Abstract
Background: Immune checkpoint inhibitors (ICIs) are now an important option for more than 14 different cancers. Recent series case reports have described that ICIs are associated with new-onset diabetes in patients, yet the definitive risk is not available. We thus performed a meta-analysis of randomized controlled trials (RCTs) to assess the incidence and risk of developing new-onset diabetes following the use of ICIs.
Methods: The PubMed, EMBASE, Cochrane Library databases, and ClinicalTrials.gov for RCTs were searched. Statistical analyses were performed using STATA 15 and R language. Fifty-two RCTs were included, and 12 did not report any events of ICI-associated diabetes.
Results: A meta-analysis of 40 trials was performed, which reported at least one diabetes-related event among 24,596 patients. Although specific diabetes-related events were rare, compared with the placebo or other therapeutic strategies, the rates of serious hyperglycemia (OR 2.41, 95% CI 1.52 to 3.82), diabetes (3.54, 1.32 to 9.51), all-grade T1D (6.60, 2.51 to 17.30), and serious-grade T1D (6.50, 2.32 to 18.17) were increased with ICI drugs. Subgroup analysis according to the type of control, type of ICIs, and the combination mode suggested that ICIs plus conventional treatments significantly decreased the risks of diabetes and serious-grade hyperglycemia. There was little heterogeneity across the studies in all results except hyperglycemic events, which in part was attributable to data from everolimus-based control group.
Conclusions: New-onset diabetes is uncommon with ICIs but the risk is increased compared with placebo or another therapeutic strategy. However, more studies are warranted to substantiate these findings across ICIs.
Keywords: immune checkpoint inhibitors, diabetes, hyperglycemia, meta-analysis, safety outcomes
Introduction
Immune checkpoint inhibitor (ICI)-based treatments that block molecules such as programmed cell death protein 1 (PD-1), PD1 ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) have emerged as powerful weapons in a growing number of cancers (Temel et al., 2018). Currently, nine ICIs have been approved for the treatment of different cancers: anti-PD-1 (nivolumab, pembrolizumab, toripalimab, sintilimab, and cemiplimab); anti-PD-L1 (atezolizumab, avelumab, and durvalumab); and anti-CTLA-4 (ipilimumab). Immune checkpoint molecules play an important role in maintaining immunological tolerance to self-antigens and preventing autoimmune disorders (Pardoll, 2012). Consequently, their blockade in cancer therapy not only promotes T cell-mediated immune destruction on tumor cells but may also facilitate autoimmune activity that affects various organ systems (Johnson et al., 2018). Thus, ICIs frequently cause toxicities related to the mechanism of action that are generally referred to as immune-related adverse events (irAEs) (Postow et al., 2018).
Among these irAEs, new-onset diabetes is receiving increased attention, as more evidence suggests the recognition of diabetes-related adverse events in patients with cancers who are treated with ICIs. A marked increase in reporting diabetes has also been seen since 2017 by analyzing the World Health Organization’s database of individual case safety reports (Wright et al., 2018). These observations raised concern as to whether ICI treatments could be associated with an increased risk of diabetes in patients with cancer. However, there has been no report of a meta-analysis of the incidence or risk of ICI-associated diabetes among the different ICIs in different tumor subtypes.
Given the dramatic growth in the number of clinical trials testing ICI agents and their clinical benefits in the increasing list of cancer types and negative influence on life quality caused by diabetes if not promptly recognized, we performed a meta-analysis of randomized controlled trials (RCTs) with ICIs in patients with cancer and evaluated the incidence and risks of diabetes-related adverse events compared with placebo or another therapeutic strategy.
Methods
Search Strategy and Selection Criteria
Scientific literature searches were performed in three databases (PubMed, EMBASE, and Cochrane Central Register of Controlled Trials) from the inception of all searched databases to March 2019. Relevant text words and medical subject headings that consisted of terms including ‘phase’ and the individual drug names (details in Supporting Information Table S1) were searched. The search was limited to RCTs and English language. We also performed a manual search using reference lists from trials and review articles to identify any other relevant data. The ClinicalTrials.gov website was searched for RCTs that were labeled as ‘completed’ with available results. This meta-analysis was performed in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher et al., 2009).
Study Selection
We included RCTs that were performed in adults with cancer and compared ICI treatment to another treatment strategy. The exclusion criteria were as follows: observational and retrospective studies; studies published in a meeting abstract without published full text original articles; quality of life studies; studies with only pediatric patients; 10 or fewer patients in any group; single dosing; cost effectiveness analyses; and those that could not assess the effect of ICI, such as when the control group was a different dose of the same ICI or another type of ICI. Two authors independently screened all titles and abstracts (HM and JZ). Two of three authors reviewed and discussed the potential full text. Any disagreements were resolved by consensus with all three (JL, HM, and JZ).
Data Extraction and Quality Assessment
Data from each study that met the inclusion criteria were independently extracted by two of the three authors (JL, HM, and YL). Any disagreement was resolved by consensus with all three. The retrieved data included author name, year of publication, trial characteristics (registry number, whether it was an international study, countries involved, study sites, and study phase), patient characteristics (sex, age, and performance status), the sizes of the intervention and control groups, ICI treatment, dose, and the outcomes of interest. We detected new-onset diabetes following treatment with ICIs using the following terms: hyperglycemia, diabetes mellitus (DM), type 2 diabetes (T2D), and type 1 diabetes (T1D). For data extracted from ClinicalTrials.gov, adverse events were reported as either serious or other; for data from published reports, we identified grades 3–5 as serious and grades 1–2 as other, according to Common Terminology of Clinical Adverse Events categorization. If data were available for both sources, we prioritized data from sources where the data were more complete. If a published study did not report diabetes-related adverse events, and the corresponding registry trial from ClinicalTrials.gov reported did, we included the registry report. For multiple reports of the same trial, only the most completely reported data were used. The quality of the included studies was independently assessed using the Cochrane Risk of Bias Tool. We considered all trials at unclear risk of incomplete outcome data and selective reporting bias as these studies were not designed primarily to assess adverse events.
Data Synthesis and Analysis
The estimated event rates in the intervention group are calculated as the total number of patients with a given adverse event divided by the total number at risk. Data were transformed using the Freeman-Tukey Double Arcsine transformation to calculate event rates. This statistical analyses were performed using R statistical software (package meta, R Foundation). For risk outcome, we pooled trials and calculated odds ratios (ORs) and their associated 95% confidence intervals (CIs) in the intervention group compared with the control group based on the number of patients with a given adverse event and sample size. Given the low rates of adverse events, we used Peto’s method to pool effect estimates across studies. The I² statistic and P value were used to examine heterogeneity across trials for each outcome. An I² statistic of 0–25%, 26–75%, and 76–100% was regarded as indicating low, moderate, and high heterogeneity, respectively. A P value of less than or equal to 0.05 was defined as significant heterogeneity. If a study included more than one intervention group (e.g. different doses or different types of ICI), we separately compared each intervention group with the control group, where the number of patients or events in the control group would be doubled. Sensitivity analyses were performed excluding an everolimus-controlled study, which was known to cause diabetes-related adverse events, to understand the reasons for the high likelihood of differences. We conducted subgroup analyses to examine studies according to the type of control group (chemotherapy vs. immunosuppressive drug vs. targeted therapy vs. placebo), the mode of intervention treatment (monotherapy vs. add-on therapy), and the type of ICI (PD-1 vs. PD-L1 vs. CTLA4 vs. combination of ICIs). Evidence of publication bias was assessed using Egger’s and Begg’s test in addition to funnel plots, and significant publication bias defined as a P < 0.1. All statistical analyses were conducted with STATA, version 15.
