Abstract
Historically, pulmonary arterial hypertension (PAH) has been considered a disease of young adults, but over the last three decades, the average age at diagnosis has increased, presenting clinicians with some unique challenges. Clinical symptoms of PAH, including shortness of breath and reduced functional capacity, are not specific for the disease and may be present in older patients because of their age or as a result of comorbid conditions. Eliminating other causes for these symptoms can delay PAH diagnosis and initiation of PAH-specific treatment compared with younger patients. Currently, there are no specific guidelines relating to PAH in older patients and existing guidelines for identifying patients at potential risk of PAH may not be appropriate for patients aged over 65 years. Even though older patients tend to be diagnosed with more advanced symptoms, and evidence suggests that they are less responsive to PAH-specific therapies, treatment is often less aggressive than in younger patients. Even after adjusting for age, survival rates remain disproportionately lower in the older vs. younger PAH populations. Specific guidelines for diagnosis and treatment of older patients with PAH are needed to improve care and outcomes in this growing population. This review aims to assess the challenges associated with diagnosing and managing PAH in older patients, based on literature searches, authors’ experiences, and expert opinions.
Keywords: Pulmonary arterial hypertension, Older patients, Diagnosis, Patient management
Introduction
Pulmonary arterial hypertension (PAH) has historically been considered to affect young adults and predominantly women. However, data from PAH registries suggest that, over recent years, the demographics of PAH populations are changing with increases in the average age at diagnosis, the number of older patients and the proportion of male patients being reported.1–3 The exact reasons for the increased incidence of PAH in older patients are unknown, but contributing factors include increased awareness and understanding of the disease; improved screening/diagnostic methods; the overall ageing of western populations; and associated increases in common age-related comorbidities linked with vascular endothelial dysfunction.4,5 Improvements in PAH survival rates over the past two decades, due to a combination of new treatments and improved patient-support strategies,6 may also have contributed to greater life expectancy for patients with PAH in general, and particularly older patients.
The diagnosis of PAH in patients aged over 65 years is impeded by a number of factors and can be clinically challenging.7 Older patients tend to present with multiple comorbidities that may mask the symptoms of PAH and delay diagnosis.4 Furthermore, as they generally have reduced exercise capacity, older patients are more likely to be diagnosed with more advanced symptoms than younger patients.4,5,8
The journey to a PAH diagnosis can have a substantial impact on a patient’s quality of life, as well as living with the disease itself.9–11 Most studies on patient-reported outcomes in PAH are generally concerned with aspects that are more relevant to non-elderly patients, such as being unable to work and the consequent financial concerns and feelings of social isolation.9,10 However, PAH adversely affects patients’ quality of life regardless of age12–14 and being mindful of its impact on patients’ daily lives can be particularly important when treating elderly patients, for whom maintenance or improvement of quality of life may be the main treatment goal. Few studies have investigated patient-reported outcomes in elderly PAH patients but a separate review in this supplement15 is devoted to the patient’s perspective and discusses measures to improve disease management in more detail.
Older patients also present unique challenges in terms of treatment and management. Treatment selection is complicated by the susceptibility of many older patients to adverse events. Moreover, these patients may already be receiving a range of concomitant medications to treat comorbidities, therefore, the potential for drug–drug interactions needs to be assessed when selecting therapy. In addition, family and social support may also present more of a challenge in this population.
Despite these difficulties, no guidelines for the management of PAH in older patients currently exist. This review aims to assess the challenges associated with diagnosing and managing PAH in older patients and address potential approaches to overcoming these challenges.
The changing profile of pulmonary arterial hypertension: registry data
For the past 30 years, PAH registries from around the globe have collected data on the demographic, clinical and haemodynamic characteristics of more than 6100 patients16–23 (Table 1). In the oldest PAH registry, established by the US National Institutes of Health (NIH) in 1981,22 patients had a mean age at diagnosis of 36 years and 118/187 (63%) were female. More recent, PAH registries have since reported higher mean ages at diagnosis (Table 1), most notably in the European COMPERA (comparative, prospective registry of newly initiated therapies for pulmonary hypertension) registry established in 2007, where 63% of the 587 patients were aged >65 years.23 One exception was in a Chinese registry, established in 1999, where the mean age at diagnosis was similar to the US NIH registry. This discrepancy has been attributed to a lack of diagnosis of PAH in older patients in China perhaps due to shortcomings in the healthcare system and poor economic conditions.25
Table 1.
