Abstract
Background
Mesenchymal stem cells (MSCs) emerged as a promising therapy for tendon pathologies. Microfragmented adipose tissue (μFAT) represents a convenient autologous product for the application of MSC-based therapies in the clinical setting. In the present study, the ability of μFAT to counteract inflammatory processes induced by IL-1β on human tendon cells (TCs) was evaluated.
Methods
Cell viability and proliferation were evaluated after 48 hours of transwell coculture of TCs and autologous μFAT in the presence or absence of IL-1β. Gene expression of scleraxis, collagen type I and type III, metalloproteinases-1 and -3, and cyclooxygenase-2 was evaluated by real-time RT-PCR. The content of VEGF, IL-1Ra, TNFα, and IL-6 was evaluated by ELISA.
Results
IL-1β-treated TCs showed augmented collagen type III, metalloproteases, and cyclooxygenase-2 expression. μFAT was able to reduce the expression of collagen type III and metalloproteases-1 in a significant manner, and at the same time, it enhanced the production of VEGF, IL-1Ra, and IL-6.
Conclusions
In this in vitro model of tendon cell inflammation, the paracrine action of μFAT, exerted by anti-inflammatory molecules and growth factors, was able to inhibit the expression of fibrosis and catabolic markers. Then, these results suggest that the application of μFAT may represent an effective conservative or adjuvant therapy for the treatment of tendon disorders.
1. Introduction
Tendon disorders represent a common condition in the field of musculoskeletal injuries. Current treatment options often lead to unsatisfactory results, with persistence of pain and reduced physical activity level [1, 2]. Among the conservative approaches, the use of mesenchymal stem cells (MSCs) from the bone marrow and adipose tissue emerged as a promising treatment, capable to counteract the pathological processes characterizing degenerative disease in the orthopedic field [3], gathering interest also in the treatment of tendon disorders. Indeed, successful applications of cultured MSCs were described for the treatment of tendon injuries in preclinical models [4]. The use of adipose-derived stem cells (ASCs), or autologous adipose-derived products, already showed efficacy in the context of tendon disorders in clinical and preclinical trials [5–8]. In vitro investigations demonstrated that the coculture of ASCs or μFAT improved proliferation, viability, and collagen and VEGF production in tendon cells (TCs) [9, 10]. In addition, in an in vitro model of TC inflammation, cultured adipose-derived MSCs were able to reduce the production of proinflammatory mediators [11]. Nevertheless, translation of MSC-based therapies implies extensive manipulation for the production of cells in good manufacturing practice (GMP) conditions and it is limited by the high costs of production and the regulatory issues related to the advanced therapy medicinal products [12, 13]. As a consequence, different solutions for adipose- and bone marrow-derived MSC isolation at the point of care have been proposed in recent years, with encouraging results [5, 14]. In particular, microfragmented adipose tissue (μFAT) represents a convenient compromise for the exploitation of MSCs in the clinical practice, avoiding extensive cell manipulation, and culture expansion [15]. μFAT is easily obtained by commercially available devices from autologous lipoaspirate, and it has been demonstrated to exert a therapeutic activity in clinical and preclinical settings, providing positive functional outcomes in particular for the treatment of knee osteoarthritis [16–20]. The procedure of μFAT production and injection is compatible with a single-stage surgery and may be combined with different surgical procedures. The aim of the present study was to investigate the paracrine effect of μFAT on the proinflammatory and catabolic response of TCs to tnterleukin-1β- (IL-1β-) mediated inflammation. This molecule has been described as the primary mediator of the inflammatory status preventing tissue healing in rotator cuff injury [21], and previous reports showed that IL-1β-treated TCs upregulated the expression of catabolic enzymes and the production of inflammatory cytokines [22–24]. Thus, the working hypothesis of the present study is that autologous μFAT would counteract the catabolic and proinflammatory response elicited by IL-1β on TCs, providing insights on the mechanism of action of this therapeutic approach and strengthening the rationale of its use in tendon disorders.
2. Materials and Methods
2.1. TCs and μFAT Isolation and Harvesting
Long head of biceps tendon biopsies and μFAT were collected from 8 patients (5 females and 3 males, mean age: 60 ± 10 y/o) undergoing arthroscopic rotator cuff repair augmented with μFAT. An informed consent was obtained from all patients as per the protocol approved by the Institutional Review Board (no. 148/INT/2015, January 13, 2016). Lipoaspirate adipose tissue was collected before surgery and processed by Lipogems® device, following the manufacturer's instruction [25]. An aliquot of μFAT was harvested for in vitro experiments. Briefly, each μFAT sample was centrifuged at 376 × g for 5 minutes at room temperature to separate the aqueous phase and the fragmented tissue. The former was stored at -20°C, while the latter was frozen at -80°C after the addition of 1 volume of freezing solution (90% FBS, 10% dimethyl sulfoxide; Sigma-Aldrich, St. Louis, MO, USA). Separation of the two phases was adopted to maintain the composition of injected μFAT even after thawing and removal of the aqueous freezing solution. This allowed for the use in the experiment of an identical product as injected during the surgical procedure.
Human tendon cells (TCs) were isolated from long head of the biceps tendon biopsies obtained during arthroscopic rotator cuff repair. Tendon tissue was digested with a 0.3% w/v collagenase type I solution (Worthington, Lakewood, NJ, USA) for 16 hours at 37°C, as previously reported [26]. TCs were cultured in a complete medium composed by high-glucose Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) with 10% v/v fetal bovine serum (FBS; Euroclone, Pero, Italy), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.29 mg/ml L-glutamine (Gibco, Waltham, MA, USA), and 5 ng/ml bFGF (Peprotech, London, UK). The medium was replaced every 3 days, and all the experiments have been performed at passage 3. After culture, TCs represent a mixed population of terminally differentiated and tendon progenitor cells at different stages of differentiation, as previously reported [26, 27].
