Abstract
The growing trend in addition to their burden, prevalence, and death has made obesity and cancer two of the most concerning diseases worldwide. Obesity is an important risk factor for common types of cancers where the risk of some cancers is directly related to the obesity. Various inflammatory mechanisms and increased level of pro-inflammatory cytokines have been investigated in many previous studies, which play key roles in the pathophysiology and development of both of these conditions. On the other hand, in the recent years, many studies have individually focused on the biomarker’s role and therapeutic targeting of microRNAs (miRNAs) in different types of cancers and obesity including newly discovered small noncoding RNAs (sncRNAs) which regulate gene expression and RNA silencing. This study is a comprehensive review of the main inflammation related miRNAs in obesity/obesity related traits. For the first time, the main roles of miRNAs in obesity related cancers have been discussed in response to the question raised in the following hypothesis; do the main inflammatory miRNAs link obesity with obesity-related cancers regarding their role as biomarkers?
Graphical abstract.
Conceptual design of inflammatory miRNAs which provide link between obesity and cancers
Keywords: Obesity, MicroRNAs, Inflammation, Cancer
Introduction
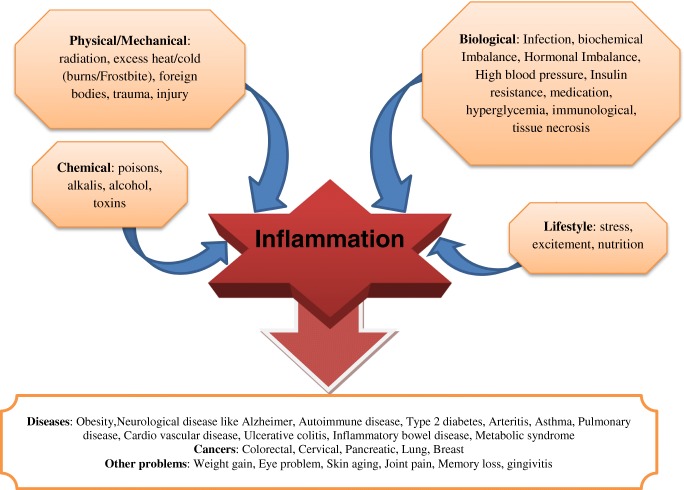
Cancer is the second deadly disease all around the world whose early diagnosis would significantly improve its prognosis and treatment [1]. Around 50% of cancer deaths are preventable by managing the main risk factors [2]. Obesity is known as a chronic low-grade inflammatory disease and is known as a risk factor in many types of cancers. Available evidence demonstrated that chronic low-grade inflammation could be one of the major causes in many chronic diseases [3], for instance, osteoarthritis [4], and obesity [5]. However, it has been suggested low grade chronic inflammation is beneficial in coronary heart disease prediction [6]. According to world health organization (WHO), the world prevalence of obesity has been increased dramatically in the last decades and in 2016 more than 39% and 13% of adults were overweight and obese, respectively [7]. Inflammation is believed to play a double-edged role in the development of cancer. Acute inflammation may play essential role in adipose tissue remodeling, expansion, and homeostasis [8]. Earlier work indicated that the acute inflammation may be useful in the inhibition of cancer development [9]. Inflammation is a major common factor mediating obesity and cancers. More than 1/4 of cancers are related to chronic infections and inflammation [10]. Inflammation is involved in protective immune response against various physical, biological, chemical, and psychological defects. Some of the main causes of inflammation and various associated conditions are summarized in Fig. 1.
Fig. 1.
Main causes and various conditions associated with inflammation
The inflammatory pathways including Jun N-terminal kinase 1 (JNK1) and IκBα kinase β (IKKβ) can activate adipose tissue [11, 12]. These signaling pathways are activated by tumor necrosis factor alpha (TNF-α), free fatty acids (FFAs), diglyceride (DAG), ceramide, reactive oxygen species (ROS), and hypoxia in subjects with obesity. miRNAs are other recently known mediators that have been extensively noted in tumor classification, cancer diagnosis, prognosis, progression, and therapy of cancers [13, 14]. Also there are several reviews about the association between obesity, inflammation, and cancer. It has been suggested that the association between obesity and inflammation may be responsible for insulin resistance and cancer [15]. The chronic inflammation is defined as a key mediator of cancer and it has been associated with obesity [16]. Another review indicated that obesity-induced metabolic disorder and immune response might be affected by functional miRNAs [17], and circulating miRNAs could have diagnostic properties and potential application as metabolic disorders biomarker [18]. Obesity and colorectal cancer“an obesity-related cancer” have been related to miRNAs dysregulation [19]. As described above, the important role of both inflammation and miRNAs in obesity and cancer is clear, and there are several reviews on the role of miRNAs in cancer or obesity. However, so far there is no review on the role of inflammation related miRNAs in obesity and cancers, thus we aimed to comprehensively review this issue. We also aimed to introduce some specific miRNAs associated with these conditions as potential biomarkers in the future studies.
Common cellular components between inflammation, obesity, and cancer
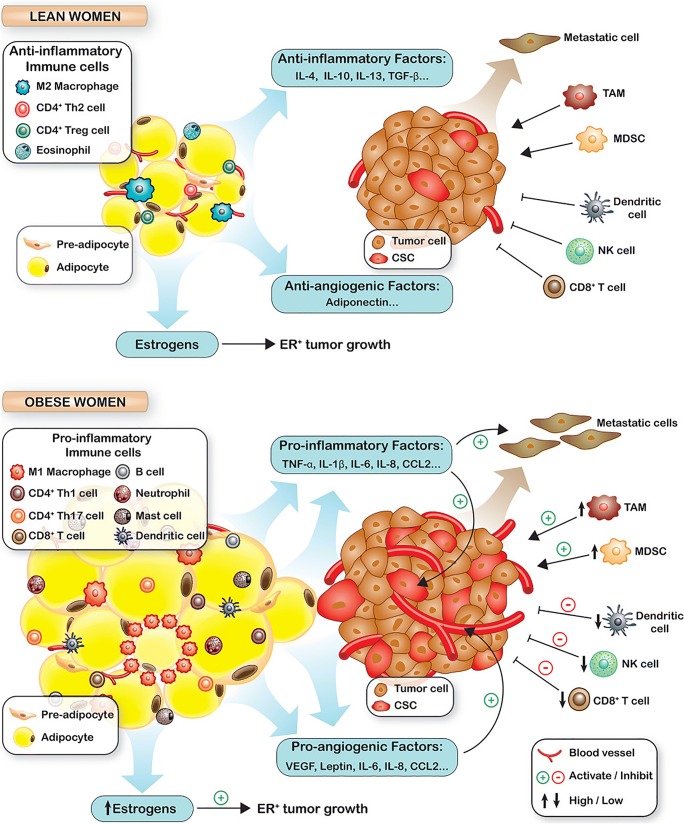
Inflammation plays an important role in obesity, fat cells, adipocytes enlargement, and remodeling [20]. Obesity-related inflammatory response in adipose tissue plays an important role in tumorigenesis. The inflammatory response elements in cells release autocrine and paracrine mediators that promote cell proliferation, prevent apoptosis, stimulate angiogenesis [21, 22]. These conditions may induce mutagenesis by stimulating proliferation of mutated cells. About 20% of all causes of cancers are related to infectious agents and inflammation, causing around 2.8 million new cancer cases and 1.7 million deaths [23]. The relationship between inflamed adipose tissue and cancer is depicted in Fig. 2.
