined as peripherally located if the center of tumor mass was in the outer one-thirds of pulmonary parenchyma and otherwise as centrally located. A lymph node was considered an enlarged one when its short axis exceeded 1 cm. The seventh edition of TNM classification was referred to in this study.
2.3. Data Analysis
All the continuous variables were described with means and standard deviations, while categorical variables were described with frequencies. In univariate analysis, we performed one-way analysis of variance for continuous variables and Pearson's chi-square tests (adjusted p values using Bonferroni method) for categorical variables. Significant variables in the univariate analysis were further analyzed in multivariate analysis using polytomous logistic regression, in order to estimate different risk factors and odds ratios for each N stage (pN0, pN1, and pN2).
The dichotomous logistic regression was performed to build a predictive model for N2 metastasis, since N2 metastasis is worse in TNM staging and requires different preoperative treatment strategies. All variables collected from HIS were analyzed with forward stepwise selection, which was based on statistics of a conditional likelihood ratio test. A significant p value for entering variables was 0.05, and the p value for excluding variables was 0.10. The optimal cutoff point of the model was set according to the highest Youden's index. A nomogram was developed using the package of rms based on the logistic regression. In addition, calibration of the model was established with the Hosmer-Lemeshow goodness-of-fit test as well as the calibration curve, and the discrimination ability of the model was assessed by receiver operating characteristic (ROC) analysis. The DeLong test was performed for the comparison of different ROC curves.
All statistical analysis was performed using SPSS Statistics 22.0 (IBM Armonk, NY, USA), EmpowerStats software (X&Y Solutions, Boston, USA, http://www.empowerstats.com/), and R 3.5.2 software (R Foundation for Statistical Computing, Vienna, Austria). We considered the differences as statistically significant when two-sided p values were less than 0.05.
3. Results
3.1. Clinicopathological Characteristics for Patients in the Development Group
The clinicopathological characteristics of 924 patients in the development group are shown in Table 1. Patients were at a mean age of 59.1 ± 9.7, and tumor sizes were 1.70 ± 0.62 cm on average. The incidence for lymph node metastasis was 10.82% (100/924), with N1 metastasis being 3.24% (30/924) and N2 metastasis being 7.58% (70/924).
Table 1.
Characteristics of patients in the development group.
| Patients with negative LNs (%) | Patients with positive N1 nodes (%) | Patients with positive N2 nodes (%) | p value∗ | |
|---|---|---|---|---|
| Age (year) | 59.1 ± 9.7 | 58.1 ± 10.0 | 58.9 ± 10.4 | 0.742 |
| Gender | ||||
| Male | 346 (85.4) | 21 (5.2) | 38 (9.4) | 0.011 |
| Female | 478 (92.1) | 9 (1.7) | 32 (6.2) | |
| Symptoms | ||||
| RCE | 456 (91.9) | 8 (1.6) | 32 (6.5) | 0.010 |
| RCRS | 256 (85.6) | 16 (5.4) | 27 (9.0) | |
| ICD | 112 (86.8) | 6 (4.7) | 11 (8.5) | |