Results
Study Search
Our search from the PubMed, EMBASE, and Cochrane Central Register databases yielded a total of 8,596 potentially relevant reports (Figure 1). After screening and eligibility assessment, we retrieved 67 reports for full text screening. We also identified 117 reports with results from ClinicalTrial.gov. After our formal search, three additional large clinical trials were published. We therefore also included these three studies. After further section, a total of 52 studies (7 from the trial registry and 45 from journals) were eligible. The included articles were published (online) between August 2010 and April 2019.
Figure 1.

Flow diagram of study selection.
Study Characteristics
All studies except one (Chih-Hsin Yang et al., 2019) were international multicenter studies. All studies were funded by the pharmaceutical industry, with sample sizes of the ICI intervention group ranging from 12 to 636 patients. Twenty-two were completed in patients with non-small-cell lung cancer, eight in melanoma, six in renal cell carcinoma, three in small-cell lung cancer, three in gastric and gastro esophageal junction cancer, two in head and neck squamous cell carcinoma, two in urothelial cancer, two in prostate cancer, two in breast cancer, one in colorectal cancer, and one in mesothelioma. Among these, patients in the intervention arm received nivolumab as monotherapy in ten studies, pembrolizumab in seven studies, atezolizumab in five studies, durvalumab in three studies, avelumab in one study, tremelimumab in three studies, combination therapy with anti-PD-1/PD-L1/CTLA-4 plus chemotherapy/radiotherapy in thirteen studies, combination therapy with anti-PD-1/PD-L1 plus anti-CTLA4 in three studies, combination therapy with anti-PD-1/PD-L1/CTLA-4 plus targeted therapy in seven studies, and combination therapy with ipilimumab plus vaccine in one study. All studies except one (Kang et al., 2017) had adverse event data on ClinicalTrials.gov. Key characteristics of these included trials are shown in Table 1.
Table 1.
Characteristics of controlled trials of ICI treatment in patients.
| NCT Author (year) | International study | No. of countries involved | No. of study sites | Phase | Group type | Drug | Dose of ICI (mg/kg) | No. of patients | Age Median (range) |
No (%) Male | Tumor type |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
NCT00527735 (Reck et al., 2013) |
Yes | 8 | NR | Phase 2 | CTLA4 | Ipilimumab Paclitaxel/carboplatin |
10 | 113 | NR | NR | NSCLC |
| CTLA4 | Ipilimumab Paclitaxel/carboplatin |
10 | 109 | NR | NR | ||||||
| Control | Paclitaxel/carboplatin | / | 109 | NR | NR | ||||||
|
NCT00861614 (Kwon et al., 2014) |
Yes | 26 | 191 | Phase 3 | CTLA4 | Ipilimumab Radiotherapy |
10 | 399 | 69 (47–86) | 399 | Prostate cancer |
| Control | Placebo radiotherapy | / | 400 | 67.5 (45–86) | 400 | ||||||
|
NCT01673867 (Borghaei et al., 2015) |
Yes | 22 | NR | Phase 3 | PD-1 | Nivolumab | 3 | 292 | 61 (37–84) | 151 (52) | NSCLC |
| Control | Docetaxel | / | 290 | 64 (21–85) | 168 (58) | ||||||
|
NCT01642004 (Brahmer et al., 2015) |
Yes | 20 | NR | Phase 3 | PD-1 | Nivolumab | 3 | 135 | 62 (39–85) | 111 (82) | NSCLC |
| Control | Docetaxel | / | 137 | 64 (42–84) | 97 (71) | ||||||
|
NCT00636168 (Eggermont et al., 2015) |
Yes | 19 | 91 | Phase 3 | CTLA4 | Ipilimumab | 10 | 475 | 51 (20–84) | 296 (62) | Melanoma |
| Placebo | Placebo | / | 476 | 52 (18–78) | 293 (62) | ||||||
|
NCT01668784 (Motzer et al., 2015) |
Yes | 24 | 146 | Phase 3 | PD-1 | Nivolumab | 3 | 410 | 62 (23–88) | 315 (77) | RCC |
| Control | Everolimus | / | 411 | 62 (18–86) | 304 (74) | ||||||
|
NCT01704287 (Ribas et al., 2015) |
Yes | 12 | 73 | Phase 2 | PD-1 | Pembrolizumab | 2 | 180 | 62 (15–87) | 104 (58) | Melanoma |
| PD-1 | Pembrolizumab | 10 | 181 | 60 (27–89) | 109 (60) | ||||||
| Control | Carboplatin/paclitaxel Dacarbazine Temozolomide |
/ | 179 | 63 (27–87) | 114 (64) | ||||||
|
NCT01721772 (Robert et al., 2015) |
Yes | 16 | 80 | Phase 3 | PD-1 | Nivolumab | 3 | 210 | 64 (18–86) | 121 (57.6) | Melanoma |
| Control | Dacarbazine | / | 208 | 66 (26–87) | 125 (60.1) | ||||||
|
NCT01721746 (Weber et al., 2015) |
Yes | 14 | 90 | Phase 3 | PD-1 | Nivolumab | 3 | 272 | 59 (23–88) | 176 (65) | Melanoma |
| Control | Dacarbazine/carboplatin/paclitaxel | / | 133 | 62 (29–85) | 85 (64) | ||||||
|
NCT01903993 (Fehrenbacher et al., 2016) |
Yes | 13 | 61 | Phase 2 | PD-L1 | Atezolizumab | 1,200 mg/dose | 144 | 62 (42–82) | 93 (65) | NSCLC |
| Control | docetaxel | / | 143 | 62 (36–84) | 76 (53) | ||||||
|
NCT02105636 (Ferris et al., 2016) |
Yes | 15 | NR | Phase 3 | PD-1 | Nivolumab | 3 | 240 | 59 (29–83) | 197 (82.1) | HNSCC |
| Control | Cetuximab/methotrexate/docetaxel | / | 121 | 61 (28–78) | 103 (85.1) | ||||||
|
NCT01905657 (Herbst et al., 2016) |
Yes | 24 | 202 | Phase 2/3 | PD-1 | Pembrolizumab | 2 | 344 | 63 (56–69) | 212 (62) | NSCLC |
| PD-1 | Pembrolizumab | 10 | 346 | 63 (56–69) | 213 (62) | ||||||
| Control | Docetaxel | / | 343 | 62 (56–69) | 209 (61) | ||||||
|
NCT02039674 (Langer et al., 2016) |
Yes | 2 | 26 | Phase 2 | PD-1 | Pembrolizumab Carboplatin/pemetrexed |
