Abstract
Polyphenols are the general designation of various kinds of phytochemicals, mainly classified as flavonoids and nonflavonoids. Polyphenolic compounds have been confirmed to exhibit numerous bioactivities and potential health benefits both in vivo and in vitro. Dietary polyphenols have been shown to significantly alleviate several manifestations of metabolic syndrome, namely, central obesity, hypertension, dyslipidemia, and high blood sugar. This review is aimed at discussing the bioprotective effects and related molecular mechanisms of polyphenols, mainly by increasing antioxidant capacity or oxygen scavenging capacity. Polyphenols can exert their antioxidative activity by balancing the organic oxidoreductase enzyme system, regulating antioxidant responsive signaling pathways, and restoring mitochondrial function. These data are helpful for providing new insights into the potential biological effects of polyphenolic compounds and the development of future antioxidant therapeutics.
1. Introduction
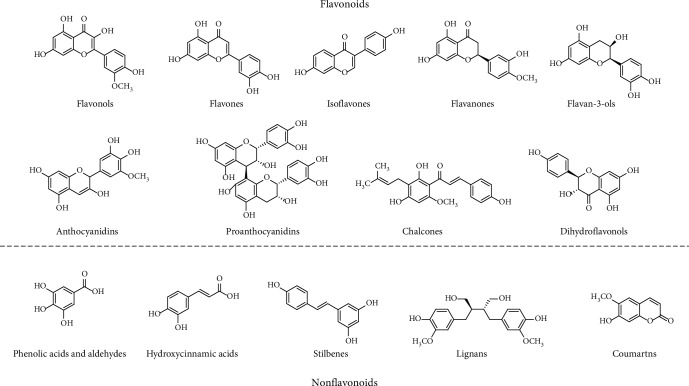
Polyphenols are compounds derived from plants, which exert protective effects against various environmental stresses in plants [1]. Polyphenols represent the largest group of phytochemicals that possess aromatic rings and ligand groups (mainly hydroxyl combined with different carbon sites). Natural polyphenols are secondary metabolites, and more than ten thousand natural polyphenols have been identified and evaluated thus far [2]. Depending on their structural features, polyphenols can be classified into flavonoids and nonflavonoids [3]. Flavonoids share a common structure of a 15-carbon skeleton, composed of two aromatic rings, connected through a heterogeneous pyrone C ring. This carbon bridge structure can be denoted as C6-C3-C6. As heterocyclic ring-linked groups, flavonoids harbor several subgroups, namely, flavonols, flavones, isoflavones, flavanones, anthocyanidins, flavan-3-ols, and minor subclasses of flavonoids, such as chalcones [3]. In contrast, nonflavonoids refer to phenolic acids, hydroxycinnamic acids, stilbenes, lignans, and coumarins [4, 5]. Representative chemical structures of flavonoids and nonflavonoids are shown in Figure 1. The classifications, compositions, and sources of polyphenols are shown in Table 1.
Figure 1.
Representative chemical structures of major groups of polyphenols, generally classified as flavonoids and nonflavonoids.
Table 1.
Summary of the classification, composition, and main sources of polyphenols.
| Classifications | Subclasses | Compositions | Main sources |
|---|---|---|---|
| Flavonoids | Flavonols | Flavonols, kaempferol, quercetin, isorhamnetin, myricetin | Onions, shallots, spinach, green and black tea, dark chocolate, various fruits, vegetables, nuts |
| Flavones | Apigenin, luteolin, tangeretin, nobiletin, wogonin, baicalein | Celery, parsley, some herbs, rooibos tea, citrus species, onion, garlic, pepper, Thai chili, citrus fruits, scutellaria, passiflora | |
| Isoflavones | Daidzein, genistein, glyciten | Leguminous plants, soybeans and soy products | |
| Flavanones | Naringenin, hesperetin, naringin, neohesperidin, rutinosides, narirutin, hesperidin | Flavedo of citrus fruits, bitter oranges, grapefruit, tomatoes, lemon, mandarin and grapefruit | |
| Anthocyanins | Pelargonidin, cyanidin, delphinidin, peonidin, petunidin, malvidin | Berries, cherries, red grapes, currants, red wines, oranges, the black varieties of soybeans, rice, beans, onions, potatoes, cabbage | |
| Flavan-3-ols | (+)-catechin, (−)-epicatechin, (+)-gallocatechin, (−)-epigallocatechin, (−)-epiafzelechin, (−)-epigallocatechin-3-O-gallate | Green tea, fruits, berries, cereals, nuts, chocolate, red wine | |
| Minor subclass of flavonoids | Chalcones | Tomatoes, licorice, shallots, and bean sprouts | |
| Dihydrochalcones (phloridzin, aspalathin, nothofagin) | Apples and apple products, rooibos tea | ||
| Aurones | Vegetables and fruits | ||
| Dihydroflavonols, flavan-3,4-diols | Biosynthetic intermediates of the flavonols and anthocyanins | ||
|
| |||
| Nonflavonoids | Phenolic acids | Hydroxybenzoic acids, gallic acid, protocatechuic acid | Gallotannins, raspberries, strawberries, blackberries, pomegranate, persimmon, walnuts, hazelnuts, grapes, wine, green and black teas, mangoes |
| Hydroxycinnamic acids | Caffeic acid, ferulic acid, p-coumaric acid, sinapic acid | Flesh of grapes, blueberries, kiwis, plums, cherries, apples, coffee | |
| Stilbenes | Resveratrol | Red wines, grapes, peanuts, peanut products, plums, pine nuts | |
| Lignans | Syringaresinol, ecoisolariciresinol, matairesinol, medioresinol, pinoresinol, lariciresino | Linseed, algae, leguminous plants, cereals, vegetables, fruits | |
| Other polyphenols | Curcumin | Turmeric | |
Although polyphenols do not belong to the category of essential nutrients, ingestion of a certain amount of polyphenolic compounds shows a positive influence on body growth and suppresses the occurrence and development of diseases. Several in vivo and in vitro studies have reported that polyphenolic compounds exhibit multiple bioactivities and potential health benefits, such as protection of cardiovascular diseases, disruption of oxidation and inflammatory response, growth inhibition of tumor cells and bacteria, and retardation of the aging process [2]. Metabolic syndrome (MetS) is a common clinical syndrome manifested as several abnormal medical phenotypes, such as central obesity, hypertension, dyslipidemia, as well as high blood sugar. MetS is a major trigger for the occurrence and progression of type II diabetes (T2D) and cardiovascular disease. With great advances in basic redox biology, the most important pathophysiology of MetS is considered to be abnormal systemic oxidative stress (OS) via increased production of free radicals and redox imbalance. Increased OS in the body triggers several diseases, such as diabetes [6], cardiovascular diseases like atherosclerosis [7], Alzheimer's disease [8], and even cancer [9]. OS is closely related to systemic inflammation, endothelial dysfunction, metabolic abnormality, and DNA damage [10]. Nowadays, the preferred management of MetS involves maintenance on a regular and standard diet with increased intake of fruits and vegetables, with reducing the consumption of high-fat, high-salt, and high-sugar substances and optimizing the diet structure, improving physical exercise training, and reducing alcohol consumption [11]. Therefore, a new preventive solution is urgently needed to reduce the morbidity and development of MetS. Until now, several studies have confirmed that supplementation with single/multiple polyphenolic compounds or specific phenolic extracts exhibits protective effects on MetS in cell and animal models. Tian et al. indicated that polyphenols that are extracted from green tea (GT) cut down fat deposition in high fat-fed rats by inhibition of extracellular signal-regulated kinase (ERK) 1/2-peroxisome proliferator-activated receptor (PPAR) γ-adiponectin pathway [12]. They also reported that intake of green tea polyphenols (GTP) reduces the prevalence of coronary heart disease in the middle-aged and elderly Chinese populations to some extent [13]. Resveratrol (RSV, a form of nonflavonoid) treatment was demonstrated to attenuate the hyperpermeability and the overexpression of cav-1 induced by high glucose in a dose-dependent manner. RSV was confirmed to downregulate the increased expressions of vascular endothelial growth factor (VEGF) and kinase insert domain receptor (KDR or VEGF receptor-2) induced by high glucose [14]. In a randomized, controlled, double-blinded clinical trial, Faghihzadeh et al. found that RSV supplementation for 12 weeks significantly decreased the level of alanine aminotransferase (ALT) and arrested hepatic steatosis in nonalcoholic fatty liver disease (NAFLD) patients compared to the control group (P < 0.05) [15]. Therefore, polyphenols, as powerful antioxidants commonly found in fruits and vegetables, are considered to be a potential therapeutic candidate for MetS [16].
The purpose of this review is to renew and supplement information about the effects of polyphenols on MetS and related antioxidative mechanisms, derived from cell studies, animal studies, and especially, interventional human studies.
2. The Potential Roles of Polyphenols in MetS
Polyphenols are biomolecules derived from plant origin and have been proven to exhibit antioxidant and anti-inflammatory activities. Several lines of evidence have suggested that polyphenols, at certain doses, might delay or prevent MetS onset by decreasing body weight, blood pressure (BP), and blood glucose, and by improving abnormal lipid metabolism [17].
2.1. Hyperglycemia and Diabetes Mellitus
The International Diabetes Federation (IDF) has predicted that there were 451 million diabetes patients globally in 2017, increasing with an alarming rate, and by 2045, diabetes patients are estimated to be drastically increased up to 693 million. Majority of those patients were classified as type 2 diabetes (T2D) patients [18]. The main clinical characteristics of T2D involve systematic insulin resistance and relative insufficiency of insulin secretion [19].