| Asymptomatic | 568 (90.9) | 14 (2.2) | 43 (6.9) | 0.008 |
| Symptomatic | 256 (85.6) | 16 (5.4) | 27 (9.0) | |
| Cancer history | ||||
| Yes | 58 (85.3) | 4 (5.9) | 6 (8.8) | 0.454 |
| No | 766 (89.5) | 26 (3.0) | 64 (7.5) | |
| Family history of cancer | ||||
| Yes | 122 (91.0) | 1 (0.8) | 11 (8.2) | 0.169 |
| No | 702 (88.9) | 29 (3.7) | 59 (7.4) | |
| Pathology | ||||
| Adenocarcinoma | 760 (91.2) | 16 (1.9) | 57 (6.9) | <0.001 |
| Squamous | 52 (75.4) | 10 (14.5) | 7 (10.1) | |
| Adenosquamous | 3 (37.5) | 1 (12.5) | 4 (50.0) | |
| Neuroendocrine | 8 (66.7) | 3 (25.0) | 1 (8.3) | |
| Other tumor type | 1 (50.0) | 0 (0.0) | 1 (50.0) | |
| Smoking history | ||||
| Yes | 220 (85.3) | 17 (6.6) | 21 (8.1) | 0.001 |
| No | 604 (90.7) | 13 (2.0) | 49 (7.3) | |
| Location | ||||
| Upper lobe | 445 (90.1) | 14 (2.8) | 35 (7.1) | 0.670 |
| Lower lobe | 270 (88.8) | 9 (3.0) | 25 (8.2) | |
| Middle lobe | 109 (86.5) | 7 (5.6) | 10 (7.9) | |
| Central | 316 (86.6) | 18 (4.9) | 31 (8.5) | 0.042 |
| Peripheral | 508 (90.9) | 12 (2.1) | 39 (7.0) | |
| Nodule size on CT | ||||
| Tumor size (cm) | 1.63 ± 0.59 | 2.25 ± 0.57 | 2.33 ± 0.50 | <0.001 |
| Consolidation size (cm) | 0.91 ± 0.83 | 2.19 ± 0.69 | 2.22 ± 0.59 | <0.001 |
| C/T ratio | 0.51 ± 0.41 | 0.95 ± 0.19 | 0.95 ± 0.15 | <0.001 |
| Chronic pulmonary disease | ||||
| Yes | 68 (78.2) | 8 (9.2) | 11 (12.6) | 0.001 |
| No | 756 (90.3) | 22 (2.6) | 59 (7.1) | |
| Clinical nodal stage on CT | ||||
| Enlarged LNs in N2 station | 72 (64.3) | 7 (6.3) | 33 (29.4) | <0.001 |
| Enlarged LNs in N1 station | 10 (38.5) | 6 (23.0) | 10 (38.5) | |
| Normal-sized LNs | 742 (94.4) | 17 (2.2) | 27 (3.4) | |
| Levels of tumor markers | ||||
| CEA (ng/ml) | 3.26 ± 4.84 | 3.55 ± 2.56 | 10.21 ± 17.58 | <0.001 |
| AFP (ng/ml) | 3.03 ± 1.80 | 2.68 ± 0.89 | 3.25 ± 2.71 | 0.542 |
| CA199 (U/ml) | 10.48 ± 15.24 | 9.99 ± 9.26 | 15.67 ± 17.16 | 0.013 |
| CA125 (U/ml) | 11.31 ± 11.22 | 13.23 ± 10.04 | 26.53 ± 77.33 | 0.093 |
| CA242 (U/ml) | 5.42 ± 4.34 | 4.80 ± 3.22 | 5.54 ± 3.31 | 0.996 |
| CA211 (ng/ml) | 1.12 ± 0.84 | 1.49 ± 1.07 | 1.36 ± 1.01 | 0.006 |
| NSE (ng/ml) | 9.58 ± 4.55 | 8.48 ± 4.87 | 9.99 ± 5.32 | 0.793 |
| SCC (ng/ml) | 0.84 ± 0.83 | 1.15 ± 0.78 | 0.93 ± 0.59 | 0.143 |
RCE: routine chest examination; RCRS: respiratory- or cancer-related symptoms; ICD: incidental chest discovery; C/T ratio: consolidation size/tumor size ratio. ∗p value acquired from one-way analysis of variance and Pearson's chi-square tests.
In univariate analysis (Table 1), lymph node metastasis was prone to be found in smoking males who suffered from chronic pulmonary diseases and were hospitalized with respiratory- or cancer-related symptoms (RCRS) and higher levels of carcinoembryonic antigen (CEA). Tumors with larger size (or consolidation size), central location, and lymph node enlargement on CT images were associated with higher likelihood of lymph node metastasis. Besides, patients with squamous carcinoma were more likely to have N1 metastasis, while N2 metastasis in patients with adenocarcinoma was three times more likely to occur than N1 metastasis.
