Abstract
Guidelines exist for management of pulmonary arterial hypertension (PAH), but information is limited for certain patient subgroups, including adults with portopulmonary hypertension (PoPH) or with PAH associated with congenital heart disease (PAH-CHD). This article discusses screening, clinical management, and prognosis in PoPH and PAH-CHD and, as such, considers the most recent clinical data and expert advice. A multidisciplinary consultation and follow-up by specialists are crucial for management of both PoPH and PAH-CHD, but each condition presents with unique challenges. Development of PoPH most commonly occurs among patients with liver cirrhosis. Initially, patients may be asymptomatic for PoPH and, if untreated, survival with PoPH is generally worse than with idiopathic PAH (IPAH), so early identification with screening is crucial. PoPH can be managed with PAH-specific pharmacological therapy, and resolution is possible in some patients with liver transplantation. With PAH-CHD, survival rates are typically higher than with IPAH but vary across the four subtypes: Eisenmenger syndrome, systemic-to-pulmonary shunts, small cardiac defects, and corrected defects. Screening is also crucial and, in patients who undergo correction of CHD, the presence of PAH should be assessed immediately after repair and throughout their long-term follow-up, with frequency of assessments determined by the patient’s characteristics at the time of correction. Early screening for PAH in patients with portal hypertension or CHD, and multidisciplinary management of PoPH or PAH-CHD are important for the best patient outcomes.
Keywords: Pulmonary arterial hypertension, Congenital heart disease, Portopulmonary hypertension, Liver transplant
Introduction
Within the World Health Organization (WHO) group classification of pulmonary hypertension (PH), Group 1—pulmonary arterial hypertension (PAH)—is subclassified as idiopathic (IPAH), heritable (HPAH), or drug- and toxin-induced, or as being associated with other conditions such as connective tissue disease, human immunodeficiency virus infection, portal hypertension, congenital heart disease (CHD), or schistosomiasis.1 Current European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines provide a treatment algorithm for PAH, as well as recommendations for the diagnosis and treatment of PAH in different patient subgroups.1
There is a relative lack of information on some PAH subgroups, and these include PAH associated with portal hypertension [portopulmonary hypertension (PoPH)] and PAH associated with CHD (PAH-CHD). These patients are typically under-represented in, or excluded from, clinical studies and have only been the focus of a small number of dedicated clinical trials.2–4 The limited amount of prospective trial data means that treatment strategies are often based on the experience of clinical experts or on findings from retrospective analyses.1,5
Portopulmonary hypertension and PAH-CHD each present a unique set of challenges to treating physicians, but both require multidisciplinary management. Resolution of PoPH can be achieved with a combination of PAH-specific therapies and liver transplantation, and, in PAH-CHD, appropriate correction of the cardiac defect can prevent development of PAH, but both of these strategies are only possible in select patients.1,6,7 A multidisciplinary strategy that tackles the development of PAH and the progression of either liver or heart disease is therefore crucial for effective management. Here, we review the challenges clinicians face in identifying and managing adult patients with PoPH or with PAH-CHD.
Portopulmonary hypertension
Typically, PoPH is defined as PAH associated with portal hypertension (hepatic venous pressure gradient >5 mmHg or indirect signs of portal hypertension) and is most commonly (but not exclusively) found in patients with cirrhotic liver disease.1,8 The proportion of patients with PoPH within Group 1 PH is in the range 6–18%9–12 but may be higher than this, as the disease is reportedly underdiagnosed.13 Treatment guidance for PoPH in the ESC/ERS treatment algorithm recommends following the algorithm used for patients with other forms of PAH, while taking into account the severity of concomitant liver disease.1 In addition, there is less evidence for the effectiveness of PAH-specific therapies in PoPH than in other PAH subgroups, because patients with liver disease are often excluded from PAH clinical trials. Thus, screening, timely diagnosis, and referral of these patients are critical. It is also recommended that patients with PoPH are managed in centres with expertise in both PH and liver disease.1
Screening
Screening for PoPH among at-risk populations with PAH is important because up to 60% of patients are asymptomatic for PoPH at the time of diagnosis,14 and early initiation of PAH-specific therapy can improve haemodynamics15 and facilitate subsequent liver transplantation (LT).16 It is particularly important that all candidates for LT or transjugular intrahepatic portosystemic shunt are screened for PH to reduce mortality risk.1 Transthoracic echocardiography (TTE) remains the best tool for screening for PH in patients with chronic liver disease. Peak tricuspid regurgitation velocity measurements associated with low, intermediate, and high risk of PH are described in the guidelines, together with echocardiographic signs of PH in the pulmonary artery, the ventricles and the inferior vena cava and right atrium.1 Among patients symptomatic for and with intermediate or high probability of PAH based on TTE, the diagnosis of PAH should be confirmed by right-heart catheterization at an expert PH centre.1
Patients with confirmed PoPH, including those not listed for LT, should undergo TTE-based screening for hypoxaemia. This can help identify associated hepatopulmonary syndrome and intracardiac shunts, such as patent foramen ovale (PFO).17 Retrospective study data suggest that the presence of PFO does not have a negative effect on short-term perioperative LT outcomes18 and is not considered a contraindication to LT.17 The utility of PFO closure in patients with PH is debated and is generally not indicated based on safety concerns.19
Prognosis
