Abstract
Background
Diabetic kidney disease (DKD) develops an end-stage renal failure and is a major cause of death in diabetic patients. A GFR below 60 ml/min per 1.73 m2 is one of the main markers of DKD. Therefore, the development of an accurate test for diagnosis and monitoring of the mentioned disease would be essential. Here, we examined the impacts of two different kits with different methods for creatinine measurement on the GFR values.
Methods
Blood samples were collected from 80 diabetic patients referring to the clinical laboratory. The levels of serum creatinine were assessed using Jaffé and enzymatic assays by kits from two different manufacturers. Then to assess the eGFR levels, the MDRD equation was used. Further descriptive parameters of both methods and correlation of methods were also calculated.
Results
Descriptive analysis of the data demonstrates a slight increase in the serum creatinine measured by Jaffé assay which leads to a substantial decrease in the levels of eGFR compared to the eGFR calculated by the enzymatic assay. Moreover, eGFR over 60 mL/min/1.73 m2in enzymatic assay was observed in 27.5% of participants while eGFR of the same participants was below 60 mL/min/1.73 m2 when it was measured by Jaffé method. Consequently, 27.5% positive discordant cases were reported by Jaffé assay followed by misclassifying them as DKD patients compared with the enzymatic assay.
Conclusion
While using Jaffé assay, a low level of eGFR is observed which generates more misclassification into the DKD group and demands to an inclusive consideration by physicians in order to diagnose and monitor the DKD patients.
Keywords: Diabetic kidney disease; Creatinine, glomerular filtration rate; Jaffé assay; Enzymatic assay
Introduction
Diabetic kidney disease (DKD) also recognized as diabetic nephropathy is increasing all over the world as a consequence of chronic type 1 or type 2 diabetes mellitus with a prevalence of 20% to 40% in these patients. DKD develops an end-stage renal failure and increases the mortality rate in diabetic patients. A prompt intervention at early stages of the disease may be sufficient to control the progression of renal damage [1–3]. Although the most accurate and reliable test for the diagnosis of such a condition would be renal biopsies, because of the high risks of the procedure, it is not a routine clinical practice [4]. Nowadays, the most clinical signs of DKD used in laboratories with the aim of diagnosis are elevated albuminuria more than 300 mg/24 h, a decrease in glomerular filtration rate (GFR) to less than 60 ml/min per 1.73 m2 and high blood pressure. Therefore, to receive an early diagnosis, staging the severity of the condition, and treatment of disease, an appropriate strategy is needed [5]. The first pathophysiological event of DKD is the albumin excretion as microalbuminuria followed by severely albuminuria through passing the time. Thus, screening the urinary albumin excretion (UAE) is a primary laboratory test to characterize DKD [6]. Another important clinical finding is GFR decline which is a common sign of DKD even in patients with normal UAE. GFR is an implication of renal function used in the diagnosis and monitoring of DKD. Among different analytical performances for determining GFR, creatinine assay is an accurate and reproducible determinant which has been used as a cheap and common marker of the disease for many years [7].
It is reported that creatinine values depend on various biological and analytical interferences including age, muscular mass, sex, nutritional habits, variable absorption, tubular secretion, and the applicable method. Then, marked limitations would be able to border its measurement which must be regarded [8]. Because of estimated GFR (eGFR) dependency on creatinine measurement, any error or miscalculation in the analysis of creatinine would impact the results of eGFR and subsequently the physician’s decision which has irreparable outcomes for patients with DKD [9]. Hence, with considering the strong effects of such differences on eGFR variability at high levels, reaching to a more reliable method for measuring creatinine is the primary goal to decrease the variability of eGFR calculations and subsequent misclassification of the DKD. Henceforth, in addition to annual screening of diabetic patients to detect DKD based on elevated albuminuria and low eGFR for starting an early disease-modifying therapy, development of an accurate method to analyze creatinine with the aim of evaluating true eGFR is critical too [10–12].