3.2. Odds Ratios of N1 and N2 Metastases versus N0 Status
In polytomous logistic regression (Table 2), significant variables in univariate analysis were further analyzed to estimate the risk factors and odds ratios of nodal metastasis stratified by the 7th TNM staging. Significantly elevated odds ratios were seen in tumors with larger consolidation size and lymph node enlargement on CT for N1 metastasis (ORconsolidation size = 5.449, 95% CI: 2.817-10.541; ORlymph node enlargement on CT = 11.424, 95% CI: 3.316-39.360) and N2 metastasis (ORconsolidation size = 8.640, 95% CI: 5.002-14.923; ORlymph node enlargement on CT = 8.703, 95% CI: 4.326-17.509) compared to N0 status. A significantly decreased odds ratio was seen in smokers for N2 metastasis (ORsmoking history = 0.217, 95% CI: 0.080-0.590) compared to N0 status in nonsmokers. Tumors with a central location seemed to have a negative correlation with N2 metastasis though there was no significant difference.
Table 2.
Odds ratios of likelihood of lymph node metastasis stratified by seventh TNM staging using polytomous logistic regression.
| Variable | N1 metastasis (n = 30) | N2 metastasis (n = 70) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value# | Odds ratio (95% CI) | p value# | |
| Male gender | 2.366 (0.726-7.707) | 0.153 | 2.019 (0.868-4.697) | 0.103 |
| Chronic pulmonary disease | 1.827 (0.665-5.021) | 0.242 | 1.231 (0.489-3.102) | 0.659 |
| Smoking history | 0.479 (0.138-1.663) | 0.246 | 0.217 (0.080-0.590) | 0.003 |
| Respiratory- or cancer-related symptoms | 1.558 (0.692-3.508) | 0.284 | 0.741 (0.374-1.467) | 0.389 |
| Adenocarcinoma histology | 1.742 (0.619-4.901) | 0.293 | 0.849 (0.330-2.181) | 0.733 |
| Consolidation size (cm) | 5.449 (2.817-10.541) | <0.001 | 8.640 (5.002-14.923) | <0.001 |
| Central location | 1.069 (0.448-2.547) | 0.881 | 0.508 (0.255-1.014) | 0.055 |
| Clinical nodal stage on CT | ||||
| Enlarged LNs in N1 station | 11.424 (3.316-39.360) | <0.001 | 14.046 (4.226-46.682) | <0.001 |
| Enlarged LNs in N2 station | 1.615 (0.582-4.480) | 0.357 | 8.703 (4.326-17.509) | <0.001 |
| Levels of serum CEA (ng/ml) | 0.932 (0.806-1.077) | 0.340 | 1.063 (1.027-1.099) | 0.001 |
# p value represented the comparison with N0 patients.
3.3. Logistic Regression Model and Predictors of N2 Metastasis
Dichotomous logistic regression identified five independent predictors for N2 metastasis: peripheral location, consolidation size, lymph node enlargement on CT, no smoking history, and levels of serum CEA (Table 3). Gender, histological type, and C/T ratio were not involved as significant factors. The formula predicting N2 metastasis for small tumor nodules was established: ex/(1 + ex), x = −0.756×central location + 1.921×consolidation size + 2.145×lymph node enlargement on CT − 1.065×smoking history + 0.064×CEA level − 6.165. The unit for “consolidation size” is cm and for “CEA level” is ng/ml. The value of “lymph node enlargement on CT,” “central location,” and “smoking history” should be 1 for yes and otherwise 0. A nomogram predicting the probability for N2 metastasis in cT1 patients was developed on the basis of multivariate logistic analysis (Figure 2).
Table 3.
Multivariate dichotomous logistic regression of the development group for predicting N2 metastasis.
| Variable | Regression coefficient | p value | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Central location | -0.756 | 0.029 | 0.469 | 0.239 | 0.924 |
| Consolidation size (cm) | 1.921 | <0.001 | 6.824 | 4.095 | 11.373 |
| Enlarged lymph node on CT | 2.145 | <0.001 | 8.546 | 4.491 | 16.262 |
| Smoking history | -1.065 | 0.003 | 0.345 | 0.169 | 0.704 |
| Level of serum CEA (ng/ml) | 0.064 | <0.001 | 1.066 | 1.031 | 1.102 |
Figure 2.