200 mg/dose | 60 | 62.5 (54–70) | 22 (37) | NSCLC |
| Control | Carboplatin/pemetrexed | / | 63 | 63.2 (58–70) | 26 (41) | ||||||
|
NCT01450761 (Reck et al., 2016a) |
Yes | 34 | 224 | Phase 3 | CTLA4 | Ipilimumab Etoposide/cisplatin/carboplatin |
10 | 478 | 62 (39–85) | 371 (66) | SCLC |
| Control | Placebo Etoposide/cisplatin/carboplatin |
/ | 476 | 63 (36–81) | 326 (68) | ||||||
|
NCT02142738 (Reck et al., 2016b) |
Yes | 16 | 142 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 154 | 64.5 (33–90) | 92 (59.7) | NSCLC |
| Control | Paclitaxel/carboplatin/pemetrexed/cisplatin/gemcitabine | / | 151 | 66 (38–85) | 95 (62.9) | ||||||
|
NCT02008227 (Rittmeyer et al., 2017) |
Yes | 31 | 194 | Phase 3 | PD-L1 | Atezolizumab | 1,200 mg/dose | 613 | NR | 378 (61.7) | NSCLC |
| Control | Docetaxel | / | 612 | NR | 379 (61.9) | ||||||
|
NCT02125461 (Antonia et al.,2017) |
Yes | 26 | 235 | Phase 3 | PD-L1 | Durvalumab | 10 | 476 | 64 (31–84) | 334 (70.2) | NSCLC |
| Control | Placebo | / | 237 | 64 (23–90) | 166 (70) | ||||||
|
NCT01057810 (Beer et al., 2017) |
Yes | 24 | NR | Phase 3 | CTLA4 | Ipilimumab | 10 | 399 | NR | 100 | Prostate cancer |
| Control | Placebo | / | 199 | NR | 100 | ||||||
|
NCT02256436 (Rogers et al., 2017) |
Yes | 120 | 29 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 270 | 67 (29–88) | 200 (74.1) | Urothelial carcinoma |
| Control | Paclitaxel/docetaxel/vinflunine | / | 272 | 65 (26–84) | 202 (74.3) | ||||||
|
NCT02041533 (Carbone et al., 2017) |
Yes | 26 | NR | Phase 3 | PD-1 | Nivolumab | 3 | 271 | 63 (32–89) | 184 (68) | NSCLC |
| Control | Gemcitabine/cisplatin Carboplatin/paclitaxel/pemetrexed | ||||||||||
| / | 270 | 65 (29–87) | 148 (55) | ||||||||
|
NCT01285609 (Govindan et al., 2017) |
Yes | 34 | 233 | Phase 3 | CTLA4 | Ipilimumab Paclitaxel/carboplatin |
10 | 479 | NR | NR | NSCLC |
| Control | Placebo Paclitaxel/carboplatin |
/ | 477 | NR | NR | ||||||
|
NCT02267343 (Kang et al., 2017) |
Yes | 3 | 49 | Phase 3 | PD-1 | Nivolumab | 3 | 330 | 62 (54–69) | 229 (69) | GEJ |
| Control | Placebo | / | 163 | 61 (53–68) | 119 (73) | ||||||
|
NCT01843374 (Llombart-Cussac et al., 2017) |
Yes | 19 | 105 | Phase 2b | CTLA4 | Tremelimumab | 10 | 382 | 66 (60–72) | 283 (74.1) | Mesothelioma |
| Control | Placebo | / | 189 | 67 (61–73) | 151 (79.9) | ||||||
|
NCT02302807 (Powles et al., 2018) |
Yes | 29 | 217 | Phase 3 | PD-L1 | Atezolizumab | 1,200 mg/dose | 467 | 67 (33–88) | 357 (76) | Urothelial bladder |
| Control | Vinflunine/paclitaxel/docetaxel | / | 464 | 67 (31–84) | 361 (78) | cancer | |||||
|
NCT02395172 (Barlesi et al., 2018) |
Yes | 31 | 173 | Phase 3 | PD-L1 | Avelumab | 10 | 396 | 64 (58–69) | 269 (67.9) | NSCLC |
| Control | Docetaxel | / | 396 | 63 (57–69) | 273 (68.9) | ||||||
|
NCT02362594 (Eggermont et al., 2018) |
Yes | 23 | 123 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 514 | 54 (19–88) | 324 (63) | Melanoma |
| Control | Placebo | / | 505 | 54 (19–83) | 304 (60.2) | ||||||
|
NCT02578680 (Gandhi et al., 2018) |
Yes | 16 | 126 | Phase 3 | PD-1 | Pembrolizumab Pemetrexed/cisplatin | 200 mg/dose | 410 | 65 (34–84) | 254 (62) | SCLC |
| Control | Pemetrexed/cisplatin | / | 206 | 63.5 (34–84) | 109 (52.9) | ||||||
| NCT02231749 (Motzer et al., 2018) | Yes | 28 | 175 | Phase 3 | PD-1/CTLA4 | Nivolumab ipilimumab |
3 1 |
550 | NR | NR | RCC |
| Control | sunitinib | / | 546 | NR | NR | ||||||
|
NCT02775435 (Paz-Ares et al., 2018) |
Yes | 17 | 137 | Phase 3 | PD-1 | Pembrolizumab Paclitaxel/nab-paclitaxel/carboplatin |
200 mg/dose | 278 | 65 (29–87) | 220 (79.1) | NSCLC |
| Control | Paclitaxel/Nab-paclitaxel/Carboplatin | / | 281 | 65 (36–88) | 235 (83.6) | ||||||
|
NCT02370498 (Shitara et al., 2018) |
Yes | 30 | 148 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 296 | 62.5 (54–70) | 202 (68) | GEJ |
| Control | Pacitraxel | / | 296 | 60.0 (53–68) | 208 (70) | ||||||
|
NCT02252042 (Cohen et al., 2019) |
Yes | 20 | 97 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 247 | 60.0 (55–66) | 207 (84) | HNSCC |
| Control | Methotrexate Docetaxel/cetuximab | / | 248 | 60.0 (54–66) | 205 (83) | ||||||
|
NCT02788279 (Eng et al., 2019) |
Yes | 11 | 73 | Phase 3 | PD-L1 | Atezolizumab Cobimetinib | 840 mg/dose | 183 | 58 (51–67) | 107 (58) | Colorectal cancer |
| PD-L1 | Atezolizumab | 1,200 mg/dose | 90 | 56 (51–64) | 59 (66) | ||||||
| Control | Regorafenib | / | 90 | 59 (52–66) | 51 (57) | ||||||
|
NCT02220894 (Mok et al., 2019) |
Yes | 32 | 213 | Phase 3 | PD-1 | Pembrolizumab | 200 mg/dose | 636 | 63 (56–69) | 450 (71) | NSCLC |
| Control | Platinum | / | 615 | 63 (57–69) | 452 (71) | ||||||
|
NCT02613507 (Wu et al., 2019) |
Yes | 3 | 32 | Phase 3 | PD-1 | Nivolumab | 3 | 338 | 60 (27–78) | 236 (78) | NSCLC |
| Control | Docetaxel | / | 166 | 60 (38–78) | 134 (81) | ||||||
|
NCT02454933 (Chih-Hsin Yang et al., 2019) |
No | 1 | 1 | Phase 3 | PD-L1 | Durvalumab Osimertinib |
10 mg/kg | 12 | 56 (41–78) | 6 (50) | NSCLC |
| Control | Osimertinib | / | 17 | 65 (41–80) | 4 (24) | ||||||
|
NCT01585987 (Squibb, 2012) |
Yes | 12 | NR | Phase 2 | CTLA4 | Ipilimumab | 10 mg/kg | 57 | NR | NR | GEJ |