In a clinical trial, 50 participants exhibiting more than one abnormal medical phenotypes of MetS were recruited and randomly administered 8 g dried grape pomace (containing mainly catechins and proanthocyanidins) or placebo daily. After six weeks of supplementation with grape pomace, basal insulin was decreased from to 8.5 to 5.5 μU/mL, showing a significant improvement in fasting insulinemia. No significant change was found in both fasting and postprandial glucose between the pre- and postintervention period. Moreover, homeostatic model assessment-insulin resistance (HOMA-IR) was decreased from 2.1 to 1.4 while quantitative insulin sensitivity check index (QUICKI) was increased from 0.35 to 0.42. Previous clinical studies have revealed that anthocyanins and RSV prevent incidence of diabetes, by reducing the levels of blood glucose and hemoglobin A1c (HbA1c), promoting secretion efficiency of pancreatic β cells, and ameliorating systematic insulin resistance [20]. In another randomized, controlled, crossover study, 12 healthy men participated in interventions with either 1.562 g gallic acid equivalent (GAE) beverage or a placebo beverage without polyphenols (CD) randomly, followed by a standard meal after 3 hours. Compared to the CD group, a 36% increase of insulin sensitivity index (SI) was found in the GAE group, while a 31% and 18% decrease was observed in the postprandial insulin incremental area (iAUC0-5h) and insulin secretion index, respectively, after polyphenol supplementation. Among the phenolic metabolites, gallic acid was positively related to the SI index (r = 0.588) but negatively with insulin secretion and response (r = −0.604) [21]. Polyphenol, added to the usual diet, could contribute to improve blood sugar metabolism but could have different influences in different individuals. In obese participants, with supplementation of mangoes that are rich in gallotannin (polymeric form of gallic acid) for 6 weeks, HbA1c was decreased by 18% [22]. Rienks et al. have systematically analyzed eight studies and the relationship between polyphenols and T2D by using a meta-analysis. In this analysis, most flavonoids, especially flavanones and anthocyanidins, exhibited nonlinear dose-response correlations with T2D. However, phenolic acids, belonging to nonflavonoids, showed a linear relationship. This difference might be attributed to the difference in their structures. The researchers concluded that additional dietary polyphenols, especially flavonoids, might exert an important influence on the improvement of T2D [23].
This section summarizes the results from various clinical trials of polyphenolic compounds as antidiabetic agents. It has been identified that natural polyphenols show antidiabetic effects, by reducing the levels of blood glucose and HbA1c, increasing the secretion efficiency of pancreatic β cells, and ameliorating systematic insulin resistance. Dietary supplements with polyphenols have been confirmed as an indispensable part of diabetes diet therapy in clinical trials. However, how diabetes alters the bioavailability and bioactivity of polyphenols is not well understood. It might contribute to an increase in their inherent effects and clinical consequences.
2.2. Obesity
In the past few decades, the global incidence rate of obesity has dramatically increased. Epidemiological survey results show that, from 1975 to 2016, the incidence rate of obesity (defined as body mass index (BMI) ≥ 25 kg/m2) was raised by affecting 40% more adults (21% in men and 24% in women). The morbidity rate of obesity (BMI ≥ 30 kg/m2) in men is growing faster than that in women, with the rate increasing for men from 3% to 12% and for women only doubling more than twice (from 7% to 16%) [24]. It has been predicted that more than 1 billion people will be obese by 2030 [25].
Marranzano et al. designed a cohort trial to elucidate the relationship between obesity and estimated dietary flavonoid intake. Intake of flavonoids was negatively correlated with BMI ≥ 25 and the odds ratio (OR) was about 66%. Surprisingly, among the subclasses of flavonoids, only flavanones showed this similar correlation (OR = 0.68) after adjustment for confounding factors. These data indicated that higher intake of flavonoid resulted in the loss of body weight [26]. In a controlled and double-blind study, 95 overweight individuals were randomly administered 900 mg of a citrus-based polyphenol (mainly catechin and naringin) extract or a placebo taken daily with meals. Compared with the placebo group, waist and abdominal fat and hip circumference in the intervention arm were significantly decreased (waist -5.71% vs. -1.56%, hip -4.71% vs. -1.35%, and fat -9.73% vs. -3.18%) compared to those in the control arm. Furthermore, OS was also lowered by increasing superoxide dismutase (SOD) and glutathione (GSH) and reducing serum malondialdehyde (MDA) levels [27]. Consistent with other clinical trials, 33 participants were recruited with either BMI of more than 25 kg/m2 or a waist circumference beyond 94 cm in men or 80 cm in women. They were randomly distributed into two groups that were administered a diet supplemented either with 500 mg C. fimbriata extract (gallic acid) or placebo. Waist to hip ratio (WHR) were measured to be decreased by 3% in the intervention group, compared to 1% in the control (P < 0.05). In addition, body weight, hip circumference, blood pressure, triglyceride levels, and fat accumulation were all found to be markedly decreased in the polyphenol-intake group compared to the placebo group (P < 0.05). These results indicated that the extract of C. fimbriata combined with diet control and adequate exercise might significantly reduce central obesity, one of the vital phenotypes of MetS [28].
Of course, there were also some studies that reported inconsistent or controversial results. The accretion of green tea (GT, mainly flavan-3-ols) for 12 weeks in sixty subjects (BMI > 18 kg/m2) did not exert a significant influence on body weight, BMI, fat deposition, and resting energy expenditure [29]. Another 12-month randomized, double-blind, placebo-controlled clinical trial recruited 937 postmenopausal females (BMI ≥ 25.0 kg/m2). These individuals were randomized into different groups, the GT extract group, containing individuals administered 843 mg (-)-epigallocatechin-3-gallate, and the remaining in the placebo group. The changes in BMI, overall fat content, percentage of body fat, or bone mineral density in 12 months were not statistically different [30].
In conclusion, this section reviewed the advances of the benefit of polyphenol supplementation in clinical trials. Consumption of polyphenols has been proposed to result in favorable improvement in fat deposition and extenuates inflammation, abnormal blood glucose metabolism, and oxidative unbalance status in overweight subjects, although some negative results were reported. In addition, polyphenol supplementation was well tolerated with no adverse effect [31–34]. Therefore, future researches are warranted to confirm these results over a longer period and discover the potential mechanisms of action of the polyphenols.
2.3. Hypertension
Elevated BP in MetS is a predominant risk factor contributing to cardiovascular damage. With an increase in blood pressure, multiple target organ dysfunctions can occur. Many studies have found that different polyphenols have different degrees of effects on BP.
Daily intake of 25 g chocolate, containing high content of polyphenols, resulted in significant decrease of about 5.93 mmHg and 6.4 mmHg in systolic and diastolic BP, respectively, after administration for 8 weeks [35]. Taubert et al. confirmed that even low habitual 6.3 g dark chocolate intake (equivalent with 30 mg polyphenols) can have a favorable clinical effect on individuals with untreated prehypertension or stage 1 hypertension not accompanied with targeted organ damage risks. From the baseline to end of trial for 18 weeks, mean diastolic BP dropped by 1.9 mmHg and systolic BP declined more prominently by 2.9 mmHg accompanied by a decreasing trend in hypertension prevalence from 86% to 68% in the experimental group. Moreover, the decline in BP was associated with a progressive elevation of S-nitrosoglutathione concentration at about 0.23 nmol/L, indicating that OS played an important role in this process [36].
It should be noted that not all polyphenols have the same effect on systolic and diastolic BP. A systemic review and meta-analysis of twenty-four randomized-controlled clinical trials (RCTs) recruited 1106 participants to explore the efficacy of flavonoid-rich cocoa (FRC) on alleviating potential cardiovascular damage and to evaluate their possible correlation. When administered with FRC for up to 2 weeks, systolic BP dropped by 1.63 mmHg (95%CI = 0.13, 3.12; P = 0.033), while diastolic BP exhibited no changes [37]. Nevertheless, a randomized, double-blind crossover study showed the purveyance of orange juice, abundant in various kinds of polyphenols (mainly anthocyanins), reduced both systolic and diastolic BP. This intervention decreased systolic BP from 128 ± 1 mmHg to 124 ± 2 mmHg (P < 0.05) and decreased diastolic BP from 79 ± 1 mmHg to 76 ± 1 mmHg (P < 0.05) [38]. Nevertheless, some studies have reported controversial results. Koli et al. evaluated the potential functions of regular ingestion of flavonoid-abundant dark chocolate on BP, while restricting snack intake intervention, for 8 weeks. Intake of dark chocolate exhibited no significant benefits on 24 h resting BP (142 ± 11.5/89 ± 8.4 mmHg in initial vs. 142 ± 14.2/88 ± 9.4 mmHg later, P > 0.05). The authors concluded that dark chocolate supplement showed no significant changes in BP or other cardiovascular risk factors during a reduced snack period [1]. As for nonflavonoids, in a randomized-controlled clinical trial, 37 middle-aged and older participants were enrolled and were either provided with 480 mL of tart cherry beverage (high in gallic acid) or control drink (only energy and sugar content) daily for 12 weeks. At the beginning, individuals with high polyphenols retained superior mean systolic BP than the control group (141.4 mmHg vs. 133.4 mmHg). At the end of this study, individuals consuming high polyphenolic compounds showed lower systolic BP than the control group (137.3 mmHg in the test group vs. 138.8 mmHg in the control group). However, no statistically significant change in diastolic BP was observed in either treated or control group [39]. Fang et al. conducted a clinical study showing that mango (full of gallotannin-derived polyphenols) supplementation for 6 weeks significantly decreased systolic BP (but not diastolic BP) in the individuals; however, no expected change in BP occurred in obese individuals [22].
Taken together, daily polyphenol-rich food intake lowers the blood pressure in adults, and it is a plausible intervention for improving cardiovascular health. However, some clinical studies have shown inconsistent results. Therefore, further clinical trials are urgently needed in a larger population and for longer durations to further elucidate the protective role of polyphenols in hypertension.
2.4. Dyslipidemia
There is no doubt that hyperlipidemia is one of the major risk factors for cardiovascular disease. In the USA, about 45.0% and 32.0% of females were found to have increased levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDLC), respectively [40].