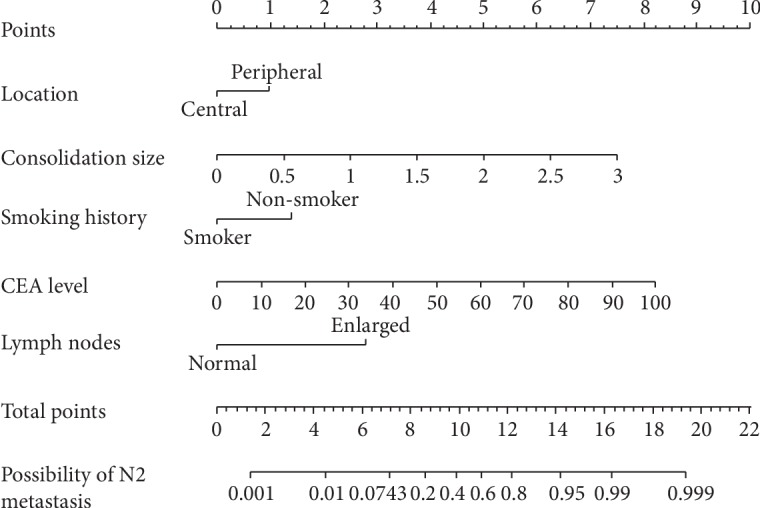
Nomogram predicting the likelihood of N2 metastasis in early lung cancers (tumor ≤ 3 cm). According to the location of value from the 2nd to the 6th axis, we can get the vertically corresponding points on the first axis. By summing up each points, we get a total point, and the vertically corresponding predicted value on the last axis shows the predicted possibility of N2 metastasis.
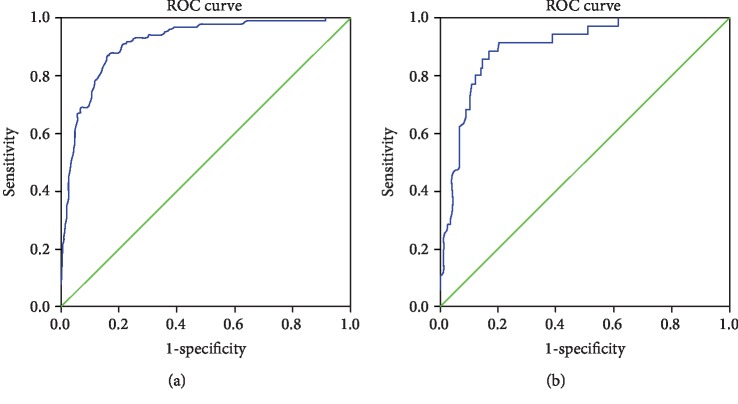
The Hosmer-Lemeshow goodness-of-fit test, which was not statistically significant (p = 0.839), indicated that the predicted probability was of high concordance to the observed probability. A calibration curve is shown in Figure 3. The area under the receiver operating characteristic curve was 0.931, with 95% confidence interval between 0.906 and 0.955 (Figure 4(a)). We selected the numerical value with the highest Youden's index as our cutoff point for the predicted probability (cutoff for probability = 7.43%).
Figure 3.
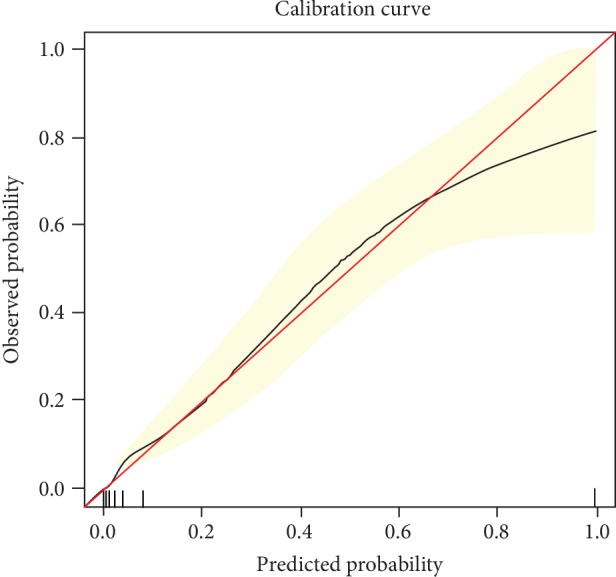
Calibration curve of the logistic regression model. The red line indicated a perfect prediction of observed possibilities. The black line represented the entire development group (n = 924).