2.2. Treatment of TCs with IL-1β and Coculture with μFAT
TCs were seeded in 12-well plates at the density of 50000 cells/well. TCs were either maintained in normal culture medium or a medium added of 1 ng/ml IL-1β. In addition, cells treated with IL-1β and untreated cells were cocultured with 250 μl of μFAT, made of125 μl of microfragmented tissue and 125 μl of the corresponding aqueous phase. The two phases were separated in order to maintain the aqueous part of μFAT, even after thawing and removal of the freezing solution. μFAT was added to the top portion of a transwell insert (pore size: 0.4 μm), while TCs were seeded on the bottom. Equal volume of phosphate-buffered saline (PBS) was added in the transwell top portion of the samples without μFAT. After 48 hours of treatment, the culture media were collected and stored at -20°C, while cells were trypsinized and the pellets stored at -80°C.
2.3. RNA Isolation and Gene Expression
RNA was obtained from cell pellets using TRI reagent (Sigma-Aldrich). Briefly, cells were lysed by the addition of 300 μl TRI reagent, and then 100 μl of 1-bromo-3-chloropropane (Sigma-Aldrich) were added to the samples. After centrifugation at 12000 × g for 10 minutes, the interphase was collected for DNA extraction while the RNA in the aqueous phase was precipitated with isopropanol, washed in 75% ethanol and then resuspended in 20 μl RNAse-free water for storage at -80°C.
Total RNA from each sample was transcribed to cDNA using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) as per manufacturer's instructions. Real-time PCR was performed starting from 10 ng of cDNA, using a PCR mix containing TaqMan® Universal PCR Master Mix and Assays-on-Demand Gene expression probes (Life Technologies, Waltham, MA, USA) for SCX (scleraxis; Hs03054634_g1), COL1A1 (collagen type I alpha 1 chain; Hs01076777_m1), COL3A1 (collagen type III alpha 1 chain; Hs00943809_m1), MMP1 (metalloproteinase-1; Hs00899658_m1), MMP3 (metalloproteinase-3; Hs00968305_m1), and PTGS2 (cyclooxygenase-2; Hs00153133_m1). Applied Biosystems StepOnePlus® (Life Technologies) was used to perform all experiments (program: 1 cycle of 2 minutes at 50°C, 1 cycle of 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, and 1 minute at 60°C). The results were normalized against the mean expression of two housekeeping genes: YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta, Hs03044281_g1) and ACTB (β-actin, Hs99999903_m1) identified in a previous study [28]. Two replicates were analyzed for each sample, and data were presented according to the ΔΔCt method [29].
2.4. DNA Isolation and Quantification
DNA was separated from the interphase (obtained as described above) by the addition of 100% ethanol and centrifugation at 2000 × g for 5 minutes. The DNA pellet was washed in 0.1 M trisodium citrate, 10% ethanol solution for 30 minutes, and then centrifuged at 2000 × g for 5 minutes. DNA was then washed in 75% ethanol, and solubilized in 50 μl 8 mM NaOH for storage at -20°C. DNA content in each sample was measured by NanoDrop spectrophotometer at 260 nm absorbance using software version 3.7.1 (NanoDrop ND-1000, ThermoFisher Scientific) [30].
2.5. TC Metabolic Activity
Viability was assessed by alamarBlue assay after 48 hours of treatment (ThermoFisher Scientific, Waltham, MA, USA). Briefly, culture medium was removed and cells were incubated with a 10% v/v solution of alamarBlue in DMEM at 37°C. After 2 hours of incubation, fluorescence was measured by a spectrophotometer (Victor X3, Perkin Elmer, Waltham, MA, USA) with excitation of 540 nm and emission of 590 nm.
2.6. ELISA
The release of interleukine-6 (IL-6), interleukine-1 receptor antigen (IL-1Ra), tumor necrosis factor α (TNFα) (Peprotech), and vascular endothelial growth factor (VEGF) (R&D Systems, Minneapolis, MN, USA) in the culture media of TCs treated for 48 hours was analyzed by ELISA assays, following the manufacturer's instruction. The detection ranges were as follows: 24–1500 pg/mL for IL-6, 23–1500 pg/mL for IL-1Ra, 31-2000 pg/ml for TNFα, and 31.3-2000 pg/ml for VEGF.
2.7. μFAT Cell Count and Viability Assays
After thawing, an aliquot of μFAT was digested by 0.075% w/v collagenase type I (Worthington) for 45 minutes at 37°C. The number of cells and viability was assessed by NucleoCounter NC-3000 using cell viability staining (Chemometech, Allerod, Denmark) [31]. The μFAT samples used in the study had a mean cell count of 2.3 ± 1.3 × 106 cells/ml, while cell viability was 53.2 ± 13.1%.
2.8. Statistical Analysis
All the analyses were performed using Prism 5.0 (Graphpad Software, La Jolla, CA, USA). Gaussian distribution of data was assessed by Shapiro-Wilk test. One-way repeated measures ANOVA test with Bonferroni's post test was applied to measure the differences among treatments when data presented a Gaussian distribution (Figures 1(a), 2(b), and 2(c)); otherwise, the Friedman's test with Dunn's post-test was used (Figures 1(b), 2(a), and 3). A level of p < 0.05 was considered statistically significant.