Erlund et al. showed that, compared to the control, serum high-density lipoprotein cholesterol (HDLC) level significantly increased in the berry-eating group (abundant flavan-3-ols, flavonols, and flavanones) compared to the control group (5.2% vs. 0.6%, P < 0.05), while TC and triacylglycerol (TG) levels remained unchanged [41]. Another RCT indicated that green tea extract consumption in individuals for 1 year led to a marked decrease in circulating TC (-2.1% vs. 0.7%; P < 0.01), LDLC (-4.1% vs. 0.9%; P < 0.0001), and non-high-density lipoprotein cholesterol (-3.1% vs. 0.4%; P < 0.01) compared with placebo. The dose of serum HDLC remained unchanged, whereas TG concentration increased to 3.6% in the experimental group compared to the control group (3.6% vs. -2.5%, P < 0.05) [42]. Another clinical study showed that intake of 122 mg Goishi tea polyphenols daily increased HDLC level and decreased TC level in the individuals, exhibiting the potential beneficial effect of reducing risks of arteriosclerosis and cardiovascular diseases [43]. A systematic review including 17 clinical trials (n = 1356) concluded that intake of green tea epigallocatechin gallate (EGCG) from 107 to 856 mg/d apparently decreased LDLC by 9.29 mg/dL (95% CI, -12.27 to -6.31), and this protective effect mainly depends on the lipid level at baseline [44]. With consumption of 500 mg aronia extract, containing anthocyanins, proanthocyanidins, and hydroxycinnamic acids, the aronia-interventional group presented a favorable effect in microlipid metabolism. After 12 weeks of treatment, in the peripheral circulating bloodstream, TC concentration dropped by 8%, while LDLC level was increased up to 11%, compared to the placebo. Even LDL receptor protein in mononuclear cells was detected to be reduced by 56% from the baseline, whereas the placebo group showed no significant changes [45].
In conclusion, favorable changes were revealed in TC, LDLC, and HDLC levels after the administration of polyphenols in several clinical trials. These findings can guide us to suggest people to consume more fruits and vegetables containing abundant polyphenols that might improve blood lipid profiles, and thereby, prevent dyslipidemia. More studies are needed to further identify the favorable effects of these polyphenolic compounds on dyslipidemia and explore their underlying molecular mechanisms.
2.5. Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease. NAFLD is closely related to the abnormal metabolism of carbohydrates and lipids and triggers insulin resistance and T2D. The favorable effects of polyphenols on NAFLD have been investigated in several clinical trials.
The supplementation of 500 mg RSV for 12 weeks significantly decreased the alanine aminotransferase (ALT) concentration and hepatic steatosis in 50 NAFLD patients compared to the placebo (P < 0.05); however, no expected improvements in anthropometric measurements, insulin resistance (IR), lipid profile, and BP were observed (P > 0.05) [15]. This group also found that RSV (at the same concentration for the same period) consumption combined with lifestyle change exhibited superior effects compared to lifestyle modification alone [46]. The reduction of AST, plasma glucose, LDLC, TC, insulin resistance index, and TNF-α was also observed in NAFLD patients after administration of edible RSV capsules [47]. A short-term (8-week) daily intake of curcumin also extenuated liver fat and serum levels of ALT and AST in patients diagnosed with NAFLD [32]. Daily consumption of 70 mg of curcumin was found to alleviate liver fat content by 78.9% in NAFLD patients, while only 27.5% improvement was observed in placebo group patients. Most physiological and biochemical features of NAFLD were improved, including levels of TC, LDLC, ALT, and AST. During the trial, curcumin was found to be safe and well tolerated in the patients [48].
In summary, increasing evidence has shown the satisfactory effectiveness of polyphenolic compounds in NAFLD. In addition, it has been shown that polyphenol (flavonoids and nonflavonoids) supplements combined with lifestyle changes ameliorated both occurrence and progression of NAFLD, which exhibited a more favorable effect than lifestyle changes alone. Therefore, polyphenol supplement plus healthy lifestyle might be a feasible choice for patients with NAFLD.
3. Antioxidant Effects of Polyphenols on MetS
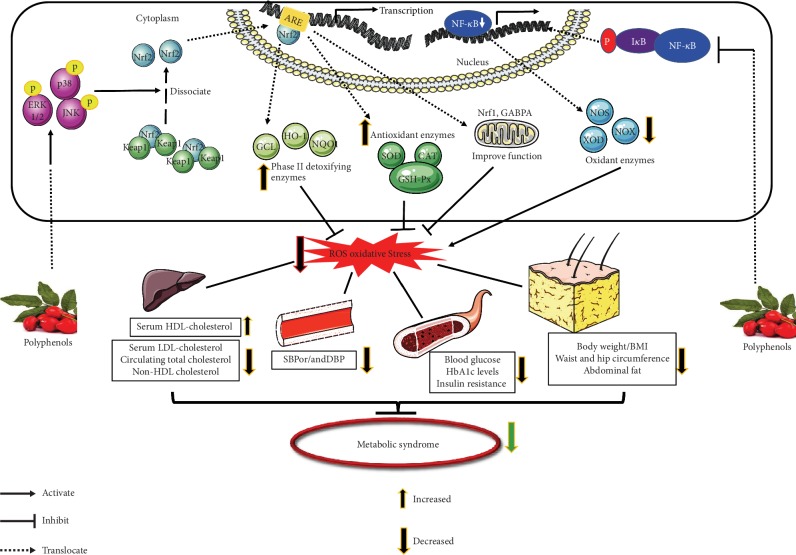
The detailed mechanism underlying the protective effects of polyphenol is not yet understood; however, oxidative stress, as one of the vital pathogeneses of MetS, suggests many targeted therapies for these diseases. For the purpose of establishing a clearer and deeper understanding of the roles of various kinds of polyphenols in MetS, we summarized the results of several in vitro and in vivo studies on antioxidant effects and related mechanisms in different models, as shown in Table 2 [49–64]. We also summarized the results of studies on the protective effects of polyphenols against MetS in clinical trials (as shown in Table 3) [15, 21, 22, 27, 28, 36, 38, 39, 41, 42, 46, 47, 65–70]. Figure 2 summarizes the schematic representation of the principal antioxidative mechanisms of polyphenols in various cellular models.
Table 2.
The antioxidant activity of polyphenols in both in vitro and in vivo models.
| Authors, year | Sources | Main polyphenols | Cell models or animal models | Antioxidant activity changes |
|---|---|---|---|---|
| (Baird and Dinkova-Kostova 2011) [49] | L. coromandelica bark | Gallic acid, caffeic acid, (-)-epigallocatechin-3-gallate, chlorogenic acid, catechin |
RAW 264.7 cells | Cellular ROS production↓, SOD, CSH-Px, and CAT activities↑ |
| (Kim, Kim et al. 2011) [50] | Pycnogenol | Procyanidins, phenolic compounds | High glucose-treated renal tubular cells | Lipid peroxidation↓, total reactive species↓, superoxide↓, nitric oxide (NO(·))↓, peroxynitrite (ONOO(-))↓, iNOS↓, COX-2↓, NF-κB nuclear translocation↓. |
| (Tsai, Hsu et al. 2017) [51] | Chemical hemisynthesis | Resveratrol | Human fibroblast-like synoviocytes | NADPH oxidase activity ↓, ROS generation↓ |
| (Adefegha, Oyeleye et al. 2018) [52] | African crocus and wonderful kola seeds | Phenolic acids, flavonoids | Rat penile homogenate | FeSO4- and SNP-induced lipid peroxidation↓ |
| (Lunder, Roskar et al. 2018) [53] | Coniferous | ND | Mouse C2C12 myoblast cells | Intracellular ROS production↓ |
| (Oliveira, Dare et al. 2018) [54] | The leaves of Nectandra hihua | Flavonoids quercitrin, avicularin, juglalin, afzelin, astragalin | L929 fibroblasts | ROS production↓, lipid peroxidation inhibition↓ |
| (Acero, Gradillas et al. 2019) [55] | Spanish local varieties of Prunus avium (L.) | ND | HepG2 cells | XOD↓, ROS↓ |
| (Sun, Tao et al. 2019) [56] | Fresh citrus fruits | Flavonoids, phenolic acids | Intestinal HepG2 cells | CAA values ↑ |
| (Nauman, Kale et al. 2018) [57] | Chemical hemisynthesis | Gallic acid, quercetin, rutin, acetylsalicylic acid | The ex-liver of mice | Peroxidative damage in microsomes↓, protein carbonyl in cytosolic fraction↓ |
| (Liu, Ren et al. 2014) [58] | ND | Resveratrol | CK-exposed mice | The MDA activity↓, SOD, CSH-Px, and CAT activities↑ |
| (Auberval, Dal et al. 2017) [59] | Red wine | Resveratrol | Wistar rats | Lipid peroxides↓, oxidative proteins↓. |
| (Jian, Ding et al. 2018) [60] | Loquat leaf | Flavonoids | PM2.5-induced NAFLD mice | Oxidative MDA↓, SOD↑ |
| (Li, Chen et al. 2018) [61] | D. loddigesii, Dendrobium | Bibenzyls, phenanthrenes | Diabetic mice | The MDA activity↓, SOD, CSH-Px, and CAT activities↑ |
| (Nauman, Kale et al. 2018) [57] | Chemical hemisynthesis | Gallic acid, acetylsalicylic acid | C57BL/6 mice | SOD, CSH-Px, and CAT activities↑, lipid peroxidation↓ |
| (Song, Park et al. 2018) [62] | Walnut, chokeberry | Anthocyanins | Balb/c mice | MDA↓, lipid peroxidation↓, SOD and CSH-Px activities↑, antioxidant enzyme gene expression↑ |
| (Zyzelewicz, Bojczuk et al. 2018) [63] | Cocoa bean | Flavan-3-ols, flavonols, phenolic acids | Male Wistar rats | GSH↑, GSSG↓ |
| (Qian, Wang et al. 2019) [64] | Bilberry and black currant | Anthocyanins | Male ICR mice | SUA level↓, XOD activity↓, XOD mRNA and protein expressions↓ |
(↓): decrease; (↑): increase; ND: not detected; ROS: reactive oxygen species; NADPH: nicotinamide adenine dinucleotide phosphate; SNP: sodium nitroprusside; CAA: cellular antioxidant activity; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; NF-κB: nuclear factor-kappa; XOD: xanthine oxidase; SOD: superoxide dismutase; CSH-Px: glutathione peroxidase; GSH: glutathione; GSSG: oxidized glutathione; CK: cigarette smoke; MDA: malondialdehyde; CAT: chloramphenicol acetyl transferase; SUA: serum uric acid; NAFLD: nonalcoholic fatty liver disease.