Demographics and pulmonary arterial hypertension characteristics of patients included in registriesa
| Registry | US NIH22 | Switzerland20 | China16 | UK/ Ireland21 | France18 | US REVEAL24 | COMPERA23b | Czech Republic17 | Spain19 |
|---|---|---|---|---|---|---|---|---|---|
| Year of initiation | 1981 | 1998 | 1999 | 2001 | 2002 | 2006 | 2007 | 2007 | 2007 |
| Year of first data report | 1987 | 2015 | 2007 | 2012 | 2006 | 2010 | 2013 | 2014 | 2012 |
| Number of patients | 187 | 517 | 72 | 482 | 674 | 2525 | 587 | 191 | 866 |
| Study cohort | |||||||||
| Incidentc | ✓ | – | ✓ | ✓ | – | – | ✓ | – | – |
| Incident and prevalentd | – | ✓ | – | – | ✓ | ✓ | – | ✓ | ✓ |
| Age (years), mean (SD) | 36 (15) | 57 (16) | 36 (12) | 50 (17) | 50 (15) | 53 (14) | 71 (16)e | 52 (17) | 45 (17) |
| Female (%) | 63.1 | 60.0 | 70.8 | 69.9 | 65.3 | 79.5 | 60.3 | 65.5 | 71.0 |
| Time from first symptoms to diagnosis (months), mean (SD) | 24.4 (58.8) | NP | 26.4 (27.6) | 18 (9–36)e | 27f | 35.6 (37.9) | NP | 39 (48) | 42 (73) |
| 6MWD (m), mean (SD) | NP | 357 (137) | NP | 292 (123)g | 329 (109) | 366 (126) | 293 (126) | 324 (120) | 363 (120) |
| Haemodynamics, mean (SD) | |||||||||
| RAP (mmHg) | 10 (6) | 9 (4) | 12.8 (5.6) | 10 (6)h | 8 (5) | 9 (6) | 8 (5) | 10 (5) | 9 (5) |
| mPAP (mmHg) | 60 (18) | 48 (15) | 64.1 (17) | 54 (14)i | 55 (15) | 51 (14) | 44 (12) | 59 (63) | 54 (16) |
| PVR (Wood units) | NP | 9.4 (5.6)j | 20.4 (8.0) | 12.8 (6.3)k | NP | NP | 9.6 (5.5) | NP | 12 (6) |
| PVRI (Wood units·m2) | 26.2 (13.8) | NP | NP | 23.1 (10.1)l | 20.5 (10.2) | 21.1 (12.5) | NP | NP | NP |
6MWD, 6-min-walk distance; COMPERA, comparative, prospective registry of newly initiated therapies for pulmonary hypertension; mPAP, mean pulmonary arterial pressure; NIH, National Institutes of Health; NP, not presented; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SD, standard deviation.
Pulmonary arterial hypertension-only where reported, idiopathic pulmonary arterial hypertension otherwise.
Austria, Belgium, Germany, Italy, the Netherlands, Switzerland, and UK.
Newly diagnosed patients.
Previously diagnosed patients.
Median (interquartile range).
SD not provided.
n = 260.
n = 439.
n = 457.
n = 440.
n = 395.
n = 355.
Results from registries that reported data by year of diagnosis or by age at diagnosis also showed increases in the mean age of diagnosis20 and the proportion of male patients being diagnosed23 (Table 2). Although older patients are more likely to have move severe symptoms and lower percent diffusing capacity of the lung for carbon monoxide (DLCO), and shorter 6-min-walk distance (6MWD),21,23 they tended to have less severe haemodynamics (Table 2).
Table 2.
Demographics and pulmonary arterial hypertension characteristics of patients included in registriesa by age
| Registry | Switzerland20 | UK/Ireland21 | COMPERA23b | |||
|---|---|---|---|---|---|---|
| Cohort | Diagnosis before 2000 | Diagnosis 2009–2012 | ≤50 years | >50 years | 18–65 years | >65 years |
| Number of patients | 24 | 171 | NP | NP | 209 | 378 |
| Age (years), mean (SD) | 42 (16) | 60 (15) | 36.5 (9.3) | 65.1 (8.3) | 54 (16)c | 75 (8)c |
| Female (%) | 66.7 | 55.6 | 73.2 | 66.5 | 69.4 | 55.3 |
| Time from first symptoms to diagnosis (months), median (IQR) | NP | NP | 12 (6–24) | 24 (12–36) | NP | NP |
| 6MWD (m), mean (SD) | 341 (120) | 366 (139) | 330 (119) | 246 (112) | 340 (131) | 266 (114) |
| Haemodynamics, mean (SD) | ||||||
| Cardiac index (L/min−1/m2) | 2.6 (0.9) | 2.6 (0.8) | 2.1 (0.7) | 2.1 (0.7) | 2.2 (0.7) | 2.2 (0.7) |
| RAP (mmHg) | 9 (4) | 9 (8) | 10.0 (5.8) | 10.2 (6.1) | 9 (5) | 8 (5) |
| mPAP (mmHg) | 55 (18) | 46 (12) | 57.2 (14.9) | 51.0 (12.2) | 50 (13) | 41 (10) |
| PVR (Wood units) | 10.1 (5.4) | 8.5 (5.2) | 13.9 (6.7) | 11.8 (5.8) | 12.0 (6.3) | 8.3 (4.5) |
6MWD, 6-min-walk distance; COMPERA, comparative, prospective registry of newly initiated therapies for pulmonary hypertension; IQR, interquartile range; mPAP, mean pulmonary arterial pressure; NP, not presented; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SD, standard deviation.