As mentioned above, the variability occurred from method nonspecificity, differing by assay type (Jaffé versus enzymatic) would alter the eGFR results and demands a full consideration by the physician when making a decision [13]. Two well-known colorimetric methods for the measurement of serum creatinine are applied in the clinical laboratories. At first, Jaffé methods were used generally with measurement of a yellow color created substance after the reaction of serum creatinine with alkaline picrate. Since the alkaline picrate used in this reaction can also react with other substances like glucose, urea, ketones, proteins, and bilirubin, it is not accepted as a sufficiently specific method to measure the serum creatinine. However, in recent years, there has been an effort to improve the precision of the Jaffé assays [14, 15]. Other known methods to quantify the serum creatinine are enzymatic assays with better specificity and sensitivity than the former. In these methods, manufacturers designed a particular enzymatic reaction to measure the exact concentration of creatinine. Still, an increased cost of this specific creatinine testing is one of the problems for its practical use in some laboratories. Additionally, enzymatic assays are not entirely free from interferences like bilirubin [15, 16]. However, several studies indicated the fewer effects of interfering substances on creatinine concentrations measured by enzymatic assays rather than Jaffé assays [13, 17].
Many studies have compared just creatinine assays specifications and many others have evaluated the eGFR values estimated from routine laboratory assays compared to reference method. Using reference method is not practical for almost all clinical laboratories so kits with different method produced by different companies are routinely used for creatinine measurement. Our study aimed to compare two different commonly used kits in Iran (MAN company and Roche Diagnostics) with principles of Jaffé and enzymatic assays for diagnosis of DKD patients regarding their measured creatinine and following eGFR values to show how differences in creatinine results can influence patient’s management in practice.
Materials and methods
Blood sample was collected from 80 white diabetic people, ages between 20 and 85 admitted to clinical laboratory of Diabetes and metabolism clinic (affiliated to Tehran University of Medical Science, Iran) for diabetes monitoring, during January and February 2018. Then all serum samples were divided into aliquots for storing with the aim of measuring by different methods. We excluded patients with concomitant infection, other severe kidney disease, malignancy, and also pregnant women from the study.
The levels of serum creatinine were determined by using two isotope dilution mass spectrometry (IDMS) -traceable methods from different manufacturers, MAN (MAN, Tehran, Iran) and Roche (Roche Diagnostics, Mannheim, Germany) using Jaffé and enzymatic assays respectively. The reference range for serum creatinine was 0.6–1.2 mg/dL for women and 0.8 to 1.4 mg/dL for men for Man kit (Jaffé assay), while it was0.5–0.9 mg/dL for women and 0.7--1.2 mg/dL for men when measured by Roche diagnostic (enzymatic assay). The difference between reference ranges obtained by two mentioned methods has been depicted in references too [18].
Then to assess the eGFR levels, we used the IDMS traceable Modification of Diet in Renal Disease (MDRD) Study equation as provided below:
Age, gender, weight, and diabetes affliction time of all participants were recorded. The HbA1c measurement was prepared by G8® from Tosoh Bioscience (Tokyo, Japan) as an HPLC method which is certified by the National Glycohemoglobin Standardization Program. Urinary albumin excretion (UAE), Fasting Blood Glucose (FBG) and Urea were measured using an automated procedure and commercial kits (Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Descriptive parameters such as the Mean, the Standard Deviation, the Maximum and the Minimum of parameters by both methods were calculated. The Normality of distribution was tested by the Kolmogorov-Smirnov test, and the independent samples t-test was used to assess the significance of differences between the two methods. Then, correlation and the simple linear regression analysis were used to determine the association of the methods. Finally, the Bland-Altman graph was depicted in order to determine the limits of agreement between the two methods. We used the SPSS statistical software version 25 for analysis of the results, and the P-values of less than 0.05 were assumed as statistically significant.
Results
The descriptive analysis of these data demonstrates a slight increase in the serum creatinine measured by Jaffé assay which leads to a substantial decrease in the levels of eGFR compared to the eGFR calculated by the enzymatic assay (Table 1). Maximum creatinine measured by Jaffé assay (10.01) was higher than the enzymatic assay (8.90) as well as the minimum measurement of the Jaffé assay (0.81) which was higher than enzymatic assay too (0.51). Table 2 shows the correlation between clinical and biochemical characteristics with eGFRs calculated by both methods. There was a significant negative correlation between urea and eGFR. Also the results indicate that the level of eGFR is decreased by increasing age. We did not observe any significant correlations between other parameters (HbA1c, FBG, weight and sex) and eGFR. The results were also evaluated for interference with glucose and HbA1c up to level 450 mg/dL and 13% respectively and found no interferences.
