Abstract
Particulate matter and polynuclear aromatic hydrocarbons are known to be cocarcinogenic. Using fluorescence spectroscopy, we determined that adsorption of benzoic] pyrene (BP) to iron oxide, silica, and asbestos (anthophyllite and Canadian chrysotile) results in a greatly enhanced rate of BP uptake into rat liver microsomes when compared to uptake from aqueous dispersions of BP microcrystals. Simple mixtures of BP microcrystals and particulates do not display enhanced microsomal uptake rates, an observation which indicates that adsorption of BP to the surface of the particle is necessary for enhanced microsomal uptake. BP was not released into microsomes from carbon black. Most importantly, the data indicate that asbestos particles are more effective than silica and iron oxide in enhancing the microsomal availability of BP. These observations suggest that particles, and especially the fibrous mineral particulates, could be cocarcinogenic as a result of their ability to adsorb polynuclear aromatic hydrocarbons and to transport these carcinogens into cells. Except for chrysotile, the particles did not disrupt microsomal integrity as determined by NADPH-dependent lipid peroxidation activity. Binding of the microsomes to the particles did not affect the BP uptake rates. In addition, these BP uptake rates were independent of both the concentrations of microsomes and of particles. These observations are consistent with the mechanism of particle-enhanced transport being an increased rate of BP solubilization from the adsorbed state into the aqueous phase, followed by rapid partitioning of BP into the microsomal membranes.
Polynuclear aromatic hydrocarbons (PAH)1 are known human and animal carcinogens, and carcinogenesis by these compounds requires metabolic activation (Heidelberger, 1975;Sims & Grover, 1974). This activation occurs in the microsomal fraction of cells (Sims et al., 1974; Yang et al., 1977). Inhaled and instilled particulate matter is known to increase the carcinogenic potency of the PAH in lung tissue. For example, intratracheal instillation of benzo [a] pyrene (BP)results in only a low incidence of lung cancer (Saffiotti et al., 1965) unless particulates are also instilled. The known cocarcinogenic particulates include hematite or iron oxide (Saffiotti et al., 1968), asbestos (Pylev & Shabad, 1973), aluminum and titanium oxide (Stenback et al., 1976), and India ink (Pylev, 1961). In humans, cigarette smoking and asbestos inhalation are known to be highly cocarcinogenic (Selikoff et al., 1968). These observations stimulated this investigation of the effects of particles on the microsomal availability of benzo [a] pyrene.
The mechanisms by which particles enhance PAH carcinogenesis have not been elucidated. The major route of PAH entry into the lungs is via retention of inhaled particulates which contain these adsorbed PAH. At present it is not known if particle-adsorbed PAH are eluted directly into lung surfactant and then transferred to cells or if the PAH are eluted after phagocytosis. However, regardless of the site of elution, particles which rapidly release adsorbed PAH could increase the effective dose of carcinogens in the lungs by elution of these compounds prior to clearance of the particles from the lungs. Phagocytosis of such particles with adsorbed PAH could also increase the intracellular availability of the PAH for microsomal activation. Although the phagocytidic macrophages may not be transformed themselves, cells are known to release mutagenic BP metabolites (Langenbach et al., 1978). In addition, an altered BP availability can alter the metabolic profile (Nemoto et al., 1978), and different BP metabolites have different carcinogenic effects (Kapitulnik et al., 1978).
In previous studies we determined that adsorption of chrysene and 1,2-benzanthracene to particulates greatly enhanced their rates of uptake into phospholipid vesicles of dipalmitoyl-L-a-phosphatidylcholine (DPPC), when compared with uptake from microcrystalline states (Lakowicz et al., 1977, 1978a,b). In addition, we demonstrated that the fibrous mineral asbestos (amosite) is superior to the nonfibrous mineral silica in adsorbing BP in the monomeric state and transporting this carcinogen into DPPC vesicles (Lakowicz & Hylden, 1978). Thus, it is clear that adsorption of PAH to particles can enhance the transport of PAH into lung surfactant, which is composed of at least 50% DPPC (Tierney, 1974; King & Clements, 1972). In this report we investigate the effects of particles on the uptake of BP into microsomes, with particular attention being given to the mechanism of enhanced uptake.
Materials and Methods
Source and Physical Properties of Particulates.
Amorphous silica was obtained from Analabs. By nitrogen adsorption, its surface area was 381 m2/g and the average particle size was 2 μm (Lakowicz et al., 1978b). Iron oxide (99.9%, lot 121377) was obtained from Ventrón Corp. (8.0 m2/g), and carbon black was from Fisher (31.1 m2/g). Microscopic examination indicated the iron oxide particles to be of varying size, with most being less than 2 μm, and the carbon particles to be about 25 μm in diameter. Anthophyllite and Canadian chrysotile were standard samples supplied by the International Union Against Cancer, Johannesburg. The reported surface areas are 11.8 and 26.8 m2/g, respectively. The particle size distributions are heterogeneous, with the average size being about 2 μm for both anthophyllite and chrysotile. For more detailed information see Timbrell (1970).
Preparation of Particulates with Adsorbed BP.