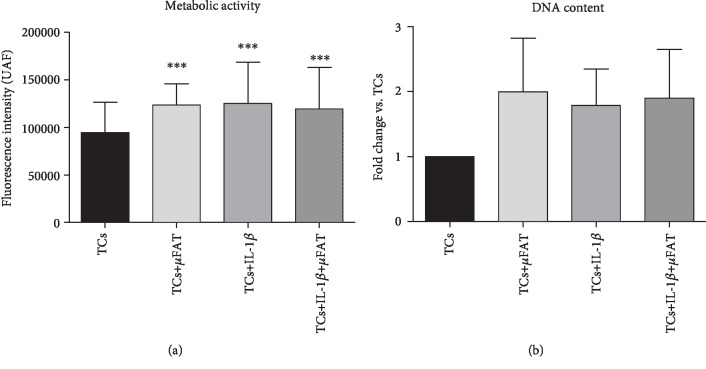
Figure 1.
Metabolic activity (a) and DNA content (b) of untreated and differently treated TC samples (n = 8). ∗p < 0.05, ∗∗∗p < 0.001 vs. TCs.
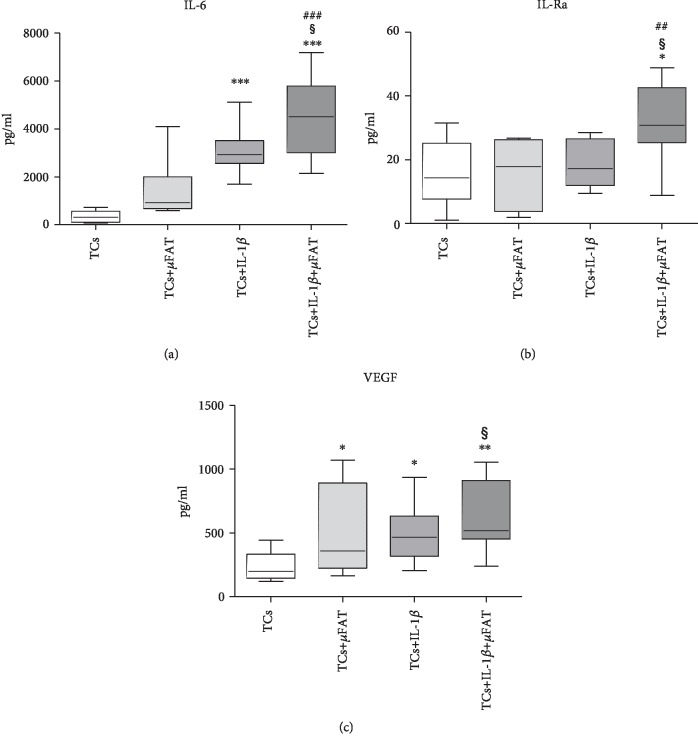
Figure 2.
IL-1Ra, IL-6, and VEGF quantification in culture media of TCs treated with IL-1β and/or μFAT and untreated TCs (n = 8). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. TCs; ##p < 0.01, ###p < 0.001 vs. +μFAT; §p < 0.05 vs. +IL-1β.
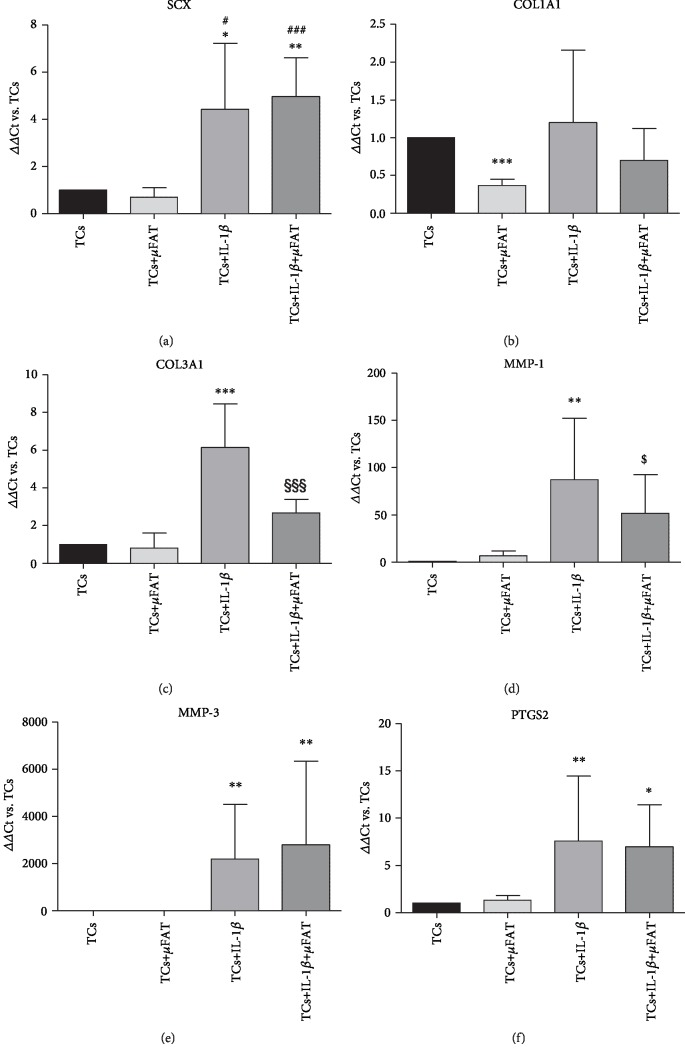
Figure 3.
Gene expression of SCX, COL1A1, COL3A1, MMP1, MMP3, and PTGS2 in TCs cultured in the presence of IL-1β and/or μFAT (n = 8). Data are expressed as mean ΔΔCt with respect to untreated controls (TCs = 1). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. TCs; #p < 0.05, ###p < 0.001 vs. +μFAT; §§§p < 0.001 vs. +IL-1β; $p < 0.1 vs. +IL-1β (tendency).
3. Results
3.1. IL-1β and μFAT Enhance TC Metabolic Activity
The metabolic activity of IL-1β-treated TCs was significantly increased with respect to untreated TCs (p < 0.01), and no further improvement was induced by the coculture with μFAT. In noninflammatory conditions, samples cocultured in transwell with μFAT demonstrated a higher metabolic activity with respect to untreated TCs (p < 0.05) (Figure 1(a)). DNA content resulted in increased in all conditions, even if in a nonsignificant manner (Figure 1(b)).