PAH-only where reported, IPAH otherwise.
Austria, Belgium, Germany, Italy, the Netherlands, Switzerland, and UK.
Median (interquartile range).
Diagnosis of pulmonary arterial hypertension in older patients
General considerations
The diagnosis of PAH is complex, and even more so as patients age. Consequently, patients may be referred to various specialists resulting in delays to diagnosis confirmation.26 Across the registries, the mean duration between symptom onset and diagnostic confirmation of PAH ranged from 24 to 36 months (Table 1) with the UK/Ireland registry reporting that the median duration of symptoms prior to diagnosis in older patients was double that for younger patients (24 vs. 12 months)21 (Table 2). Older patients often present with multiple comorbidities21 that may complicate and delay a diagnosis of PAH. Systemic hypertension, ischaemic heart disease, dyslipidaemia, diabetes, and arrhythmia are among the most common comorbidities reported in older patients,21,27,28 and they are more prevalent in this population compared with younger adults, as evidenced across two studies that defined older as >50 years of age,21 and ≥75 years of age.27 Comorbid cardiovascular and metabolic diseases are also commonly observed in the general PAH population24 and management of PAH in these cases is fully discussed in a separate review in this supplement.29
Identification of PAH as the leading pulmonary hypertension (PH) diagnosis in older patients is important so as not to mix the true PAH population with patients in whom disorders relating to PH Group 2 (left heart disease), Group 3 (lung pathology/hypoxia), or even Group 4 [chronic thromboembolic PH (CTEPH)] are the primary condition responsible for disease progression.26 Incorrect diagnoses could result in inappropriate treatment choices being made, particularly as therapies for treating PAH are often contraindicated for Group 2 and Group 3 PH.26 In order to form a correct diagnosis, it is also important to discriminate PAH from secondary causes commonly seen in older populations, such as coronary artery disease (CAD), left ventricular diastolic dysfunction, or dyspnoeic conditions like chronic obstructive pulmonary disease.4,30,31
Differentiating clinical signs of PAH from those related to ageing or comorbidities add to the time required for diagnosis. PAH is a progressive disease and greater deterioration as a consequence of delayed diagnosis may be one of the reasons older patients with PAH are typically less responsive to therapy. However, older patients with PAH do tend to present with less severe haemodynamic measures associated with worse functional impairment than in younger adults with PAH, suggesting that a slightly different mechanism of disease progression may be at work, and it is also possible that this is the reason for poorer response to PAH treatment in older populations. Nevertheless, timely diagnosis is important for this vulnerable population.3,21,23
Pulmonary arterial hypertension should, therefore, always be considered in older patients presenting with appropriate symptoms, but care must be taken to ensure that appropriate steps are taken to determine the correct diagnosis.
Diagnostic process
The diagnostic process for PAH in older patients should follow that recommended in the current guidelines for PAH,32,33 but with additional considerations specific for older patients. The recommended process involves clinical examination, electrocardiogram, chest radiography, pulmonary function tests, 6MWD, arterial blood gas analysis, echocardiography, ventilation/perfusion scanning, and high-resolution computed tomography prior to mandatory confirmation via right heart catheterization (RHC).32 In older patients, challenges in undertaking some of these examinations need to be taken into consideration.
Clinical examination
The main clinical symptoms of PAH at initial presentation (e.g. shortness of breath, reduced exercise tolerance) are non-specific and shared with a variety of other, much more common, age-related comorbid conditions, including left heart disease, aortic stenosis, and impaired lung function.4,28,30 Consequently, PAH may not be an immediate consideration for the cause of the symptoms. Physician awareness of PAH is, therefore, particularly important when dealing with older patients so that further diagnostic testing and potential specialist referral can take place in a timely manner.32
Echocardiography
Echocardiography is a vital tool for the screening and monitoring of PAH.32 However, the measurement of certain echocardiographic parameters can be less reliable in older patients. Atrial fibrillation, which is often seen in older patients who have cardiac/valvular comorbidities, can complicate echocardiographic analyses, reducing the reliability of atrial size measurements and estimates of left ventricular filling pressures and mitral annular velocities.34–38 In addition, distinguishing between echocardiographic changes due to PAH from those due to, for example, age or left heart disease can be challenging in older patients.4
Exercise testing
The physical condition of older patients should be considered when interpreting 6MWD data. Age-related changes in a number of pulmonary function parameters (e.g. progressive decline in lung function), as well as pulmonary and systemic haemodynamic measures, may lead to reduced exercise tolerance in older patients and should be considered separately from changes that may be due to pulmonary vasculopathy. It should also be considered that older patients often exhibit reduced mobility due to additional factors such as osteoporosis, arthritis, or other musculoskeletal pathology (e.g. hip/knee fractures or hip replacement).