Particulates containing adsorbed BP were prepared as described previously (Lakowicz & Hylden, 1978). Briefly, the particulates were mixed with a benzene solution of BP, followed by evaporation of the benzene under reduced pressure. A total of 0.3 mg of BP was added for each gram of particulate. These samples were stored in the dark under an argon atmosphere and were used within 1 week of their preparation. The fluorescence emission spectra of BP which was extracted from the particles by using benzene was identical with that of the starting material. Thus, there did not appear to be any degradation of BP on these particulates which interfered with our measurements.
Aqueous dispersions of BP crystals were prepared by evaporation of a benzene solution of BP to dryness, addition of buffer, and sonication for 30 min at 40 W using a Cole-Parmer Model 8845–2 bath-type sonicator. Microscopic examination of these preparations reveals a heterogeneous size distribution with 90% of the crystals being less than 15 μm and 50% being less than 5 μm. We refer to this preparation as being microcrystalline.
Preparation of Rat Liver Microsomes.
Microsomes were prepared according to Ames et al. (1975) 5 days after inducing the rats by peritoneal injection of Aroclor 1254. The 105000g pellet was resuspended in buffer at a concentration of 0.6 mg of protein per mL (Lowry et al., 1951).
Fluorescence Spectral Data.
Fluorescence spectral data were obtained by using a computerized, photon-counting spectrofluorometer (SLM Instruments, Inc., Urbana, IL) as described previously (Lakowicz & Hylden, 1978). The background fluorescence seen above 395 nm (shorter wavelengths are not transmitted by the emission filters, Corning 0–52 and 2 mm of 1 M NaN02) was typically less than 10% of the total intensity. Since a similar intensity and spectral distribution for this background were observed for all particulates, we conclude that it results from stray light scattered off the turbid suspensions of particulates. These backgrounds were quantified by using particles without BP and subtracted from the spectra shown. The fluorescence of the microsomes was minor in relation to that resulting from the BP and did not interfere with our observations. In spite of the turbidity of the suspensions of particles, the scattered light did not interfere with our measurements. In addition, BP emission from microsome-bound BP could be observed even in opaque suspensions of iron oxide and carbon black.
Measurement of Microsomal Uptake of BP.
BP uptake into microsomes was quantified by the increase in fluorescence intensity at 405 nm which occurs upon transfer of BP from the surface of the particle into the microsomes. For all the BP uptake kinetics reported here, we used 5 μg of BP and 1 mL of rat liver microsomes, which was equivalent to 0.6 mg of microsomal protein. The buffer used was 0.1 M potassium phosphate, pH 7.7, containing 3 mM MgCl2 and 0.1 mM EDTA. An amount of particulate (16.7 mg) containing 5 μg of adsorbed BP was suspended in 10 mL of buffer and dispersed by sonication in a bath-type sonicator for 30 min at room temperature. In addition to dispersing the particles, this procedure facilitated equilibration of BP with the aqueous phase. After measurement of the initial fluorescence spectrum and intensity, microsomes were added in 1 mL of buffer to initiate the reaction. Complete BP transfer to the microsomes was obtained by heating the sample to 50 °C for 60 min. The final fluorescence intensity was measured after reequilibration at the experimental temperature of 25 °C. BP uptake rates from the microcrystalline state, and from BP microcrystals in the presence of particulates, were obtained in a similar manner, except that the 5 μg of BP microcrystals was suspended in 10 mL of aqueous buffer.
Measurement of Microsomal Integrity in the Presence of Particulates.
Microsomal integrity was assayed by lipid peroxidation activity (Ernster & Nordenbrand, 1967; Wills, 1969). NADPH and oxygen are consumed in lipid peroxidation, and we quantified this activity by the loss of the NADPH fluorescence which occurs upon its oxidation. The fluorescence of NADPH was convenient because we found it is possible to quantify consumption of NADPH even in the optically dense particulate suspensions which are used in the BP uptake measurements.
The reported measurements were made in 0.1 M potassium phosphate, pH 7.7, 25 °C, but equivalent activities were obtained in this same buffer when it also contained 3 mM MgCl2 and 0.1 mM EDTA. The same front face illumination was used as for the BP uptake measurements. The instrumental conditions were as follows: excitation wavelength and filter, 340 nm and 7–54; emission filters, Corning 3–144 and 2 mm of 1 M NaN02; emission wavelength, 464 nm. The 10-mL assay mixture contained 16.7 mg of particles and 0.6 mg of microsomal protein. To simulate the conditions used in the BP uptake studies, we stirred this mixture for 30 min at 25 °C prior to initiation of lipid peroxidation by addition of 0.5 mL of 5 s 10−4 M NADPH. The activity was obtained from the loss of NADPH fluorescence which occurred during the first 2 min. At this time 50 μL of 0.2 M ADP in 5 mM FeCl3 was added, and the fluorescence intensity was monitored for an additional 2 min. Essentially identical activities were obtained during both incubation periods, probably as a result of our use of phosphate buffer (Wills, 1969). Upon incubation of particles and NADPH in the absence of microsomes, the fluorescence intensity of NADPH was constant. This control indicates the particles themselves did not adsorb the NADPH, they did not catalyze reduction of the NADPH, nor did they cleave the phosphodiester bond. Cleavage of this bond results in an approximate fourfold increase in the fluorescence yield of NADPH.
Results
Fluorescence Emission Spectra of Benzo[a]pyrene.