Figure 4.
The receiver operating characteristic curve for the development and validation groups. (a) The ROC curve for the development group. The AUC was 0.931 (95% CI: 0.906-0.955). (b) The ROC curve for the validation group. The AUC was 0.906 (95% CI: 0.857-0.956).
3.4. Validation of the Model and Comparison with Previous Models
The characteristics of patients in the validation group were shown in Supplementary . In the external validation, the AUC of our model was 0.906 (95% CI: 0.857-0.956, Figure 4(b)). With the cutoff point set above (cutoff = 7.43%), we tested our model in the validation group. The sensitivity and specificity were 60.0% and 90.3%, respectively. The negative and positive predictive values (NPV and PPV) were 97.6% and 25.5%, respectively. In a subgroup analysis of adenocarcinoma (ADC) and squamous cell carcinoma (SCC), the validated AUC of ADC patients was 0.856 (95% CI: 0.790-0.922) and the validated AUC of SCC patients was 0.864 (95% CI: 0.777-0.952) (p = 0.885, DeLong test).
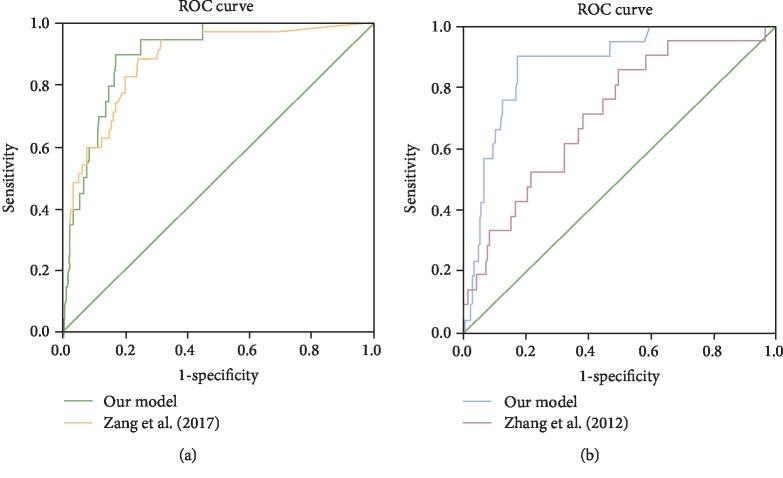
We also compared our model with the Fudan model [16] and Beijing model [17], as all three studies included clinical T1 NSCLC. Analyzed with all the data from our validation group, the validated AUC of the Beijing model was 0.879 (95% CI: 0.821-0.937) compared with 0.906 (95% CI: 0.857-0.956) of our model (p = 0.405, DeLong test). Based on the inclusion criteria of the Fudan model, patients with cT1N0M0 lung cancers were selected from the validation group of our study. And the validated AUC of the Fudan model was 0.712 (95% CI: 0.602-0.822) while the AUC of our model was 0.885 (95% CI: 0.820-0.949) (p = 0.002, DeLong test). Our model showed a larger area under the ROC curve compared to other models (Figure 5).
Figure 5.
Comparison of our model and other published models using data from the same validation group. (a) Comparison with Zang et al. (2017) in cT1NxM0 patients. The AUC was 0.879 validated by our data (95% CI: 0.821-0.937). DeLong test for comparing two ROC curves: p = 0.405. (b) Comparison of our model with Zhang et al. (2012) in cT1N0M0 patients. The AUC was 0.712 validated by our data (95% CI: 0.602-0.822). DeLong test for comparing two ROC curves: p = 0.002.
4. Discussion
Lymph node status, especially the assessment of N2 metastasis, largely affects the treatment strategies in the clinic. Therefore, it is of great significance to make an accurate and noninvasive assessment of lymph nodes before operation. In this study, we established a five-variable formula predicting N2 metastasis for malignant nodules within 3 cm. Our model showed a high negative predictive value of 97.6% and specificity of 90.3%, which can select patients with low risks of N2 metastasis and help with the clinical decision-making.