Analysis of the US-based REVEAL Registry that included data from 174 patients with PoPH enrolled between 2006 and 2009 demonstrated that these patients had significantly reduced survival at 5 years from diagnosis compared with patients with IPAH or HPAH (40% vs. 64%; P < 0.001).7 Similarly, fewer than half of PoPH patients (49%) were free from all-cause hospitalization after 2 years, compared with 59% of patients with IPAH or HPAH (P = 0.019).7 Consistent with these findings, a UK registry study of 110 treatment-naïve patients diagnosed with PoPH between 2001 and 2010 revealed a 5-year survival rate of 35%.20
Approximately 82–88% of cases of PoPH are thought to be caused by underlying liver cirrhosis11,12,20; other cases are typically attributable to extrahepatic portal hypertension. Some evidence exists to suggest that survival in PoPH is related to the severity of cirrhosis, based on Child–Pugh category. Analysis of the UK registry showed a trend towards increased mortality among those with PoPH in Child–Pugh Category C (n = 10); hazard ratio (HR) 2.116 (P = 0.055), compared with 0.716 (P = 0.248) for those in Child–Pugh Category A (n = 45).20 However, the analysis could not conclude that severity of cirrhosis was predictive of mortality, and it also found no significant difference in survival between cirrhotic and non-cirrhotic causes of portal hypertension.20 In contrast, an analysis of 154 cirrhotic and non-cirrhotic patients with PoPH referred to the French Referral Center for PAH between 1984 and 2004 did find that cirrhosis and its severity by Child–Pugh category were predictive of mortality.12 Multivariate analysis determined mortality HR to be 0.2 among patients with no cirrhosis (P = 0.003), 2.05 among those in Child-Pugh Category B (P = 0.007), and 2.42 for those in Child–Pugh Category C (P = 0.008). Survival in Categories A, B, and C, respectively, was: 97%, 76%, and 73% at 1 year; 79%, 59%, and 73% at 3 years; and 71%, 55%, and 58% at 5 years.12
Management
Patients with PoPH are treated with pharmacological therapy. LT may be indicated in patients with PoPH and liver disease, and PAH-specific therapy can help to stabilize some patients before LT.1
Pharmacological therapy
Goals of pharmacological therapy in PoPH are to improve haemodynamics to a level that improves exercise tolerance and quality of life, and, where indicated, to enable LT.21 The 2015 ESC/ERS Guidelines recommend that the treatment algorithm for patients with other forms of PAH is applied to patients with PoPH, taking into account the severity of liver disease.1 Treatment strategy and possible subsequent escalation are based on a multi-parameter assessment of risk.22 Clinical, exercise, right ventricular function, haemodynamic parameters, and biomarkers are used to define low, intermediate, or high risk of mortality at 1 year.22 When choosing first-line therapy for PoPH, physicians must consider the severity of PAH and whether there is an indication for LT on the basis of hepatic disease. The treatment algorithm recommends either initial monotherapy or combination therapy for patients with WHO functional class (FC) II–III, and initial combination therapy including intravenous prostacyclin analogues for patients in WHO FC IV.1
The first trial of a PAH-specific therapy has recently been conducted in PoPH.4 Clinical trials of most PAH-specific therapies that have excluded patients with PoPH owing to potential risks associated with administering investigational agents to individuals with liver complications.23 In PORTICO, 85 patients were randomized to macitentan or placebo for 12 weeks, followed by a 12-week open-label period during which all patients received macitentan.4 For the primary endpoint, compared with placebo, there was a 35% reduction in pulmonary vascular resistance (PVR) from baseline to week 12 with macitentan, and significant improvements were demonstrated in secondary endpoints of mean pulmonary arterial pressure (mPAP), cardiac index, and total pulmonary resistance.4 No worsening of liver function or portal hypertension was observed with macitentan.4 In fact, the hepatic safety profile of macitentan in patients with PoPH was similar to that observed in other PAH populations.24 In the Phase III, placebo-controlled PATENT trial of riociguat (n = 443), positive outcomes were noted in a subgroup of 13 patients with PoPH.25 However, the small sample size prevented any conclusions from being drawn about the efficacy and safety of riociguat in PoPH.26
Prospective and retrospective analyses have been undertaken of therapies approved in PAH. Such therapies include phosphodiesterase type 5 (PDE-5) inhibitors, endothelin receptor antagonists (ERAs), and prostaglandin I2 (PGI2; prostacyclin) analogues. Improvements in haemodynamics have been shown in patients with PoPH receiving PDE-5 inhibitors, intravenous epoprostenol, inhaled iloprost, ambrisentan, and bosentan (Table 1).11,27–30 Real-world data from 209 patients with PoPH from a French centre showed that, between 2007 and 2017, 44% of patients initiated treatment with PDE-5 inhibitor monotherapy, 16% with ERA monotherapy, 19% with dual oral-combination therapy, and 3% with a PGI2 analogue.31
Table 1.
Studies of endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostaglandin I2 analogues in patients with portopulmonary hypertension
| Drug | Study type, recruitment | Duration | N | Age (years) | mPAP (mmHg) | PVR (dynes/s/cm5) |
|---|---|---|---|---|---|---|
| Ambrisentan ≤10 mg/day27 | Prospective, consecutive | 2 years; median 390 days on treatment | 13 | Median, 57 | BL: 58 EoS: 41 | BL: 445 EoS: 174 |
| Bosentan 62.5 mg b.i.d. (4 weeks), 125 mg b.i.d thereafter11 | Retrospective, consecutive | Mean, 5 monthsa | 34 | Mean, 50 | BL: 50 EoS: 43 | BL: 8.7 WU EoS: 5.7 WU Equivalent to BL: 696 EoS: 456 |
| Epoprostenol i.v., 4–10 ng/kg/min28 | Prospective, consecutive | Single dose over 60 mina | 15 | Mean, 50 | BL: 50 60 min: 41 | BL: 525 60 min: 359 |
| Iloprost (inhaled), 2.8 μg29 | Prospective, consecutive | Acute dose, 60 min follow-upa | 22 | Mean, 47 | BL: 49 15 min: 42b | BL: 564 15 min: 471b |
| PDE-5 inhibitor therapy (sildenafil 20 mg t.i.d., sildenafil 25 mg t.i.d., or tadalafil 40 mg q.d.)30 | Retrospective analysis of hospital records | No minimum duration of treatment or dose | 20 | Not reported | BL: 47.5 6 months of post-PDE-5 inhibitor treatment: 38.6 | BL: 683.3 6 months of post-PDE-5 inhibitor treatment: 447.2 |
b.i.d., twice daily; BL, baseline; ERA, endothelin receptor antagonist; i.v., intravenous; mPAP, mean pulmonary arterial pressure; PDE-5, phosphodiesterase-5; PGI2, prostaglandin I2; PVR, pulmonary vascular resistance; q.d., once daily; t.i.d., three times daily.