Fig. 2.
The role of obesity in tumorigenesis; Reproduced from reference [24] (obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention)
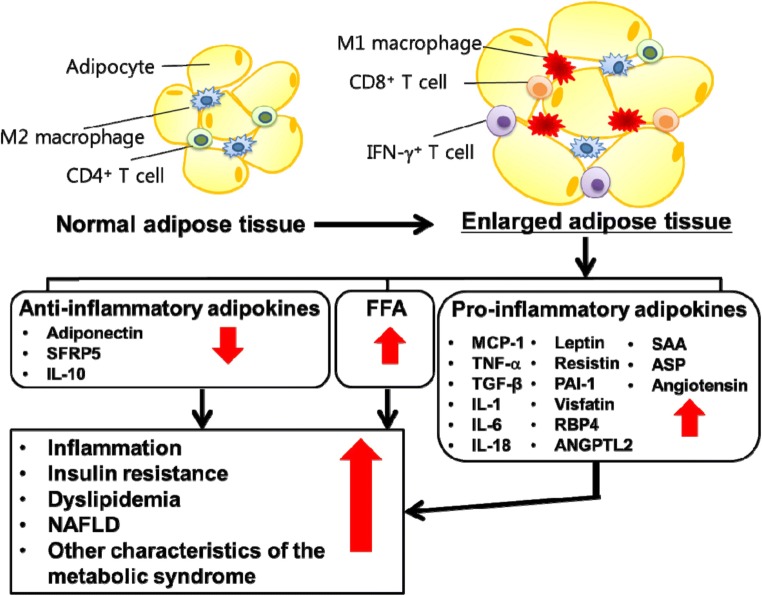
Obesity and overweight are associated with 13 types of cancers [25]. These cancers are related to inflamed adipose tissues. Adipose tissue is a source of many endocrine hormones and high metabolically active organs [26]. White adipose tissue (WAT) makes cytokines that can induce inflammation. Visceral fat is the accumulation of WAT in the visceral area which includes numerous immune and inflammatory cells [27]. Obesity associated lipolysis [28] increases saturated fatty acids and induce macrophage activation [29]. Consequently, they initiate cytokines and adipokines productions and in turn inflammation. The inflammation is known to play a key role in progression or promotion of cancers [30]. For instance, cancer may progress in inflammatory sites such as inflammation of colon and rectum in ulcerative colitis. A predisposing factor of esophageal cancer is Barrett esophagus which is an inflammatory condition [31]. Cervical inflammation is the result of lesions which may aid HPV infection to develop to high-grade cervical intraepithelial neoplasia and clear cell adenocarcinoma [32]. The cytokines or chemokines secreted from lymphocytes switch cellular activities towards neoplasm [33]. The elevated cytokine levels such as CRP, IL- 6, IL-8, IL-1β, TNF-α, and level of anti-inflammatory cytokines such as IL-4 and IL-10 have been reported in cancer [34, 35]. In adipose tissue, changes in the level of adipokines such as leptin are involved in cancer initiation and progression [27]. Furthermore, the accumulation of pro-inflammatory macrophages is a basic characteristic of adipose tissue in obesity linking the adipose inflammation to systemic complications [36]. Adipose tissue hypoxia may stimulate the expression of pro-inflammatory cytokines such as TNF-α, interleukin (IL) 1, IL-6, Monocyte Chemoattractant Protein-1(MCP-1), and plasminogen activator inhibitor-1(PAI-1) in the fat tissue and circulation in obesity. Adipose tissues secrete different proteins signaling as adipokines. Alteration in the expression of these adipokines leads to the development of chronic inflammatory and metabolic dysfunction [37]. The following figure (fig. 3) shows the secretion of pro- and anti-inflammatory adipokines in adipose tissue.
Fig. 3.
Reproduced with permission from reference [38] (Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia, and nonalcoholic fatty liver disease)
Adipokines are more than 50 polypeptide hormones which are mainly produced from visceral fat. Some pro- and anti-inflammatory components such as leptin, adiponectin, IL-6, TNF-α, IL-1B, TGF-β, IL-10, FFAs play critical roles in obesity and cancers, as depicted in Figs. 1 and 2. For instance, Leptin level is higher in bodies with more fat and in women compared with men [39, 40]. It has a prominent role in the initiation of adipose pro-inflammatory pathway [41]. Pro-inflammatory cytokines expression is positively correlated with the plasma levels of leptin. It has various pro-inflammatory effects including stimulating innate immune cells for producing IL-1, IL-6, IL-12, and TNF-α, as well as enhancing the production of ROS, cyclooxygenase 2 (COX2), leukotriene B4, and nitric oxide [42, 43]. This hormone is responsible for modulating food intake, homeostasis, as well as proliferation of normal and cancer cells. High level of leptin can cause cancer initiation and progression, based on its proinflammatory, pro-angiogenic, mitogenic, anti-apoptotic, and oxidative effects [27]. Studies suggest that leptin receptor is also expressed in some cancers like breast and colon cancers [44, 45]. The long variant of leptin receptor (LRb) induces MAPK, PI3 kinase, and STAT signaling pathways which are responsible for proliferation, survival, and differentiation in both normal and cancer cells [46]. It has been previously demonstrated that cancer patients have serum leptin values at low concentrations and leptin is considered to be correlated with the stage of disease [47]. It has been reported that leptin is an important factor in signaling pathways initiation which is activated by estradiol stimulation. Leptin affects cancer risk by different signaling pathways such as JAK-STAT, MAPK, and PI3K Pathways which regulate cancer cell growth, migration, survival and cellular apoptosis [48].