As a truly multidisciplinary process, preoperative evaluation of lymph node evaluation has confused clinical physicians for many years. An algorithm that integrates imaging, endoscopic, and surgical techniques recommended by ESTS guidelines has been widely practiced and prospectively validated, with the negative predictive value as high as 0.94 [18]. However, some researchers are more interested in creating a predictive model ahead of biopsy strategy [16, 17, 19–21], because the accuracy of preoperative invasive staging such as TBNA may largely depend on the experience of operators.
Shafazand and Gould reported the first quantitative model to pretest the probability for N2 metastasis in NSCLC of all stages [20]. The formula consisted of six independent predictors, which were age, tumor size, central location, adenocarcinoma histology, onset of primary symptoms, and abnormal mediastinum on chest X-ray. However, their data was directly collected from a previous randomized controlled trial and no CT images were included at that time. After that, Zhang and colleagues reported a four-predictor model for N2 metastasis in CT-defined T1N0M0 NSCLC in 2012 [16]. Younger patients with a central-located and larger-sized lung adenocarcinoma had higher risks of N2 disease. However, patients with a histology of AIS (adenocarcinoma in situ) and MIA (microinvasive adenocarcinoma) were excluded from their study, despite the fact that the pathology of AIS or MIA could only be confirmed from a resected specimen. In that case, the percentage of adenocarcinoma might be underestimated in their model because there will be AIS and MIA patients in reality. More recently, there were predictive models evaluating N2 metastasis for NSCLC of all stages [21] and models estimating nodal metastasis in clinical T1a stages [17].
No models above have referred to the different risk factors of N1 and N2 metastases. Analyzed by polytomous logistic regression, we found that consolidation tumor size and lymph node enlargement on CT scan were the most related factors to both N1 and N2 metastases in patients with early malignant nodules (diameter ≤ 3 cm, stage T1). Though it is difficult to differentiate benign lymphadenectasis from lymph node metastasis on CT, our results showed that lymphadenectasis in N1 station was of higher correlation to N2 metastasis. This could be explained by the lymphatic drainage, and the rate for skip N2 metastasis was only 29% [22]. This result was partly in accordance with the previous literature and the ESTS recommendation [13, 23].
In both polytomous and dichotomous logistic analyses, consolidation tumor size and lymph node enlargement on CT and CEA levels were correlated to N2 metastasis, which is consistent with previous studies [16, 17, 24, 25]. Smoking history seemed to be negatively associated with N2 disease, as the odds ratio was less than 1 in both analyses. Despite the lack of molecular mechanisms, nonsmokers are more prone to a delayed or incidental detection of lung cancer than smokers and thus are more likely to progress into nodal metastasis, as supported by data from Lee et al. [26]. Apart from that, tumors with peripheral location were found with a higher likelihood of N2 metastasis in this study. The inconsistency between different research studies [27, 28] could result from the different criteria of the definition as “central location” and the different target population. Takeda et al. also found that peripheral tumors are more likely to have N2 metastasis by subpleural lymph drainage pathways [29].
Compared with previous logistic analysis, this study exhibited a larger sample size and reduced selective bias by enrolling patients with all pathological type including AIS and MIA, which constituted 8.2% and 16.7% of ground-glass nodules in the development group. Our data suggested that consolidation size was a stronger predictive factor of nodal metastasis compared with tumor size and C/T ratio in the multivariate analysis. Squamous cell carcinoma also fit in with this model though it was a minority type of histology. Besides, pathological type was not an independent factor in this multivariate model, suggesting that preoperative histology might not be a necessity for predicting N2 metastasis.
Nevertheless, this study also had several limitations. Firstly, it was a retrospective study and there was no standard on the number of resected lymph nodes. In 2014, American College of Surgeons Commission on Cancer recommended at least 10 regional lymph nodes to be removed and pathologically examined for resectable NSCLC [30]. Thus, a diagnostic bias might occur in our study. Secondly, we only collected data from a single-center institution and reflected patient characteristics in local areas. Finally, in order to ensure the general use of the model, the proportion of lymph node metastasis in this study was coherent with the prevalence in reality, which was insufficient and influenced the positive predictive value of the model. Therefore, a larger-sized study with more positive data from multiple medical centers will be needed to carry out a more practical model for clinical use.