3.2. μFAT Reduces the Gene Expression of Catabolic and Fibrosis Markers
The presence of IL-1β significantly enhanced the TC expression of SCX (p < 0.05), MMP1 (p < 0.01), MMP3 (p < 0.01), COL3A1 (p < 0.001), and PTGS2 (p < 0.01) with respect to untreated TCs, while it did not exert any effect on COL1A1 expression (Figure 3). In this inflammatory condition, the coculture with μFAT decreased the expression of COL3A1 (-57%, p < 0.001) and MMP1 (-41%, p = 0.08) (Figures 3(c) and 3(d)), with little effect on the other parameters. No effect of μFAT on MMP3 expression was observed, while it was strongly induced by IL-1β treatment (Figure 3(e)). Moreover, TCs cocultured with μFAT, with or without IL-1β, showed a reduced expression of COL1A1 with respect to untreated TCs (p = 0.06 and p < 0.001 in the presence or absence of IL-1β, respectively) (Figure 3(b)). A slight increase in SCX expression was observed in TCs cocultured with μFAT and treated with IL-1β, with respect to IL-1β only treated cells (+12%, n.s.) (Figure 3(a)).
A fivefold increase in overall COL3A1/COL1A1 expression ratio was observed in IL-1β-treated TCs with respect to untreated TCs (p < 0.05, data not shown). In inflammatory conditions (+IL-1β), the coculture with μFAT was able to reduce the ratio, even if in a nonstatistically significant manner (-27%, n.s.), while in TCs+μFAT, the ratio was increased in a nonsignificant manner in comparison to untreated TCs samples, in both inflammatory and noninflammatory conditions.
3.3. Il-1β and μFAT Enhance the Production of Cytokines and VEGF
In the presence of IL-1β, an enhanced production of IL-6 (p < 0.001) was observed with respect to untreated TCs, and it was further increased by the coculture with μFAT (+45%, p < 0.05 with respect to TCs+IL-1β) (Figure 2(a)). On the contrary, IL-1Ra production was not induced by IL-1β or μFAT alone, while the contemporary presence of these factors significantly increased the production of this molecule (+217% and +290%, with respect to both untreated TCs and IL-1β-treated TCs; p < 0.05) (Figure 2(b)). The production of VEGF was induced by IL-1β (+109% with respect to untreated cells, p < 0.05) or μFAT coculture (+116% with respect to untreated cells, p < 0.05); the combination of both factors further increased VEGF production (+29% in comparison with TCs treated with IL-1β, p < 0.05) (Figure 2(c)). The content of TNFα was undetectable in all samples (data not shown).
4. Discussion
The present study aimed to test the ability of autologous μFAT in favouring tissue healing in the context of tendon cell inflammation. Our results demonstrated that the paracrine action of μFAT effectively reduces the expression of catabolic and fibrosis markers in pair-matched TCs cultured in inflammatory conditions. The strength of this work is the use of patient-matched TCs and μFAT, allowing for taking into account the inter-donor variability in both elements.
More in details, in cells exposed to IL-1β, the paracrine action of μFAT was able to significantly reduce the COL3A1/COL1A1 ratio which positively correlates with the deposition of rupture-prone fibrotic tendon matrix [32, 33], confirming previous findings obtained in cocultures of TCs and adipose-derived MSCs [34, 35]. IL-1β has been described as the key effector of tendon inflammation leading to the inhibition of the proper healing process [21, 23, 24], also enhancing the production of catabolic enzymes such as MMP1 and MMP3 involved in tendon matrix degradation [36, 37], and increasing collagen type III expression over collagen type I. Moreover, IL-1β directly induces the production of the enzyme cyclooxygenase-2 (COX-2), typical marker of inflammation [38]. Interestingly, cells cultured in inflammatory condition in presence of μFAT showed an overall reduction of the absolute expression of both collagen type I and III. The increase in collagen type III after injury is associated with the formation of scar tissue, which allows for prompt healing at the expense of tissue quality and functionality [39, 40]. In this view, a slower matrix deposition, where the proportion between the different types of collagen is similar to the physiological level, represents a therapeutic goal aimed to improve the quality of regenerated tissue.
In addition, μFAT was able to inhibit MMP1 expression, suggesting a protective role towards tendon ECM integrity. Metalloproteases are crucial for the physiological maintenance of tendon extracellular matrix (ECM) homeostasis [41], to the extent that the inhibition of MMPs results in pathological changes and pain [42]. On the other hand, an excess of MMP expression is associated with aberrant matrix degradation, typical of degenerative tendon disorders [43]. In particular, MMP1 is clearly involved in tendon pathology in association with the action of IL-1β [44, 45]. The effect of μFAT on MMP expression is consistent with previous observations reported in a model of inflamed synoviocytes, supporting the inhibition of MMP-related matrix degradation as a mechanism of action of this product [46]. Moreover, when inflammatory processes are not involved, μFAT has little effect on the expression of MMPs, indicating that it would not inhibit the physiological remodeling of tendon ECM.
In this study, the influence of IL-1β on the expression of the tendon-specific transcription factor SCX was also assessed. While previous reports showed an inhibition of its transcription in inflammatory conditions [23, 47, 48], in our model, the presence of IL-1β significantly increased SCX expression. The discrepancy between the results described in literature and those reported in the present study is probably due to the different sources of TCs and to the inflammatory protocol. Since adipose-derived MSCs are known to produce trophic mediator-specific for TCs [49], a slight reduction of SCX expression was observed when TCs were cocultured with μFAT in basal conditions, and a downregulation of this marker have been already reported by other authors after TCs-MSCs coculture [34]. These observations suggest that μFAT (or MSCs) trophic action on TCs may be independent of SCX upregulation.