Ventilation/perfusion scanning
Ventilation/perfusion should be used to rule out a diagnosis of CTEPH.32
Right heart catheterization
A 2018 study concluded that RHC could be successfully used in the diagnosis of PH regardless of age.39 However, it should be noted that certain difficulties can present in the recording and interpretation of RHC results in older patients.
Measurement of pulmonary artery wedge pressure (PAWP) allows differentiation between PAH (pre-capillary PH) and PH due to heart failure with preserved left ejection fraction, which is common in elderly patients and those with Group 2 PH classification (post-capillary). However, PAWP can be falsely low/normal in patients who do actually have elevated left ventricle end-diastolic pressure (LVEDP), for example with patients treated with diuretics.40 Therefore, it is often necessary to use exercise or fluid challenge during RHC to determine the true resistive properties of the pulmonary vessels. However, measuring PAWP during exercise testing is a complex procedure and, moreover, as exercise capacity and mobility may be reduced in the elderly, fluid challenge may be preferable.41 In patients with an increased PAWP, the diastolic pulmonary gradient can be used to help distinguish patients with a true pre-capillary component in a post-capillary setting.1,4 Similarly, the transpulmonary pressure gradient can be used to discriminate between ‘passive’ PH and ‘reactive’ PH, but this can be influenced by pulmonary circulation characteristics and age-related vascular stiffness, so should be interpreted with caution.1,4
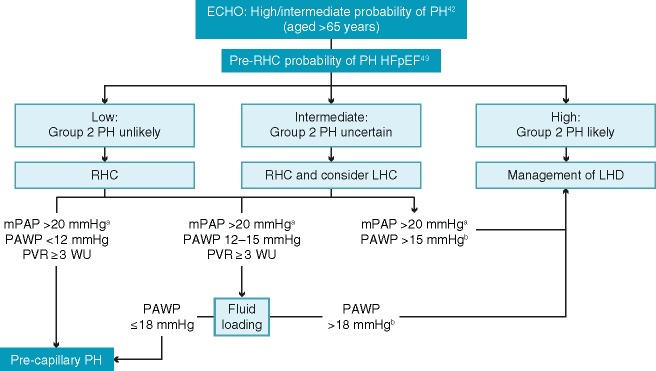
Taking all these factors into account, Figure 1 illustrates a proposed algorithm for diagnosing PAH in older patients based on haemodynamic evaluations.
Figure 1.
Algorithm for haemodynamic evaluation of suspected pulmonary arterial hypertension in patients aged >65 years.42,43aThis reflects the recommendation from the 6th World Symposium on Pulmonary Hypertension to consider this threshold as the upper limit of normal value.44 Please note the 2015 ESC/ERS Guidelines use the threshold value of 25 mmHg for mean pulmonary arterial pressure measurements.32bConsider left ventricle end-diastolic pressure validation. ECHO, echocardiography; HFpEF, heart failure with preserved ejection fraction; LHC, left heart catheterization; LHD, left heart disease; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; WU, Wood units.
Management of pulmonary arterial hypertension in older patients
General considerations and treatment goals
Older patients with PAH are more likely to have more severe symptoms compared with a younger cohort, although haemodynamic impairments tend to be less extensive. While younger patients with PAH are more likely to be diagnosed in New York Heart Association (NYHA)/World Health Organization (WHO) functional Class I/II, older patients generally have a more severe functional class (III/IV), and lower exercise tolerance.21,23 Furthermore, a subgroup of PAH patients who present with low DLCO (<45% predicted) has been identified. These patients were more frequently older, male, and smokers, had more comorbidities (including coronary disease), a poorer prognosis, and worse pulmonary function, exercise capacity and survival rate than other patients with PAH.45 However, their haemodynamic profile is similar to other age-matched patients and they are without bone morphogenetic protein receptor Type 2 gene (BMPR2) mutations, the most important genetic predisposition factor for PAH.46 This low DLCO patient subgroup, who present with different risk factors and comorbidities to patients with higher DLCO values, may be enriched in older populations and could account for the increased mortality seen in older vs. younger patients.
Another reason for poorer survival in older patients may be that treatment goals differ from those for younger patients. The primary focus of treatment in older patients is likely to be improvement or maintenance of quality of life and palliative care. It should also be considered that, in general, the systemic and pulmonary vasculature of older patients is less responsive to vasodilation due to vascular stiffening and reduced left heart compliance (with progressive left ventricular diastolic dysfunction)30,47 and may be less responsive to PAH-specific therapies.23,48 In addition, improvement in exercise capacity should be individualized based on patient age and take into account any comorbidities present, particularly those affecting mobility. Given the reduced survival times seen in older patients, there is a need to individually optimize treatment, especially in those patients with very low DLCO, which could represent covert pulmonary veno-occlusive disease.