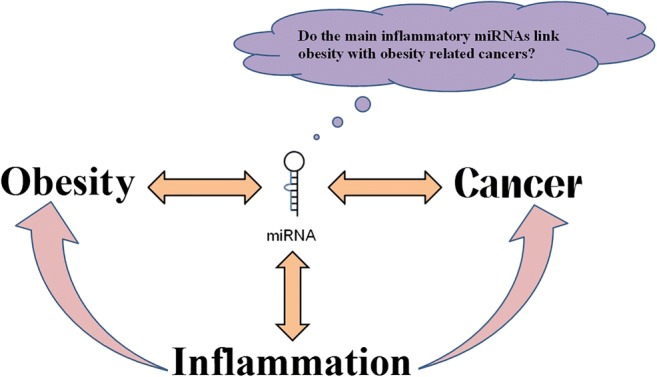
Do the main inflammatory miRNAs link obesity with obesity related cancers regarding their role as biomarkers?
miRNAs are small noncoding RNAs which regulate gene expression and RNA silencing by target mRNAs. While the first miRNA was discovered in 1993, their regulatory effects were suggested seven years later [49–51]. Thereafter, they attracted the attention of researchers. A study in 2002 in chronic lymphoid leukemia (CCL) revealed that miR-15 and miR-16 genes are located in a region which is deleted in a majority of CCL cases [52]. In 2003, in a review, McManus for the first time used the terms of miRNA and cancer together as “microRNAs and cancer”. Based on this review, miRNAs target and regulate tumor suppressors such as cell cycle control factors. Any impairments concerning miRNAs or their targets site in mRNA gene might ensue cancer. Concerning the role of miRNAs in obesity, in 2003 Xu et al. indicated that miR-14 in Drosophila is related to fat metabolism [53]. In 2004. Esau et al. found that miR-143 regulates adipocyte differentiation and may by a target of ERK5 gene [54]. Today, a large number of studies are focusing on the role of miRNAs in obesity and cancer. There are several new reviews on relationship between obesity and cancers [19, 55]. Given the importance of inflammation in obesity and cancer (discussed in the previous section), this section was considered to represent the main inflammation related miRNAs in obesity, adipose tissue, lipid metabolism, and adiposeness. The main effects of these miRNAs and inflammation on obesity related cancers are discussed in the following sections.
cmiRNA1s in obesity and their role in inflammation
The role of cmiRNAs as a biomarker was firstly used in lymphoma, in 2007 [56]. After those years, many studies have focused on the role of cmiRNAs in metabolic diseases. Today, several cmiRNA profiles have been identified in obesity, diabetes, and other inflammation related diseases. For instance in 2018 a review article explained the role of circulating miRNAs on some inflammatory diseases such as Cystic Fibrosis, inflammatory bowel disease [57]. In another review role of circulating miRNAs such as miR-192, miR-375, miR-15a, miR-21, miR-126, and miR29b were discussed in type 2 diabetes mellitus [58]. Here we aimed to investigate circulating microRNAs in obesity and investigate their role in inflammation.
Several cmiRNAs may be dysregulated in obesity. miR-132 is significantly reduced in the blood of people with obesity [59]. This miRNA plays a critical role in inflammation. Its expression level is remarkably related to the number of macrophages infiltrating in adipose tissue [60], NF-κB pathway, as well as production of IL-8 and MCP-1 [61]. miR-132 decreases lipopolysaccharide (LPS)-induced inflammation through targeting acetylcholinesterase (AChE) and enhances the acetylcholine-mediated cholinergic anti-inflammatory response [62]. The circulating levels of miR-140-5p, miR-125b, miR-15a, and miR-221 are dysregulated in morbid patients with obesity [63]. These miRNAs play critical roles in inflammation. On example is the regulation of the pro-inflammatory function of monocyte-derived dendritic cells in SLE [64]. miR-140-5p inhibits secretion of inflammatory cytokines such as IL-6 and IL-8. This may be related to the role of TLR4 gene as miR-140 target gene [65]. In this way, miR-125b is elevated in chronic inflammation which controls the expression of genes involved in inflammatory and apoptotic functions such as IL-6 and MCP-1 [66]. Also, it plays a significant role in inflammation by controlling mitochondria integrity via BIK and MTP18 silencing [67]. miR-15a reduces inflammation [68] and negatively affects the LPS-induced inflammatory response in neonatal sepsis [69]. miR-221 facilitates inflammation in white adipose tissue and reduces insulin sensitivity in obesity, through suppressing Sirtuin1 (SIRT1) [70]. Its reduced expression in chronic inflammation is associated with high levels of TNF-α [71].
cmiRNAs also regulates adipogenic processes for instance by let-7b and miR-221 as anti-adipogenic and miR-143 as promoting miRNAs [54, 72]. They are involved in inflammatory pathways and regulated by TNF-α. Existing data demonstrate that let-7 miRNA family has a key role in regulating inflammatory responses. Let-7b modulates the inflammatory response [73, 74]. TNF-α could regulate let-7 expression through Lin28b, an RNA binding protein (RBP) which negatively regulates let-7 [75]. miR-143 is involved in ERK5 signaling and works as a positive regulator of human adipocyte differentiation. miR-143 level grows in the mesenteric adipose of high-fat diet mice. Also, reduction of miR-143 expression by TNF-α treatment indicates its dysregulation by obesity-associated inflammation process. Insulin sensitivity and inflammatory components such as FFAs, resistin, and leptin influence its expression. Therefore, miR-143 may be an important regulator in the occurrence of obesity-related insulin resistance [76–79].
Some cmiRNAs (miR-146, miR-378, miR-143, miR-145) are related to obesity through inflammation or other correlated traits [80]. miR-146b is an inflammatory miRNA involved in cytokine signaling via the NF-κB pathway, as well as through cytokine production and inflammatory response. It may be overexpressed in response to pro-inflammatory cytokines in adipose tissue inflammation [81–83]. miR-146a/b level rises in mice models of obesity with increased fat mass [84]. This miRNA negatively controls inflammatory response and cytokine production such as TNF-α plus IL-1β, IL-8, and IL-6 [85, 86]. miR-146a-5p suppresses adipogenesis through targeting insulin receptor (IR) and plays a role in insulin signaling pathway through reducing tyrosine phosphorylation of IRS-1 [87]. Also, this miRNA acts as a negative regulator for the inflammatory process. It also represses the target gene translation such as IL-1-receptor-associated kinase-1 (IRAK1) and TRAF6 [88]. miR-378 is encoded by peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β) gene and participates in adipocyte gene expression, lipogenesis, control of mitochondrial metabolism, and systemic energy homeostasis. This miRNA is induced by adipokines [89, 90] and regulates adiponectin expression [91]. Pro-inflammatory cytokines, such as TNF-α, IL-1b, and IL-6 play crucial roles in adipocyte biology and regulation of miR-378 expression [89]. Finally, miR-145 decreases Arf6 and cytokines in macrophages (anti-inflammatory role), which may be related to NF-κB pathway. Further, the expression of this miRNA is attenuated in subjects with obesity [92].