These studies also investigated longer-term, continuous dosing.
Maximum effect at 15 min post-dose, with subsequent values returning towards the mean.
Analysis of liver function in the studies summarized in Table 1 revealed no change from baseline in levels of alanine and aspartate aminotransferases with the ERA ambrisentan,27 but elevated levels of liver enzymes were observed in a small number of patients during long-term use of bosentan.11 Laboratory values and liver enzyme levels were stable during a 1-year follow-up of daily inhaled iloprost,29 and long-term use of epoprostenol was not associated with notable toxicity issues but patients experienced some side effects, including facial flushing, jaw pain, lower leg pain, and diarrhoea.28 In the study of patients receiving PDE-5 inhibitors, tests of liver function and enzymes were not conducted, but all PDE-5 inhibitors were well-tolerated and no hepatic adverse events were reported.30
In addition to PAH-specific medications, supportive therapies such as administration of diuretics and oxygen, and supervised exercise rehabilitation play an important role in PoPH management. Beta-blockers are generally not recommended in PAH and should be avoided in PoPH owing to their potentially deleterious effect on exercise capacity and haemodynamics.32 Oral anticoagulants are neither indicated in, nor recommended for, PoPH,1 but patients taking oral anticoagulants for concomitant conditions can continue treatment after PoPH diagnosis and therapy initiation, unless precluded by known or suspected drug–drug interactions.
Treatment of co-existing hepatitis must also be considered when managing PoPH. Interferon therapy is a possible risk factor for inducing PAH,33,34 and case reports have suggested an association between the use of sofosbuvir for hepatitis C and the induction or exacerbation of PAH in patients with existing PAH-associated comorbidities, including portal hypertension.35 Ongoing development of new antiviral agents may yield effective hepatitis therapies that do not exacerbate PAH.
Transplantation
Currently, adult patients with PoPH in whom PAH is controlled and whose liver-disease status meets transplantation criteria may be eligible for LT. As well as addressing the liver disease, LT offers the possibility of curing PoPH, although outcomes are difficult to predict.6,7 Recent data support the use of PAH-specific therapy to stabilize patients with PoPH in anticipation of LT,16 and, given the potentially beneficial long-term outcomes with PAH-specific therapy, it is possible that these drugs could eventually broaden the indications for LT.36
Assessment of patients for LT should be based on haemodynamic severity and comorbidities.36 Transplantation is contraindicated when mPAP is persistently >50 mmHg despite PAH-specific treatment,36 and LT is contraindicated in patients with severe PoPH.13 Before LT is undertaken, International Liver Transplantation Society practice guidelines recommend that PAH-specific therapy should be administered to patients with mPAP >35 mmHg to decrease mPAP and PVR, and to improve right ventricular function.37 Optimal haemodynamic values that could permit LT are not clearly established, but the risk of LT may be acceptable if mPAP is <35 mmHg or is in the range 35 to <50 mmHg with good right ventricular function and PVR <3–4 Wood units (WU).36 In a prospective study of 49 patients with PoPH,16 a high proportion attained these haemodynamic criteria. In this study, 39 patients with PoPH received PAH-specific therapy as a bridge to LT. There were significant improvements in mPAP, cardiac output, cardiac index, and PVR after 5 months of therapy in 34 patients re-assessed before LT, 70% (n = 24) of whom met the haemodynamic criteria for transplantation.16 At baseline, only two patients had either an mPAP <35 mmHg or an mPAP in the range 35 to <50 mmHg with PVR <3 WU. Overall survival following LT was 80% at 6 months, 77% at 1 year, and 77% at 3 years.16
When patients attain haemodynamic criteria suitable for LT, waiting-list time can be decreased by implementing Model for End-stage Liver Disease (MELD) exception rules.13,16 In the USA, a MELD exception rule has been implemented that ranks patients with PoPH higher on the transplant waiting list than their MELD score would usually allow.38 In the prospective study described above, six patients who underwent LT received MELD exception points.16
On the day of LT, haemodynamic measurements should be taken before surgery commences. If mPAP is >50 mm Hg or PVR >4 WU, LT should be cancelled and further PAH-specific therapy considered.36 Haemodynamic monitoring is essential during surgery to check for large variations in pressure and cardiac output, and if right ventricular failure occurs, right ventricular afterload can be decreased with inhaled nitric oxide combined with catecholamines as necessary.36 Extracorporeal life support may be considered in the event of refractory right ventricular failure, but the effectiveness of this is ill-defined.39 During the 6-month post-operative period, PAH should be monitored closely for worsening, and additional PAH-specific therapy prescribed if needed. The likelihood of PoPH improvement or even cure is good among patients surviving beyond this period.36
Pulmonary arterial hypertension associated with congenital heart disease
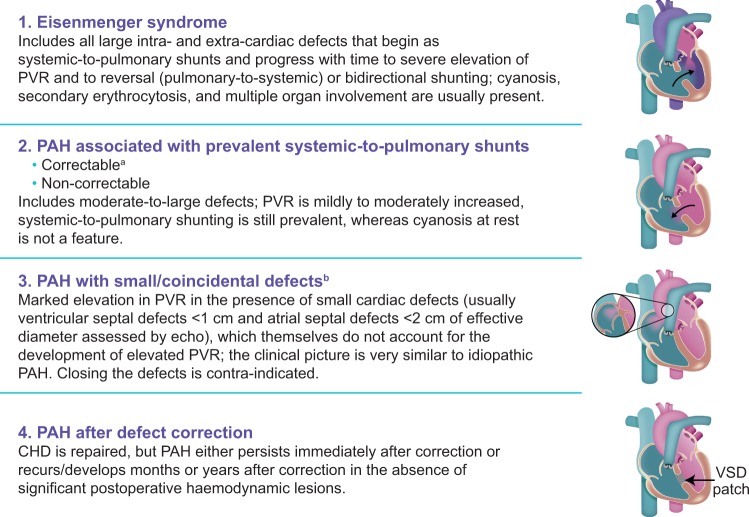
The prevalence of PAH in the general population is estimated to be 10–52 cases per million.40 Approximately 10% of PAH patients are estimated to have PAH-CHD,9,10 and 3% of patients included in a large CHD registry had PAH-CHD.41 Patients with PAH-CHD can be classified into one of four subgroups, as summarized in the latest ESC/ERS guidelines1 (Figure 1). The relative prevalence of each subgroup is unclear, but estimates based on single-centre analyses show that Eisenmenger syndrome is the most common (Group A; 38–58%), followed by PAH-associated systemic-to-pulmonary shunts (Group B; 11–25%), PAH after defect correction (Group D; 15–47%), and PAH with small/coincidental defects (Group C; 4–5%).41,43,44
Figure 1.