Table 1.
Descriptive analysis of study population
| Creatinine | GFR | |||
|---|---|---|---|---|
| Enzymatic | Jaffé | Enzymatic | Jaffé | |
| Mean | 1.70 | 2.12 | 57.72 | 41.84 |
| Std. Deviation | 1.40 | 1.57 | 30.20 | 19.84 |
| Maximum | 8.90 | 10.01 | 140 | 96.6 |
| Minimum | 0.51 | 0.81 | 4.9 | 4.3 |
Table 2.
Correlation analysis of GFR level with clinical and biochemical characteristics
| GFR (Enzymatic) | GFR (Jaffé) | |
|---|---|---|
| Sex (male/female) | 0.198 | 0.111 |
| Age (years) | −0.355** | −0.358** |
| GFR (Jaffé) | 0.892** | – |
| Creatinine_ Enzymatic(mg/dl) | −0.723** | −0.738** |
| Creatinine_ Jaffé(mg/dl) | −0.727** | −0.747** |
| Weight (kg) | 0.095 | 0.101 |
| Urea (mg/dl) | −0.791** | −0.804** |
| FBG (mg/dl) | 0.082 | 0.114 |
| HbA1c(%) | 0.018 | 0.057 |
| Diabetes time (Years) | −0.272* | −0.294* |
*.Correlation is significant at the 0.05 level (2-tailed)
**.Correlation is significant at the 0.01 level (2-tailed)
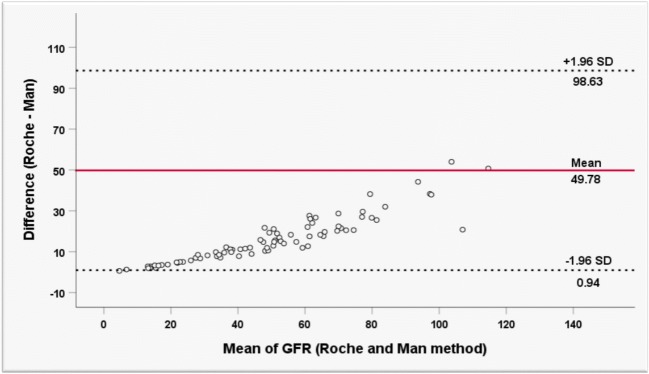
27.5% of participants showed eGFR over 60 mL/min/1.73 m2 in enzymatic assay whereas eGFR of the same individuals was below 60 mL/min/1.73 m2when measured by the Jaffé method. Therefore, 27.5% positive discordant cases were reported by Jaffé assay followed by misclassifying them as DKD patients compared with the enzymatic assay. The Jaffé assay was 41.66% sensitive and also had 100% specificity compared to the enzymatic assay. The negative predictive value (NPV) and the positive predictive value(PPV) were equal to 67.69% and 100%, respectively. Figure 1 represents the strong association between the eGFR level calculated with Jaffé and enzymatic methods (R = 0.98, R2 = 96). The level of agreement between the two methods is depicted in Fig. 2.
Fig. 1.