In general, it can be concluded that the majority of aforementioned cmiRNAs in obesity play important roles in inflammation.
Adipose tissue miRNAs related to inflammation
miRNAs secreted by fat cells in the circulation can act as a biomarker of disturbed adipose tissues [93]. The number of miRNAs in tissue and blood are different; blood contains 30% of tissues’ miRNAs. For instance, 28.8% of adipocyte miRNAs are found in the whole blood [94]. Thus, many tissues’ potential miRNAs may not be detected as a biomarker in blood. miRNAs originating from adipose tissue can be applied for management and categorization of obesity. Adipose tissue is the main contributor to the pathophysiology of obesity and plays a significant role in the progress of complications associated with obesity [93]. The main action of white adipose tissue (WAT) is storing and releasing energy-rich lipids. It is remodeled in obesity by endothelial cell overactivation, adipocyte hypertrophy, hyperplasia, immune cell infiltration, and extracellular matrix overproduction. Hypoxic and metabolic stress leads to the activation of multiple inflammatory signaling pathways. Further, the lipolytic activity of adipose tissue is increased in people with obesity. miRNAs in adipose tissue stimulate or inhibit differentiation of adipocytes and regulate metabolic as well as endocrine actions. Here, the key roles of miRNAs in inflammation mechanisms related to adipose tissue are discussed in detail.
White and subcutaneous adipose tissue
Some miRNAs are up-regulated or downregulated in human adipose tissue and influence adipocyte differentiation. miR-150 [95], and miR-139-5p [96], are downregulated in subcutaneous adipose tissue (SAT). On the other hand, miR-143, miR-378 [97], miR-26a, and miR-145 [98] as biomarkers are downregulated in WAT. Note that they may act conversely; for instance, miR-26a inhibits lipolysis and TNF-α secretion while miR-145 stimulates them [99]. miR-335 expression is associated with adipogenesis and is up-regulated in response to leptin, resistin, TNF-α, and IL-6 in human mature adipocytes [100]. MiR-155 expression in WAT is associated with the number of macrophages in the fat depot [60], and inhibits differentiation of brown adipose tissue and enhances WAT transition [101]. miR-221 and miR-222, which are related to adipocytokines, are negatively associated with adiponectin and positively with TNF-α gene expression [102]. miR-99a, miR-125b, miR-22 [96, 103], and miR-222 [97] are up-regulated in WAT/SAT. As discussed earlier, these miRNAs play roles in the level of important obesity related cytokines, inflammatory response and pathways. The main association of these miRNAs with inflammation is summarized in Table 1.
Table 1.
Major roles of white and subcutaneous adipose tissue miRNAs in inflammation
| Adipose tissue microRNAs | Position | Main roles in inflammation | Ref |
|---|---|---|---|
| miR-150 | SAT | Reducing inflammatory cytokines (such as TNF-α and IL-2) by targeting Akt/IKK/NF-κB pathway | [104] |
| miR-139-5p | SAT | Playing anti- inflammatory effects | [105] |
| MiR-26a | WAT | Its upregulation/suppression results in reduction/elevation of production of inflammatory cytokines such as TNFα and IL-6 | [106] |
| miR-335 | SAT |
Upregulated by stimulation of cytokines (leptin, resistin, TNF-α and IL 6), Involved in adipose tissue inflammation |
[100] |
| miR-155 | WAT | Involved in the macrophage inflammatory response and progress of chronic inflammation | [107–109] |
| miR-222 | WAT |
Correlated with adipocytokines (TNF-α and adiponectin), Targeting CXCL12 in macrophages |
[102, 110] |
| miR-99a | WAT | Blocking the inflammation by targeting mTOR/NF-κB signaling | [111] |
| miR-125a | SAT | Negative modulator of macrophage-related inflammatory responses | [112] |
WTA = white adipose tissue, SAT = subcutaneous adipose tissue.
Visceral adipose tissue (VAT)
SAT and visceral adipose tissue are related to macrophage-associated inflammation [113]. VAT adipocyte compared to SAT adipocyte is more metabolically active and sensitive to lipolysis [114]. Furthermore, VAT is considered as an active endocrine organ where macrophage infiltration in VAT is considered as a low-grade inflammatory condition [115]. Dysregulation of several miRNAs in VAT is related to obesity [113]. Among them, miR-223 has a major impact on the regulation of VAT in subjects with obesity. miRNA-223 up-regulation can cause a suppressive effect on the inflammatory cascade in VAT macrophages [113]. For instance, it can negatively regulate IL-1β production [116]. Also, miR-223-FBXW7-TLR4 axis has a significant role in the macrophage inflammatory phenotype [113]. miR-146b is known as a new regulator for visceral pre-adipocyte proliferation and differentiation in humans [117], which is also involved in cytokine signaling via the NF-κB pathway. It is highly expressed in mature adipocytes while its expression is low in visceral preadipocytes [117]. Its expression grows by stimulation of pro-inflammatory cytokines [80]. Circulating levels of miR-27a are significantly associated with VA and body mass index [118]. This miRNA is up-regulated by leptin, while the down-regulation of miR-27a reduces macrophages activation by TLR2/4 induction and results in elevated IL-10 expression. miR-27a overexpression enhances the expression of pro-inflammatory cytokines including IL-6, IL-12, and TNF-α, and is also related to TNF-α-induced inflammatory damage. miR-181 family, miR-181a, acts as a new marker for inflammatory response, where TLR-4 signaling may play a role increased expression of miR-181a during this response. Further, miR-181a level is correlated with IL-1b, IL-6, and TNF-α expression [119]. miR-181a-5p modulates the expression of PTEN/S6K and prevents TNF-α induced insulin resistance in subjects with obesity [102]. miR-181a-3p is negatively related to adiponectin and expression of SIRT1 in VAT [119]. Also, miR-181a expression declines in monocytes of subjects with obesity [113], which is regulated in the inflammatory responses [119]. miR-378 facilitates adipogenesis in SC fat [120]. Adipokines and cytokines such as leptin, IL-6, and TNF-α promote its expression via SREBP and C/EBP. This miRNA may be a target for adipose tissue inflammation [89], and obesity-associated insulin resistance [90]. Additionally, the levels of miR-132 and miR-150 from the VAT samples are negatively correlated with the levels of IL-6 as a pro-inflammatory cytokines [121]. The role of this miRNA in inflammation is described in more detail in the following sections.