| Control | Fluoropyrimidine | / | 57 | NR | NR | ||||||
|
NCT01984242 (Roche, 2014) |
Yes | 9 | NR | Phase 2 | PD-L1 | Atezolizumab Bevacizumab | 1,200 mg/dose | 101 | NR | 74 (73.3) | RCC |
| PD-L1 | Atezolizumab | 1,200 mg/dose | 103 | NR | 77 (74.8) | ||||||
| Control | Sunitinib | / | 101 | NR | 79 (78.2) | ||||||
|
NCT02367781 (Roche, 2015b) |
Yes | 36 | NR | Phase 3 | PD-L1 | Atezolizumab Nab-paclitaxel/carboplatin |
1,200 mg/dose | 483 | NR | NR | NSCLC |
| Control | Nab-paclitaxel/carboplatin | / | 240 | NR | NR | ||||||
|
NCT02352948 (AstraZeneca, 2015) |
Yes | NR | 82 | Phase 3 subA | PD-L1 | Durvalumab | 10 | 62 | NR | 42 (67.7) | NSCLC |
| PD-L1/CTLA4 | Durvalumab Tremelimumab |
20 1 |
174 | NR | 115 (66.1) | ||||||
| Yes | NR | 143 | Phase 3 subB | Control | Eerlotinib/gemcitabine/vinorelbine | / | 64 | NR | 48 (75.0) | ||
| PD-L1 | Durvalumab | 10 | 117 | NR | 73 (62.4) | ||||||
| CTLA4 | Tremelimumab | 10 | 60 | NR | 39 (65.0) | ||||||
| Control | Gemcitabine/vinorelbine | / | 118 | NR | 81 (68.6) | ||||||
|
NCT02420821 (Roche, 2015a) |
Yes | 21 | NR | Phase 3 | PD-L1 | Atezolizumab Bevacizumab | 1,200 mg/dose | 451 | NR | NR | RCC |
| Control | Sunitinib | / | 446 | NR | NR | ||||||
|
NCT00094653 (Hodi et al., 2010) |
Yes | 13 | 125 | Phase 3 | CTLA4 | Ipilimumab gp100 |
3 | 403 | 55.6a | 247 (61.3) | Melanoma |
| CTLA4 | Ipilimumab | 3 | 137 | 56.8a | 81 (59.1) | ||||||
| Control | gp100 | / | 136 | 57.4a | 73 (53.7) | ||||||
| NCT00324155 (Robert et al., 2011) | Yes | NR | 25 | Phase 3 | CTLA4 | Ipilimumab Dacarbazine | 10 | 250 | 57.5a | 152 (60.8) | Melanoma |
| Control | Dacarbazine | / | 252 | 56.4a | 149 (59.1) | ||||||
| (Lynch et al., 2012) | Yes | NR | NR | Phase 2 | CTLA4 | Ipilimumab Paclitaxel/carboplatin |
10 | 70 | NR | NR | NSCLC |
| CTLA4 | Ipilimumab Paclitaxel/carboplatin |
10 | 68 | NR | NR | ||||||
| Control | Paclitaxel/carboplatin | / | 66 | NR | NR | ||||||
|
NCT00257205 (Ribas et al., 2013) |
Yes | 24 | 114 | Phase 3 | CTLA4 | Tremelimumab | 15 | 328 | 57a | 190 (58) | Melanoma |
| Control | Dacarbazine/temozolomide | / | 327 | 56a | 182 (56) | ||||||
| NCT02477826 (Hellmann et al., 2017) | Yes | 36 | NR | Phase 3 | PD-1/ CTLA4 |
Nivolumab/ipilimumab | 3 1 |
396 | NR | NR | PD-L1 expression≥1% NSCLC |
| PD-1 | Nivolumab | 240 mg/dose | 396 | NR | NR | ||||||
| Control | Platinum | / | 397 | NR | NR | ||||||
| PD-1/CTLA4 | Nivolumab/ipilimumab | 3 1 |
187 | NR | NR | PD-L1 expression | |||||
| PD-1 | Nivolumab | 360 mg/dose | 177 | NR | NR | <1% NSCLC | |||||
| Control | Platinum | / | 186 | NR | NR | ||||||
| NCT02763579 (Horn et al., 2018) | Yes | 21 | 106 | Phase 3 | PD-L1 | Atezolizumab Carboplatin/etoposide | 1,200 mg/dose | 201 | 64 (28-90) | 129 (64.2) | SCLC |
| Control | Carboplatin/etoposide | / | 202 | 64 (26-87) | 132 (65.3) | ||||||
| NCT02425891 (Schmid et al., 2018) | Yes | 41 | 246 | Phase 3 | PD-L1 | Atezolizumab Nab-paclitaxel | 840 mg/dose | 451 | 55 (20-82) | 3 (0.7) | Breast cancer |
| Control | Placebo nab-paclitaxel | / | 451 | 56 (26-86) | 1 (0.2) | ||||||
| NCT02366143 (Socinski et al., 2018) | Yes | 26 | 240 | Phase 3 | PD-L1 | Atezolizumab Bevacizumab/barboplatin/paclitaxel | 1,200 mg/dose | 400 | 63 (31-89) | 240 (60.0) | NSCLC |
| PD-L1 | Atezolizumab Carboplatin/paclitaxel | 1,200 mg/dose | 402 | NR | NR | ||||||
| Control | Bevacizumab/carboplatin/paclitaxel | / | 400 | 63 (31-90) | 239 (59.8) | ||||||
| NCT02684006 (Motzer et al., 2019) | Yes | 21 | 144 | Phase 3 | PD-L1 | Avelumab Axitinib |
10 | 442 | 62 (29-83) | 316 (71.5) | RCC |
| Control | Sunitinib | / | 444 | 61 (27-88) | 344 (77.5) | ||||||
| NCT02853331 (Rini et al., 2019) | Yes | 16 | 129 | Phase 3 | PD-1 | Pembrolizumab Axitinib |
200 mg/dose | 432 | 62 (30-89) | 308 (71.3) | RCC |
| Control | Sunitinib | / | 429 | 61 (26-90) | 320 (74.6) | ||||||
| NCT02250326 (Celgene, 2015) | Yes | 7 | 34 | Phase 2 | PD-L1 | Nab-Paclitaxel Durvalumab | 1,125 mg/m2 | 79 | NR | 54 (68.4) | NSCLC |
| Control | Nab-paclitaxel | / | 80 | NR | 50 (62.5) | ||||||
| Control | Nab-paclitaxel CC-486 |
/ | 81 | NR | 50 (61.7) | ||||||
| NCT02924883 (Roche, 2016) | Yes | 9 | Phase 3 | PD-L1 | Atezolizumab Trastuzumab/emtansine | 1,200 mg/dose | 133 | NR | 2 (1.5) | Breast cancer | |
| Control | Placebo Trastuzumab/emtansine |
/ | 69 | NR | 0 (0.0) |
GEJ, gastric and gastroesophageal junction cancer; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitors; NR, not reported; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; SCLC, small-cell lung cancer. aAge mean.
Quality of the Included Studies
Table S2 shows the risk of bias assessment of the included studies for meta-analysis. All studies were RCTs with adequate reported randomization, and all studies were funded by the pharmaceutical industry with a high risk of sponsorship bias. Of the 40 included studies for meta-analysis, 26 (65%) were open labels with a high risk of blinding participants and personnel. None of the included studies specifically stated blinded assessment or collection of diabetes-related adverse events. We classified all trials at unclear risk of incomplete outcome data and selective reporting bias.