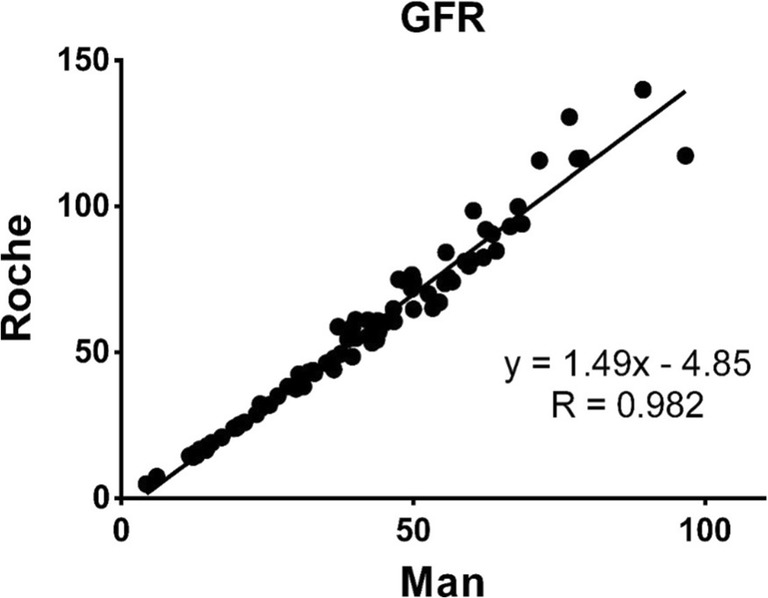
Regression line and regression equation for GFR calculated by Enzymatic and Jaffé methods
Fig. 2.
Bland-Altman Plot of differences in GFR calculated by Enzymatic (Roche) and Jaffé (Man) methods
Discussion
Most clinical analyses used in clinical laboratories to diagnose and monitor DKD are elevated albumin excretion, a decrease in GFR and high blood pressure. Among these clinical findings, GFR decline is a well-known implication of renal function even in patients with normal UAE [5, 7].
There are several analytical performances for evaluating GFR. However, creatinine assay as a common determining factor of renal function has been used more routinely in determining eGFR over the years [7]. Considering the dependency of eGFR level on creatinine measurement, and also the influence of different measurement methods and analytical interferences on creatinine values, any error in the creatinine analysis would impact the eGFR results too. Therefore, developing a more reliable and accurate method for evaluating eGFR based on creatinine analysis would decrease the variability of results and subsequent misclassification of DKD patients [9, 10].
Then, in this study, we evaluated the influence of two commercial kits (MAN and Roche) with the principles of Jaffé and enzymatic assays on measured creatinine and following eGFR levels with the aim of diagnosing DKD patients to find how GFR is estimated in practice. Accordingly, the blood sample was collected for creatinine analysis with particular methods. Next, based on appropriate predictive formulas considering the relationship between creatinine and eGFR [19], the equation GFR was acquired.
The most critical finding of our study by using individual patient samples is that creatinine values measured by Jaffé assay were higher than enzymatic method. This leads to a lower eGFR and more diagnosis of DKD (n = 22 characterized as DKD patients by Jaffé assay vs. enzymatic method) and also better analytical precision (CVA for the analytical coefficient of variation) of the enzymatic method compared with Jaffé method.
Similar studies were conducted to examine the differences in creatinine methods with the principles of Jaffé and enzymatic assays. In agreement with our data, it was shown that in comparison of two Jaffé and enzymatic assays (both Roche) in samples from type 2 diabetes patients, the method using Jaffé assay yielded lower eGFR. However, the value of FBG as interference was not determined [20].In a study, creatinine value was measured by four different methods using Jaffé and enzymatic assays in the serum of patients with no medications used, in order to compare their accuracies. The results presented no appropriate specifications for methods using Jaffé assays compared to enzymatic assays with showing a higher level of creatinine [21]. Another study demonstrated a lower precision for three Jaffé methods compared to four enzymatic methods in control and clinical disease samples. Accordingly, a “significant bias” with a range of more than 10% was detected more in patient samples using Jaffé assays rather than those using enzymatic assays [22]. In a cross-sectional study, by using Jaffé assay to estimate eGFR, there were more positively discordant cases (8%) of advanced CKD stages in a large cohort of diabetic patients. Although, the negatively discordant cases were only 1% in this condition. FBG was also determined as a significant cause for the bias between methods [13].
In contrast, there was a low proportion of CKD misclassification (4%) by using the Jaffé assay in a study on the general population [23]. But an investigation on the interlaboratory variability revealed that eGFR evaluated by Jaffé assays was lower than those assessed by enzymatic assays in individual patients with chronic kidney disease [17]. Hence, the methodology of Jaffé measured higher levels of creatinine and subsequent lower eGFR than enzymatic assay with an enzymatic method. So, this finding reflects a persistent bias between two methods despite IDMS standardization which may increase false DKD diagnoses.