Inflammation related microRNAs in lipid metabolism
miRNAs as a new type of posttranscriptional regulators of gene expression are highly involved in several mechanisms including regulation of lipid metabolism, fatty acid oxidation, lipoprotein formation, and secretion [122, 123]. Therefore, they may be a potential target for treatment of obesity or obesity-related diseases. They also might be regarded as potential biomarkers in adult or childhood obesity, promote obesity, and increase food intake [59, 124, 125]. The roles of miRNAs in lipid and lipoprotein metabolism are reported in Table 2.
Table 2.
Summary of the function of miRNAs in lipid metabolism
| miRNAs | Target | Function | Ref |
|---|---|---|---|
| miR-335 | – | During adipose differentiation, its levels correlate with lipid accumulation and PPARγ or FAS levels | [126] |
| miR-9 | ACAT1 | Regulating formation of foam cells | [127] |
| miR-122 | HMGCR | Effectiveness on lipid metabolism | [128, 129] |
| miR-27b | PPARγ and C/EBPα | blocking adipocyte differentiation, regulating lipid metabolism | [130, 131] |
| miR-144 | ABCA1 |
Regulating cholesterol metabolism Inhibition of HDL formation |
[132, 133] |
| miR-33 and miR-33* | NPC1, ABCA1, IRS2, CPT1A and CROT, HADHB | Cholesterol export, lipid and fatty acid metabolism, fatty acid oxidation | [134, 135] |
| miR-378 | CAT, ------- | Over-expression of miR-378/378∗ encourages lipogenesis, regulates cholesterol homeostasis and adipocyte gene expression | [136–138] |
| miR-155 | FAAD | Role in lipid metabolism by targeting liver X receptor XLR, lipid uptake | [139, 140] |
| miR-125a | ORP9 | It is up-regulated in macrophages treated with oxLDL mediating lipid uptake and inhibits the secretion of inflammatory cytokines | [141] |
| miR-223 | HMGCS1 and SC4MOL | Regulating HDL-C uptake, lipoprotein metabolism, and cholesterol biosynthesis | [142] |
The miRNAs described in Table 2, are involved in inflammation and inflammatory response, as mentioned in the following sentences. miR-9 is known as an LPS-responsive miRNA in monocytes and polymorphonuclear neutrophils (PMNs). This miRNA regulates inflammatory responses and pathways [143]. In this regard, NF-κB pathway may induce miR-9 in macrophages and control inflammation by feedback loop [144]. miR-122 has an anti-inflammatory function [145] and inhibits production of inflammatory cytokines [146]. miR-27b regulates lipid metabolism, modifies dyslipidemia [131], and inhibits inflammatory response in the NF-κB signaling by targeting PPARγ [147]. It also downregulates PPARγ and C/EBPα and blocks adipocyte differentiation [130]. miR-144 suppresses the expression of cytokines in immune cells and promotes inhibition of TNF-α plus IL-1β as well as secretion of IL-6 via activating ERK signaling [148]. miR-33 regulates peripheral inflammatory Ly6Chighmonocytes [149] and NLRP3 inflammasome pathways [150]. The roles of other lipid metabolism miRNAs in inflammation were described in the previous sections.
Inflammatory microRNAs in adipogenesis
Inflammation changes miRNA profiles in adipocytes and macrophages. Here, we discuss important inflammatory miRNAs involved in adipogenesis.
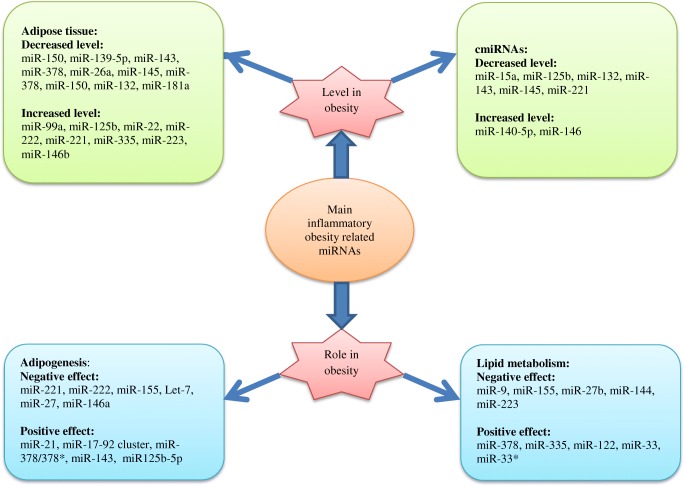
Some of the obesity-related miRNAs such as miR-221, miR-222, and miR-155 are significantly elevated in differentiated adipocytes during inflammation and obesity. miR-155 inhibits adipogenesis by targeting CREB and C/EBPβ [151]. Ectopic expression of miR-155, miR-221, and miR-222 leads to inhibition of adipogenesis, and play a significant role in adipocyte differentiation [152]. miR-155 upregulation may diminish the expression levels of lipogenic and adipogenic markers in adipocytes [153]. miR-21, miR-17-92 cluster, plus miR-378/378* promote adipogenesis in adipose tissue of subjects with obesity through over-expression of adipogenic markers and elevated triglycerides [154]. miR-17-92 cluster increases adipocyte differentiation by negatively modifying Rb2/p130 [155]. Meanwhile, miR-21is a key factor in inflammatory response [156]. It may induce T-cells to produce pro-inflammatory cytokines such as TNF and IFNγ [157], TGF-β signaling pathway prevents adipogenesis and miR-21 by inhibiting interaction of this pathway with adipogenesis [158]. Let-7 and miR-27 suppress adipogenic differentiation, induce down-regulation of adipogenic factors; for instance, miR-27 targets C/EBPα and PPARγ [159, 160]. Let-7 prevents adipogenesis, which is overexpressed during adipogenesis and suppresses 3 T3-L1 differentiation by suppressing HMGA2 [159]. Let-7, as a pro-inflammatory mediator, is involved in regulating inflammatory responses [73, 161–163], and can be probably considered as a regulatory marker in NF-κB pathway [163–165]. On the other hand, hypoxia dysregulates inflammatory adipocytokines and elevates miR-27 in adipose tissues of mice with obesity which is associated with deficient adipogenesis [160, 166]. miR-146a regulates immune functions [144]. TNF-α and IL-1β in NF-κB pathway induce miR-146a which are imperative factors in inflammation [167]. This miRNA decreases the inflammatory response in adipocytes by targeting IRAK1 and TRAF-6 response [88] and suppresses adipogenesis through targeting insulin receptor [168]. During human adipogenesis, miR125b-5p is upregulated which is involved in the regulation of adipocyte differentiation. Additionally, miR-125b-5p affects adipogenesis by regulating MMP11 which is an adipogenesis inhibitor [169]. miR-143 is involved in ERK5 signaling and is a positive regulator of human adipocyte differentiation. Finally, miR-375 is correlated to obesity by regulating 3 T3-L1 adipocyte differentiation through ERK–PPARγ2–aP2 pathway [170]. This miRNA attenuates inflammatory response [171], inhibits inflammatory cytokines, and regulates adipokines in non-alcoholic fatty liver disease [172]. The above described inflammatory obesity related miRNAs were listed in fig. 4, based on their role and level in obesity.