Incidence of Diabetes-Related Adverse Events
Of the 52 clinical controlled trials assessing the effects of ICIs, 40 trials described ICI-associated diabetes events during the course of study. Hyperglycemia events were described in 32 studies; 303 cases of all-grade hyperglycemia and 55 serious-grade hyperglycemia events occurred in 10,393 patients. Pooling the data showed that the rates of all-grade and serious-grade hyperglycemia events were 2.26% (95% CI, 1.28 to 3.48) and 0.28% (95% CI, 0.16 to 0.42), respectively. The rates of hyperglycemia events differed by the type of ICI and tumor. In particular, patients treated with ICI combination therapy were more likely to report hyperglycemia: 3.37% for all-grade hyperglycemia events, 0.47% for serious-grade hyperglycemia. Patients with RCC showed a trend toward higher rates of both all-grade and serious-grade hyperglycemia, with rates of 6.82% and 0.66%, respectively. High dose of ICIs was not associated with high rates of hyperglycemia events (Table 2).
Table 2.
Incidence of hyperglycemia events in patients treated with immune checkpoint inhibitors. Values are percentages (95% confidence intervals).
| Characteristic | All-grade hyperglycemia | Serious-grade hyperglycemia |
|---|---|---|
| Total | 2.26 (1.28, 3.48) | 0.28 (0.16, 0.42) |
| ICI type | ||
| PD-1 inhibitors | 4.86 (2.86, 7.32) | 0.49 (0.26, 0.78) |
| PD-L1 inhibitors | 0.81 (0.07, 2.06) | \ |
| CTLA-4 inhibitors | 0.52 (0.09, 1.18) | 0.06 (0.00, 0.28) |
| Combination therapy | 3.37 (0.00, 21.49) | 0.47 (0.00, 2.01) |
| Tumor type | ||
| NSCLC | 2.54 (1.10, 4.43) | 0.22 (0.06, 0.45) |
| Melanoma | 1.75 (0.31, 4.15) | 0.35 (0.09, 0.73) |
| RCC | 6.82 (2.00, 14.05) | 0.66 (0.27, 1.18) |
| Prostate cancer | 0.12a | 0.12a |
| Colorectal cancer | 0.37a | / |
| GEJ | 0.57a | 0.53a |
| HNSCC | 5.42a | 0.42a |
| Mesothelioma | 0.52a | 0.52a |
| SCLC | 0.63a | 0.63a |
| Dose | ||
| High dose | 1.33 (0.27, 2.99) | 0.22 (0.00, 0.80) |
| Normal dose | 2.52 (1.32, 4.03) | 0.28 (0.15, 0.44) |
GEJ, gastric and gastroesophageal junction cancer; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; SCLC, small-cell lung cancer.
High doses: including Ipilimumab 10 mg/kg and pembrolizumab 10 mg/kg.
Raw event rate.
Due to the smaller number of other ICI-associated diabetes events, no statistical inferences of the rates were made. Overall, 13 cases of DM occurred in 5,655 patients (raw event rate 0.23%), five cases of T2D occurred in 3,117 patients (raw event rate 0.16%), and 17 cases of all-grade T1D occurred in 3,899 patients (raw event rate 0.44%), and 15 cases of serious-grade T1D events occurred in 3,603 patients (raw event rate 0.42%).
Risk of Diabetes-Related Adverse Events
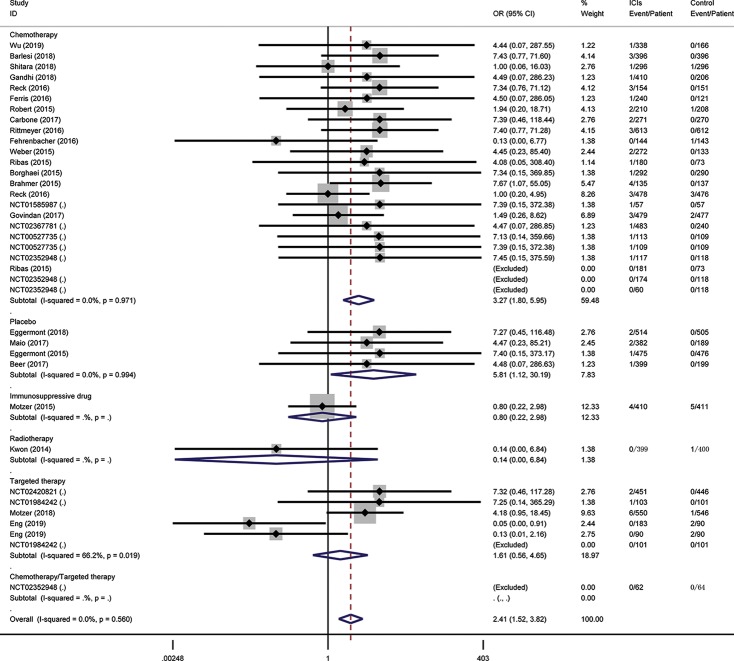
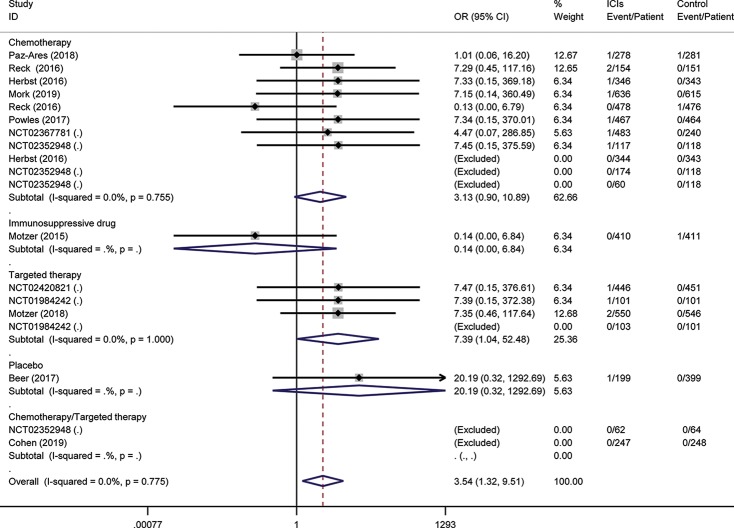
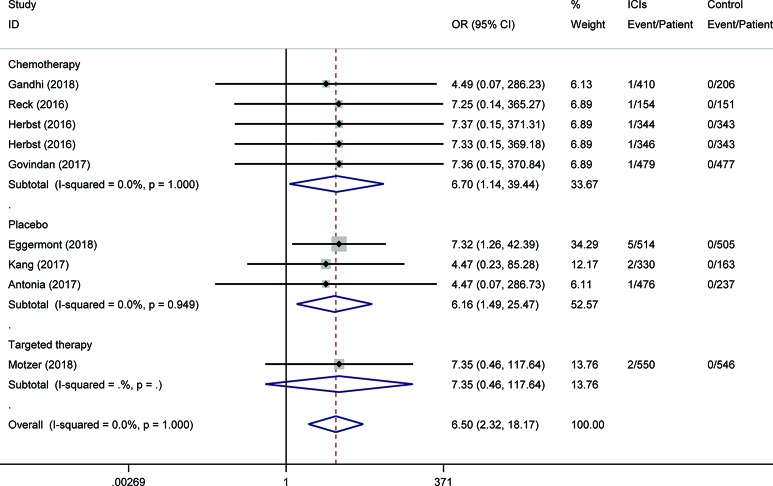
To assess the relative rate of ICI-associated diabetes compared with those in control arms, we calculated the OR of developing diabetes in the RCTs. Pooling the data of these studies showed that patients treated with ICIs were at higher risk for serious-grade hyperglycemia (OR 2.41, 95% CI 1.52 to 3.82, Figure 2), DM (OR 3.54, 95% CI 1.32 to 9.51, Figure 3), all-grade T1D (OR 6.60, 95% CI 2.51 to 17.30, Figure S1), and serious-grade T1D (OR 6.50, 95% CI 2.32 to 18.17, Figure 4) than those treated with other regimens. ICIs showed a trend toward an increased risk of all-grade hyperglycemia (OR 1.38, 95% CI 1.15 to 1.66, Figure S2), but no increased risk of T2D (OR 0.92, 95% CI 0.24 to 3.52, Figure S3). Excluding the study in which the control group was everolimus, a drug known to cause diabetes, the risk of ICI-associated diabetes events were also higher than the control: OR 4.42 for DM, OR 1.75 for all-grade hyperglycemia, OR 2.81 for serious-grade hyperglycemia (Figures S4–S6).