Our findings showed no effect of μFAT on PTGS2 expression, neither in inflammatory nor in noninflammatory conditions. As expected, PTGS2 transcription was enhanced by IL-1β treatment with respect to basal conditions, being IL-1β the main inducer of this gene encoding for COX2 [38]. Despite its role in inflammation, COX2 has been reported exerting an important role in the maintenance of tendon homeostasis and ECM maturation [50, 51]. Therefore, the expression of this enzyme may represent not only as a symptom of inflammation but also as a response of TCs towards tissue healing.
IL-1β and μFAT both demonstrated an enhancing effect on TC metabolic activity and DNA content, even if no statistical significance was found for the latter. Interestingly, this effect was not further enhanced by the use of IL-1β on TCs cocultured with μFAT, showing a lack of additive/synergistic action of the two elements on these parameters.
In our model, due to the use of a transwell system, the modifications induced by μFAT on TC gene expression and metabolic activity are ascribed to its paracrine activity. In particular, μFAT increased the content of soluble IL-1Ra, IL-6, and VEGF in the culture medium. IL-1Ra is a direct inhibitor of IL-1β, and it acts by competing with the cytokines for the binding to IL-1 receptor 1 [52], to the extent that IL-1Ra-based treatments have been developed for rheumatoid arthritis and other autoinflammatory diseases [53]. IL-1Ra production was elicited in μFAT samples only in the presence of inflammatory conditions, suggesting it is a reaction of the μFAT-embedded adipose-derived MSCs to this condition. Indeed, these cells are known to respond to IL-1β stimulation by releasing IL-1Ra [54], as well as MSCs from other sources [55].
As expected, in our model, the content of IL-6 was enhanced in the presence of IL-1β [56] and μFAT further increased its production. This effect is possibly related to the IL-1β-mediated induction of IL-6 in the cells contained in μFAT [54], and even if there are little evidences of direct pathological changes mediated by IL-6 on TCs and tendon matrix production [57], this aspect should be taken into account as a possible side effect when applying cell-based regenerative medicine products. The role of IL-6 in tendon pathologies has been confirmed by several studies, and it is considered one of the evidences of inflammation involvement in tendinopathy [58]. Indeed, IL-6 increased after tears and ruptures, as well as after intense exercise and injuries, in both humans and animals [59]. Nevertheless, despite its role in inflammation, IL-6 exhibits an immunoregulatory activity and it demonstrated to support tenocyte proliferation and survival, thus resulting among the effectors of the early phases of tendon healing [60–62]. In addition, IL-6 induces the production of VEGF [63], a growth factor mainly known for its role in angiogenesis, that is also involved in tissue regeneration, exerting a homeostatic function [64]. Different to what is observed in IL-6, VEGF is induced by both IL-1β and μFAT treatments at similar intensities, and it was further enhanced by the combination of these factors. Then, since IL-6 was just slightly enhanced by μFAT treatment in comparison to untreated TCs, the μFAT-induced VEGF production appears to be at least partially independent from the IL-6 pathway. Despite the promotion of angiogenesis which may result detrimental for tendon ECM integrity [65], VEGF was able to improve tendon healing strength in several studies [66–68].
The ability of MSCs and μFAT paracrine mediators to counteract inflammation is well described, and it has been reported in several models involving different cells and tissues, comprising chondrocytes, synoviocytes, and the central nervous system [46, 69, 70]. Indeed, the increased production of VEGF and the augmented expression of SCX and, to some extent, COL3A1, demonstrate that IL-1β also elicits an initial reparative response in TCs [71]. This observation is consistent with the presence of a subpopulation of progenitor cells, demonstrating features of mesenchymal stem/stromal cells, within TCs [26, 72]. In pathological conditions and aging, the presence of progenitor cells may be reduced, leading to a loss of homeostatic function and thus tissue degeneration [73]. Then, the idea to supply the injured tissue with a convenient source of autologous MSCs with homeostatic and immunomodulatory activity perfectly represents the rationale of the application of cell concentrates in degenerative disorders.
Limitations of the present study are represented by the use of a nonphysiological inflammatory stimulus that may result in considerably stronger than the one occurring in vivo, and thus partially masking the ability of μFAT to counteract the catabolic response. In addition, the quantity of μFAT was chosen based on previous reports [9], but the proportion between cells and tissue hardly corresponds to the physiological ratio. Another limitation is given by the possible influence of patients' characteristics on the activity of μFAT, which was not taken into account in the present work while it may indeed identify a fraction of individuals who are nonresponders to the μFAT treatment due to pathology or adipose tissue-specific features [74] and thus representing a source of bias.
The results obtained in this work sustain the rationale of μFAT application to tendon disorders and they may provide evidences for the interpretation of future clinical data, identifying the possible mechanisms involved in μFAT-mediated effects on tendon healing. A clinical trial is now ongoing for the evaluation of the possible clinical benefit of μFAT injection as adjuvant therapy in the arthroscopic rotator cuff repair, especially in terms of possible reduction of retears.
5. Conclusions
In an inflammatory context, TCs demonstrated a catabolic and profibrosis pattern of gene expression, while at the same time, producing molecules with a role in tissue healing. The coculture with μFAT not only reduced the expression of fibrosis and catabolic markers but it also enhanced the production of cytokines and growth factors able to counteract the inflammatory process and to contribute to tissue regeneration. These observations provide a rationale for the clinical application of μFAT in the treatment of tendon disorders.
Acknowledgments
This research was funded by the Italian Ministry of Health “Ricerca Corrente.”