Table 3.
Summary of results from clinical trials of polyphenols in individuals with MetS.
| Authors, year | Sources | Main polyphenols | Individuals | Duration | Bioactivities | Improvements |
|---|---|---|---|---|---|---|
| (Faghihzadeh, Adibi et al. 2015) [15] | Resveratrol capsules | Resveratrol | 50 NAFLD patients | 12 weeks | ND | ALT↓, hepatic steatosis↓ |
| (Costabile, Vitale et al. 2018) [21] | Red grape pomace | Anthocyanins, flavan-3-ol, procyanidins | 12 healthy men | 1 week | ND | Postprandial insulin incremental area↓, insulin secretion index↓, IS index↑ |
| (Fang, Kim et al. 2018) [22] | Mango | Gallic acid, gallotannin, galloylglycosides, flavonoids |
12 lean and 9 obese participants | 6 weeks | Anti-inflammation: IL-8↓ and MCP-1 ↓. | In lean participants: systolic BP↓ In obese participants: HbA1c and PAI-1↓ |
| (Dallas, Gerbi et al. 2014) [27] | Sinetrol-XPur | Catechin, naringin | 95 overweight subjects | 12 weeks | Antioxidation: MDA↓, SOD↑, GSH↑ Anti-inflammation: CRP↓, fibrinogen↓ |
Waist and hip circumference↓, abdominal fat↓, body weight↓ |
| (Astell, Mathai et al. 2013) [28] | C. fimbriata extract | Gallic acid | 43 overweight and obese subjects | 12 weeks | ND | Waist circumference↓, WHR↓ palatability of the test meal↓, sodium intake↓, body weight↓, BMI↓, hip circumference↓, systolic BP↓, HR↓, TG↓, total fat and saturated fat intake↓ |
| (Taubert, Roesen et al. 2007) [36] | Cocoa-containing foods | Ericatechin, procyanidin dimer, flavonols | 44 untreated upper-range prehypertension or stage 1 hypertension | 18 weeks | Antioxidation: fasting plasma levels of S-nitrosoglutathione↑ | Systolic BP↓, diastolic BP↓, hypertension prevalence↓ |
| (Rangel-Huerta, Aguilera et al. 2015) [38] | Orange juice | Anthocyanins | 100 obese or overweight adults | 12 weeks | Antioxidation: 8-OHdG↓, GR↓, erythrocyte catalase↓, 8-isoPGF2α↓, SOD↑ |
Body mass index↓, waist circumference↓, leptin↓, systolic and diastolic BP↓ |
| (Chai, Davis et al. 2018) [39] | Tart cherry juice | Gallic acid | 17 men and 20 women | 12 weeks | ND | Systolic BP↓, LDLC↓, TC↓ |
| (Erlund, Koli et al. 2008) [41] | Berry | Quercetin, caffeic acid, vanillic acid, | 72 unmedicated subjects with cardiovascular risks | 8 weeks | ND | HDLC↑, systolic BP↓ |
| (Samavat, Newman et al. 2016) [42] | Green tea | Catechins | 936 women | 12 months | ND | Circulating TC↓, LDLC↓ Non-HDLC↓. |
| (Faghihzadeh, Adibi et al. 2014) [46] | Resveratrol capsules | Resveratrol | 50 NAFLD patients | 12 weeks | Anti-inflammation: IL-6↓, NF-κB activity↓, serum CK-18 ↓ |
Weight↓, body mass index↓, waist circumference↓, ALT↓, hepatic steatosis grade↓ |
| (Chen, Zhao et al. 2015) [47] | Resveratrol capsules | Resveratrol | 60 NAFLD patients | 3 months | Anti-inflammation: NF-κB activity ↓, serum CK-18 ↓ | AST↓, glucose↓, LDLC↓, ALT↓, TC↓HOMA-IR↓TNF-α↓, CK-18 fragment↓, FGF21↓, adiponectin level↑ |
| (Martinez-Maqueda, Zapatera et al. 2018) [65] | Dried grape pomace | Anthocyanins, flavan-3-ol | 50 participants with at least one phenotype of MetS | 6 weeks | ND | IR↓, fasting insulinemia↓, Is↑ |
| (Tynkkynen, Mursu et al. 2012) [66] | Chocolate | Epicatechin | 45 nonsmoking volunteers | 3 weeks | Antioxidation: oxidation susceptibility of serum lipids↓ |
HDL↑ |
| (Mursu, Voutilainen et al. 2005) [67] | Polyphenol-rich phloem | Catechins | 75 nonsmoking hypercholesterolemic men | 4 weeks | Antioxidation: Oxidation resistance of total serum lipids↑ | Lipid peroxidation↓ |
| (Espinosa-Moncada, Marin-Echeverri et al. 2018) [68] | Vaccinium meridionale Swartz (agraz) | Anthocyanins | 40 women with MetS | 4 weeks | Antioxidation: serum antioxidant capacity↑, urinary 8-OHdG ↓ Anti-inflammation: Hs-CRP levels ↓ |
Serum antioxidant capacity↑, DNA oxidative damage↓ |
| (Vetrani, Vitale et al. 2018) [69] | ND | Flavonoids, flavan-3-ols, phenolic acids, flavonols | 78 individuals with at least one features of the MetS | 8 weeks | Antioxidation: urinary isoprostanes↓ |
Postprandial lipid response↓, VLDL↓, early insulin secretion↑ |
| (Chiva-Blanch, Urpi-Sarda et al. 2013) [70] | Red wine | Catechin, epicatechin, malvidin-3-glucoside, gallic acid |
73 male moderate alcohol consumers | 4 weeks | ND | Plasma insulin↓, HOMA-IR↓, HDLC↑, Apo A-I↑, Apo A-II↑, lipoprotein↓ |
(↓): decrease; (↑): increase; ND: not detected. MetS: metabolic syndrome; IR: insulin resistance; IS: insulin sensitivity; IL: interleukin; MCP-1: monocyte chemotactic protein-1; HbA1c: glycosylated hemoglobin; PAI-1: plasminogen activator inhibitor 1; BP: blood pressure; MDA: malondialdehyde; SOD: superoxide dismutase; GSH: glutathione; CRP: C-reactive protein; WHR: waist to hip ratio; BMI: body mass index; HR: heart rate; TG: triacylglycerol; LDLC: low-density lipoprotein cholesterol; 8-OHdG: 8-hydroxydeoxyguanosine; GR: glutathione reductase; HDLC: high-density lipoprotein cholesterol; TC: total cholesterol; VLDL: very low-density lipoprotein; NAFLD: nonalcoholic fatty liver disease; ALT: alanine aminotransferase; NF-κB: nuclear factor-kappa B; CK: cytokeratin; FGF: fibroblast growth factor; 8-isoPGF2α: 8-iso-prostaglandin F2α; AST: aspartate aminotransferase; HOMA-IR: homeostasis model assessment insulin resistance index; TNF-α: tumor necrosis factor-alpha; hs-CRP: high-sensitivity C-reactive protein; Apo: apolipoprotein.
Figure 2.
Schematic representation of the main antioxidative mechanisms responsible for MetS regulated by polyphenols.
3.1. Antioxidant Properties of Polyphenols
The antioxidant properties of polyphenols and secondary metabolites depend on the chemical construction of attached functional groups, more specifically, the permutation of functional groups about the nuclear structure. The number of hydroxyl residues greatly affects antioxidant activity by scavenging radicals and disrupting metal ion chelation [71]. In addition, other structures like O-methylation, 2–3 double bond, and 4-oxo, carbohydrate moieties, and degree of polymerization play roles in antioxidant activity [71]. Antioxidant activities of polyphenols are associated with their capacity to eliminate high levels of reactive oxygen species (ROS).
Bai et al. performed a study to investigate the biological characteristics of polyphenolic extracts from apple pomace using antioxidant assays and high-performance liquid chromatography (HPLC) analysis. The study identified that the antioxidant ability among apple polyphenolic extracts was highest in procyanidins B2, followed by chlorogenic acid, hyperin, quercetin, caffeic acid, syringin, cinnamic acid, and phloridzin [72]. Another study showed that O-methylation of the hydroxyls of the catechol B ring resulted in a decrease of the antioxidant activity of the parent compounds. Antioxidant activity from strong to weak is as follows: quercetin>epicatechin>catechin. In addition, pH value also affected antioxidant activity of polyphenols in this assay; lower antioxidant activity was observed at acidic pH. It suggested that the same polyphenols might exhibit different antioxidant capacities in different parts of the body and also provided a possible strategy to enhance the efficacy of dietary polyphenolic compounds [73]. Lemańska et al. reported that O-methylation modification of the quercetin and luteolin undermined the radical scavenging capability by affecting the donating property of electron and hydrogen atom. This study also provided a new insight into the mechanisms of catechol O-methylation on radical scavenging [74].