5. Conclusions
In this study, we analyzed the clinical features of patients with lymph node metastasis and produced a model predicting the possibility of N2 nodal metastasis for early lung cancers (tumor ≤ 3 cm). Stratified by the cutoff point, a low predicted probability may suggest an operation directly without neoadjuvant therapies, while a relatively high predicted probability needs support from further invasive and expensive examinations. Our model will provide some clues for clinical decision-making.
Acknowledgments
The study was supported by the General Project (U1609220, 81470212, 81472171, and 81502565) and the Key Science Project (2016YFC0902300) from the National Natural Science Foundation of China.
Contributor Information
Pingli Wang, Email: pingliwang@zju.edu.cn.
Kai Wang, Email: kaiw@zju.edu.cn.
Data Availability
All relevant data are within the article and the supplementary materials.
Ethical Approval
This study was approved by the Institutional Ethics of Committee of SAHZU (2017-031).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Authors' Contributions
Chengyan Zhang and Guanchao Pang contributed equally to this work.
Supplementary Materials
Supplementary Table 1: characteristics of patients in the validation group.
References
- 1.Bilfinger T., Keresztes R., Albano D., Nemesure B. Five-year survival among stage IIIA lung cancer patients receiving two different treatment modalities. Medical Science Monitor. 2016;22:2589–2594. doi: 10.12659/msm.898675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Meerkbeeck J., Van Schil P., Kramer G., et al. Pr5 A randomized trial of radical surgery (S) versus thoracicradiotherapy (TRT) in patients (pts) with stage IIIA-N2 non-small cell lung cancer (NSCLC) after response to induction chemotherapy (ICT) (EORTC 08941) Lung Cancer. 2005;49, article S4(Supplement 2) doi: 10.1016/S0169-5002(05)80131-X. [DOI] [Google Scholar]
- 3.Albain K. S., Swann R. S., Rusch V. R., et al. Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer (NSCLC): outcomes update of North American Intergroup 0139 (RTOG 9309) Journal of Clinical Oncology. 2005;23(16):7014–7014. doi: 10.1200/jco.2005.23.16_suppl.7014. [DOI] [Google Scholar]
- 4.Bueno R., Richards W. G., Swanson S. J., et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. The Annals of Thoracic Surgery. 2000;70(6):1826–1831. doi: 10.1016/s0003-4975(00)01585-x. [DOI] [PubMed] [Google Scholar]
- 5.Betticher D. C., Hsu Schmitz S. F., Tötsch M., et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. Journal of Clinical Oncology. 2003;21(9):1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Lorent N., de Leyn P., Lievens Y., et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Annals of Oncology. 2004;15(11):1645–1653. doi: 10.1093/annonc/mdh435. [DOI] [PubMed] [Google Scholar]
- 7.Roth J. A., Atkinson E. N., Fossella F., et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21(1):1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R., Gomez-Codina J., Camps C., et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer. 1999;26(1):7–14. doi: 10.1016/s0169-5002(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 9.Depierre A., Westeel V., Milleron B., et al. O-211 5-year results of the French randomized study comparing preoperative chemotherapy followed by surgery and primary surgery in resectable stage I (except T1N0), II and IIIA non-small cell lung cancer. Lung Cancer. 2003;41:S62–S62. doi: 10.1016/S0169-5002(03)91869-1. [DOI] [Google Scholar]
- 10.Gilligan D., Nicolson M., Smith I., et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. The Lancet. 2007;369(9577):1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 11.NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. The Lancet. 2014;383(9928):1561–1571. doi: 10.1016/s0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Leyn P., Dooms C., Kuzdzal J., et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. European Journal of Cardio-Thoracic Surgery. 2014;45(5):787–798. doi: 10.1093/ejcts/ezu028. [DOI] [PubMed] [Google Scholar]