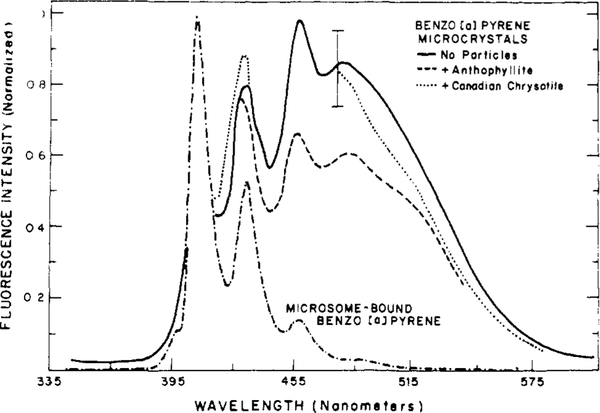
The fluorescence spectral distribution of benzo[a]pyrene is dependent upon its environment. In Figure 1 we compare the emission spectrum of BP when bound to rat liver microsomes and when present as a microcrystalline dispersion in aqueous buffer. The spectrum of the microsome-bound BP is seen to be highly structured, and it is essentially identical with that observed in dilute benzene solution (Lakowicz & Hylden, 1978).
figure 1:
Fluorescence emission spectra of benzo[a]pyrene. Microsomes containing BP were obtained by incubation of microsomes (equivalent to 0.6 mg of microsomal protein) with 5 Mg of BP microcrystals, in a total volume of 11 mL. BP microcrystals (5 Mg) were suspended in 10 mL of buffer. Anthophyllite and Canadian chrysotile(16.7 mg) were added after formation of the microcrystals.
Microcrystalline aqueous dispersions of BP display markedly different emission spectra compared to microsome-bound BP. These spectra, when normalized with spectra of microsome-bound BP, show a greatly increased relative intensity in the region of 490 nm (Figure 1). This structureless long-wavelength emission, which we will call the excimer emission, may result from the formation of a charge-transfer complex between a BP molecule in the excited state and an adjacent BP molecule in the ground state (Birks & Cameron, 1959). The apparently large contribution of the excimer emission in these normalized spectra could result from (1) excimer formation between adjacent BP molecules in the microcrystalline aggre-gates, (2) the quenching of the structured monomer emission which results upon excimer formation, or (3) reabsorption of the fluorescence emission from the monomeric state prior to its escape from the crystal. In addition, BP is known to form two types of crystal structures (Stevens, 1962), each with different fluorescence spectral properties. Sublimation or rapid removal of solvent, which is the procedure we used to form BP microcrystals, could result in the formation of a metastable crystal form of BP in which excimer formation does not occur. In this crystal state only reabsorption processes would be expected to affect the observed emissions.
At present we do not know the proportion of each crystal type which we obtain by solvent evaporation. We note that the relative intensities of the monomer (405 nm) and excimer (490 nm) emission from these aqueous dispersions are some what variable, as is indicated by the error bar on Figure 1. This variability could be a result of variations in the crystal size, the proportion of each crystal form, and the amount of BP which is dissolved in the aqueous phase. These parameters are likely to be strongly dependent upon the rate of solvent evaporation, temperature, and other factors such as the size and surface properties of the container used in preparation of the BP microcrystals. More detailed spectroscopic investigations are required to quantitate the relative importance of these excited-state processes, and different forms of BP, as determinants of the observed emission spectra.
Also shown in Figure 1 are the emission spectra of BP microcrystals to which anthophyllite or Canadian chrysotile was added. These spectra are essentially identical with that of BP microcrystals alone. The smaller excimer contribution of the anthophyllite-containing sample is not significant in comparison with the variability observed in the spectra of BP microcrystals alone. The spectra of microcrystalline dispersions of BP which contain silica or iron oxide are also similar to that of microcrystals alone and are not shown for the sake of clarity. Thus, addition of particulates to preformed BP microcrystals does not result in a shift of the BP emission spectra to that observed for BP which is adsorbed to the surface of these particulates. These spectra for particle-adsorbed BP will be described below. We conclude that, upon addition of particles to microcrystalline dispersions of BP, the carcinogen does not readily adsorb to the surface of the particulates. As a result we are able to compare the microsomal availabilities of particle-adsorbed BP with the availability of BP in the presence of, but not adsorbed to, these same particles.
Emission Spectra of Particle-Adsorbed. Benzo[a]pyrene.