Despite a generally worse prognosis for older patients, they tend to be treated less aggressively than younger patients. In the UK/Ireland registry, significantly fewer patients aged >50 years vs. ≤50 years received sequential combination therapy (34.5% vs. 57.6%, P < 0.001), triple combination therapy (1.3% vs. 10.6%, P < 0.001) or prostacyclin analogues (28.1% vs. 51.8%, P < 0.001).21 Similarly, in the COMPERA registry, in the first 3 months following diagnosis, fewer patients aged >65 years (compared with those aged 18–65 years) received prostacyclin analogues (6.3% vs. 14.4%, P = 0.003), endothelin receptor antagonists (ERAs) (63.2% vs. 71.3%, P = 0.056), and combination therapy (12.7% vs. 19.2%; P = 0.07).23
These differences in therapeutic approaches between older and younger patients may have contributed to the corresponding disparities seen in the COMPERA registry in the improvement in 6MWD after 1 year (median increases of 30 and 50 m, respectively, P = 0.028) and overall mortality rates (22.0% vs. 12.0%, P = 0.003).23 Although the smaller improvement in 6MWD could be due to other age-related mobility issues, the difference in mortality rates remained statistically significant (P = 0.001) after adjustment for the survival estimates of age-/gender-matched populations.23
Each patient’s expectations of any prescribed treatment should always be considered. Discussions between patients and their physicians on considerations such as life expectancy, quality of life and the benefits and risks of treatment need to be undertaken throughout the disease course and particularly before any change in treatment.
Treatment approach and risk stratification
Treatments used in the management of PAH focus on three separate signalling pathways: the prostacyclin pathway targeted with prostacyclin analogues and prostacyclin receptor agonists, the endothelin pathway, targeted with ERAs, and the nitric oxide pathway targeted with phosphodiesterase-5 (PDE-5) inhibitors and soluble guanylate cyclase stimulators.32 In younger patients, combinations of these therapies may be prescribed, but in older patients, a sequential monotherapy approach to treatment is frequently adopted with combinations of treatments only administered to patients showing little or no improvement.8,49,50 This less aggressive approach taken with older patients may be because current risk scoring/stratification assessments for combination treatment of PAH are based on younger cohorts of patients and cannot reliably be extrapolated directly to older patients.8 For example, assessments of peripheral oedema/fluid management are more important in older patients, who are more likely to have decompensated right heart failure and/or renal dysfunction.8 Although it is not possible to tailor risk scoring/stratification assessments so that they are applicable to older patients in general, it is important that the severity of PAH and any comorbid conditions are evaluated in each patient to aid in the decision of what, if any, combination of therapies can be used.
In general, the range and frequency of follow-up assessments in older patients receiving PAH-specific therapies are those recommended in the current European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines (i.e. to follow-up 3 months after treatment initiation).32 Detailed reassessments, particularly with catheterization, may be beneficial in the older patient to assess for unmasking of left heart disease. A final treatment consideration is how the age of the patient may affect planning of treatment and future treatment options. Lung transplantation may eventually be required, but the upper age limit for such operations is not globally consistent (e.g. 60 years in UK, but 70 years in France). All potential medicinal options should have been assessed before surgery is contemplated, taking into account the age limit and donor organ availability.
Tolerability
In the COMPERA registry, a significantly greater percentage of patients aged >65 years discontinued treatment with the ERAs bosentan, sitaxentan, and ambrisentan compared with patients aged 18–65 years (17.2% vs. 8.1%, P = 0.014), mainly because of an apparent lack of efficacy (65.9% vs. 58.3% of patients who discontinued these treatments). A non-significantly higher percentage of discontinuations was also seen from treatment with PDE-5 inhibitors in the older patient group (6.7% vs. 4.5%).23 Although the number of patients reporting side effects was not presented in the COMPERA registry manuscript, there was an age discrepancy in discontinuations related to side effects following treatment with PDE-5 inhibitors (47.4% vs. 14.3% of patients receiving PDE-5 who discontinued).23
It has been reported that older patients can be more sensitive to side effects related to PAH-specific treatments, in particular, peripheral oedema resulting from fluid retention.21 As side effects of medications can have a significant impact on patient quality of life, treatment decisions need to be thoroughly considered and discussed with patients. This is particularly true of intravenous therapies, which can be particularly challenging for patients with arthritis, visual impairment, or social isolation.
The increased prevalence of comorbidities and associated co-medication in older patients may also increase the risk of drug–drug interactions with PAH-specific medications.1 This, combined with the potentially greater risk of side effects, could result in a greater impact on patient quality of life compared with younger patients. These issues need to be thoroughly considered and discussed with patients, along with careful monitoring for any treatment-related complications.