Previous studies demonstrated that many biological substances including glucose, urea, ketones, proteins, and bilirubin might interfere with different creatinine assays, and as detailed earlier, the impact would be significantly higher when the measured creatinine value is low [10]. Then, with considering these pieces of evidence, we also examined the correlation between different analytical interferences such as glucose, HbA1c, urea and measured eGFR regarding two methods.
In this study, we found a negative correlation between the eGFR evaluated by two methods and urea levels in DKD patients which means that an increase in the level of urea yields significant differences between the enzymatic and Jaffé results, then interference by urea will contribute to inaccurate and variable creatinine result. Moreover, the positive correlation between the values of FBG and HbA1c with eGFR was not significant in our study. There was no significant correlation between the patient’s weight and the eGFRs by both Jaffé and enzymatic assays (all data provided in Table 2).
There is also a significant agreement between the eGFR measured by Jaffé assay and the eGFR measured by enzymatic assay in low levels of eGFR. But with raising its value, a more noticeable difference between the two methods would be observed.
Other studies also examined the effects of interfering biological substances on the eGFR results. One of the most known interferences is glucose which can react with alkaline picrate as pseudocreatinines like the way creatinine reacts. In a study, Haugen HN [24] demonstrated that both glucose and acetone are interferences in creatinine measurement in diabetic patients. Moreover, it was reported that a greater value of glucose leads to a higher error in creatinine evaluated by Jaffé assay [25]. We evaluated the results for interference with glucose and HbA1c up to level 450 mg/dL and 13% respectively and found no interferences.
In this study, our main goal was reporting this idea that eGFR below 60 mL/min/1.73 m2is indicative of DKD only in case of using the right kit and the right method. To declare this point, we specifically showed the differences of creatinine values measured by two kits which are used most commonly in IRAN. We revealed that calculating eGFR by MAN kit is followed by an error in diagnosing process of DKD patients. Therefore, the physician needs to know the exact type of method and reference ranges of kits by which eGFR value was calculated in order to make the right decision. In summary, it seems likely that a lower level of eGFR is calculated when Jaffé assay (MAN kit) is used which results in a more misclassification into the DKD group. However, compared to a very low percentage of negatively discordant cases, Jaffé assay does not present a significant burden for the laboratories and more importantly, the DKD patients. Putting these findings together, in the diagnosis and monitoring of diabetic patients, a physician must consider the used method for eGFR, however the diagnosis is not only made by GFR results alone.
Acknowledgements
This work was supported by Diabetes Research Center, EMRI, Tehran University of Medical Sciences andis acknowledged by authors.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author.
Compliance with ethical standards
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Endocrinology and Metabolism Research Institute (EMRI) affiliated with Tehran University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farshad Niazpour, Email: f-niazpour@alumnus.tums.ac.ir.
Alireza Bahiraee, Email: Bahiraee.alireza96@gmail.com.
Ensieh Nasli Esfahani, Email: n.nasli@yahoo.com.
Maryam Abdollahi, Email: m_abdollahi1981@yahoo.com.
Fatemeh Bandarian, Email: fbandarian@yahoo.com.
Farideh Razi, Email: f-razi@tums.ac.ir.
References
- 1.Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49. [PMC free article] [PubMed]
- 2.Anders H-J, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 3.Abrishami Z, Nasli-Esfahani E, Razmandeh R, NI BL, Bandarian F. Iran diabetes research roadmap (Idrr) study; gap analysis of diabetes complications in iran: a review article. Iranian Journal of Public Health. 2017;46(Suppl 1):32–38.