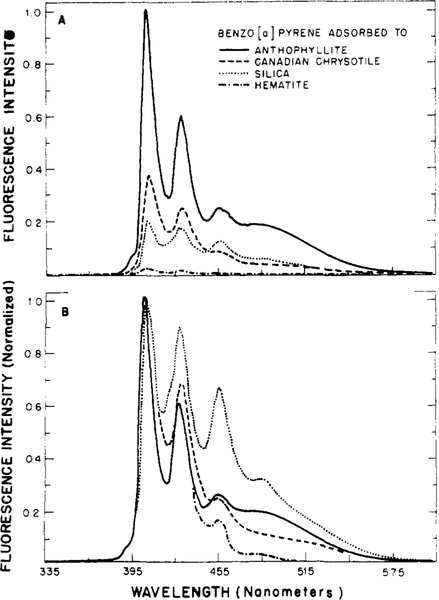
The emission spectra of particle-adsorbed BP are dependent upon the type of particle and the surface density of BP on the particles (Lakowicz et al., unpublished observation). From the intensity-normalized spectra (Figure 2B), we see that the contribution of the excimer fluorescence is greater for silica than for anthophyllite and chrysotile. Precise interpretation of these spectra is complicated by the effects of the particles themselves on the fluorescence intensities, the existence of a heterogeneous population of BP molecules on the surface of the particles, and the contribution of the BP which is solubilized in the aqueous phase to the total emission. We investigated the effects of the particles themselves on the fluorescence intensities by examining the emission spectra of BP microcrystals in the absence and presence of particles. Relative to BP microcrystals alone (1.0), the intensities of these crystals in the presence of anthophyllite, Canadian chrysotile, silica, and iron oxide were 1.5, 1.4, 2.0, and 0.12, respectively. We interpret the increased intensities as being a result of sample turbidity. Light scattering by the turbid samples may intensify the exciting light at that location in the sample where the fluorescence emission is collected. However, small quantities of BP adsorbed to the surface of the particles could also account for the observed enhancements. On the other hand, hematite, which is red in color, probably attenuates the fluorescence intensity by absorption processes. Only minor changes in spectral distribution of the microcrystals occur upon addition of particles. Thus, the filtering or enhancing effects of the particles appear to be fairly uniform across the emission bands of BP.
figure 2:
Fluorescence emission spectra of benzo[a]pyrene adsorbed to particulates. Emission spectra of adsorbed BP are shown at the same instrumental amplification (A) and normalized to the same peak intensities (B). Each 10-mL suspension in buffer contained 16.7 mg of particles and 5 μg of BP, i.e., 0.3 mg of BP per g of particulate.
We attempted to compensate for these effects on the fluorescence emissions by normalizing the emission spectra of particle-bound BP to equivalent intensities at 405 nm. This peak is probably due to both water-solubilized BP and BP which is adsorbed in the monomeric state. The larger contribution of the excimer emission for silica-adsorbed BP than for BP which is adsorbed to anthophyllite and chrysotile suggests that the latter fibrous minerals have a superior ability to adsorb BP in the monomeric state.
Note from Figure 2B that hematite-adsorbed BP does not display significant excimer emission. This absence appears to be a result of the highly quenched nature of BP which is adsorbed to hematite (Figure 2A). This conclusion is based on the spectra of the dry BP-particle powders. These spectra are essentially identical with those shown in Figure 2B, except that no spectrum was observable for the dry hematite-BP powder and somewhat less excimer emission was observed from dry BP-silica than from the aqueous suspension. That is, in the dry state the fluorescence of BP adsorbed to hematite is completely quenched. Thus, the observed emission in aqueous suspension is due to BP which is solubilized in the aqueous phase. Quenching of BP fluorescence by energy transfer to the hematite seems a likely quenching mechanism in that hematite adsorbs light strongly in the region of BP emission. This overlap of BP fluorescence with hematite absorption favors energy transfer (Forster, 1948).
No fluorescence emission was observed for BP bound to carbon black, but the emission of microsome-bound BP was observed in suspensions of carbon black. For the latter samples, the BP fluorescence decreased with time, presumably as a result of BP adsorption by the carbon black. Thus, our inability to observe fluorescence from carbon black bound BP is not a result of the inner filter effects in these opaque samples. We conclude that the fluorescence of carbon black bound BP is also quenched by an energy-transfer mechanism. This is not surprising in light of the broad absorption bands of carbon black. Unlike the emission spectrum observed for suspensions of hematite-bound BP, no water-solubilized spectrum was visible for suspensions of carbon black adsorbed BP, probably as a result of the high affinity of carbon black for polynuclear aromatic hydrocarbons.
The relative fluorescence intensities of particle-adsorbed BP are revealed by the unnormalized emission spectra (Figure 2A). The most intense monomer emission was observed for BP adsorbed to the fibrous minerals anthophyllite and chrysotile. The emission of hematite-adsorbed BP is seen to be weak in comparison with that of BP which is adsorbed to the other particles. As indicated above, only water-solubilized BP appears to contribute significantly to the fluorescence of the BP-hematite suspensions. Since the presence of hematite results in a 10-fold attenuation of the BP fluorescence, it appears that water-solubilized BP accounts for about 0.2 unit of the “monomer” fluorescence observed in Figure 2A for the other particles. Hence, these spectra result primarily from BP which is adsorbed to the surface of these particles. The larger relative intensities of the monomeric emission of BP on the asbestos samples as compared to silica are probably indicative of their superior ability to adsorb BP in the monomeric state. This ability is surprising in light of the surface areas of these particles. By nitrogen adsorption these areas are 381,26.8, and 11.8 m2/g for silica, chrysotile, and anthophyllite, respectively (see Materials and Methods). However, we stress that our conclusions must be regarded as tentative until more accurate methods are developed to quantify the fluorescence emissions of compounds which are adsorbed to surfaces.
Fluorescence Spectral Changes of Benzo[a\pyrene upon Its Microsomal Uptake.