Clinical subgroups of pulmonary arterial hypertension associated with congenital heart disease.1,42aWith surgery or intravascular percutaneous procedure. bThe size applies to adult patients. However, in adults, the diameter may be insufficient to define the haemodynamic relevance of the defect; the pressure gradient, the shunt size and direction, and the pulmonary-to-systemic flow ratio should be considered. CHD, congenital heart disease; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; VSD, ventricular septal defect. Reproduced from Gatzoulis et al.42 with permission from Elsevier.
Screening
Initially, patients with some types of CHD may be asymptomatic for PAH, so screening for PAH-CHD, which can develop late in life, is crucial.45 Among patients with CHD, PAH is present by definition in patients with Eisenmenger syndrome (Group A) and in patients with small/coincidental defects (Group C). Thus PAH screening in the CHD population can be applied to patients with prevalent systemic-to-pulmonary shunts and in patients in whom shunts or other cardiac defects have been repaired.45
Echocardiography represents the most valuable and convenient screening tool routinely used to assess the probability of PH diagnosis by predicting pulmonary arterial systolic pressure (PASP) values and describing ventricular morphology and function. In PAH-CHD, echocardiography-based screening as a precursor to diagnosis can be more complex, since the presence of shunts alters haemodynamic parameters. For example, in patients with a systemic-to-pulmonary shunt, increased PASP may be associated with normal PVR and elevated pulmonary (Qp):systemic blood flow (Qs) ratio. In addition, patients with elevated right ventricular systolic pressure may have normal PASP in the presence of right ventricular outflow tract obstruction or congenital pulmonary artery stenosis. In patients with additional conditions such as thoracic abnormalities or comorbidities (e.g. chronic obstructive pulmonary disease or left heart disease), the non-invasive estimation of PAP by TTE is associated with a significant risk of overestimating PAH prevalence. Thus, if a patient with CHD develops echocardiographic signs of PH, additional investigations including cardiac magnetic resonance imaging and right-heart catheterization are required to confirm PAH diagnosis and determine the optimal treatment strategy.
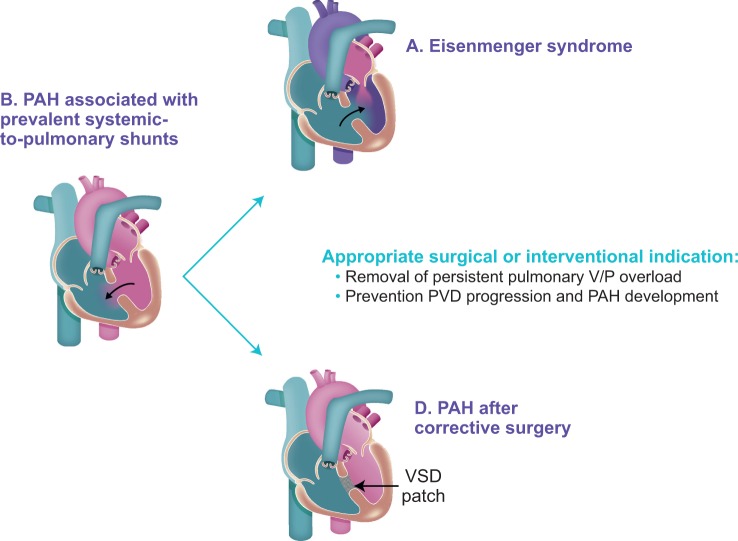
In patients with systemic-to-pulmonary shunts, PAH (Group B) may develop because of persistent and substantial volume and/or pressure pulmonary vascular overload, leading to pulmonary vascular disease (PVD) and potentially evolving to Eisenmenger syndrome.42 Thus, timely correction of the cardiac shunt can prevent the development of PAH due to irreversible PVD (Group A). In patients with corrected CHD, PAH (Group D) develops when irreversible PVD is already present at the time of correction, and/or if PVD progresses over time despite the closure of the cardiac shunt (Figure 2); Group D PAH-CHD is characterized by worse prognosis when compared with Group B. Thus, screening should be focused on the correct identification of patients with systemic-to-pulmonary shunts who will benefit from closure and the exclusion of those who may develop PAH after defect correction (Group D).
Figure 2.