- 4.Persson F, Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl. 2018;8(1):2–7. doi: 10.1016/j.kisu.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fioretto P. Diabetic kidney disease: natural history and pathophysiology. Medicographia. 2016;38:49–55. [Google Scholar]
- 6.Chang SS. Albuminuria and diabetic nephropathy. Pediatr Endocrinol Rev. 2008;5(Suppl 4):974–979. [PubMed] [Google Scholar]
- 7.Tang Sydney C.W., Chan Gary C.W., Lai Kar Neng. Recent advances in managing and understanding diabetic nephropathy. F1000Research. 2016;5:1044. doi: 10.12688/f1000research.7693.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerard SK, Khayam-Bashi H. Characterization of creatinine error in ketotic patients. A prospective comparison of alkaline picrate methods with an enzymatic method. Am J Clin Pathol. 1985;84(5):659–664. doi: 10.1093/ajcp/84.5.659. [DOI] [PubMed] [Google Scholar]
- 9.Stevens PE, Levin A, M. for the Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 10.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, National Kidney Disease Education Program Laboratory Working Group Recommendations for improving serum creatinine measurement: a report from the laboratory working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 11.Cobbaert CM, Baadenhuijsen H, Weykamp CW. Prime time for enzymatic creatinine methods in pediatrics. Clin Chem. 2009;55(3):549–558. doi: 10.1373/clinchem.2008.116863. [DOI] [PubMed] [Google Scholar]
- 12.Association AD. Standards of medical care in diabetes—2014. Diabetes care. 2014;37(Supplement 1):S14–S80. [DOI] [PubMed]
- 13.Lovrencic MV, et al. Impact of creatinine methodology on glomerular filtration rate estimation in diabetes. World J Diabetes. 2017;8(5):222–229. doi: 10.4239/wjd.v8.i5.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delanghe JR, Speeckaert MM. Creatinine determination according to Jaffe—what does it stand for? NDT Plus. 2011;4(2):83–86. doi: 10.1093/ndtplus/sfq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisawasdi P, Chaichanajarernkul U, Teerakanjana N, Vanavanan S, Kroll MH. Exogenous interferences with Jaffe creatinine assays: addition of sodium dodecyl sulfate to reagent eliminates bilirubin and total protein interference with Jaffe methods. J Clin Lab Anal. 2010;24(3):123–133. doi: 10.1002/jcla.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen LJ, Keevil BG. Does bilirubin cause interference in Roche creatinine methods? Clin Chem. 2007;53(2):370–371. doi: 10.1373/clinchem.2006.075846. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Collier CP, White CA. Interlaboratory variability in plasma Creatinine measurement and the relation with estimated glomerular filtration rate and chronic kidney disease diagnosis. Clin J Am Soc Nephrol. 2017;12(1):29–37. doi: 10.2215/CJN.05400516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.rd, et al., editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: ButterworthsButterworth Publishers, a division of Reed Publishing; 1990. [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cheuiche AV, Soares AA, Camargo EG, Weinert LS, Camargo JL, Silveiro SP. Comparison between IDMS-traceable Jaffe and enzymatic creatinine assays for estimation of glomerular filtration rate by the CKD-EPI equation in healthy and diabetic subjects. Clin Biochem. 2013;46(15):1423–1429. doi: 10.1016/j.clinbiochem.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 21.Boutten A, et al. Enzymatic but not compensated Jaffe methods reach the desirable specifications of NKDEP at normal levels of creatinine. Results of the French multicentric evaluation. Clin Chim Acta. 2013;419:132–135. doi: 10.1016/j.cca.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg N, Roberts WL, Bachmann LM, Wright EC, Dalton RN, Zakowski JJ, Miller WG. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and Jaffe method principles. Clin Chem. 2012;58(2):391–401. doi: 10.1373/clinchem.2011.172288. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt RL, Straseski JA, Raphael KL, Adams AH, Lehman CM. A risk assessment of the Jaffe vs enzymatic method for Creatinine measurement in an outpatient population. PLoS One. 2015;10(11):e0143205. doi: 10.1371/journal.pone.0143205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugen HN. Glucose and acetone as sources of error in plasma “Creatinine” determinations. Scand J Clin Lab Invest. 1954;6(1):17–21. doi: 10.1080/00365515409134823. [DOI] [PubMed] [Google Scholar]
- 25.Husdan H, Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem. 1968;14(3):222–238. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author.