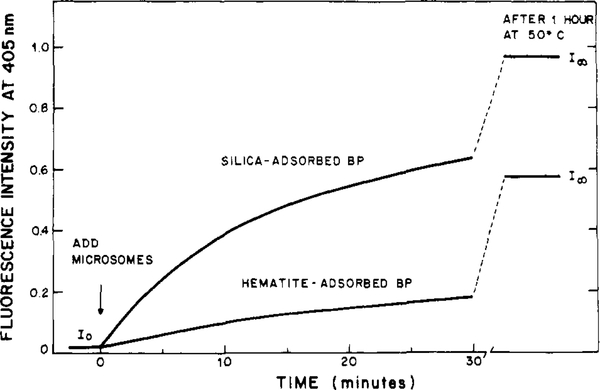
The fluorescence spectral distributions and intensities of microcrystalline and particulate-adsorbed BP remain constant over a period of hours when suspended in aqueous buffer. Upon addition of microsomes a rapid increase in fluorescence intensity occurs at 405 nm (Figure 3), the excimer emission becomes less significant, and the emission spectra become similar to that of the solution form of BP (Figure 1). We attribute these spectral changes to the transfer of BP from the particles to the microsomes.
figure 3:
Fluorescence intensity of benzo[a] pyrene during transfer from particulates to rat liver microsomes.
Calculation of the percentage of BP which is transferred to the microsomes requires knowledge of the fluorescence intensity after complete microsomal uptake of BP. These final fluorescence intensities (/∞) are obtained by heating the samples to 50 °C for 1 h, followed by reequilibration at the experimental temperature of 25 °C. Other studies (Lakowicz et al., unpublished observation) indicated that increased temperatures resulted in increased transfer rates of BP off particles into vesicles of dipalmitoyl-L-α-phosphatidylcholine (DPPC). However, unlike DPPC vesicles, the microsomes are likely to be unstable at these high temperatures. Inactivation of the microsomes by heating could result in an alteration in the observed value for I∞. We tested the validity of this heating procedure for obtaining the final fluorescence intensity in two ways. First, uptake of BP from anthophyllite was followed to completion without heating. Subsequent heating to 50 °C did not alter the spectral distribution or intensity of BP. Second, longer incubation periods at 50 °C did not affect the fluorescence intensity. We conclude that the I∞ values are representative of complete BP transfer to microsomes even if the heating procedure results in loss of microsomal activity.
If less than 0.4 mg of microsomes (as protein) is added, the full fluorescence enhancements are not observed. That is, after complete transfer of BP, subsequent addition of more microsomes results in an additional rapid increase in fluorescence intensity. At higher microsome concentrations the final fluorescence intensities are independent of the amount of microsomes added, except for iron oxide (see below). This independence indicates that the amount of microsomes added is adequate to bind all the BP. We attribute the lower final intensities at lower microsome concentrations to excimer formation in the microsomes themselves which results in a decreased relative intensity at 405 nm.
For iron oxide the final fluorescence intensity is dependent upon the amount of microsomes added even under conditions where excimer formation within the microsomes does not occur. Microsomes bind to all these particulates (see below) and, hence, a portion of the microsome-bound BP remains in close proximity to the surface of these particles. We suspect that BP fluorescence can be quenched by energy transfer from BP in the microsomes to the surface of hematite. By adding more microsomes, we can increase the average distance between the BP and this surface, decrease the probability of energy transfer, and, hence, increase the fluorescence yield.
Microsomal Uptake of Benzo[a]pyrene.
We used the time-dependent changes in the fluorescence intensity at 405 nm [I(t)] to quantify the amount of BP transferred to the microsomes. In particular, we assumed
| (1) |
where I0 and I∞ are the fluorescence intensities prior to the addition of microsomes and after complete BP transfer, respectively.
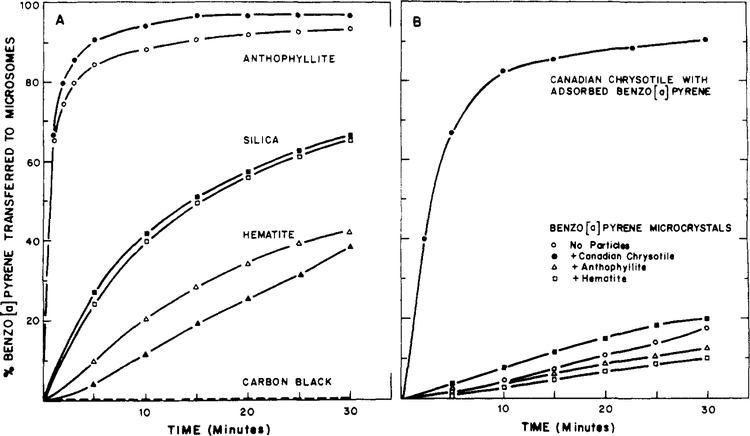
We compared the microsomal uptake rates of BP from the microcrystalline and particulate-adsorbed states (Figure 4). Adsorption of BP to all four mineral particulates results in enhanced microsomal uptake when compared with the microcrystalline state. Perhaps most importantly, chrysotile- and anthophyllite-adsorbed BP show the highest rates of microsomal uptake. The nonfibrous minerals silica and hematite also enhance BP uptake but are less effective than the asbestos particles. BP was not released into the microsomes from carbon black.
figure 4:
Benzo[a]pyrene uptake into rat liver microsomes. (A) The open symbols represent BP uptake into microsomes from 16.7 mg of particles, and the closed symbols represent BP uptake into microsomes in an identical fashion, except that the microsomes were preincubated for 30 min with an additional 16.7 mg of particles which did not contain BP. Similar results were obtained upon addition of microsomes to a suspension containing both 16.7 mg of unlabeled particles and 16.7 mg of particles with adsorbed BP. (B) As indicated, the particles were added to preformed BP microcrystals.