Fig. 4.
Main inflammatory-obesity related miRNAs
The main roles of the most important inflammatory obesity miRNAs in cancers
miR-132
The expression of miR-132 differs in various types of cancers given the role of its target gene. Via targeting SOX-4 [173] and MUC13 [174], miR-132 inhibit lung and gastric cancer, respectively. By binding to 3UTR of HN1 and ZEB2 transcript, miR-132 downregulates their expression thereby inhibiting cell invasion and metastasis in breast cancer and colorectal cancers [175, 176]. It has also a negative role in pancreatic cancer by targeting retinoblastoma protein (pRb) tumor suppressor [177].
miR-9
miR-9 acts as a putative tumor suppressor gene and potential biomarker in different types of cancers including recurrent ovarian cancer [178]. In breast and gastric cancer, aberrant hypermethylation of miR-9 gene occurs which epigenetically inactivates miR-9 [179, 180]. This miRNA targets MTHFD2 in breast cancer which is involved in its suppressor effect on cancer [181]. It is also downregulated in colon cancer which may promote proliferation and survival [182]. In contrast, the role of miR-9 on metastatic highly malignant cells has shown that its inhibition leads reserved metastasis [183].
miR-29
This miRNA has a dual effect on cancer which is downregulated or upregulated in many types of cancers. Its tumor suppressor effect is related to activating tumor suppressor genes. For instance, the members of miR-29 family upregulate P53 as an important tumor suppressor and also suppress p85α and CDC42 (two regulators that negatively regulate P53 [184]), inhibiting cancer cell proliferation and involving apoptosis. In contrast, it is also observed that this miRNA regulates apoptosis by increasing the level of Mcl-1. This anti-apoptotic protein is increased in cancer whose recurrence and upregulation in colon and breast cancers have a positive effect on the tumor metastasis [185].
miR-145
This miRNA acts as a tumor suppressor in different types of cancers like breast cancer [186], which may be due to various reasons. For example, this miRNA regulates P53 which negatively controls c-Myc [187]. It also targets RTKN and decreases its expression inhibiting cell growth in breast cancer [188]. In bladder cancer, miR-145 targets FSCN1 mRNA, an oncogene which suppresses tumor progression [189]. The expression of this miRNA is reduced in colorectal cancer [190]. miR-145, by targeting p70S6K1 gene, inhibits tumor progress in colorectal cancer [191]. In contrast, in metastatic colorectal cancer, this miRNA plays an oncogenic role depending on its effect on target genes [192].
miR-150
This miRNA promotes cancer progression by targeting different genes. For instance, in breast cancer, miR-150 targets P2X7 mRNA and downregulates the expression of P2X7 receptor thereby regulating cell growth and apoptosis [193]. In lung and gastric cancers, this miRNA targets P53 andEGR2 mRNA, respectively, and negatively regulates their expression [194, 195]. In contrast, it targets IGF-1R to induce apoptosis in prostate cancer [196]. Further, in colorectal cancer, the level of miR-150 drops with lower levels being associated with to shorter survival and weaker response to therapy [197].
miR-99a
miR-99a diminishes the inflammation by targeting mTOR/NF-κB signaling [111]. It is believed that miR-99a plays an inhibiting role in many cancers. Reduced number of breast cancer cells is the result of miR-99a over-expression through induced accumulation of cells at the sub-G1 phase and inhibiting tumorigenesis. It targets the mTOR/p-4E-BP1/p-S6K1 pathway [198, 199]. Further, it has a critical role in regulating the radio-sensitivity of non-small lung cancer by targeting mTOR signaling pathways [200].
miR-143
miR-143 inhibits inflammatory cytokines which are induced by IL-13 in nasal epithelial mucosal cells [201]. MiR-143 plays an inhibiting role in gastric cancer. Nevertheless, its precise mechanism is uncertain. It is believed that up-regulation of miR-143 is suppressed by proliferation of gastric cells via targeting DNMT3A. Wu et al. have shown that miR-143 inhibited gastric cells through COX-2 and induced apoptosis [202]. It has been observed that proliferation of cells, invasion, and cell cycle-related protein levels including Cyclin D1, CDK4, and CDK6 in gastric cancer are suppressed by miR-143 up-regulation. Also, transfection of miR-143 inhibitor to gastric cancer cells can boost cell proliferation [202]. He et al. concluded that miR-143 plays an inhibiting role by targeting QKI-5 in esophageal squamous cell carcinoma (ESCC). Therefore, it seems that it can be considered as a potential biomarker for the treatment of ESCC [203].
miR-221/222
miR-221/222 facilitates inflammation in white adipose tissue and reduces insulin sensitivity in obesity, through suppressing SIRT1 [204]. miR-221/222 is considered as an oncogene in various human cancers such as breast cancer. miR-221/222 enhanced growth of breast cancer, migration, and invasion, which acts through PTEN/Akt pathway. Furthermore, recent studies have revealed that miR221/222 over-expression leads to proliferation and cell cycle phase distribution in glioblastomas, thyroid papillary carcinomas, breast cancer, as well as in hepatocellular and lung cancer [205]. Furthermore, aggressive prostate cancer tissue outperformed non-aggressive forms in terms of miR-221/222 expression. Seemingly, there is a strong correlation between increased miR-221/222 level and poor overall survival in patients with prostate cancer.
miR-181
Researchers have not reached a consensus regarding the role of miR-181a in breast cancer [206]. Some studies have revealed that miR-181a expression is associated with anti-breast cancer effects via inhibiting tumor invasion and metastasis, decreasing the formation of mammosphere, inducing the death of cancer cells, and increasing sensitivity of drugs. On the other hand, the Oncomir activity of miR-181a intensifies metastasis (via Bax targeting), decreases apoptosis (via Bim targeting), increases tumorigenesis (via ATM targeting). Furthermore, miR-181a is associated with progression of ovarian cancer through epithelial-mesenchymal transition regulation [207] and of gastric cancer [208]. It has been shown that miR-181a plays an inhibiting role against oral squamous cell carcinoma cells through downregulation of K-ras [209]. miR-181 family may be involved in vascular inflammation by targeting NF-κB signaling and other important signaling pathways [210]. miR-181b plays a mediating role between inflammation and malignancy. STAT-3 is a transcription factor which activates miR181b. It has been shown that chromobox homolog 7 (CBX7) and p27 are suppressed by miR-181b induced cell cycle controls. Further, miR-181b regulates BCL2, TIMP3, p53, and other important targets and pathways thus influencing cell proliferation, adhesion, chemosensitivity, and apoptosis. There was a significant association between miR-181b levels and pancreatic, head and neck, and bladder cancer. Furthermore, its downregulation was observed in gastric, lung, and prostate cancer. It is believed that miR-181b acts uniquely based on the tumor type and cellular context [211].