Figure 2.
Risk of serious-grade hyperglycemia following the use of ICIs versus control treatment, stratified by the type of control group.
Figure 3.
Risk of diabetes mellitus following the use of ICIs versus control treatment, stratified by the type of control group.
Figure 4.
Risk of serious-grade type 1 diabetes following the use of ICIs versus control treatment, stratified by the type of control group.
Subgroup analysis for these outcomes was stratification by the type of control, the mode of treatment, and type of ICI. Regarding the type of control, there were apparent differences across subgroups for the risk of ICI-associated diabetes events. Within the placebo-controlled group, ICIs were associated with a higher risk in hyperglycemia (OR 5.81). Subgroup analysis based on the mode of treatment (monotherapy vs. add-on therapy) suggests that add-on therapy decreased the risk of ICI-associated diabetes, with OR 1.77 for DM, 1.31 for serious-grade hyperglycemia, 0.58 for T2D, and 5.83 for T1D (Figures S7–S11). The subgroup analysis by the type of ICI suggests the risk of these events was increased in the subset of trials in which anti-PD-1 or anti-PD-L1 was combined with anti-CTLA-4, with OR 7.35 for DM, 2.51 for all-grade hyperglycemia, 4.18 for serious-grade hyperglycemia (Figures S12–S17).
The funnel plot and statistical test showed no evidence of publication bias for DM (Egger’s test P = 0.994), all-grade hyperglycemia (Egger’s test P = 0.128), serious-grade hyperglycemia (Egger’s test P = 0.325), T2D (Egger’s test P = 0.310), all-grade T1D (Egger’s test P = 0.300), and serious-grade T1D (Egger’s test P = 0.334) (Table S3, Figures S18–S23). We noted no heterogeneity in the effects of ICI on DM, serious-grade hyperglycemia, T2D, all-grade T1D, and serious-grade T1D (I² = 0.0%). However, we noted substantial heterogeneity for the outcome of all-grade hyperglycemia (I² = 88.2%), which was considerably reduced in the analyses of data excluding the everolimus-controlled study (I² = 8.0%).
Discussion
We completed a systematic analysis of new-onset diabetes following treatment with ICIs versus other therapeutic regimens to further our understanding of the safety of these agents. We used data from 40 RCTs that included 13,787 patients treated with ICIs, and also extracted data from the ClinicalTrials.gov results database to supplement the published studies. To our knowledge, this is the largest and most comprehensive meta-analysis on the incidence and risk of ICI-associated diabetes events following the use of ICI regimens published to date, although previous case series analyses showed that there is an increased reporting of rapidly progressive ICI-associated diabetes (Wright et al., 2018; Kotwal et al., 2019; Perdigoto et al., 2019). This meta-analysis shows that the risk of serious-grade hyperglycemia, DM, and T1D following ICIs is significantly higher compared with patients treated with other regimens, but provides no support that ICI treatment is associated with an increased risk of all-grade of hyperglycemia. Among patients on each different ICI regimens, patients on combination therapy were more likely to develop hyperglycemia.
Although the incidence was low, T1D has emerged as the highest risk associated with ICI therapy compared with other diabetes-related adverse events. The pathogenesis of T1D in the populations of patients receiving ICIs is not currently well understood. Several case reports have shown that the presence of autoantibodies before ICIs-based therapy might be at risk of developing diabetes, particularly in treated with anti-PD-1/anti-PD-L1 (Gauci et al., 2017; Usui et al., 2017; Way et al., 2017). Further support for autoimmune-based mechanism has been shown by Clotman et al. (2018), who overviewed the reported cases and demonstrated that approximately half of the tested cases of ICI-associated T1D had detectable diabetes-related autoantibodies. Other studies have shown that anti-PD-1 resulted in a rapid progression of autoimmune diabetes in patients with a high underlying genetic predisposition to T1D (Mellati et al., 2015), raising the concern for genetic factors as a possible mechanism in patients with diabetes-prone HLA genotypes. Similar to what has been described in humans, the study demonstrated that PD-1 or PD-L1 blockade rapidly precipitated diabetes in prediabetic nonobese diabetic (NOD) mice (Ansari et al., 2003). Taken together, these studies reveal a potential mechanism of ICI-associated T1D that involves in both diabetes-related immunologic and genetic factors.
The subgroup analysis showed that the risk of ICI-associated T1D was different among the different type of ICIs. One possible explanation for this would be the mechanistic link to each target. Unlike the PD-1 pathway, which modulates effector cells, CTLA-4 functions in early immune responses during T cell priming and activation (Topalian et al., 2016). As such, the distinct function of the PD-1 and CTLA4 potentially contributed to different rates of T1D following the use of ICIs. In NOD mice, CTLA-4 blockade negatively physiologically regulated diabetes in only the early stages of life compared with the PD-1 pathway (Ansari et al., 2003). Additionally, there was strong PD-L1 expression in the inflamed islets of NOD mice, which suggested that the PD-1-mediated regulation of autoreactive immune cells played an important role at the site of islet inflammation (Ansari et al., 2003). However, this finding should be interpreted cautiously; more data are needed for definitive conclusions given the low absolute number of T1D in patients receiving ICIs.
ICIs plus conventional treatments have been tested in multiple solid tumors, which achieved synergetic effects and overcame the resistance to immunotherapy (Yan et al., 2018). When we combined all non-ICI therapy into one control category, the ICI-based regimens substantially increased the risk of ICI-associated diabetes compared with control group. However, this magnitude was reduced when ICIs were used as an add-on therapy. The risk of DM was 200% lower in the add-on therapy than in the ICI monotherapy. There was also a substantial reduction (over 175%) in ICI-associated serious-grade hyperglycemia in the setting of conventional treatments. These results consistently suggested that compared with ICI therapy, ICIs plus traditional therapy could result in a decreased risk of diabetes-related adverse events.