As controls we investigated the effects of particles on the microsomal uptake of BP when the particles are added to preformed microcrystals (Figure 4B). We previously indicated that such mixtures displayed the fluorescence spectral properties of BP microcrystals and not the properties of particle-adsorbed BP. As a result we expected, and found, that all these mixtures displayed BP uptake rates which were identical with that of BP microcrystals to within our experimental errors. These errors are probably a result of the variability in the microcrystalline dispersions of BP. We conclude that adsorption of BP to the surface of particulates is necessary for particle-enhanced uptake to occur.
Mechanism of Particle-Enhanced Microsomal Uptake of Benzo[a]pyrene.
Particle-enhanced uptake of BP could result from various processes. Among these are binding of the microsomes to the particulates, disruption of the integrity of the microsomes by the particulates, and an increased rate of solubilization of the BP into the aqueous phase from the surface of the particulates as compared to that of solubilization from the microcrystalline state.
We investigated the role of particle-microsome binding on the microsomal uptake rates of BP. We reasoned that, if binding were important for BP transport, then the presence of particles in the reaction mixture which do not contain BP should decrease its microsomal uptake rate. A decreased rate would be observable under conditions where the amount of unlabeled particulates is adequate to bind a significant fraction of the microsomes.
From Table I we see that 16.7 mg of anthophyllite, chrysotile, or iron oxide is adequate to bind essentially all the microsomes under our experimental conditions, and 16.7 mg of silica binds approximately half of the microsomes. We assumed that microsomes would bind randomly to both the labeled and the unlabeled particles. Hence, if binding were important for BP uptake, a decreased uptake rate would be observed in the presence of excess unlabeled particles. However, identical uptake kinetics were observed in the presence of 16.7 or 33.4 mg of unlabeled particulates.
Table I:
Binding of Microsomes to Particulates
| particulate | Amount (mg) | % of microsomes bounda |
|---|---|---|
| anthophyllite | 4.2 | 44 |
| anthophyllite | 8.3 | 73 |
| anthophyllite | 16.7 | 91 |
| anthophyllite | 20.0 | 96 |
| anthophyllite | 33.4 | 91 |
| iron oxide | 4.2 | 38 |
| iron oxide | 8.3 | 63 |
| iron oxide | 16.7 | 83 |
| iron oxide | 20.0 | 87 |
| iron oxide | 33.4 | 89 |
| silica | 16.7 | 40 |
| Canadian chrysotile | 16.7 | 84 |
| carbon black | 16.7 | 53 |
Binding of microsomes to particulates was quantitated by incubation of microsomes (equivalent to 0.6 mg of microsomal protein) with the amount of particles shown for 30 min at 25 °C with continual stirring. The 480g supernatant was then assayed for protein to determine the extent of binding.
Second, to increase further the potential sensitivity of this experiment to binding, we preincubated the microsomes with unlabeled particles prior to their addition to the BP-particulate suspensions. In particular, we incubated microsomes with the unlabeled particles (16.7 mg) for 30 min at 25 °C. These microsomes were subsequently added to the 10-mL suspension of particles which contained adsorbed BP. If binding were important for BP uptake, we expect a decreased uptake rate under these conditions in which the microsomes are bound to particles not containing BP. The preincubation with unlabeled particles had no significant effect on the BP uptake rate (Figure 4A). We conclude that microsome-particle binding is not a significant determinant of the microsomal uptake rate of BP. We note that a rapid reequilibration of the microsomes between the particles with and without adsorbed BP would invalidate our conclusion. Since the initial rates of BP uptake were identical within our experimental limits, such redistribution must be complete within 30 s. Assuming this redistribution requires times in excess of 30 s, these data indicate that particle-microsome binding is not a significant factor in determining the rates of BP uptake.
Because of the known cytotoxic effects of particulates (Harington, 1974; Wade et al., 1976), we investigated the possibility that disruption of the microsomes was responsible for particle-enhanced uptake of BP. We assayed microsomal integrity by measuring lipid peroxidation activity. This activity was lost upon addition of deoxycholate and, therefore, provides a measure of the integrity of the microsomal membranes. Lipid peroxidation consumes both NADPH and oxygen. We monitored this activity fluorometrically by the oxidation of NADPH to NADP+, a measurement we could make in the presence of particulates. Anthophyllite, silica, and hematite had no significant effect on the rate of lipid peroxidation (Table II), but chrysotile was found to have a significant inhibitory effect. These results indicate no correlation between the rates of BP uptake and disruption of the microsomes. That is, anthophyllite and chrysotile show nearly identical enhancements of uptake, but anthophyllite does not disrupt the microsomes and chrysotile does. Likewise, the enhancements of anthophyllite, hematite, and silica all differ, yet none of these particles significantly affect the integrity of the microsomes.