Let-7
There are thirteen members in human let-7 family which are found on 9 different chromosomes. Let-7 is a pro-inflammatory mediator and is considered as a new regulatory molecule for the NF-κB pathway via ceRNA crosstalk [163]. Let-7 as a tumor suppressive biomarker can inhibit carcinogenesis and progression of the tumor. Further, its overexpression inhibits cancer cell proliferation [212]. Let-7 family expression changes in different types of cancers including gastric, breast, and ovarian cancer [213].
miR-335
A new role for miR-335 in adipose tissue inflammation has been observed. Apparently, miR-335 is probably involved in the pathogenesis of obesity by regulating its transcription [100]. It has been reported that miR-335 regulates target genes in several oncogenic signal-pathways like p53, MAPK, TGF-β, Wnt, ERbB, mTOR, Toll-like receptor, and focal adhesion [214]. However, in breast cancer, miR-335 inhibits migration of cancer cells via negative regulation of HGF/c-Met pathways [215]. Another study reported that miRNA downregulation in lung cancer facilitated the proliferation of cells via upregulation of Tra2B, through activating the AKT/mTOR signaling [216].
miR-26
miR-26 regulates inflammation and tumorigenicity via downregulation of IL-6production [217]. Studies suggest that miR-26 decreases TNF-α-activated expression of some genes in inflammatory NF-κB pathway [217]. Some studies have highlighted the suppressing role of miR-26b expression in cancer [218, 219]. It is reduced in cancers including T-ALL and colorectal sample. Therefore, miR-26b lowers cell proliferation and increases apoptosis. Further, miR-26b reduction leads to fibroblast migration and invasion [219].
miR-223
It has been shown that miR-223 acts as a mediator in morbid obesity adipocyte associated inflammation [220]. Expression of miR-223 is deregulated in several types of cancer. Although it is not expressed significantly in acute myeloid leukemia (AML) and several other types of leukemia, its expression is detected in breast, gastric, hepatocellular cancer via targeting MEF2C, EPB4IL3, and STMN1 [221]. Further, miR-223 affects ovarian cancer through KRAS, EGF, EGFR2, MMP9, and SEPTIN6 [178].
miR-125b
It has been shown that the expression of genes involved in inflammatory and apoptotic functions is regulated by miR-125b [58, 222–225]. miR-125b has a key role in inflammation by controlling mitochondria integrity via BIK and MTP18 silencing [226, 227]. In addition, the double role of miR-125b as a tumor suppressor [228] (for cutaneous and head/neck squamous cell carcinoma) and an oncomiR) [226] (in hematopoiesis) has been observed.
miR-21
miR-21 is significantly involved in inflammatory response [156]. miR-21 is overexpressed in most human cancers. Studies have confirmed its role as an oncogene [229]. Oncogenic miR-21 reduces FBXO11, as a novel miR-21 target gene (a tumor suppressor) thereby promoting tumorigenesis [229]. miR-21, as an oncogene, is involved in tumor growth, invasion, and metastasis [230].
miR-155
Studies by Xiaoyi and Ye J have confirmed that miR-155 has anti-inflammatory and inflammatory features [231, 232]. Over-expression of miR-155 inhibits cell proliferation, induces cell cycle arrest, and enhances apoptosis in colorectal cancer [233], which could be potentially considered as a tumor suppressor. Note that the oncogenic role of miR-155 in breast cancer has also been detected [234].
miR-146a
Tumor suppression effect of miR-146a has been reported by Bleau et al. [235]. miR-146a downregulation was observed in breast tissues which is probably associated with the development and deterioration of breast cancer [236]. Anti-inflammatory effect of miR-146b has been observed by downregulating IL-6 and IL-8 and influencing the HSP10 expression in the activated endothelium [237]. Furthermore, miR-146b plays an inhibiting role and suppresses NF-kB dependent production of IL-6 and STAT3 activation.
Further perspectives
According to the important role of inflammatory components in cancers and obesity, the role of major inflammatory miRNAs in obesity and obesity-related cancers, as well as the profiles of miRNAs related to these components, miRNAs could be considered as noninvasive biomarkers for pre-clinical diagnosis or prognosis of cancers which are associated with obesity and overweight. Consequently, further studies are required to be performed on the function and mechanisms of inflammation-related miRNAs in obesity-related cancers, as well as on miRNAs target genes.
Conclusion
This study aimed to investigate the relationship between miRNAs and obesity-related cancers based on inflammation. After comparing the findings for miRNAs involved in inflammations, it was observed that the main pro- and anti-inflammatory miRNAs such as miR-9, miR-21, miR-26, miR-29, miR-125b, miR-99a, miR-132, miR-143, miR-145, miR-146a/b, miR-150, miR-155, miR-181a, miR-221/222, miR-223, miR-335, and let-7 are involved in obesity or adipose tissues formation. It was also found that obesity linked miRNAs are also involved in inflammatory pathways through affecting adipokines.
It seems that miRNAs link obesity with cancer through inflammation and immune related mechanisms. Overweight and obesity-related cancers such as colorectal, breast, esophageal, pancreatic, liver, ovarian, myeloma, gastric, and thyroid cancers seem to have a greater association with inflammatory miRNAs. miR-150, miR-155, mir-181a, miR-125b, and miR-21 are mentioned in many studies, in which they proved to be highly involved in cell growth, proliferation, migration, apoptosis, invasion, therapy, and survival in these types of cancers. It can be concluded that the main inflammatory obesity related miRNAs are involved in common types of cancers which are remarkably caused by obesity and overweight status in individuals developing certain types of cancer.
Acknowledgements
This study was supported by Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (Grant No. 1395-02-105-2087). The authors would like to express special gratitude to Dr. Kamyar Khoshnevisan and Dr. Vahid Haghpanah for their valuable insights.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Circulating micro RNA
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weir HK, Anderson RN, King SMC, Soman A, Thompson TD, Hong Y et al. Peer Reviewed: Heart Disease and Cancer Deaths—Trends and Projections in the United States, 1969–2020. Preventing chronic disease. 2016;13. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization. Cancer prevention. http://www.who.int/cancer/prevention/en/.