We found little heterogeneity across studies for all results except hyperglycemia, which strengthens the primary conclusion that ICIs increased risks of diabetes events. A sensitivity analysis identified that everolimus-based control group is responsible for this heterogeneity. Everolimus is an mTOR inhibitor, which is known to influence insulin signaling pathway in peripheral tissues and insulin secretion in pancreatic β cells (Tuo and Xiang, 2018). It has described that mTOR inhibitors resulted in a 5-fold increase in the risk for severe hyperglycemia in patients with cancer (Verges, 2018). Thus, when everolimus was presented separately, the heterogeneity was reduced.
There are several limitations in the present study. We conducted this analysis in study-level, rather than individual patient data. It is not possible to assess potential risk factors that are associated with higher risk of new-onset diabetes, due to the lack of detailed clinical data such as sex, diabetes-prone HLA genotypes, presence of autoantibodies, and islet function in patients receiving ICIs therapy. Secondly, subgroup effects could not be evaluated when there were less than two trials in each subgroup, which could not allow assessing whether the rates of ICI-associated diabetes are varied based on the type of tumor and the dose of ICIs. Our results showed that high dose of ICIs did not contribute to high rates of hyperglycemia events, while the type of tumor showed association of treatment effects. However, regarding other diabetes symptoms, we pooled data across studies together, which might result in the missed difference in dose-dependent and tumor-dependent effect on the risk for these adverse events. Thirdly, whether the increased risk of hyperglycemic events were caused, at least partly, by the use of corticosteroids for the management of irAEs is unclear. Moreover, the results of the present analysis are unable to address potential associations between the incidence of new-onset diabetes and other irAEs in the individual-level. Lastly, only very recent publications have noted T1D after ICI therapy; our study therefore may have underestimated the prevalence of ICI-associated diabetes with only a focus on clinical trials. As emerging case reports that described new-onset diabetes were seen in clinical practice (Hughes et al., 2015; Martin-Liberal et al., 2015; Wright et al., 2018), these adverse events may become more accurately diagnosed and recorded in future trials.
In summary, the use of ICIs compared with placebo or other treatment strategies was associated with an increased risk of new-onset diabetes, especially autoimmune diabetes, although the overall event rates remained low. In contrast, compared with the control group, the risk of T2D was not increased. As the widespread awareness of these events increases, additional large, well-designed randomized trials are needed to definitively determine the risks of new-onset diabetes following the use of ICIs.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
JL, JY, and XZ conceived and designed the study. YL, HM, JZ, JL, and JY reviewed the literatures, extracted and analyzed the data. JL, JY, and XZ wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81603122 to JL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01453/full#supplementary-material
References
- Ansari M. J., Salama A. D., Chitnis T., Smith R. N., Yagita H., Akiba H., et al. (2003). The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198 (1), 63–69. 10.1084/jem.20022125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S. J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. (2017). Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377 (20), 1919–1929. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- AstraZeneca (2015). “A Global Study to Assess the Effects of MEDI4736 (Durvalumab), Given as Monotherapy or in Combination With Tremelimumab Determined by PD-L1 Expression Versus Standard of Care in Patients With Locally Advanced or Metastatic Non Small Cell Lung Cancer”. https://ClinicalTrials.gov/show/NCT02352948).
- Barlesi F., Vansteenkiste J., Spigel D., Ishii H., Garassino M., de Marinis F., et al. (2018). Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 19 (11), 1468–1479. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- Beer T. M., Kwon E. D., Drake C. G., Fizazi K., Logothetis C., Gravis G., et al. (2017). Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients With metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35 (1), 40–47. 10.1200/JCO.2016.69.1584 [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D. R., Steins M., Ready N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K. L., Baas P., Crino L., Eberhardt W. E., Poddubskaya E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone D. P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. (2017). First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376 (25), 2415–2426. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celgene (2015). “Safety and Efficacy Study of Nab®-Paclitaxel With CC-486 or Nab®-Paclitaxel With Durvalumab, and Nab®-Paclitaxel Monotherapy as Second/Third-line Treatment for Advanced Non-small Cell Lung Cancer”. https://ClinicalTrials.gov/show/NCT02250326).
- Chih-Hsin Yang J., Shepherd F. A., Kim D. W., Lee G. W., Lee J. S., Chang G. C., et al. (2019). Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J. Thorac. Oncol. 14 (5), 933–939. 10.1016/j.jtho.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Clotman K., Janssens K., Specenier P., Weets I., De Block C. E. M. (2018). Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 103 (9), 3144–3154. 10.1210/jc.2018-00728 [DOI] [PubMed] [Google Scholar]
- Cohen E. E. W., Soulieres D., Le Tourneau C., Dinis J., Licitra L., Ahn M. J., et al. (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393 (10167), 156–167. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- Eggermont A. M., Chiarion-Sileni V., Grob J. J., Dummer R., Wolchok J. D., Schmidt H., et al. (2015). Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16 (5), 522–530. 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- Eggermont A. M. M., Blank C. U., Mandala M., Long G. V., Atkinson V., Dalle S., et al. (2018). Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378 (19), 1789–1801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- Eng C., Kim T. W., Bendell J., Argiles G., Tebbutt N. C., Di Bartolomeo M., et al. (2019). Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 20 (6), 849–861. 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030), 1837–1846. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- Ferris R. L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A. D., Licitra L., et al. (2016). Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375 (19), 1856–1867. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., et al. (2018). Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378 (22), 2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- Gauci M. L., Laly P., Vidal-Trecan T., Baroudjian B., Gottlieb J., Madjlessi-Ezra N., et al. (2017). Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol. Immunother. 66 (11), 1399–1410. 10.1007/s00262-017-2033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R., Szczesna A., Ahn M. J., Schneider C. P., Gonzalez Mella P. F., Barlesi F., et al. (2017). Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J. Clin. Oncol. 35 (30), 3449–3457. 10.1200/JCO.2016.71.7629 [DOI] [PubMed] [Google Scholar]
- Hellmann M. D., Rizvi N. A., Goldman J. W., Gettinger S. N., Borghaei H., Brahmer J. R., et al. (2017). Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 18 (1), 31–41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Baas P., Kim D. W., Felip E., Perez-Gracia J. L., Han J. Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387 (10027), 1540–1550. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Hodi F. S., O’Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363 (8), 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L., Mansfield A. S., Szczesna A., Havel L., Krzakowski M., Hochmair M. J., et al. (2018). First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379 (23), 2220–2229. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- Hughes J., Vudattu N., Sznol M., Gettinger S., Kluger H., Lupsa B., et al. (2015). Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 38 (4), e55–e57. 10.2337/dc14-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. B., Chandra S., Sosman J. A. (2018). Immune checkpoint inhibitor toxicity in 2018. JAMA 320 (16), 1702–1703. 10.1001/jama.2018.13995 [DOI] [PubMed] [Google Scholar]