Adherence to pharmacotherapy
Treatment adherence is an important issue in any chronic illness but can be especially challenging for older patients with PAH. Treatment often requires combination therapies to target multiple pathways and older patients are more likely to already be taking multiple medications for comorbid pathologies. A systematic literature review identified several variables that were significantly related to medication adherence in older patients.51 These included self-efficacy (belief of being able to perform a specific task under differing conditions), belief in the efficacy of the treatment, confidence in the prescribing physician, views on natural products and home remedies, beliefs on control over their own health and perceptions of the illness. More technologically minded patients may benefit from the use of on-line or mobile phone apps to aid adherence to medications. Specialist nurses can be a vital point of contact, providing information and advice for older patients with PAH who might need help with adhering to therapy.
Outcomes in older patients
While overall patient clinical outcomes and survival have improved over recent years, in part due to the introduction of PAH-specific therapies, survival rates remain disproportionately lower in the older PAH population vs. younger patients.2
Progressively worsening survival rates were reported in older vs. younger patients over time in the COMPERA registry (1 year: 89.8% vs. 96.0%; 2 years: 78.6% vs. 90.9%; 3 years: 68.0% vs. 83.3%), and this finding persisted following adjustment for an age-/gender-matched population.23 In the UK/Ireland registry,21 patients aged >50 years had a three-fold greater mortality risk vs. younger patients. These inferior survival rates may be related to a more limited response to pharmacotherapy in older patients, but it is not possible to confirm this due to the less aggressive treatment approach typically adopted in these patients.
Previous studies have shown that patients with PAH and risk factors for left ventricular diastolic dysfunction, such as CAD or with cardiac comorbidities do not display a reduced response to treatment.52,53 However, another study in PAH has shown that in addition to older age, cardiovascular risk factors may predict a reduced response to treatment.54 The elucidation of whether treatment response in older, comorbid patients is similar or reduced when compared with younger patients requires further investigation.
The lower survival rate among older patients with PAH may be due to the right ventricle in older patients having less capacity to adapt following stress loading as seen by their lower pulmonary vascular resistance compared with younger patients. Lower survival rates are also likely to be related to a longer delay in diagnosis and a higher burden of comorbid conditions,27 such as cardiac or lung disease associated with secondary PH.28,30 It has even been postulated that there is a different natural history of PAH in late-onset cases,55 and this might be associated with older patients presenting with low DLCO values, which carry a particularly poor prognosis.45 Furthermore, it is not known why older patients should have worse symptoms but less severe haemodynamics. PAH symptom severity may be directly related to age itself, the number and severity of confounding comorbidities or physiological changes with age may decrease the response of the right ventricle to afterload. More research into these age-related differences is required.
Conclusions
The diagnosis and management of PAH in older patients are complicated by ageing per se and comorbid conditions associated with ageing, resulting in delays in starting treatment and worse outcomes compared with younger patients. The goals, expectations, and treatment tolerability in older patients should be assessed on an individual basis, as they may differ from those usually experienced in a younger patient cohort. Importantly, guidelines relating specifically to older patients are needed to improve care and outcomes in this growing PAH population.
Funding
Medical writing and editorial support were provided by Victoria Atess and Richard McDonald of Watermeadow Medical, an Ashfield Company, funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Conflict of interest: O.S. has served as a steering committee member for Actelion Pharmaceuticals Ltd; has served as an advisory board member for and received research grants from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has received consultancy fees from Actelion Pharmaceuticals Ltd, Arena, Bayer, GlaxoSmithKline and Merck Sharp & Dohme; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has served on a scientific advisory board for Arena Pharmaceuticals and Gossamer Bio; and has received writing assistance from Actelion Pharmaceuticals Ltd and GlaxoSmithKline. L.H. reports lecture fees, travel/accommodation and meeting expenses, departmental funding for service review and that he is a steering committee and advisory board member for Actelion Pharmaceuticals Ltd; lecture fees, travel/accommodation, and meeting expenses, grant/research support and that he is an advisory board member for Bayer PLC; travel/accommodation and meeting expenses and that he is an advisory board member for Merck; consultancy fees from Endotronix; he is a steering committee member for Bristol-Myers Squibb, BTG PLC, and United Therapeutics; provision of scientific advice for Third Pole Therapeutics and Gossamer Bio; and that he is an advisory board member for Arena Pharmaceuticals and GlaxoSmithKline.