Evolution of pulmonary arterial hypertension associated with congenital heart disease subgroup B.42 CHD, congenital heart disease; PAH, pulmonary arterial hypertension; PVD, pulmonary vascular disease; V/P, volume/pressure; VSD, ventricular septal defect. Reproduced from Gatzoulis et al.42 with permission from Elsevier.
PAH after defect correction is not easily predictable since it can occur when the repair was performed in a timely manner and when short-term outcomes were positive. The following characteristics (assessed at time of correction) are associated with risk to develop PAH after defect corrections and should be taken into account when considering operability: size and type of defect; patient age at correction; extracardiac abnormalities; and PVR at time of repair. Patients presenting with combinations of these features need individualized evaluation for possible development of PAH.
Since post-operative PAH can persist or develop in the weeks, months or years after defect repair, both short-term (3–6 months) and long-term regular monitoring for development of PAH is important.1,45 Postoperative monitoring should also account for patient characteristics at the time of defect correction, including age, type, and size of defect, and comorbidities, and for clinical and echocardiographic findings during follow-up. If suspected, diagnosis of PAH should be confirmed with right-heart catheterization.45
Prognosis
Overall, patients with PAH-CHD have a higher survival rate than patients with IPAH; one study reported 5-year survival rates of 91% for PAH-CHD compared with 63% for IPAH.43 However, it is important to note that survival rates in PAH-CHD vary by subgroup. Available data are somewhat limited except for Eisenmenger syndrome (Group A). Group A patients have the most advanced form of PAH, with the most severe exercise intolerance, but also have the best survival rates, which are matched only by patients in Group B.1 Group A survival rates up to 5 years are >90%,43,46 and were estimated at 89% after 10 years, and at 87% after 20 years.43 Survival estimates in Group B are >90% at up to 10 years, and at 86% after 20 years.43 Explanations for the apparently contradictory observation that survival is best in patients with the most advanced disease (Group A) include the possible presence of a cardiac defect that allows a pulmonary-to-systemic shunt, which may maintain adequate systemic flow and pressure.43 In addition, these patients may have enhanced right ventricular function owing to early development of PAH, which is responsible for maintenance of the foetal type of right ventricular hypertrophy.43,47
In patients with small/coincidental defects and severe PAH, the shunts are considered too small to be producing high pulmonary blood flow and to be causing deleterious effects on the pulmonary vasculature, so development of PAH is not generally attributable to such defects.1 Thus, these patients are considered to have IPAH and are treated as such.43 Data regarding prognosis in Group C are limited owing to low prevalence, but, in one study (n = 10), the survival rate was 88% at 5 years and at 10 years, and reduced to 66% at 20 years.43
Patients who develop PAH after defect correction have significantly worse survival rates than those in Groups A and B, and it would be useful in future to compare outcomes between patients who do or do not undergo defect closure (Group D vs. Group B). Among 44 patients in Group D, the first right-heart catheterization diagnostic for PAH was at a median of 17 years after defect correction; survival rate at 5 years was 83%, reducing to 65% at 10 years and 36% at 20 years.43 The reasons for poorer prognosis in Group D than in other groups are unclear but could be attributable to the lack of a possible pulmonary-to-systemic shunt in the case of elevated PVR, or could be owing to impaired right ventricle adaptation to an after-load that increases after the first months or years of life.43
Management
All patients with PAH-CHD should be managed at a PH centre. Patient education, behavioural modifications, and awareness of medical risk factors are all important aspects of management. The optimal care regimen requires a multidisciplinary team approach, which includes the collaboration of cardiologists and pulmonologists. Patients with CHD can have thoracic structural abnormalities that lead to restrictive pulmonary disease, and patients can also have obstructive upper airway breathing disorders,48 so the ventilatory contribution to PH development should always be assessed by a pulmonologist.
Operability should be considered in patients with prevalent systemic-to-pulmonary shunting (Group B). Defect correction may prevent the development of Eisenmenger’s syndrome (Figure 1); however, PAH after defect correction (Group D) is characterized by worse prognosis than uncorrected PAH-CHD (Group B).43 Unfortunately, PAH after defect correction is not easily predictable and evidence-based criteria for defining operability are still lacking. Baseline PVR reflects the extent of PVD, and guidelines recommend its measurement to identify the appropriate therapeutic approach in Group B patients.1 In adults, closure of prevalent systemic-to-pulmonary defects (Group B) should be considered when PVR is <2.3 WU and PVR index is <4 WU/m2.1 Closure of defects is generally not indicated when PVR >4.6 WU and PVR index is >8 WU/m2; for patients with haemodynamic parameters between these values, PVR levels alone do not allow the identification of the appropriate treatment strategy.1 Additional criteria should be taken into account when considering operability in patients with Group B PAH-CHD, including size and type of defect, patient age, PVR:systemic vascular resistance ratio, Qp:Qs ratio, genetic factors, and extracardiac syndromes (e.g. Down syndrome).1 No prospective data are available on the utility of vasoreactivity testing with nitric oxide, closure test or lung biopsy for operability assessment.1 The decision to repair any defect in patients with increased PVR should be made at tertiary centres with expertise in PAH-CHD, considering not just haemodynamic data but all clinical data available and the patient’s medical history.