Table II.
Microsomals Lipid Peroxidase Activity in the Presence of Particulates
| sp act. (nmol of NADPH oxidized per min per mg of protein) | % of act. with no particles | |
|---|---|---|
| particulatea | ||
| none | 25 | (100) |
| anthophyllite | 21 | 84 |
| chrysotile | 9 | 36 |
| silica | 23 | 92 |
| hematite | 34 | 136 |
| controls | ||
| deoxycholate (0.3%) | 5 | 20 |
| 100 °C, 5 min | 2 | 8 |
| p-(chloromercuri)-benzoate (1 mM) | 8 | 32 |
| NADH (no NADPH) | 9 | 36 |
A total of 16.7 mg of each particulate was used.
The disruptive effects of chrysotile are easily understood in terms of its unique surface chemistry (Spiel & Leineweber, 1969). As opposed to the other particulates whose surface charges are neutral or slightly negative, the surface of chrysotile is strongly positive (the isoelectric point is 11.8) and highly basic. In addition, chrysotile has a stronger affinity for polar molecules than the other particles and is known to be more highly cytotoxic.
In control experiments we showed that deoxycholate and p-(chloromercuri)benzoate inhibited the lipid peroxidase activity, as did boiling of the microsomes (Wills, 1969). In addition, NADH did not support the lipid peroxidation activity to the same extent as did NADPH. These experiments demonstrate that the activity we measured had the properties of the NADPH-dependent lipid peroxidation described by Wills (1969) and was not a nonspecific oxidation of NADPH. We conclude that disruption of the microsomes by the particles cannot account for the particle-enhanced uptake of BP.
Having eliminated binding of microsomes to the particulates and disruption of the microsomes by the particulates as important factors in the particle-enhanced uptake of BP, we felt that an increased rate of solubilization of BP in the aqueous phase may be the most important factor in enhanced uptake. We quantified the rates of BP uptake under conditions where both the particle and microsome concentrations were increased twofold. By increasing both concentrations, we were able to keep the particle-to-microsome and BP-to-microsome ratios constant. If collisional encounters between particles and microsomes were responsible for transfer of BP, then the BP uptake rate should increase fourfold under these conditions. The observed rates for all particles were identical with those shown in Figure 4. These observations indicate that collisional encounters between particles and microsomes are not of mechanistic significance for the transfer of BP from particles to microsomes. Due to the low water solubility of BP in aqueous solutions (about 3.8 μg/L; Davis et al., 1942) only a small fraction of the total BP could be dissolved in the aqueous phase of our 10-mL samples. Thus, it appears unlikely that the rate-limiting step for BP uptake into microsomes would be its rate of entry into the microsomal membranes. The zero-order dependence of the BP uptake rate on both particle and microsome concentrations probably indicates that the desorption of BP from the surface of the particles is the rate-limiting step for BP uptake into microsomes. Similarly, the rate of solubilization of 1,2-benzanthracene into the aqueous phase of silica was found to control the rate of benzanthracene uptake by lipid vesicles (Lakowicz et al., 1978b).
Discussion
We demonstrated that fibrous minerals are superior to nonfibrous minerals in their ability to transport carcinogens into microsomes. We suggest that this ability may provide a partial explanation for the cocarcinogenic effects of asbestos inhalation and cigarette smoking in humans and for the cocarcinogenic effects between particles and PAH found in animal carcinogenicity testing. However, we point out that mechanisms other than particle-enhanced transport may be operative in particle-PAH cocarcinogenesis. These mecha nisms include (1) tissue damage caused by smoking (Auerbach et al., 1961) or by the inhaled particles, (2) inhibited clearance of smoke particles resulting from the presence of inhaled mineral particulates (Blenkinsopp, 1968; Ferin & Leach, 1976), and (3) altered metabolic profiles of the PAH in the presence of particulates.
However, the known cocarcinogenic effects of particles and PAH which are observed in experimental animals correlate well with the microsomal availabilities described in this paper. For example, iron oxide with adsorbed BP is more carcinogenic than simple mixtures of BP and iron oxide (Henry et al., 1975). Similar results were found with asbestos-adsorbed BP and asbestos-BP mixtures (Pylev & Shabad, 1973). Our results indicate that BP must be adsorbed to the particle for enhanced transport to occur. Carbon black with adsorbed BP has been found to be less carcinogenic than BP alone (Davis et al., 1975; Steiner, 1956), although, depending on particle size, cocarcinogenic effects have also been observed (Pylev, 1961). The former results agree with our own, in that carbon black did not release BP into microsomes.
In summary, our results seem to be relevant to carcinogen testing in animals, but alternative mechanisms of cocarcinogenesis may also be of importance. We hope our methods, which allow the PAH delivery rates to be quantified, will facilitate the design of experiments which further elucidate the mechanism of particle-PAH cocarcinogenesis and thereby increase our understanding of the multiple etiology of human cancer.
Acknowledgments
We express our appreciation to the Freshwater Foundation and especially to its founder, Richard Gray, Sr., without whose support this work would not have been possible.
These studies were supported by Grant BC-261 from the American Cancer Society. D.R.B. was supported in part by a Postdoctoral Fellowship (CA-6405) from the National Cancer Institute. This work was done during the tenure of an Established Investigatorship (to J.R.L.) of the American Heart Association.