Cellular antioxidant activity (CAA) assay, combined with in vitro digestion, is notably more effective in detecting antioxidant activity than a simple chemical assay alone [75]. Sun et al. reported that flavonoids and phenolic acids in fresh citrus fruits significantly increased CAA values in a human intestinal HepG2 cell model [56]. In ex vivo studies, the order of ability to donate electron and antioxidant activity was gallic acid>quercetin>rutin>vanillin>acetylsalicylic acid. These compounds were considered to play roles in the antioxidant properties of S. aegyptiaca [57].
Increasing evidence has shown that polyphenols have strong antioxidant properties. Currently, it has been speculated that the antioxidant efficiency of polyphenols suppressed ROS generation by means of inhibiting the catalytic activity of related synthetic enzymes, scavenging of ROS, and upregulating the protective antioxidant defensive network [76, 77].
3.2. Antioxidant Signal Pathway Regulation
The antioxidant response elements (AREs), namely, cis-acting regulatory elements, participate in regulating the expression of special gene encoding enzymes involved in antioxidant and phase II detoxifying reactions [78]. The nuclear transcription factor, erythroid 2-like 2 (Nrf2), a central conditioner of AREs, is commonly associated with the Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm, and subsequently, degraded by the proteasome. However, in the stimulation of oxidative stress, Keap1 cysteine residues were oxidized, which led to Nrf2 dissociation and translocation into the nucleus, where it interacted with AREs, and thus, upregulated expression of downstream genes [79].
Polyphenols, as powerful antioxidants, were confirmed to promote the Nrf2 nuclear translocation under oxidative stress. On one hand, polyphenols protected against tert-Butyl hydroperoxide- (t-BHP-) induced oxidative injury via upregulation of the expression of primary and phase II detoxifying genes, especially enzyme-encoding genes. Heme oxygenase-1 (HO-1), genetically encoded by Hmox-1, is a rate-limiting enzyme of heme catabolism, which gets transformed into carbon monoxide (CO), biliverdin, and ferrous iron. The interaction of biliverdin and bilirubin constituted an effective antioxidant, namely, biliverdin reductase (BVR), to reduce OS [80]. The polyphenol-rich extract of the Nymphaea nouchali flower (NNF) was shown to reduce the adverse effects of t-BHP by attenuating oxidative DNA damage and reducing cellular ROS formulation. This process was accompanied by phosphorylation of MAP kinase (p38 kinase and ERK) as a downstream effect of upregulating Nrf2 nuclear translocation [81]. Polyphenol-rich Nymphaea nouchali leaf extract (NNLE) was also observed to act against OS by increasing the mRNA and protein level of antioxidant and phase II detoxifying enzymes, especially HO-1. NNLE supplement promoted Nrf2 translocation into the nucleus and the level of phosphorylated p38 kinase and ERK [82]. These results were consistent with other in vitro results reporting that quercetin [83], flavon-3-ols [84, 85], puerarin [86], and phenolic acids [87] activate HO-1 by suppressing the MAPK pathway to mediate antioxidant activity.
However, multiple potential mechanisms have been hypothesized for the separation of Keap1 and Nrf2 and Nrf2 nuclear translocation. According to the first mechanism, polyphenols were able to increase cellular Nrf2 levels. Alam et al. had shown that NNF extract supplement increased the protein level of Nrf2 in a time-dependent manner, reaching the maximum level at 12 h after treatment [81]. In addition, quercetin also suppressed the ubiquitination and proteasomal turnover of Nrf2 to maintain its stability [83]. Secondly, NNF extract supplement dose-dependently decreased the transcriptional and translational levels of Keap1 [81]. Quercetin was also found to significantly reduce posttranslational levels of Keap1 protein [83]. However, the main bioactive polyphenols, agrimonolide (AM) and desmethylagrimonolide (DM), have been proved to facilitate Keap1 degradation to further accelerate the release of Nrf2 [88].
3.3. Regulation of Oxidoreductase Enzyme System
3.3.1. Induction of Antioxidant Enzymes
Cellular ROS levels are regulated by an enzymatic antioxidant system, such as SOD, catalase (CAT), and glutathione peroxidase (GSH-Px). SOD can transform superoxide into H2O2, and then, H2O2 is catalyzed by CAT and GSH-Px. The Nrf2-ARE pathway was found to activate the endogenous protective system, facilitating antioxidant capacity in cells [88]. Song et al. found that anthocyanin supplement upregulated the expressions of SOD and GSH-Px, and subsequently, attenuated lipid peroxidation in the serum, kidney, and liver in an aging mouse model [62]. Psotova et al. had detected the inhibitory ability of some flavonoids on lipid peroxidation. This study showed that the order of the inhibitory ability of flavonoids on lipid peroxidation was as follows: quercetin>baicalein>kaempferol>luteolin>apigenin [89]. RSV supplementation significantly restored the activities of SOD, GSH-Px, and CAT. When administered with resveratrol at the dose of 1 mg/kg, the activities of the above enzymes increased 1.4-, 1.5-, and 1.3-fold, respectively, compared to the control group [58]. Glutathione, as a major antioxidant, exists in almost every cell in reduced (GSH) and oxidized (GSSG) forms. The above enzymes play key protective functions against reactive species. Pereira et al. compared the structure-antioxidant activity of GSH combined with different kinds of flavonoids. All of tested flavonoids exhibited antioxidant properties; however, those carrying the catechol in B ring showed synergistic effects with GSH, except those with -OH group at C6, such as quercetin, (+)-catechin, fisetin, luteolin-7-O-glucoside, and taxifolin. In addition, adducts formed at C′2 and C′5 of the B ring exhibited a notably stronger antioxidant activity than those at C′6 and C′8 of the A ring [90].
3.3.2. Inhibition of Oxidant Enzymes
Oxidant enzymes, a large collection of multiple enzymes, include nicotinamide adenine dinucleotide phosphate oxidase (NOX), xanthine oxidase (XOD), nitric oxide synthase (NOS), cyclooxygenase (COX), and lipoxygenase (LOX). They not only play essential roles in redox reactions but also promote cellular reactive oxygen and nitrogen (ROS/RNS) [91]. The NOX family members exhibit multiple cellular localizations, activated patterns, and types of produced ROS. However, as major producers of ROS, all those members could generate hydrogen peroxide and superoxide [92].
RSV has been found to attenuate particular matter-enhanced COX-2/prostaglandin E2 (PGE2) expression, NOX activity, and ROS generation by inhibiting the activation of oxidative stress signaling pathways (NF-κB/NOX/ROS) in human fibroblast-like synoviocytes [51]. Nuclear factor κB (NF-κB) is a nuclear transcription factor participating in the regulation of various inflammation-related genes, which can be activated by several stimulations through different forms of radiation to oxidative stress. Resveratrol was shown to impair p65 NF-κB translocation into the nucleus, and subsequently, inhibit binding with DNA in a dose-dependent manner [51, 58]. Resveratrol was confirmed to protect from oxidative stress by downregulation of protein kinase C-α (PKC-α) activation and NOX in A549 cell line and isolated human neutrophils [93, 94]. Gang et al. found that puerarin decreased NOX activity by suppressing activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) and membrane translocation of oxidase subunits, and then, reduced ROS production [86]. RSV was shown to suppress NOX activity, including an O-methylation group, a 4′-OH group, and an extra -OH group. RSV was confirmed to inhibit 11β-hydroxysteroid dehydrogenase type 1 directly by decreasing cortisol production [95]. With supplementation of anthocyanins, both mRNA and protein level of XOD as well as XOD activity in the serum and liver were inhibited significantly in a dose-dependent manner [64]. Consistent with previous reports, polyphenol extracts of sweet cherry, containing hydroxycinnamic acids and flavonoids, were found to inhibit the XO system to reduce intracellular ROS [55]. Lin et al. compared the effects of a series of phenolic acids on XOD activity. A biochemical method was utilized to study the inhibitory ability of phenolic acids on XOD, and the order of inhibition was as follows: sinapic acid>ferulic acid>syringic acid>p-coumaric acid>chlorogenic acid>caffeic acid [96]. Based on previous studies, flavonoids inhibit XOD activity not only in a dose-dependent manner but also in a structure-activity manner. Hydroxyl attached to C′5 and C′7 and the double bond nitric oxide, as a common type of reactive nitrogen, participated in several physiological and pathological processes. RSV was found to be able to disrupt the formation of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production in macrophages [94, 97]. Another polyphenol, quercetin, was also shown to reduce the production of NO by downregulating the NF-κB signaling pathway [98].
3.4. Restoration of Mitochondrial Function
Mitochondria, as one of the important organelles, are considered to play a vital role in energy production and the regulation of cellular homeostasis and energy production. However, extra ROS produced by impaired mitochondria, especially superoxide anion (O2−) and hydrogen peroxide (H2O2), contributes to a burden of oxidative stress. In turn, extra ROS leads to rapid depolarization of mitochondrial inner membrane potential and impairment of oxidative phosphorylation, further accelerating ROS generation and damage.
Cocultured with catechin-rich cocoa flavanol, both rat islets and pancreatic β cells have been demonstrated to exhibit enhanced insulin secretion and mitochondrial respiration. The activities of mitochondrial components, III, IV, and V, were also increased, along with induced production of adenosine triphosphate (ATP) [99]. Catechins were demonstrated to promote Nrf2 nuclear translocation, initiate the expression of related target genes, like nuclear respiratory factor 1 (Nrf1) and GA-binding protein transcription factor alpha subunit (GABPA), improve the cell's mitochondrial function, and reduce OS [99]. Using electron microscopy, (-)-epicatechin(EPI) was shown to alleviate the decline in quantity and volume densities of mitochondria induced by ischemia/reperfusion compared to sham-operated animals [100]. EPI was also proved to significantly increase the stability of mitochondrial membranes and defend calcium-triggered mitochondrial deformation and swelling by activating δ-opioid receptor (DOR) [101]. DOR was confirmed to activate extracellular signaling-regulated kinase (ERK) signaling and decrease the cytochrome c release, protecting neurons against oxidative damage [102]. At the microscopic level, the protection of EPI on the mitochondrial membrane was prominent when exhibiting less mitochondrial respiration suppression, lowering Ca2+ accumulation in mitochondria, along with a certain amount of NADPH [100]. Ca2+ has been confirmed to enhance the activity of the mitochondrial respiratory chain by activating a Ca2+-dependent dehydrogenase in the tricarboxylic acid (TCA) cycle, thus producing ATP by oxidative phosphorylation and stabilizing the mitochondrial membrane potential. However, upon excess accumulation of mitochondrial Ca2+, the permeability of the mitochondrial inner membrane increased significantly, impairing inner and outer membrane potential difference and mitochondrial respiration, eventually leading to failure of mitochondrial function [103].