Medical management of patients with PAH-CHD is to an extent hindered by the fact that the four subgroups are not uniformly represented in randomized controlled trials (RCTs) of PAH-specific therapies. Trials of PAH-specific therapies have only been conducted in patients in Group A. Patients in Groups B and C are usually excluded from trials, and patients in Group D have been represented only as a population subgroup. These differences in patient enrolment are reflected in the ESC/ERS guidelines, which include recommendations specifically for Group A, and for PAH-CHD in general, but none specifically for Groups B, C, or D.1 Consequently, treatment decisions in these groups are based on clinical experience and on cohort studies conducted in expert PH centres.1,5
Studies of bosentan (BREATHE-5)2 and macitentan (MAESTRO)3 have been performed in patients with Eisenmenger syndrome (Group A). The PHIRST study of tadalafil included patients with systemic-to-pulmonary shunts (Group B),49 and a few patients from PAH-CHD Group C were enrolled in the EARLY study of bosentan.50 The prostacyclin analogue treprostinil has also been investigated in a trial for which PAH-CHD patients (Group A or D) comprised ∼25% of the population.51 A subgroup of patients with PAH after defect correction (Group D), typically >10% of the trial population, have been included in several PAH-specific studies: SERAPHIN (macitentan),24 AMBITION (ambrisentan plus tadalafil),52 COMPASS-2 (sildenafil plus bosentan),53 PATENT-1 and PATENT-2 (riociguat),25,54 and GRIPHON (selexipag).55
Bosentan is recommended for patients with Eisenmenger syndrome,1 based on findings from the placebo-controlled BREATHE-5 trial.2 Patients in BREATHE-5 (n = 54) who received bosentan for 16 weeks had improved PVR index and exercise capacity compared with controls.2 No improvement in exercise capacity relative to placebo was found in patients with Eisenmenger syndrome in the 16-week MAESTRO trial (n = 226) of macitentan, but MAESTRO enrolled a more heterogeneous patient population than did BREATHE-5.3 No safety concerns were raised in MAESTRO, and exploratory analysis in a subgroup of 39 patients with simple atrial or ventricular septal defects found that levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) were reduced, and both PVR index and exercise capacity had improved relative to controls.3 A retrospective study that compared patients with Eisenmenger syndrome receiving or not receiving PAH-specific therapy (bosentan, sildenafil, or epoprostenol) found a substantial unadjusted survival benefit associated with treatment (HR 0.16; P = 0.015).56
Owing to a lack of evidence for the effectiveness of pharmacological therapy in Group B patients, management focuses on preventing evolution to Group A by correcting defects when possible, or on regular monitoring in non-correctable patients. Despite the lack of evidence, many PAH centres utilize PAH-targeted treatments in non-correctable patients to prevent the development or the progression of obstructive remodelling of the pulmonary vessels. Due to poor prognosis in CHD patients who develop Group D PAH-CHD,43 specialist clinicians should determine which patients will benefit from closure. Patients with small/coincidental defects in Group C are often treated the same as patients with IPAH, as the clinical picture is very similar other than for the defect, and prescribed PAH-specific therapies.43 As noted above, patients in Group C are seldom enrolled in RCTs, but a small number of patients with small/coincidental defects were enrolled in the placebo-controlled EARLY study of bosentan, which demonstrated a significant reduction in PVR in the overall population.50 All groups may benefit from non-pharmacological management strategies including psychological support, exercise advice, and lifestyle counselling, including family planning.
As noted earlier, several RCTs of PAH-specific therapies have included patients (<10%) with PAH-CHD after defect correction (Group D) as a population subgroup, including RCTs investigating PAH-specific and combination therapies.24,52–55,57 Analyses of the Group D subgroup in the GRIPHON study of selexipag55 and overall in the SERAPHIN study of macitentan24 both suggested reductions in the rate of morbidity/mortality compared with placebo. In GRIPHON (n = 110), the HR for morbidity/mortality on selexipag vs. placebo was 0.58 in Group D55 and 0.60 in the overall population.58 Overall HR for morbidity/mortality in SERAPHIN was 0.55 for macitentan 10 mg vs. placebo.24 Improvements in exercise capacity, PVR, NT-proBNP, and WHO FC were demonstrated in the PATENT-1 study (n = 35) after treatment with riociguat for 12 weeks vs. placebo.54
Overall, the evidence from clinical trials supports the use of PAH-specific therapies in Group A and Group D patients, and this is reflected in the guidelines1. While there is limited evidence for the use of PAH therapies in Group C patients specifically, these patients are usually classified as having IPAH, for which there is abundant evidence on the efficacy of these therapies.1 The lack of evidence for the use of PAH-specific therapies in Group B PAH-CHD patients should be addressed by enrolling subgroups of these patients in future clinical trials or real-world analysis.
Conclusion
Screening to detect PAH and to initiate treatment as early as possible is important in both portal hypertension and CHD. Patients with PoPH or with PAH-CHD typically require lifelong PAH-specific treatment, although, in a small number of cases, PAH may be resolved completely as a consequence of organ transplant. There is evidence to support the use of PAH-specific therapy to improve haemodynamics before LT in PoPH, and to treat patients in PAH-CHD Groups A and D. Owing to the complexity of both PoPH and PAH-CHD, referral networks and multidisciplinary collaborations are essential for effective management of these patients.
Funding
Medical writing and editorial support were provided by Shuna Gould, Zoe Schafer and Richard McDonald of Watermeadow Medical, an Ashfield Company, funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Conflict of interest: L.S. has received personal fees and non-financial support from Actelion Pharmaceuticals Ltd. A.M. has received grants, personal fees, and non-financial support from Actelion Pharmaceuticals Ltd.