Footnotes
A preliminary account of this work was presented at the 23rd Annual Meeting of the Biophysical Society, Atlanta, GA, Feb 1979.
PAH, polynuclear aromatic hydrocarbons; AHH, aryl hydrocarbon hydroxylase; BP, benzo[a]pyrene; DPPC, dipalmitoyl-L-af-phosphatidyl-choline.
References
- Ames BN, McCann J, & Yamasaki E (1975) Mutat. Res 31, 347–364. [DOI] [PubMed] [Google Scholar]
- Auerbach O, Stout AP, Hammond OC, & Garfinkel L (1961) N. Engl. J. Med 265, 253–267. [DOI] [PubMed] [Google Scholar]
- Birks JB, & Cameron JW (1959) Proc. R. Soc. London,Ser. A 249, 297–317. [Google Scholar]
- Blenkinsopp WK (1968) J. Pathol. Bacteriol 96, 297–304. [DOI] [PubMed] [Google Scholar]
- Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, & Roe FJR (1975) Br. J. Cancer 31, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WW, Krahl ME, & Clowes GHA (1942) J.Am. Chem. Soc 64, 108–110. [Google Scholar]
- Ernster L, & Nordenbrand K (1967) Methods Enzymol. 10, 574–580. [DOI] [PubMed] [Google Scholar]
- Ferin J, & Leach LJ (1976) Environ. Res 12, 250–254. [DOI] [PubMed] [Google Scholar]
- Forster T (1948) Ann. Phys. (Leipzig) 2, 55–75. [Google Scholar]
- Harington JS (1974) Environ. Health Perspect 9, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C (1975) Annu. Rev. Biochem 44, 79–121. [DOI] [PubMed] [Google Scholar]
- Henry MC, Port CD, & Kaufman DG (1975) Cancer Res. 35, 207–217. [PubMed] [Google Scholar]
- Kapitulnik J, Wislocki PG, Levin W, Yagi H, Thakker DR, Akagi H, Koreeda M, Jerina DM, & Conney AH (1978) Cancer Res. 38, 2661–2665. [PubMed] [Google Scholar]
- King RJ, & Clements JA (1972) Am. J. Physiol 223,715–726. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR, & Hylden JL (1978) Nature (London) 275,446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, McNamara M, & Steenson L (1977) Science 199, 305–307. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR, Englund F, & Hidmark A (1978a) J. Natl. Cancer Inst 61, 1155–1159. [PubMed] [Google Scholar]
- Lakowicz JR, Englund F, & Hidmark A (1978b) Biochim. Biophys. Acta 543, 202–216. [DOI] [PubMed] [Google Scholar]
- Langenback R, Freed HJ, Raveh D, & Huberman E(1978) Nature (London) 276, 277–279. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, & Randall RJ (1951) J. Biol. Chem 193, 265–275. [PubMed] [Google Scholar]
- Nemoto N, Hirakawa T, & Takayama S (1978) Chem.-Biol. Interact 22, 1–14. [DOI] [PubMed] [Google Scholar]
- Pylev LN (1961) Bull. Exp. Biol. Med. (Engl. Transí.) 52,1316–1319. [Google Scholar]
- Pylev LN, & Shabad KM (1973) IARC Sci. Publ 8,99–106. [Google Scholar]
- Saffiotti U, Cefis F, Kolb LH, & Shubik P (1965) J. Air Pollut. Control Assoc 15, 23–25. [DOI] [PubMed] [Google Scholar]
- Saffiotti U, Cefis F, & Kolb LH (1968) Cancer Res. 28,104–124. [PubMed] [Google Scholar]
- Selikoff IJ, Hammond EC, & Churg J (1968) J. Am. Med. Assoc 204, 104–110. [Google Scholar]
- Sims P, & Grover PL (1974) Adv. Cancer Res 20,165–274. [DOI] [PubMed] [Google Scholar]
- Sims P, Grover PL, Swaisland A, Pal K, & Hewer A(1974) Nature (London) 252, 326–328. [DOI] [PubMed] [Google Scholar]
- Spiel S, & Leineweber JP (1969) Environ. Res 2,166–208. [DOI] [PubMed] [Google Scholar]
- Steiner PE (1956) Cancer Res 14, 103–110. [PubMed] [Google Scholar]
- Stenback F, Rowland J, & Sellakumar A (1976) Oncology 33, 29–34. [DOI] [PubMed] [Google Scholar]
- Stevens B (1962) Spectrochim. Acta, Part A 18, 439–448. [Google Scholar]
- Tierney DF (1974) Annu. Rev. Biochem 36, 209–231. [DOI] [PubMed] [Google Scholar]
- Timbrell V (1970) Pneumoconiosis, Proc. Int. Conf, 3rd, 1969, 28–36. [Google Scholar]
- Wade MJ, Lipkin LE, Tucker RW, & Frank AL (1976) Nature (London) 264, 444–446. [DOI] [PubMed] [Google Scholar]
- Wills ED (1969) Biochem. J 113, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SK, McCourt DW, Leutz JC, & Gelboin HV (1977) Science 196, 1199–1201. [DOI] [PubMed] [Google Scholar]