RSV has been investigated in several studies, showing a significant protective effect on mitochondrial function. RSV was demonstrated to enhance the production of ATP and phosphocreatine in T2D female rat hearts in the reperfusion period. Biochemical analysis confirmed that RSV improved the levels of indicators of mitochondrial function, including an increase in total adenine nucleotide, creatine, and citrate synthase activity [104]. It should be noted that the effect of RSV on mitochondria depends very strictly on drug concentration. At low concentration (<50 μM), RSV promoted cellular antioxidant ability, activating AMP-activated protein kinase- (AMPK-) and sirtuin 1- (SIRT1-) related signaling pathways to enhance the network formation of mitochondria against toxins and disease-related stress. However, when its concentration increased to more than 50 μM, RSV destroyed cellular Ca2+ homeostasis, impaired mitochondrial membrane potential, and selectively activated caspases to induce cell death [103].
Taken together, this section provided an overview of these findings and is aimed at evaluating the potential medical feasibility of mitochondrial manipulation by polyphenols. We believe that the main pharmacological potential of polyphenols is closely associated with mitochondria, thus highlighting the advantage of these special cellular organelles as promising future therapeutic targets.
4. Challenges and Future Directions
This article reviews the classifications, chemical structures, sources, and potential roles of various kinds of polyphenols in metabolic disorders. Furthermore, acting against the oxidative stress is confirmed to be a major potential mechanism for the bioprotective effect of polyphenols in MetS. Polyphenols are naturally occurring drugs that rely on their various biological activities, not just antioxidant, and have gradually become an integral part of the dietary preventive treatment of many diseases, including MetS. However, various challenges still exist and are arduous. First, improving the bioavailability of polyphenols more effectively in order to promote their effectiveness is challenging. From several animal models and clinical trials, researchers have reached a consensus that polyphenols possess low bioavailability/high bioactivity paradox [3]. For example, the daily dosage of resveratrol in humans is much lower than that in mice (150 mg/day vs. 200-400 mg/kg/day) [105, 106]; however, the plasma resveratrol levels is about 2-23 folds higher in humans than in mice (231 ng/mL vs. 10-120 ng/mL) [106]. These differences may be attributed to different metabolic rates of resveratrol between humans and mice, suggesting that resveratrol may exert its effects at different concentration ranges in different mammals [107]. Due to these differences between humans and animals, we emphasize that clinical research is more desirable and informative. Second, a linear dose-response relationship existed in the polyphenol extract of D. loddigesii (DJP) and the improvement of diabetes [61], while a quadratic relationship was shown between flavonoid-rich cocoa (FRC) dose and flow-mediated vascular dilation (FMD) (P‐nonlinearity = 0.004), and its maximum effect was observed at 500 mg/d [37]. Moreover, a quadratic relationship was also observed between polyphenolic dose and HDLC levels (P‐nonlinearity = 0.06) [37]. Third, if the polyphenols are extracted as the medicine or as health supplements, attention should be paid to the activity loss and degradation of polyphenols during the extraction process. Fourth, the effects cannot be generalized for all kinds of polyphenols, because each polyphenol has its own unique features. In view of the fact that dietary polyphenols are a mixture, the research on potential mechanism is more complicated and difficult. Hence, it is difficult to compare doses achieved from polyphenolic supplements and polyphenol-rich food. The dose-response relationship between polyphenols and diseases is still largely unknown, requiring further studies to provide reasonable standards or critical doses for daily polyphenol intake.
Current lines of evidences suggest that polyphenols have the potential to alleviate phenotypes of MetS. However, further studies are required to identify the therapeutic potential of polyphenols in MetS progression. Safe doses also need to be determined, as the effects greatly vary among polyphenols and food sources, and no specific food or polyphenol is able to improve all phenotypes of MetS [17]. In addition, several undesirable properties of polyphenols have been disclosed, including genotoxicity [108], thyroid damage [109], hormone disorder [110], antinutritional activity [111], and interactions with other drugs [112]. Therefore, the potential cytotoxicity of polyphenols will become one of the important study directions in the future.
Acknowledgments
This work was supported by funding from the National Natural Science Foundation of China (No.81772477 and No. 81201848) awarded to Dr. Shuang Wei.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Kui Liu and Miao Luo contributed equally to this paper.
References
- 1.Koli R., Köhler K., Tonteri E., Peltonen J., Tikkanen H., Fogelholm M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: an intervention study. Nutrition Journal. 2015;14(1):p. 84. doi: 10.1186/s12937-015-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li A. N., Li S., Zhang Y. J., Xu X. R., Chen Y. M., Li H. B. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Rio D., Rodriguez-Mateos A., Spencer J. P. E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & Redox Signaling. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantini M., Benvenuto M., Masuelli L., et al. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment. International Journal of Molecular Sciences. 2015;16(12):9236–9282. doi: 10.3390/ijms16059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selma M. V., Espín J. C., Tomás-Barberán F. A. Interaction between phenolics and gut microbiota: role in human health. Journal of Agricultural and Food Chemistry. 2009;57(15):6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 6.Sottero B., Gargiulo S., Russo I., Barale C., Poli G., Cavalot F. Postprandial dysmetabolism and oxidative stress in type 2 diabetes: pathogenetic mechanisms and therapeutic strategies. Medicinal Research Reviews. 2015;35(5):968–1031. doi: 10.1002/med.21349. [DOI] [PubMed] [Google Scholar]
- 7.Wu L., Noyan Ashraf M. H., Facci M., et al. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proceedings of the National Academy of Sciences. 2004;101(18):7094–7099. doi: 10.1073/pnas.0402004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui H., Diaz F., Garcia S., Moraes C. T. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2007;104(35):14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxidative Medicine and Cellular Longevity. 2016;2016:4. doi: 10.1155/2016/8589318.8589318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonomini F., Rodella L. F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging and Disease. 2015;6(2):109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy S. M. Metabolic syndrome update. Trends in Cardiovascular Medicine. 2016;26(4):364–373. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Tian C., Ye X., Zhang R., et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARγ-adiponectin pathway. PLoS One. 2013;8(1, article e53796) doi: 10.1371/journal.pone.0053796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian C., Huang Q., Yang L., et al. Green tea consumption is associated with reduced incident CHD and improved CHD-related biomarkers in the Dongfeng-Tongji cohort. Scientific Reports. 2016;6(1, article 24353) doi: 10.1038/srep24353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian C., Zhang R., Ye X., et al. Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes & Nutrition. 2013;8(2):231–239. doi: 10.1007/s12263-012-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faghihzadeh F., Adibi P., Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. British Journal of Nutrition. 2015;114(5):796–803. doi: 10.1017/S0007114515002433. [DOI] [PubMed] [Google Scholar]
- 16.Amiot M. J., Riva C., Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obesity Reviews. 2016;17(7):573–586. doi: 10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- 17.Chiva-Blanch G., Badimon L. Effects of polyphenol intake on metabolic syndrome: current evidences from human trials. Oxidative Medicine and Cellular Longevity. 2017;2017:18. doi: 10.1155/2017/5812401.5812401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogurtsova K., da Rocha Fernandes J. D., Huang Y., et al. Idf diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Hameed I., Masoodi S. R., Mir S. A., Nabi M., Ghazanfar K., Ganai B. A. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World Journal of Diabetes. 2015;6(4):598–612. doi: 10.4239/wjd.v6.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H., Ou J., Chen L., et al. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Critical Reviews in Food Science and Nutrition. 2018;59:1–9. doi: 10.1080/10408398.2018.1492900. [DOI] [PubMed] [Google Scholar]
- 21.Costabile G., Vitale M., Luongo D., et al. Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: a pilot study. Clinical Nutrition. 2019;38(6):2727–2734. doi: 10.1016/j.clnu.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Fang C., Kim H., Barnes R. C., Talcott S. T., Mertens-Talcott S. U. Obesity-associated diseases biomarkers are differently modulated in lean and obese individuals and inversely correlated to plasma polyphenolic metabolites after 6 weeks of mango (mangifera indicaL.) consumption. Molecular Nutrition & Food Research. 2018;62(14, article 1800129) doi: 10.1002/mnfr.201800129. [DOI] [PubMed] [Google Scholar]
- 23.Rienks J., Barbaresko J., Oluwagbemigun K., Schmid M., Nöthlings U. Polyphenol exposure and risk of type 2 diabetes: dose-response meta-analyses and systematic review of prospective cohort studies. The American Journal of Clinical Nutrition. 2018;108(1):49–61. doi: 10.1093/ajcn/nqy083. [DOI] [PubMed] [Google Scholar]
- 24.Sung H., Siegel R. L., Torre L. A., et al. Global patterns in excess body weight and the associated cancer burden. CA: A Cancer Journal for Clinicians. 2018;69(2):88–112. doi: 10.3322/caac.21499. [DOI] [PubMed] [Google Scholar]