References
- 1. Rothbard N, Agrawal A, Fischer C, Talwar A, Sahni S.. Pulmonary arterial hypertension in the elderly: clinical perspectives. Cardiol J 2018;doi:10.5603/CJ.a2018.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, Pulido T, Rich S, Rosenkranz S, Suissa S, Humbert M.. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62:D51–D59. [DOI] [PubMed] [Google Scholar]
- 3. Hoeper MM, Simon R.. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014;23:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berra G, Noble S, Soccal PM, Beghetti M, Lador F.. Pulmonary hypertension in the elderly: a different disease? Breathe (Sheff) 2016;12:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akdeniz B, Ozpelit E.. Which prognostic factors should be used in pulmonary arterial hypertension in elderly patients? J Geriatr Cardiol 2017;14:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD.. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448–456. [DOI] [PubMed] [Google Scholar]
- 7. Lador F, Herve P.. A practical approach of pulmonary hypertension in the elderly. Semin Respir Crit Care Med 2013;34:654–664. [DOI] [PubMed] [Google Scholar]
- 8. Hjalmarsson C, Radegran G, Kylhammar D, Rundqvist B, Multing J, Nisell MD, Kjellstrom B; SveFPH and SPAHR. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J 2018;51:1702310.. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong I, Billings C, Kiely DG, Yorke J, Harries C, Clayton S, Gin-Sing W.. The patient experience of pulmonary hypertension: a large cross-sectional study of UK patients. BMC Pulm Med 2019;19:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guillevin L, Armstrong I, Aldrighetti R, Howard LS, Ryftenius H, Fischer A, Lombardi S, Studer S, Ferrari P.. Understanding the impact of pulmonary arterial hypertension on patients' and carers' lives. Eur Respir Rev 2013;22:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Pulmonary Hypertension Association (PHA). The impact of pulmonary arterial hypertension (PAH) on the lives of patients and carers: results from an international survey. 2012. www.phaeurope.org\\wp-content\\uploads\\International-PAH-patient-and-Carer-Survey-Report-FINAL1.pdf (8 August 2019).
- 12. Kukkonen M, Puhakka A, Halme M.. Quality of life among pulmonary hypertension patients in Finland. Eur Clin Respir J 2016;3:26405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taichman DB, Shin J, Hud L, Archer-Chicko C, Kaplan S, Sager JS, Gallop R, Christie J, Hansen-Flaschen J, Palevsky H.. Health-related quality of life in patients with pulmonary arterial hypertension. Respir Res 2005;6:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zlupko M, Harhay MO, Gallop R, Shin J, Archer-Chicko C, Patel R, Palevsky HI, Taichman DB.. Evaluation of disease-specific health-related quality of life in patients with pulmonary arterial hypertension. Respir Med 2008;102:1431–1438. [DOI] [PubMed] [Google Scholar]
- 15. Ferrari P, Skåra H. My life with pulmonary arterial hypertension: a patient perspective. Eur Heart J Suppl 2019;21:K54–K59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jing Z-C, Xu X-Q, Han Z-Y, Wu Y, Deng K-W, Wang H, Wang Z-W, Cheng X-S, Xu B, Hu S-S, Hui R-T, Yang Y-J.. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007;132:373–379. [DOI] [PubMed] [Google Scholar]
- 17. Jansa P, Jarkovsky J, Al-Hiti H, Popelova J, Ambroz D, Zatocil T, Votavova R, Polacek P, Maresova J, Aschermann M, Brabec P, Dusek L, Linhart A.. Epidemiology and long-term survival of pulmonary arterial hypertension in the Czech Republic: a retrospective analysis of a nationwide registry. BMC Pulm Med 2014;14:45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G.. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 19. Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, Barbera JA; REHAP Investigators. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012;40:596–603. [DOI] [PubMed] [Google Scholar]
- 20. Mueller-Mottet S, Stricker H, Domeninghetti G, Azzola A, Geiser T, Schwerzmann M, Weilenmann D, Schoch O, Fellrath J-M, Rochat T, Lador F, Beghetti M, Nicod L, Aubert J-D, Popov V, Speich R, Keusch S, Hasler E, Huber LC, Grendelmeier P, Tamm M, Ulrich S.. Long-term data from the Swiss pulmonary hypertension registry. Respiration 2015;89:127–140. [DOI] [PubMed] [Google Scholar]
- 21. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, Fisher AJ, Lordan JL, Gaine S, Coghlan JG, Wort SJ, Gatzoulis MA, Peacock AJ.. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186:790–796. [DOI] [PubMed] [Google Scholar]
- 22. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK.. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987;107:216–223. [DOI] [PubMed] [Google Scholar]
- 23. Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Grohe C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D.. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013;168:871–880. [DOI] [PubMed] [Google Scholar]
- 24. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD.. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 25. Zhang R, Dai LZ, Xie WP, Yu ZX, Wu BX, Pan L, Yuan P, Jiang X, He J, Humbert M, Jing ZC.. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 2011;140:301–309. [DOI] [PubMed] [Google Scholar]