- Kang Y. K., Boku N., Satoh T., Ryu M. H., Chao Y., Kato K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 (10111), 2461–2471. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- Kotwal A., Haddox C., Block M., Kudva Y. C. (2019). Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res. Care 7 (1), e000591. 10.1136/bmjdrc-2018-000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E. D., Drake C. G., Scher H. I., Fizazi K., Bossi A., van den Eertwegh A. J., et al. (2014). Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15 (7), 700–712. 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer C. J., Gadgeel S. M., Borghaei H., Papadimitrakopoulou V. A., Patnaik A., Powell S. F., et al. (2016). Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17 (11), 1497–1508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llombart-Cussac A., Cortes J., Pare L., Galvan P., Bermejo B., Martinez N., et al. (2017). HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 18 (4), 545–554. 10.1016/S1470-2045(17)30021-9 [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., et al. (2012). Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 30 (17), 2046–2054. 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- Martin-Liberal J., Furness A. J., Joshi K., Peggs K. S., Quezada S. A., Larkin J. (2015). Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol. Immunother. 64 (6), 765–767. 10.1007/s00262-015-1689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellati M., Eaton K. D., Brooks-Worrell B. M., Hagopian W. A., Martins R., Palmer J. P., et al. (2015). Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care 38 (9), e137–e138. 10.2337/dc15-0889 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern Med. 151 (4), 264–269, W264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Mok T. S. K., Wu Y. L., Kudaba I., Kowalski D. M., Cho B. C., Turna H. Z., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393 (10183), 1819–1830. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Escudier B., McDermott D. F., George S., Hammers H. J., Srinivas S., et al. (2015). Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373 (19), 1803–1813. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J., Tannir N. M., McDermott D. F., Aren Frontera O., Melichar B., Choueiri T. K., et al. (2018). Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 378 (14), 1277–1290. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J., Penkov K., Haanen J., Rini B., Albiges L., Campbell M. T., et al. (2019). Avelumab plus Axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380 (12), 1103–1115. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 (4), 252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gumus M., Mazieres J., et al. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379 (21), 2040–2051. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- Perdigoto A. L., Quandt Z., Anderson M., Herold K. C. (2019). Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinol. 7(6), 421–423. 10.1016/S2213-8587(19)30072-5 [DOI] [PubMed] [Google Scholar]
- Postow M. A., Sidlow R., Hellmann M. D. (2018). Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378 (2), 158–168. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- Powles T., Duran I., van der Heijden M. S., Loriot Y., Vogelzang N. J., De Giorgi U., et al. (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391 (10122), 748–757. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- Reck M., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., et al. (2013). Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann. Oncol. 24 (1), 75–83. 10.1093/annonc/mds213 [DOI] [PubMed] [Google Scholar]
- Reck M., Luft A., Szczesna A., Havel L., Kim S. W., Akerley W., et al. (2016. a). Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 34 (31), 3740–3748. 10.1200/JCO.2016.67.6601 [DOI] [PubMed] [Google Scholar]
- Reck M., Rodriguez-Abreu D., Robinson A. G., Hui R., Csoszi T., Fulop A., et al. (2016. b). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375 (19), 1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Ribas A., Kefford R., Marshall M. A., Punt C. J., Haanen J. B., Marmol M., et al. (2013). Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31 (5), 616–622. 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C., et al. (2015). Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16 (8), 908–918. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B. I., Plimack E. R., Stus V., Gafanov R., Hawkins R., Nosov D., et al. (2019). Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380 (12), 1116–1127. 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066), 255–265. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364 (26), 2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Robert C., Long G. V., Brady B., Dutriaux C., Maio M., Mortier L., et al. (2015). Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372 (4), 320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Roche H.-L. (2014). “A Study of Atezolizumab (an Engineered Anti-Programmed Death-Ligand 1 PD-L1 Antibody) as Monotherapy or in Combination With Bevacizumab (Avastin®) Compared to Sunitinib (Sutent®) in Participants With Untreated Advanced Renal Cell Carcinoma”. https://ClinicalTrials.gov/show/NCT01984242).
- Roche H.-L. (2015. a). “A Study of Atezolizumab in Combination With Bevacizumab Versus Sunitinib in Participants With Untreated Advanced Renal Cell Carcinoma (RCC)”. https://ClinicalTrials.gov/show/NCT02420821).
- Roche H.-L. (2015. b). “A Study of Atezolizumab in Combination With Carboplatin Plus (+) Nab-Paclitaxel Compared With Carboplatin+Nab-Paclitaxel in Participants With Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC)”. https://ClinicalTrials.gov/show/NCT02367781).
- Roche H.-L. (2016). “A Study to Evaluate the Efficacy and Safety of Trastuzumab Emtansine in Combination With Atezolizumab or Atezolizumab-Placebo in Participants With Human Epidermal Growth Factor-2 (HER2) Positive Locally Advanced or Metastatic Breast Cancer (BC) Who Received Prior Trastuzumab and Taxane Based Therapy”. https://ClinicalTrials.gov/show/NCT02924883).
- Rogers J. G., Pagani F. D., Tatooles A. J., Bhat G., Slaughter M. S., Birks E. J., et al. (2017). Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 376 (5), 451–460. 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- Schmid P., Adams S., Rugo H. S., Schneeweiss A., Barrios C. H., Iwata H., et al. (2018). Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379 (22), 2108–2121. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- Shitara K., Ozguroglu M., Bang Y. J., Di Bartolomeo M., Mandala M., Ryu M. H., et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392 (10142), 123–133. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- Socinski M. A., Jotte R. M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., et al. (2018). Atezolizumab for first-line treatment of Metastatic nonsquamous NSCLC. N. Engl. J. Med. 378 (24), 2288–2301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- Squibb B.-M. (2012). “An Efficacy Study in Gastric and Gastroesophageal Junction Cancer Comparing Ipilimumab Versus Standard of Care Immediately Following First Line Chemotherapy”. https://ClinicalTrials.gov/show/NCT01585987).
- Temel J. S., Gainor J. F., Sullivan R. J., Greer J. A. (2018). Keeping expectations in check with immune checkpoint inhibitors. J. Clin. Oncol. 36 (17), 1654–1657. 10.1200/JCO.2017.76.2146 [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Taube J. M., Anders R. A., Pardoll D. M. (2016). Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16 (5), 275–287. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Y., Xiang M. (2018). mTOR: A double-edged sword for diabetes. J. Leukoc. Biol. 106 (2), 385–395. 10.1189/JLB.3MR0317-095RR [DOI] [PubMed] [Google Scholar]
- Usui Y., Udagawa H., Matsumoto S., Imai K., Ohashi K., Ishibashi M., et al. (2017). Association of serum Anti-GAD antibody and HLA haplotypes with Type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J. Thorac. Oncol. 12 (5), e41–e43. 10.1016/j.jtho.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Verges B. (2018). mTOR and Cardiovascular Diseases: Diabetes Mellitus. Transplantation 102 (2S Suppl 1), S47–S49. 10.1097/TP.0000000000001722 [DOI] [PubMed] [Google Scholar]
- Way J., Drakaki A., Drexler A., Freeby M. (2017). Anti-PD-L1 therapy and the onset of diabetes mellitus with positive pancreatic autoantibodies. BMJ Case Rep. 2017, 1–3. 10.1136/bcr-2017-220415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. S., D’Angelo S. P., Minor D., Hodi F. S., Gutzmer R., Neyns B., et al. (2015). Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16 (4), 375–384. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- Wright J. J., Salem J. E., Johnson D. B., Lebrun-Vignes B., Stamatouli A., Thomas J. W., et al. (2018). Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care 41 (12), e150–e151. 10.2337/dc18-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. L., Lu S., Cheng Y., Zhou C., Wang J., Mok T., et al. (2019). Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J. Thorac. Oncol. 14 (5), 867–875. 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Yan Y., Kumar A. B., Finnes H., Markovic S. N., Park S., Dronca R. S., et al. (2018). Combining immune checkpoint inhibitors with conventional cancer therapy. Front. Immunol. 9, 1739. 10.3389/fimmu.2018.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.