- 13.De Leyn P., Lardinois D., Van Schil P. E., et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. European Journal of Cardio-Thoracic Surgery. 2007;32(1):1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 14.Meyers B. F., Haddad F., Siegel B. A., et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage I lung cancer. The Journal of Thoracic and Cardiovascular Surgery. 2006;131(4):822–829.e2. doi: 10.1016/j.jtcvs.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Lee P. C., Port J. L., Korst R. J., Liss Y., Meherally D. N., Altorki N. K. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. The Annals of Thoracic Surgery. 2007;84(1):177–181. doi: 10.1016/j.athoracsur.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Sun Y., Xiang J., Zhang Y., Hu H., Chen H. A prediction model for N2 disease in T1 non-small cell lung cancer. The Journal of Thoracic and Cardiovascular Surgery. 2012;144(6):1360–1364. doi: 10.1016/j.jtcvs.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Zang R. C., Qiu B., Gao S. G., He J. A model predicting lymph node status for patients with clinical stage T1aN0-2M0 nonsmall cell lung cancer. Chinese Medical Journal. 2017;130(4):398–403. doi: 10.4103/0366-6999.199838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Leyn P., Dooms C., Kuzdzal J., et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Translational Lung Cancer Research. 2014;3(4):225–233. doi: 10.3978/j.issn.2218-6751.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connell O. J., Almeida F. A., Simoff M. J., et al. A prediction model to help with the assessment of adenopathy in lung cancer: HAL. American Journal of Respiratory and Critical Care Medicine. 2017;195(12):1651–1660. doi: 10.1164/rccm.201607-1397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafazand S., Gould M. K. A clinical prediction rule to estimate the probability of mediastinal metastasis in patients with non-small cell lung cancer. Journal of Thoracic Oncology. 2006;1(9):953–959. [PubMed] [Google Scholar]
- 21.Chen K., Yang F., Jiang G., Li J., Wang J. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. The Annals of Thoracic Surgery. 2013;96(5):1761–1768. doi: 10.1016/j.athoracsur.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Misthos P., Sepsas E., Athanassiadi K., Kakaris S., Skottis I. Skip metastases: analysis of their clinical significance and prognosis in the IIIA stage of non-small cell lung cancer. European Journal of Cardio-Thoracic Surgery. 2004;25(4):502–508. doi: 10.1016/j.ejcts.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Martínez A. F. H., Jiménez M. D. G., Vicente A. G., et al. Ratio between maximum standardized uptake value of N1 lymph nodes and tumor predicts N2 disease in patients with non-small cell lung cancer in 18F-FDG PET-CT scan. Revista Española de Medicina Nuclear e Imagen Molecular. 2016;35(3):159–164. doi: 10.1016/j.remn.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Koike T., Koike T., Yamato Y., Yoshiya K., Toyabe S. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. Journal of Thoracic Oncology. 2012;7(8):1246–1251. doi: 10.1097/JTO.0b013e31825871de. [DOI] [PubMed] [Google Scholar]
- 25.Xiong J., Wang R., Sun Y., Chen H. Clinical analysis of sixty-four patients with T1aN2M0 stage non-small cell lung cancer who had undergone resection. Thorac Cancer. 2016;7(2):215–221. doi: 10.1111/1759-7714.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J. Y., Na I. I., Jang S. H., et al. Differences in clinical presentation of non-small cell lung cancer in never-smokers versus smokers. Journal of Thoracic Disease. 2013;5(6):758–763. doi: 10.3978/j.issn.2072-1439.2013.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao S. J., Kim A. W., Puchalski J. T., et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer. 2017;109:36–41. doi: 10.1016/j.lungcan.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Caro A., Garcia S., Reguart N., et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. European Journal of Cardio-Thoracic Surgery. 2010;37(5):1168–1174. doi: 10.1016/j.ejcts.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Takeda A. H., Watanabe Y., Nagata T., et al. Detection of alternative subpleural lymph flow pathways using indocyanine green fluorescence. Surgery Today. 2018;48(6):640–648. doi: 10.1007/s00595-018-1631-1. [DOI] [PubMed] [Google Scholar]
- 30.American College of Surgeons Commission on Cancer. CoC quality of care measures, non-small cell lung cancer, 2015. American College of Surgeons; 2018. March 2019, https://www.facs.org/quality%20programs/cancer/ncdb/qualitymeasures. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: characteristics of patients in the validation group.
Data Availability Statement
All relevant data are within the article and the supplementary materials.