Data Availability
All data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Snedeker J. G., Foolen J. Tendon injury and repair – a perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomaterialia. 2017;63:18–36. doi: 10.1016/j.actbio.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Wu F., Nerlich M., Docheva D. Tendon injuries. EFORT Open Reviews. 2017;2(7):332–342. doi: 10.1302/2058-5241.2.160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy M. B., Moncivais K., Caplan A. I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine. 2013;45(11):p. e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhury S. Mesenchymal stem cell applications to tendon healing. Muscles Ligaments Tendons Journal. 2012;2(3):222–229. [PMC free article] [PubMed] [Google Scholar]
- 5.Usuelli F. G., Grassi M., Maccario C., et al. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surgery, Sports Traumatology, Arthroscopy. 2018;26(7):2000–2010. doi: 10.1007/s00167-017-4479-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee S. Y., Kwon B., Lee K., Son Y. H., Chung S. G. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. The American Journal of Sports Medicine. 2017;45(6):1429–1439. doi: 10.1177/0363546517689874. [DOI] [PubMed] [Google Scholar]
- 7.Canapp S. O., Canapp D. A., Ibrahim V., Carr B. J., Cox C., Barrett J. G. The use of adipose-derived progenitor cells and platelet-rich plasma combination for the treatment of supraspinatus tendinopathy in 55 dogs: a retrospective study. Frontiers in Veterinary Science. 2016;3:p. 61. doi: 10.3389/fvets.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora M. V., Antuña S. A., Arranz M. G., Carrascal M. T., Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45(Supplement 4):S22–S27. doi: 10.1016/s0020-1383(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 9.Randelli P., Menon A., Ragone V., et al. Lipogems product treatment increases the proliferation rate of human tendon stem cells without affecting their stemness and differentiation capability. Stem Cells International. 2016;2016:11. doi: 10.1155/2016/4373410.4373410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long C., Wang Z., Legrand A., Chattopadhyay A., Chang J., Fox P. M. Tendon tissue engineering: mechanism and effects of human tenocyte coculture with adipose-derived stem cells. The Journal of Hand Surgery. 2018;43(2):183.e1–183.e9. doi: 10.1016/j.jhsa.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Manning C. N., Martel C., Sakiyama-Elbert S. E., et al. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Research & Therapy. 2015;6(1):p. 59. doi: 10.1186/s13287-015-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmikangas P., Schuessler-Lenz M., Ruiz S., et al. Marketing regulatory oversight of advanced therapy medicinal products (ATMPs) in Europe: the EMA/CAT perspective. Advances in Experimental Medicine and Biology. 2015;871:103–130. doi: 10.1007/978-3-319-18618-4_6. [DOI] [PubMed] [Google Scholar]
- 13.Guess A. J., Daneault B., Wang R., et al. Safety profile of good manufacturing practice manufactured interferon γ-primed mesenchymal stem/stromal cells for clinical trials. Stem Cells Translational Medicine. 2017;6(10):1868–1879. doi: 10.1002/sctm.16-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S. J., Kim E. K., Kim S. J., Song D. H. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. Journal of Orthopaedic Surgery. 2018;13(1):p. 1. doi: 10.1186/s13018-017-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremolada C., Colombo V., Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Current Stem Cell Reports. 2016;2:304–312. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeira O., Scaccia S., Pettinari L., et al. Intra-articular administration of autologous micro-fragmented adipose tissue in dogs with spontaneous osteoarthritis: safety, feasibility, and clinical outcomes. Stem Cells Translational Medicine. 2018;7(11):819–828. doi: 10.1002/sctm.18-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouglé A., Rocheteau P., Hivelin M., et al. Micro-fragmented fat injection reduces sepsis-induced acute inflammatory response in a mouse model. British Journal of Anaesthesia. 2018;121(6):1249–1259. doi: 10.1016/j.bja.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo G., De Caro A., Napoli F., Chiapale D., Trada P., Camera A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskeletal Disorders. 2018;19(1):p. 176. doi: 10.1186/s12891-018-2105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo A., Condello V., Madonna V., Guerriero M., Zorzi C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. Journal of Experimental Orthopaedics. 2017;4(1):p. 33. doi: 10.1186/s40634-017-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavone Panni A., Vasso M., Braile A., et al. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: identification of a subpopulation with greater response. International Orthopaedics. 2019;43(1):7–13. doi: 10.1007/s00264-018-4182-6. [DOI] [PubMed] [Google Scholar]
- 21.Thankam F. G., Roesch Z. K., Dilisio M. F., et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Scientific Reports. 2018;8(1):p. 8918. doi: 10.1038/s41598-018-27250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuzaki M., Guyton G., Garrett W., et al. IL-1β induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. Journal of Orthopaedic Research. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K., Asai S., Yu B., Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochemical and Biophysical Research Communications. 2015;463(4):667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo C. H., Lee S. Y., Yoon K. S., Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1β-induced tendinopathic conditions. The American Journal of Sports Medicine. 2017;45(5):1141–1150. doi: 10.1177/0363546516681294. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi F., Maioli M., Leonardi E., et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplantation. 2013;22(11):2063–2077. doi: 10.3727/096368912X657855. [DOI] [PubMed] [Google Scholar]