- 25.Kelly T., Yang W., Chen C.-S., Reynolds K., He J. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity. 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 26.Marranzano M., Ray S., Godos J., Galvano F. Association between dietary flavonoids intake and obesity in a cohort of adults living in the mediterranean area. International Journal of Food Sciences and Nutrition. 2018;69(8):1020–1029. doi: 10.1080/09637486.2018.1452900. [DOI] [PubMed] [Google Scholar]
- 27.Dallas C., Gerbi A., Elbez Y., Caillard P., Zamaria N., Cloarec M. Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (sinetrol-xpur) on weight management and metabolic parameters in healthy overweight individuals. Phytotherapy Research. 2014;28(2):212–218. doi: 10.1002/ptr.4981. [DOI] [PubMed] [Google Scholar]
- 28.Astell K. J., Mathai M. L., McAinch A. J., Stathis C. G., Su X. Q. A pilot study investigating the effect of Caralluma fimbriata extract on the risk factors of metabolic syndrome in overweight and obese subjects: a randomised controlled clinical trial. Complementary Therapies in Medicine. 2013;21(3):180–189. doi: 10.1016/j.ctim.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Janssens P. L. H. R., Hursel R., Westerterp-Plantenga M. S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. The Journal of Nutrition. 2015;145(5):864–870. doi: 10.3945/jn.114.207829. [DOI] [PubMed] [Google Scholar]
- 30.Dostal A. M., Arikawa A., Espejo L., Kurzer M. S. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women. The Journal of Nutrition. 2016;146(2):256–264. doi: 10.3945/jn.115.219238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seyyedebrahimi S. S., Khodabandehloo H., Nasli Esfahani E., Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetologica. 2018;55(4):341–353. doi: 10.1007/s00592-017-1098-3. [DOI] [PubMed] [Google Scholar]
- 32.Panahi Y., Kianpour P., Mohtashami R., Jafari R., Simental-Mendía L., Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Research. 2017;67(4):244–251. doi: 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- 33.Rassaf T., Rammos C., Hendgen-Cotta U. B., et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial. Clinical Journal of the American Society of Nephrology : CJASN. 2016;11(1):108–118. doi: 10.2215/CJN.05560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas R., Williams M., Sharma H., Chaudry A., Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer--the UK NCRN Pomi-T study. Prostate Cancer and Prostatic Diseases. 2014;17(2):180–186. doi: 10.1038/pcan.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostami A., Khalili M., Haghighat N., et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atherosclerosis. 2015;11(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 36.Taubert D., Roesen R., Lehmann C., Jung N., Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. Jama. 2007;298(1):49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 37.Shrime M. G., Bauer S. R., McDonald A. C., Chowdhury N. H., Coltart C. E. M., Ding E. L. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. The Journal of Nutrition. 2011;141(11):1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 38.Rangel-Huerta O. D., Aguilera C. M., Martin M. V., et al. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. The Journal of Nutrition. 2015;145(8):1808–1816. doi: 10.3945/jn.115.213660. [DOI] [PubMed] [Google Scholar]
- 39.Chai S. C., Davis K., Wright R. S., Kuczmarski M. F., Zhang Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food & Function. 2018;9(6):3185–3194. doi: 10.1039/C8FO00468D. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D., Benjamin E. J., Go A. S., et al. Heart disease and stroke Statistics—2015 Update. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 41.Erlund I., Koli R., Alfthan G., et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. The American Journal of Clinical Nutrition. 2008;87(2):323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 42.Samavat H., Newman A. R., Wang R., Yuan J. M., Wu A. H., Kurzer M. S. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. The American Journal of Clinical Nutrition. 2016;104(6):1671–1682. doi: 10.3945/ajcn.116.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida N., Iizuka M., Kataoka K., et al. Improvement of blood lipid profiles by goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. International Journal of Food Sciences and Nutrition. 2018;69(5):598–607. doi: 10.1080/09637486.2017.1386629. [DOI] [PubMed] [Google Scholar]
- 44.Momose Y., Maeda-Yamamoto M., Nabetani H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. International Journal of Food Sciences and Nutrition. 2016;67(6):606–613. doi: 10.1080/09637486.2016.1196655. [DOI] [PubMed] [Google Scholar]
- 45.Xie L., Vance T., Kim B., et al. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial. Nutrition Research. 2017;37:67–77. doi: 10.1016/j.nutres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Faghihzadeh F., Adibi P., Rafiei R., Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutrition Research. 2014;34(10):837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Chen S., Zhao X., Ran L., et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Digestive and Liver Disease. 2015;47(3):226–232. doi: 10.1016/j.dld.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Rahmani S., Asgary S., Askari G., et al. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytotherapy Research. 2016;30(9):1540–1548. doi: 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- 49.Baird L., Dinkova-Kostova A. T. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of Toxicology. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y. J., Kim Y. A., Yokozawa T. Pycnogenol modulates apoptosis by suppressing oxidative stress and inflammation in high glucose-treated renal tubular cells. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49(9):2196–2201. doi: 10.1016/j.fct.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Tsai M.-H., Hsu L.-F., Lee C.-W., et al. Resveratrol inhibits urban particulate matter-induced cox-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. The International Journal of Biochemistry & Cell Biology. 2017;88:113–123. doi: 10.1016/j.biocel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Adefegha S. A., Oyeleye S. I., Oboh G. African crocus (Curculigo pilosa) and wonderful kola (Buchholzia coriacea) seeds modulate critical enzymes relevant to erectile dysfunction and oxidative stress. Journal of Complementary & Integrative Medicine. 2018;15(4) doi: 10.1515/jcim-2016-0159. [DOI] [PubMed] [Google Scholar]
- 53.Lunder M., Roškar I., Hošek J., Štrukelj B. Silver fir (Abies alba) extracts inhibit enzymes involved in blood glucose management and protect against oxidative stress in high glucose environment. Plant Foods for Human Nutrition. 2019;74(1):47–53. doi: 10.1007/s11130-018-0698-6. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira M. M., Daré R. G., Barizão É. O., et al. Photodamage attenuating potential of Nectandra hihua against UVB-induced oxidative stress in L929 fibroblasts. Journal of Photochemistry and Photobiology B: Biology. 2018;181:127–133. doi: 10.1016/j.jphotobiol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Acero N., Gradillas A., Beltran M., García A., Muñoz Mingarro D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chemistry. 2019;279:260–271. doi: 10.1016/j.foodchem.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y., Tao W., Huang H., Ye X., Sun P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chemistry. 2019;279:321–327. doi: 10.1016/j.foodchem.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Nauman M., Kale R. K., Singh R. P. Polyphenols of Salix aegyptiaca modulate the activities of drug metabolizing and antioxidant enzymes, and level of lipid peroxidation. BMC Complementary and Alternative Medicine. 2018;18(1):p. 81. doi: 10.1186/s12906-018-2143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H., Ren J. Z., Chen H., et al. Resveratrol protects against cigarette smoke-induced oxidative damage and pulmonary inflammation. Journal of Biochemical and Molecular Toxicology. 2014;28(10):465–471. doi: 10.1002/jbt.21586. [DOI] [PubMed] [Google Scholar]
- 59.Auberval N., Dal S., Maillard E., et al. Beneficial effects of a red wine polyphenol extract on high-fat diet-induced metabolic syndrome in rats. European Journal of Nutrition. 2017;56(4):1467–1475. doi: 10.1007/s00394-016-1192-2. [DOI] [PubMed] [Google Scholar]
- 60.Jian T., Ding X., Wu Y., et al. Hepatoprotective effect of loquat leaf flavonoids in PM2.5-induced non-alcoholic fatty liver disease via regulation of Irs-1/Akt and CYP2E1/JNK pathways. International Journal of Molecular Sciences. 2018;19(10):p. 3005. doi: 10.3390/ijms19103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X.-W., Chen H.-P., He Y.-Y., et al. Effects of rich-polyphenols extract of Dendrobium loddigesii on anti-diabetic, anti-inflammatory, anti-oxidant, and gut microbiota modulation in db/db mice. Molecules. 2018;23(12):p. 3245. doi: 10.3390/molecules23123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song E.-K., Park H., Kim H.-S. Additive effect of walnut and chokeberry on regulation of antioxidant enzyme gene expression and attenuation of lipid peroxidation in d-galactose-induced aging-mouse model. Nutrition Research. 2018;(18):30142–30148. doi: 10.1016/j.nutres.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Żyżelewicz D., Bojczuk M., Budryn G., et al. Influence of diet based on bread supplemented with raw and roasted cocoa bean extracts on physiological indices of laboratory rats. Food Research International. 2018;112:209–216. doi: 10.1016/j.foodres.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 64.Qian X., Wang X., Luo J., et al. Hypouricemic and nephroprotective roles of anthocyanins in hyperuricemic mice. Food & Function. 2019;10(2):867–878. doi: 10.1039/C8FO02124D. [DOI] [PubMed] [Google Scholar]
- 65.Martínez-Maqueda D., Zapatera B., Gallego-Narbón A., Vaquero M. P., Saura-Calixto F., Pérez-Jiménez J. A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers. Food & Function. 2018;9(11):6010–6019. doi: 10.1039/C8FO01323C. [DOI] [PubMed] [Google Scholar]
- 66.Tynkkynen T., Mursu J., Nurmi T., Tuppurainen K., Laatikainen R., Soininen P. NMR protocol for determination of oxidation susceptibility of serum lipids and application of the protocol to a chocolate study. Metabolomics. 2012;8(3):386–398. doi: 10.1007/s11306-011-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mursu J., Voutilainen S., Nurmi T., et al. Polyphenol-rich phloem enhances the resistance of total serum lipids to oxidation in men. Journal of Agricultural and Food Chemistry. 2005;53(8):3017–3022. doi: 10.1021/jf048448x. [DOI] [PubMed] [Google Scholar]