- 26. Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 27. Ginoux M, Turquier S, Chebib N, Glerant JC, Traclet J, Philit F, Senechal A, Mornex JF, Cottin V.. Impact of comorbidities and delay in diagnosis in elderly patients with pulmonary hypertension. ERJ Open Res 2018;4:00100-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pugh ME, Sivarajan L, Wang L, Robbins IM, Newman JH, Hemnes AR.. Causes of pulmonary hypertension in the elderly. Chest 2014;146:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl 2019;21:K21–K28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shapiro BP, McGoon MD, Redfield MM.. Unexplained pulmonary hypertension in elderly patients. Chest 2007;131:94–100. [DOI] [PubMed] [Google Scholar]
- 31. Shimony A, Fox BD, Afilalo J, Rudski LG, Hirsch A, Langleben D.. Pulmonary arterial hypertension in the elderly-clinical characteristics and long-term survival. Lung 2012;190:645–649. [DOI] [PubMed] [Google Scholar]
- 32. Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–975. [DOI] [PubMed] [Google Scholar]
- 33. McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–1619. [DOI] [PubMed] [Google Scholar]
- 34. Hemnes AR, Opotowsky AR, Assad TR, Xu M, Doss LN, Farber-Eger E, Wells QS, Brittain EL.. Features associated with discordance between pulmonary arterial wedge pressure and left ventricular end diastolic pressure in clinical practice: implications for pulmonary hypertension classification. Chest 2018;154:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wanamaker B, Cascino T, McLaughlin V, Oral H, Latchamsetty R, Siontis KC.. Atrial arrhythmias in pulmonary hypertension: pathogenesis, prognosis and management. Arrhythm Electrophysiol Rev 2018;7:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park K, Park T-H, Kim S-J, Cho Y-R, Park J-S, Kim M-H, Kim Y-D.. Changes in mitral annular velocities after cardioversion of atrial fibrillation. Echocardiography 2018;35:1782–1787. [DOI] [PubMed] [Google Scholar]
- 37. Nagueh SF. Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail 2018;20:38–48. [DOI] [PubMed] [Google Scholar]
- 38. Al-Omari MA, Finstuen J, Appleton CP, Barnes ME, Tsang TS.. Echocardiographic assessment of left ventricular diastolic function and filling pressure in atrial fibrillation. Am J Cardiol 2008;101:1759–1765. [DOI] [PubMed] [Google Scholar]
- 39. Ginoux M, Cottin V, Glerant JC, Traclet J, Philit F, Senechal A, Mornex JF, Turquier S.. Safety of right heart catheterization for pulmonary hypertension in very elderly patients. Pulm Circ 2018;8:204589401879927.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franssen C, Paulus WJ.. Normal resting pulmonary artery wedge pressure: a diagnostic trap for heart failure with preserved ejection fraction. Eur J Heart Fail 2015;17:132–134. [DOI] [PubMed] [Google Scholar]
- 41. Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP, Newman JH.. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014;7:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, Oudiz R, Satoh T, Torres F, Torbicki A.. Diagnosis of pulmonary hypertension. Eur Respir J 2019;53:1801904.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T.. Pulmonary hypertension due to left heart disease. Eur Respir J 2018;53:1801897.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R.. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trip P, Nossent EJ, de Man FS, van den Berk IAH, Boonstra A, Groepenhoff H, Leter EM, Westerhof N, Grünberg K, Bogaard H-J, Vonk-Noordegraaf A.. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013;42:1575–1585. [DOI] [PubMed] [Google Scholar]
- 46. Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA.. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D.. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 48. Ozpelit E, Akdeniz B, Sezgin D, Sevinc C, Tertemiz KC, Ozpelit ME, Baris M, Baris N.. Clinical and hemodynamic profiles of elderly patients with pulmonary arterial hypertension: a single center, prospective study. J Geriatr Cardiol 2017;14:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD.. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354–362. [DOI] [PubMed] [Google Scholar]
- 50. Benza RL, Miller DP, Foreman AJ, Frost AE, Badesch DB, Benton WW, McGoon MD.. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant 2015;34:356–361. [DOI] [PubMed] [Google Scholar]
- 51. Chia LR, Schlenk EA, Dunbar-Jacob J.. Effect of personal and cultural beliefs on medication adherence in the elderly. Drugs Aging 2006;23:191–202. [DOI] [PubMed] [Google Scholar]
- 52. Galiè N, Barberà JA, Frost AE, Ghofrani H-A, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiéry J-L, Grünig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JHN, Langley J, Rubin LJ.. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–844. [DOI] [PubMed] [Google Scholar]
- 53. Opitz CF, Hoeper MM, Gibbs JSR, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grünig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S.. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016;68:368–378. [DOI] [PubMed] [Google Scholar]
- 54. Charalampopoulos A, Howard LS, Tzoulaki I, Gin-Sing W, Grapsa J, Wilkins MR, Davies RJ, Nihoyannopoulos P, Connolly SB, Gibbs JS.. Response to pulmonary arterial hypertension drug therapies in patients with pulmonary arterial hypertension and cardiovascular risk factors. Pulm Circ 2014;4:669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Orem C. Epidemiology of pulmonary hypertension in the elderly. J Geriatr Cardiol 2017;14:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]