- 26.Stanco D., Viganò M., Perucca Orfei C., et al. Multidifferentiation potential of human mesenchymal stem cells from adipose tissue and hamstring tendons for musculoskeletal cell-based therapy. Regenerative Medicine. 2015;10(6):729–743. doi: 10.2217/rme.14.92. [DOI] [PubMed] [Google Scholar]
- 27.Viganò M., Perucca Orfei C., Colombini A., et al. Different culture conditions affect the growth of human tendon stem/progenitor cells (TSPCs) within a mixed tendon cells (TCs) population. Journal of Experimental Orthopaedics. 2017;4(1):p. 8. doi: 10.1186/s40634-017-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viganò M., Perucca Orfei C., de Girolamo L., et al. Housekeeping gene stability in human mesenchymal stem and tendon cells exposed to tenogenic factors. Tissue Engineering. Part C, Methods. 2018;24(6):360–367. doi: 10.1089/ten.TEC.2017.0518. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):45e–445. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desjardins P., Conklin D. NanoDrop Microvolume Quantitation of Nucleic Acids. Journal of Visualized Experiments. 2010;45(1) doi: 10.3791/2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah D., Naciri M., Clee P., Al-Rubeai M. NucleoCounter-an efficient technique for the determination of cell number and viability in animal cell culture processes. Cytotechnology. 2006;51(1):39–44. doi: 10.1007/s10616-006-9012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksen H. A., Pajala A., Leppilahti J., Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. Journal of Orthopaedic Research. 2002;20(6):1352–1357. doi: 10.1016/s0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 33.Shirachi I., Gotoh M., Mitsui Y., et al. Collagen production at the edge of ruptured rotator cuff tendon is correlated with postoperative cuff integrity. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2011;27(9):1173–1179. doi: 10.1016/j.arthro.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 34.Veronesi F., Della Bella E., Torricelli P., Pagani S., Fini M. Effect of adipose-derived mesenchymal stromal cells on tendon healing in aging and estrogen deficiency: an in vitro co-culture model. Cytotherapy. 2015;17(11):1536–1544. doi: 10.1016/j.jcyt.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Costa-Almeida R., Calejo I., Reis R. L., Gomes M. E. Crosstalk between adipose stem cells and tendon cells reveals a temporal regulation of tenogenesis by matrix deposition and remodeling. Journal of Cellular Physiology. 2018;233(7):5383–5395. doi: 10.1002/jcp.26363. [DOI] [PubMed] [Google Scholar]
- 36.Baroneza J. E., Godoy-Santos A., Massa B. F., de Araujo Munhoz F. B., Fernandes T. D., dos Santos M. C. L. G. MMP-1 promoter genotype and haplotype association with posterior tibial tendinopathy. Gene. 2014;547(2):334–337. doi: 10.1016/j.gene.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Castagna A., Cesari E., Gigante A., Conti M., Garofalo R. Metalloproteases and their inhibitors are altered in both torn and intact rotator cuff tendons. Musculoskeletal Surgery. 2013;97(S1) Supplement 1:39–47. doi: 10.1007/s12306-013-0264-1. [DOI] [PubMed] [Google Scholar]
- 38.Kordulewska N. K., Cieślińska A., Fiedorowicz E., Jarmołowska B., Kostyra E. High Expression of IL-1RI and EP2 receptors in the IL-1β/COX-2 pathway, and a new alternative to non-steroidal drugs—osthole in inhibition COX-2. International Journal of Molecular Sciences. 2019;20(1):p. 186. doi: 10.3390/ijms20010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thankam F. G., Evan D. K., Agrawal D. K., Dilisio M. F. Collagen type III content of the long head of the biceps tendon as an indicator of glenohumeral arthritis. Molecular and Cellular Biochemistry. 2019;454(1-2):25–31. doi: 10.1007/s11010-018-3449-y. [DOI] [PubMed] [Google Scholar]
- 40.Pajala A., Melkko J., Leppilahti J., Ohtonen P., Soini Y., Risteli J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histology and histopathology. 2009;24(10):1207–1211. doi: 10.14670/HH-24.1207. [DOI] [PubMed] [Google Scholar]
- 41.Sbardella D., Tundo G., Fasciglione G., et al. Role of metalloproteinases in tendon pathophysiology. Mini Reviews in Medicinal Chemistry. 2014;14(12):978–987. doi: 10.2174/1389557514666141106132411. [DOI] [PubMed] [Google Scholar]
- 42.Millar A. W., Brown P. D., Moore J., et al. Results of single and repeat dose studies of the oral matrix metalloproteinase inhibitor marimastat in healthy male volunteers. British Journal of Clinical Pharmacology. 1998;45(1):21–26. doi: 10.1046/j.1365-2125.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Buono A., Oliva F., Longo U. G., et al. Metalloproteases and rotator cuff disease. Journal of Shoulder and Elbow Surgery. 2012;21(2):200–208. doi: 10.1016/j.jse.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Riley G. P., Curry V., DeGroot J., et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biology. 2002;21(2):185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 45.Gotoh M., Hamada K., Yamakawa H., Tomonaga A., Inoue A., Fukuda H. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. Journal of Orthopaedic Research. 1997;15(1):33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- 46.Paolella F., Manferdini C., Gabusi E., et al. Effect of microfragmented adipose tissue on osteoarthritic synovial macrophage factors. Journal of Cellular Physiology. 2019;234(4):5044–5055. doi: 10.1002/jcp.27307. [DOI] [PubMed] [Google Scholar]
- 47.McClellan A., Evans R., Sze C., Kan S., Paterson Y., Guest D. A novel mechanism for the protection of embryonic stem cell derived tenocytes from inflammatory cytokine interleukin 1 beta. Scientific Reports. 2019;9(1):p. 2755. doi: 10.1038/s41598-019-39370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busch F., Mobasheri A., Shayan P., Lueders C., Stahlmann R., Shakibaei M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. The Journal of Biological Chemistry. 2012;287(45):38050–38063. doi: 10.1074/jbc.M112.377028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polly S. S., Nichols A. E. C., Donnini E., et al. Adipose-Derived Stromal Vascular Fraction and Cultured Stromal Cells as Trophic Mediators for Tendon Healing. Journal of Orthopaedic Research. 2019;37(6):1429–1439. doi: 10.1002/jor.24307. [DOI] [PubMed] [Google Scholar]