- 68.Espinosa-Moncada J., Marín-Echeverri C., Galvis-Pérez Y., et al. Evaluation of agraz consumption on adipocytokines, inflammation, and oxidative stress markers in women with metabolic syndrome. Nutrients. 2018;10(11):p. 1639. doi: 10.3390/nu10111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vetrani C., Vitale M., Bozzetto L., et al. Association between different dietary polyphenol subclasses and the improvement in cardiometabolic risk factors: evidence from a randomized controlled clinical trial. Acta Diabetologica. 2018;55(2):149–153. doi: 10.1007/s00592-017-1075-x. [DOI] [PubMed] [Google Scholar]
- 70.Chiva-Blanch G., Urpi-Sarda M., Ros E., et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clinical Nutrition. 2013;32(2):200–206. doi: 10.1016/j.clnu.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 72.Bai X., Zhang H., Ren S. Antioxidant activity and hplc analysis of polyphenol-enriched extracts from industrial apple pomace. Journal of the Science of Food and Agriculture. 2013;93(10):2502–2506. doi: 10.1002/jsfa.6066. [DOI] [PubMed] [Google Scholar]
- 73.Dueñas M., González-Manzano S., González-Paramás A., Santos-Buelga C. Antioxidant evaluation of O -methylated metabolites of catechin, epicatechin and quercetin. Journal of Pharmaceutical and Biomedical Analysis. 2010;51(2):443–449. doi: 10.1016/j.jpba.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Lemańska K., van der Woude H., Szymusiak H., et al. The effect of CatecholO-methylation on radical scavenging characteristics of quercetin and luteolin-a mechanistic insight. Free Radical Research. 2004;38(6):639–647. doi: 10.1080/10715760410001694062. [DOI] [PubMed] [Google Scholar]
- 75.Wolfe K. L., Liu R. H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 76.Mishra A., Sharma A. K., Kumar S., Saxena A. K., Pandey A. K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/915436.915436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar S., Sharma U. K., Sharma A. K., Pandey A. K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cellular and Molecular Biology (Noisy-le-Grand, France) 2012;58(1):174–181. [PubMed] [Google Scholar]
- 78.Hyung J.-H., Ahn C.-B., IL Kim B., Kim K., Je J.-Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. European Journal of Pharmacology. 2016;793:43–48. doi: 10.1016/j.ejphar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Levonen A. L., Landar A., Ramachandran A., et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. The Biochemical Journal. 2004;378(2) Part 2:373–382. doi: 10.1042/bj20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alam M., Ju M.-K., Lee S.-H. DNA protecting activities of Nymphaea nouchali (Burm.f) flower extract attenuate t-BHP-induced oxidative stress cell death through Nrf2-mediated induction of heme oxygenase-1 expression by activating MAP-kinases. International Journal of Molecular Sciences. 2017;18(10):p. 2069. doi: 10.3390/ijms18102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajpai V. K., Alam M. B., Ju M.-K., et al. Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomedicine & Pharmacotherapy. 2018;103:1397–1407. doi: 10.1016/j.biopha.2018.04.186. [DOI] [PubMed] [Google Scholar]
- 83.Tanigawa S., Fujii M., Hou D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Biology and Medicine. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 84.Zou B., Xiao G., Xu Y., Wu J., Yu Y., Fu M. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chemistry. 2018;255:23–30. doi: 10.1016/j.foodchem.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 85.Ma Y. F., Zhao L., Coleman D. N., Gao M., Loor J. J. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative damage in vitro by activating NFE2L2/HMOX1 pathways. Journal of Dairy Science. 2019;102(2):1658–1670. doi: 10.3168/jds.2018-15047. [DOI] [PubMed] [Google Scholar]
- 86.Gang C., Qiang C., Xiangli C., Shifen P., Chong S., Lihong L. Puerarin suppresses angiotensin ii-induced cardiac hypertrophy by inhibiting NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. Journal of Pharmacy & Pharmaceutical Sciences. 2015;18(2):235–248. doi: 10.18433/J3N318. [DOI] [PubMed] [Google Scholar]
- 87.Peng J., Hu T., Li J., et al. Shepherd's Purse Polyphenols Exert Its Anti-Inflammatory and Antioxidative Effects Associated with Suppressing MAPK and NF- κB Pathways and Heme Oxygenase-1 Activation. Oxidative Medicine and Cellular Longevity. 2019;2019:14. doi: 10.1155/2019/7202695.7202695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L., Teng H., Zhang K. Y., Skalicka-Woźniak K., Georgiev M. I., Xiao J. Agrimonolide and desmethylagrimonolide induced HO-1 expression in HepG2 cells through Nrf2-transduction and p38 inactivation. Frontiers in Pharmacology. 2017;7:p. 513. doi: 10.3389/fphar.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Psotová J., Chlopcíková S., Miketová P., Hrbác J., Simánek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part iii. Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytotherapy Research. 2004;18(7):516–521. doi: 10.1002/ptr.1462. [DOI] [PubMed] [Google Scholar]
- 90.Pereira R. B., Sousa C., Costa A., Andrade P. B., Valentão P. Glutathione and the antioxidant potential of binary mixtures with flavonoids: Synergisms and antagonisms. Molecules. 2013;18(8):8858–8872. doi: 10.3390/molecules18088858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.López-Alarcón C., Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Analytica Chimica Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 92.Salazar G. NADPH oxidases and mitochondria in vascular senescence. International Journal of Molecular Sciences. 2018;19(5, article 1327) doi: 10.3390/ijms19051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsu H.-T., Tseng Y.-T., Wong W.-J., Liu C.-M., Lo Y.-C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial a549 cells. BMC Complementary and Alternative Medicine. 2018;18(1):p. 211. doi: 10.1186/s12906-018-2278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nosáľ R., Drábiková K., Jančinová V., et al. On the molecular pharmacology of resveratrol on oxidative burst inhibition in professional phagocytes. Oxidative Medicine and Cellular Longevity. 2014;2014:9. doi: 10.1155/2014/706269.706269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szelényi P., Somogyi A., Sarnyai F., et al. Microsomal pre‐receptor cortisol production is inhibited by resveratrol and epigallocatechin gallate through different mechanisms. Biofactors. 2018;45(2):236–243. doi: 10.1002/biof.1477. [DOI] [PubMed] [Google Scholar]
- 96.Lin L., Yang Q., Zhao K., Zhao M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chemistry. 2018;253:108–118. doi: 10.1016/j.foodchem.2018.01.139. [DOI] [PubMed] [Google Scholar]
- 97.Kim M. H., Yoo D. S., Lee S. Y., et al. The TRIF/TBK1/irf-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects. Die Pharmazie. 2011;66(4):293–300. [PubMed] [Google Scholar]
- 98.Qureshi A. A., Tan X., Reis J. C., et al. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by δ-tocotrienol and quercetin. Lipids in Health and Disease. 2011;10(1):p. 239. doi: 10.1186/1476-511X-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rowley T. J., Bitner B. F., Ray J. D., et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. The Journal of Nutritional Biochemistry. 2017;49:30–41. doi: 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 100.Yamazaki K. G., Andreyev A. Y., Ortiz-Vilchis P., et al. Intravenous (−)-epicatechin reduces myocardial ischemic injury by protecting mitochondrial function. International Journal of Cardiology. 2014;175(2):297–306. doi: 10.1016/j.ijcard.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panneerselvam M., Ali S. S., Finley J. C., et al. Epicatechin regulation of mitochondrial structure and function is opioid receptor dependent. Molecular Nutrition & Food Research. 2013;57(6):1007–1014. doi: 10.1002/mnfr.201300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma M.-C., Qian H., Ghassemi F., Zhao P., Xia Y. Oxygen-sensitive δ-opioid receptor-regulated survival and death Signals. The Journal of Biological Chemistry. 2005;280(16):16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 103.Madreiter-Sokolowski C., Sokolowski A., Graier W. Dosis facit sanitatem—concentration-dependent effects of resveratrol on mitochondria. Nutrients. 2017;9(10):p. 1117. doi: 10.3390/nu9101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fourny N., Lan C., Sérée E., Bernard M., Desrois M. Protective effect of resveratrol against ischemia-reperfusion injury via enhanced high energy compounds and enos-sirt1 expression in type 2 diabetic female rat heart. Nutrients. 2019;11(1):p. 105. doi: 10.3390/nu11010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baur J. A., Pearson K. J., Price N. L., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lagouge M., Argmann C., Gerhart-Hines Z., et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Timmers S., Konings E., Bilet L., et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barbonetti A., Castellini C., Di Giammarco N., Santilli G., Francavilla S., Francavilla F. In vitro exposure of human spermatozoa to bisphenol A induces pro- oxidative/apoptotic mitochondrial dysfunction. Reproductive Toxicology. 2016;66:61–67. doi: 10.1016/j.reprotox.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 109.Jomaa B., de Haan L. H., Peijnenburg A. A., Bovee T. F., Aarts J. M., Rietjens I. M. Simple and rapid in vitro assay for detecting human thyroid peroxidase disruption. Altex. 2015;32(3):191–200. doi: 10.14573/altex.1412201. [DOI] [PubMed] [Google Scholar]
- 110.Mumford S. L., Kim S., Chen Z., Barr D. B., Buck Louis G. M. Urinary phytoestrogens are associated with subtle indicators of semen quality among male partners of couples desiring pregnancy. The Journal of Nutrition. 2015;145(11):2535–2541. doi: 10.3945/jn.115.214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bitzer Z. T., Elias R. J., Vijay-Kumar M., Lambert J. D. (-)-epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Molecular Nutrition & Food Research. 2016;60(10):2267–2274. doi: 10.1002/mnfr.201501042. [DOI] [PubMed] [Google Scholar]
- 112.Boušová I., Skálová L., Souček P., Matoušková P. The modulation of carbonyl reductase 1 by polyphenols. Drug Metabolism Reviews. 2015;47(4):520–533. doi: 10.3109/03602532.2015.1089885. [DOI] [PubMed] [Google Scholar]