References
- 1. Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, Chiossi E, Landzberg M.. Bosentan randomized trial of endothelin antagonist therapy I. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48–54. [DOI] [PubMed] [Google Scholar]
- 3. Gatzoulis MA, Landzberg M, Beghetti M, Berger RM, Efficace M, Gesang S, He J, Papadakis K, Pulido T, Galiè N, Investigators MS.. Evaluation of macitentan in patients with Eisenmenger syndrome. Circulation 2019;139:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sitbon O, Bosch J, Cottreel E, Csonka D, de Groote P, Hoeper MM, Kim NH, Martin N, Savale L, Krowka M.. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med 2019;7:594–604. [DOI] [PubMed] [Google Scholar]
- 5. Dardi F, Manes A, Palazzini M, Bachetti C, Mazzanti G, Rinaldi A, Albini A, Gotti E, Monti E, Bacchi Reggiani ML, Galiè N.. Combining bosentan and sildenafil in pulmonary arterial hypertension patients failing monotherapy: real-world insights. Eur Respir J 2015;46:414–421. [DOI] [PubMed] [Google Scholar]
- 6. Klupp J, Kohler S, Pascher A, Neuhaus P.. Liver transplantation as ultimate tool to treat portal hypertension. Dig Dis 2005;23:65–71. [DOI] [PubMed] [Google Scholar]
- 7. Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD.. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest 2012;141:906–915. [DOI] [PubMed] [Google Scholar]
- 8. Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol 2014;20:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G.. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 10. Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD.. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013;144:169–176. [DOI] [PubMed] [Google Scholar]
- 11. Savale L, Magnier R, Le Pavec J, Jais X, Montani D, O'Callaghan DS, Humbert M, Dingemanse J, Simonneau G, Sitbon O.. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur Respir J 2013;41:96–103. [DOI] [PubMed] [Google Scholar]
- 12. Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jais X, Yaici A, Humbert M, Simonneau G, Sitbon O.. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 2008;178:637–643. [DOI] [PubMed] [Google Scholar]
- 13. Porres-Aguilar M, Gallegos-Orozco JF, Garcia H, Aguirre J, Macias-Rodriguez RU, Torre-Delgadillo A.. Pulmonary vascular complications in portal hypertension and liver disease: a concise review. Rev Gastroenterol Mex 2013;78:35–44. [DOI] [PubMed] [Google Scholar]
- 14. Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP.. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991;100:520–528. [DOI] [PubMed] [Google Scholar]
- 15. Awdish RL, Cajigas HR.. Early initiation of prostacyclin in portopulmonary hypertension: 10 years of a transplant center's experience. Lung 2013;191:593–600. [DOI] [PubMed] [Google Scholar]
- 16. Savale L, Sattler C, Coilly A, Conti F, Renard S, Francoz C, Bouvaist H, Feray C, Borentain P, Jaïs X, Montani D, Parent F, O'Connell C, Hervé P, Humbert M, Simonneau G, Samuel D, Calmus Y, Duvoux C, Durand F, Duclos-Vallée JC, Sitbon O.. Long-term outcome in liver transplantation candidates with portopulmonary hypertension. Hepatology 2017;65:1683–1692. [DOI] [PubMed] [Google Scholar]
- 17. Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, Flaherty JD.. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol 2011;58:223–231. [DOI] [PubMed] [Google Scholar]
- 18. Werlang ME, Palmer WC, Boyd EA, Cangemi DJ, Harnois DM, Taner CB, Stancampiano FF.. Patent foramen ovale in liver transplant recipients does not negatively impact short-term outcomes. Clin Transplant 2016;30:26–32. [DOI] [PubMed] [Google Scholar]
- 19. Layoun ME, Aboulhosn JA, Tobis JM.. Potential role of patent foramen ovale in exacerbating hypoxemia in chronic pulmonary disease. Tex Heart Inst J 2017;44:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sithamparanathan S, Nair A, Thirugnanasothy L, Coghlan JG, Condliffe R, Dimopoulos K, Elliot CA, Fisher AJ, Gaine S, Gibbs JSR, Gatzoulis MA, E. Handler C, Howard LS, Johnson M, Kiely DG, Lordan JL, Peacock AJ, Pepke-Zaba J, Schreiber BE, Sheares KKK, Wort SJ, Corris PA; National Pulmonary Hypertension Service Research Collaboration of the United Kingdom and Ireland. Survival in portopulmonary hypertension: outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant 2017;36:770–779. [DOI] [PubMed] [Google Scholar]
- 21. Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, Ramsay M, Davis GL.. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant 2007;7:1258–1264. [DOI] [PubMed] [Google Scholar]
- 22. Galiè N, McLaughlin VV, Rubin LJ, Simonneau G.. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J 2019;53:doi:10.1183/13993003.02148-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sitbon O, O'Callaghan DS, Savale L.. Portopulmonary hypertension: light at the end of the tunnel? Chest 2012;141:840–842. [DOI] [PubMed] [Google Scholar]
- 24. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BK, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G, Investigators S.. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809–818. [DOI] [PubMed] [Google Scholar]
- 25. Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ.. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369:330–340. [DOI] [PubMed] [Google Scholar]
- 26. Cartin-Ceba R, Halank M, Ghofrani HA, Humbert M, Mattson J, Fritsch A, Krowka M.. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ 2018;8:204589401876930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cartin-Ceba R, Swanson K, Iyer V, Wiesner RH, Krowka MJ.. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest 2011;139:109–114. [DOI] [PubMed] [Google Scholar]
- 28. Krowka MJ, Frantz RP, McGoon MD, Severson C, Plevak DJ, Wiesner RH.. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): a study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999;30:641–648. [DOI] [PubMed] [Google Scholar]
- 29. Melgosa MT, Ricci GL, Garcia-Pagan JC, Blanco I, Escribano P, Abraldes JG, Roca J, Bosch J, Barbera JA.. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transpl 2010;16:348–356. [DOI] [PubMed] [Google Scholar]