- 50.Hammerman M., Blomgran P., Ramstedt S., Aspenberg P. COX-2 inhibition impairs mechanical stimulation of early tendon healing in rats by reducing the response to microdamage. Journal of Applied Physiology. 2015;119(5):534–540. doi: 10.1152/japplphysiol.00239.2015. [DOI] [PubMed] [Google Scholar]
- 51.Rundle C. H., Chen S.-T., Coen M. J., Wergedal J. E., Stiffel V., Lau K.-H. W. Direct lentiviral-cyclooxygenase 2 application to the tendon-bone interface promotes osteointegration and enhances return of the pull-out tensile strength of the tendon graft in a rat model of biceps tenodesis. PLoS One. 2014;9(5, article e98004) doi: 10.1371/journal.pone.0098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dabrowski M. P., Stankiewicz W., Płusa T., Chciałowski A., Szmigielski S. Competition of IL-1 and IL-1ra determines lymphocyte response to delayed stimulation with PHA. Mediators of Inflammation. 2001;10(3):107. doi: 10.1080/09629350124376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palomo J., Dietrich D., Martin P., Palmer G., Gabay C. The interleukin (IL)-1 cytokine family – balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 54.De Luca P., Kouroupis D., Viganò M., et al. Human diseased articular cartilage contains a mesenchymal stem cell-like population of chondroprogenitors with strong immunomodulatory responses. Journal of Clinical Medicine. 2019;8(4):p. 423. doi: 10.3390/jcm8040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortiz L. A., DuTreil M., Fattman C., et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z., Simpson R. J., Cheers C. Interaction of interleukin-6, tumour necrosis factor and interleukin-1 during Listeria infection. Immunology. 1995;85(4):562–567. [PMC free article] [PubMed] [Google Scholar]
- 57.Katsma M. S., Patel S. H., Eldon E., et al. The influence of chronic IL-6 exposure, in vivo, on rat Achilles tendon extracellular matrix. Cytokine. 2017;93:10–14. doi: 10.1016/j.cyto.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Legerlotz K., Jones E. R., Screen H. R. C., Riley G. P. Increased expression of IL-6 family members in tendon pathology. Rheumatology. 2012;51(7):1161–1165. doi: 10.1093/rheumatology/kes002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morita W., Dakin S. G., Snelling S. J. B., Carr A. J. Cytokines in tendon disease. Bone & Joint Research. 2017;6(12):656–664. doi: 10.1302/2046-3758.612.bjr-2017-0112.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S., Deng G., Li K., et al. Interleukin-6 promotes proliferation but inhibits tenogenic differentiation via the Janus kinase/signal transducers and activators of transcription 3 (JAK/STAT3) pathway in tendon-derived stem cells. Medical Science Monitor. 2018;24:1567–1573. doi: 10.12659/MSM.908802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin T. W., Cardenas L., Glaser D. L., Soslowsky L. J. Tendon healing in interleukin-4 and interleukin-6 knockout mice. Journal of Biomechanics. 2006;39(1):61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 62.John T., Lodka D., Kohl B., et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. Journal of Orthopaedic Research. 2010;28:n/a–1077. doi: 10.1002/jor.21079. [DOI] [PubMed] [Google Scholar]
- 63.Cohen T., Nahari D., Cerem L. W., Neufeld G., Levi B. Z. Interleukin 6 induces the expression of vascular endothelial growth factor. The Journal of Biological Chemistry. 1996;271(2):736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 64.Luo J., Xiong Y., Han X., Lu Y. VEGF non-angiogenic functions in adult organ homeostasis: therapeutic implications. Journal of Molecular Medicine. 2011;89(7):635–645. doi: 10.1007/s00109-011-0739-1. [DOI] [PubMed] [Google Scholar]
- 65.Pufe T., Petersen W. J., Mentlein R., Tillmann B. N. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scandinavian Journal of Medicine & Science in Sports. 2005;15(4):211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 66.Mao W. F., Wu Y. F., Yang Q. Q., et al. Modulation of digital flexor tendon healing by vascular endothelial growth factor gene transfection in a chicken model. Gene Therapy. 2017;24(4):234–240. doi: 10.1038/gt.2017.12. [DOI] [PubMed] [Google Scholar]
- 67.Kaux J.-F., Janssen L., Drion P., et al. Vascular endothelial growth factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons Journal. 2014;4(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- 68.Tang J. B., Wu Y. F., Cao Y., et al. Basic FGF or VEGF gene therapy corrects insufficiency in the intrinsic healing capacity of tendons. Scientific Reports. 2016;6(1):p. 20643. doi: 10.1038/srep20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marfia G., Navone S. E., Hadi L. A., et al. The adipose mesenchymal stem cell secretome inhibits inflammatory responses of microglia: evidence for an involvement of sphingosine-1-phosphate signalling. Stem Cells and Development. 2016;25(14):1095–1107. doi: 10.1089/scd.2015.0268. [DOI] [PubMed] [Google Scholar]
- 70.Jin R., Shen M., Yu L., Wang X., Lin X. Adipose-derived stem cells suppress inflammation induced by IL-1β through down-regulation of P2X7R mediated by miR-373 in chondrocytes of osteoarthritis. Molecules and Cells. 2017;40(3):222–229. doi: 10.14348/molcells.2017.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millar N. L., Murrell G. A. C., McInnes I. B. Inflammatory mechanisms in tendinopathy - towards translation. Nature Reviews Rheumatology. 2017;13(2):110–122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 72.Bi Y., Ehirchiou D., Kilts T. M., et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 73.Kohler J., Popov C., Klotz B., et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013;12(6):988–999. doi: 10.1111/acel.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caplan A. I. Cell-based therapies: the nonresponder. Stem Cells Translational Medicine. 2018;7(11):762–766. doi: 10.1002/sctm.18-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request.