- 30. Fisher JH, Johnson SR, Chau C, Kron AT, Granton JT.. Effectiveness of phosphodiesterase-5 inhibitor therapy for portopulmonary hypertension. Can Respir J 2015;22:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savale L, Ebstein N, Jevnikar M, Jaïs X, Parent F, Boucly A, Simonneau G, Montani D, Humbert M, Sitbon O.. Survival in portopulmonary hypertension (PoPH) in the era of modern PAH-targeted therapy. Eur Respir J 2018;52(Suppl 62):PA3081. [Google Scholar]
- 32. Provencher S, Herve P, Jais X, Lebrec D, Humbert M, Simonneau G, Sitbon O.. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006;130:120–126. [DOI] [PubMed] [Google Scholar]
- 33. Savale L, Chaumais MC, Sitbon O, Humbert M.. Pulmonary arterial hypertension in patients treated with interferon. Eur Respir J 2015;46:1851–1853. [DOI] [PubMed] [Google Scholar]
- 34. Savale L, Sattler C, Gunther S, Montani D, Chaumais MC, Perrin S, Jais X, Seferian A, Jovan R, Bulifon S, Parent F, Simonneau G, Humbert M, Sitbon O.. Pulmonary arterial hypertension in patients treated with interferon. Eur Respir J 2014;44:1627–1634. [DOI] [PubMed] [Google Scholar]
- 35. Renard S, Borentain P, Salaun E, Benhaourech S, Maille B, Darque A, Bregigeon S, Colson P, Laugier D, Gaubert MR, Habib G.. Severe pulmonary arterial hypertension in patients treated for hepatitis C with sofosbuvir. Chest 2016;149:e69–73. [DOI] [PubMed] [Google Scholar]
- 36. Savale L, Watherald J, Sitbon O.. Portopulmonary hypertension. Semin Respir Crit Care Med 2017;38:651–661. [DOI] [PubMed] [Google Scholar]
- 37. Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MA, Sitbon O, Sokol RJ.. International Liver Transplant Society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation 2016;100:1440–1452. [DOI] [PubMed] [Google Scholar]
- 38. Goldberg DS, Batra S, Sahay S, Kawut SM, Fallon MB.. MELD exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant 2014;14:2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stratta C, Lavezzo B, Ballaris MA, Panio A, Crucitti M, Andruetto P, Fanelli V, Grosso Marra W, Ranieri MV, Salizzoni M.. Extracorporeal membrane oxygenation rescue therapy in a case of portopulmonary hypertension during liver transplantation: a case report. Transplant Proc 2013;45:2774–2775. [DOI] [PubMed] [Google Scholar]
- 40. Hoeper MM, Simon R. Gibbs J.. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014;23:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Riel AC, Schuuring MJ, van Hessen ID, Zwinderman AH, Cozijnsen L, Reichert CL, Hoorntje JC, Wagenaar LJ, Post MC, van Dijk AP, Hoendermis ES, Mulder BJ, Bouma BJ.. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol 2014;174:299–305. [DOI] [PubMed] [Google Scholar]
- 42. Gatzoulis MA, Beghetti M, Landzberg MJ, Galiè N.. Pulmonary arterial hypertension associated with congenital heart disease: recent advances and future directions. Int J Cardiol 2014;177:340–347. [DOI] [PubMed] [Google Scholar]
- 43. Manes A, Palazzini M, Leci E, Bacchi Reggiani ML, Branzi A, Galiè N.. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J 2014;35:716–724. [DOI] [PubMed] [Google Scholar]
- 44. Drakopoulou M, Nashat H, Kempny A, Alonso-Gonzalez R, Swan L, Wort SJ, Price LC, McCabe C, Wong T, Gatzoulis MA, Ernst S, Dimopoulos K.. Arrhythmias in adult patients with congenital heart disease and pulmonary arterial hypertension. Heart 2018;104:1963–1969. [DOI] [PubMed] [Google Scholar]
- 45. Dimopoulos K, Wort SJ, Gatzoulis MA.. Pulmonary hypertension related to congenital heart disease: a call for action. Eur Heart J 2014;35:691–700. [DOI] [PubMed] [Google Scholar]
- 46. Diller GP, Dimopoulos K, Broberg CS, Kaya MG, Naghotra US, Uebing A, Harries C, Goktekin O, Gibbs JS, Gatzoulis MA.. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737–1742. [DOI] [PubMed] [Google Scholar]
- 47. Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis 2005;16:19–25. [DOI] [PubMed] [Google Scholar]
- 48. Healy F, Hanna BD, Zinman R.. Pulmonary complications of congenital heart disease. Paediatr Respir Rev 2012;13:10–15. [DOI] [PubMed] [Google Scholar]
- 49. Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ; Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119:2894–2903. [DOI] [PubMed] [Google Scholar]
- 50. Galiè N, Rubin L, Hoeper M, Jansa P, Al-Hiti H, Meyer G, Chiossi E, Kusic-Pajic A, Simonneau G.. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008;371:2093–2100. [DOI] [PubMed] [Google Scholar]
- 51. Simonneau G, Barst RJ, Galiè N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ.. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165:800–804. [DOI] [PubMed] [Google Scholar]
- 52. Galiè N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiéry JL, Grunig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ, Investigators A.. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–844. [DOI] [PubMed] [Google Scholar]
- 53. McLaughlin V, Channick RN, Ghofrani HA, Lemarie JC, Naeije R, Packer M, Souza R, Tapson VF, Tolson J, Al Hiti H, Meyer G, Hoeper MM.. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015;46:405–413. [DOI] [PubMed] [Google Scholar]
- 54. Rosenkranz S, Ghofrani HA, Beghetti M, Ivy D, Frey R, Fritsch A, Weimann G, Saleh S, Apitz C.. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart 2015;101:1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beghetti M, Channick RN, Chin KM, Di Scala L, Gaine S, Ghofrani H-A, Hoeper MM, Lang IM, McLaughlin VV, Preiss R, Rubin LJ, Simonneau G, Sitbon O, Tapson VF, Galiè N.. Selexipag treatment for pulmonary arterial hypertension associated with congenital heart disease after defect correction: insights from the randomised controlled GRIPHON study. Eur J Heart Fail 2019;21:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dimopoulos K, Inuzuka R, Goletto S, Giannakoulas G, Swan L, Wort SJ, Gatzoulis MA.. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010;121:20–25. [DOI] [PubMed] [Google Scholar]
- 57. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G; Sildenafil Use in Pulmonary Arterial Hypertension Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 58. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV, Investigators G.. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015;373:2522–2533. [DOI] [PubMed] [Google Scholar]