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Dean E, Gormsen Hansen R. Prescribing optimal nutrition and physical activity as “first-line” interventions for best practice management of chronic low-grade inflammation associated with osteoarthritis: evidence synthesis. Arthritis. 2012;2012. [DOI] [PMC free article] [PubMed]
- 5.Lee Y-H, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Current diabetes reports. 2005;5(1):70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Obesity and overweight. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 8.Asterholm IW, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Irish Journal of Medical Science (1971-) 2017;186(1):57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vendramini-Costa BD, Carvalho EJ. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 11.Ye J. Mechanisms of insulin resistance in obesity. Frontiers of Medicine. 2013;7(1):14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5(10):1122. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93. doi: 10.1016/j.addr.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Amin MN, Hussain MS, Sarwar MS, Moghal MMR, Das A, Hossain MZ et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019. [DOI] [PubMed]
- 16.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annual Review of Pathology: Mechanisms of Disease. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 17.Zhong H, Ma M, Liang T, Guo L. Role of microRNAs in obesity-induced metabolic disorder and immune response. Journal of immunology research. 2018;2018. [DOI] [PMC free article] [PubMed]
- 18.Iacomino G, Siani A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr. 2017;12(1):23. doi: 10.1186/s12263-017-0577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo F, Catellani C, Sartori C, Lazzeroni P, Amarri S, Street ME. Obesity, insulin resistance, and colorectal Cancer: could miRNA dysregulation play a role? Int J Mol Sci. 2019;20(12):2922. doi: 10.3390/ijms20122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sargent J. Rethinking inflammation and adipocyte homeostasis. Nat Rev Endocrinol. 2014;10(8):446–447. doi: 10.1038/nrendo.2014.103. [DOI] [PubMed] [Google Scholar]
- 21.Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin R-Z, Klagsbrun M, et al. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15(3):481–495. doi: 10.1007/s10456-012-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7(15):2346. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada F. Inflammation-related carcinogenesis: current findings in epidemiological trends, causes and mechanisms. Yonago Acta Medica. 2014;57(2):65. [PMC free article] [PubMed] [Google Scholar]
- 24.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute NC. Obesity and Cancer. 2017.
- 26.Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue-an update. Horm Metab Res. 2007;39(05):314–321. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 27.Ungefroren H, Gieseler F, Fliedner S, Lehnert H. Obesity and cancer. Horm Mol Biol Clin Investig. 2015;21(1):5–15. doi: 10.1515/hmbci-2014-0046. [DOI] [PubMed] [Google Scholar]
- 28.Reynisdottir S, Langin D, Carlström K, Holm C, Rössner S, Arner P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin Sci. 1995;89(4):421–429. doi: 10.1042/cs0890421. [DOI] [PubMed] [Google Scholar]
- 29.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research. 2013:clincanres. 2603.013. [DOI] [PMC free article] [PubMed]
- 30.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Thun MJ, Henley SJ, Gansler T, editors. Inflammation and cancer: an epidemiological perspective. Novartis Foundation symposium; 2004: Chichester; New York; John Wiley; 1999. [PubMed]
- 32.Zhang Q, Xie W, Wang F, Li RH, Cui L, Wang H, et al. Epidemiological investigation and risk factors for cervical lesions: cervical Cancer screening among women in rural areas of Henan Province China. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. 2016;22:1858–1865. doi: 10.12659/MSM.894663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karan D, Dubey S. From inflammation to prostate Cancer: the role of Inflammasomes. Advances in Urology. 2016;2016:5. doi: 10.1155/2016/3140372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171(2 Pt 2):S36–S40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 38.Jung U, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäffler A, Schölmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Rev Endocrinol. 2007;3(4):345. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 40.Harvie M, Howell A. Energy balance adiposity and breast cancer–energy restriction strategies for breast cancer prevention. Obes Rev. 2006;7(1):33–47. doi: 10.1111/j.1467-789X.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 41.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17(3):411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94(10):2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Ekström M, Söderberg S, Tornvall P. Acute systemic inflammation is unlikely to affect adiponectin and leptin synthesis in humans. Frontiers in Cardiovascular Medicine. 2015;2:7. doi: 10.3389/fcvm.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snoussi K, Strosberg AD, Bouaouina N, Ahmed SB, Helal AN, Chouchane L. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6(1):38. doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uddin Shahab, P.Bavi Prashant, Hussain Azhar R., Alsbeih Ghazi, Al-Sanea Nasser, AbdulJabbar Alaa, Ashari Luai H., Alhomoud Samar, Al-Dayel Fouad, Ahmed Maqbool, Al-Kuraya Khawla S. Leptin receptor expression in Middle Eastern colorectal cancer and its potential clinical implication. Carcinogenesis. 2009;30(11):1832–1840. doi: 10.1093/carcin/bgp145. [DOI] [PubMed] [Google Scholar]
- 46.Dieudonne M-N, Machinal-Quelin F, Serazin-Leroy V, Leneveu M-C, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293(1):622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 47.Mantovani G, Macciò A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Quantitative evaluation of oxidative stress, chronic inflammatory indices and leptin in cancer patients: correlation with stage and performance status. Int J Cancer. 2002;98(1):84–91. doi: 10.1002/ijc.10143. [DOI] [PubMed] [Google Scholar]
- 48.Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Frontiers in oncology. 2019;9. [DOI] [PMC free article] [PubMed]
- 49.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 50.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 51.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 52.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13(9):790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 54.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279(50):52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 55.Kasiappan R, Rajarajan D. Role of microRNA regulation in obesity-associated breast cancer: nutritional perspectives. Adv Nutr. 2017;8(6):868–888. doi: 10.3945/an.117.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121(5):1156–1161. doi: 10.1002/ijc.22800. [DOI] [PubMed] [Google Scholar]
- 57.Cirillo F, Lazzeroni P, Catellani C, Sartori C, Amarri S, Street ME. MicroRNAs link chronic inflammation in childhood to growth impairment and insulin-resistance. Cytokine Growth Factor Rev. 2018;39:1–18. doi: 10.1016/j.cytogfr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Yan Y, Xv W, Qian G, Li C, Zou H et al. A New Insight into the Roles of MiRNAs in Metabolic Syndrome. BioMed Research International. 2018;2018. [DOI] [PMC free article] [PubMed]
- 59.Heneghan H, Miller N, McAnena O, O'brien T, Kerin M. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. The Journal of Clinical Endocrinology & Metabolism. 2011;96(5):E846–EE50. doi: 10.1210/jc.2010-2701. [DOI] [PubMed] [Google Scholar]
- 60.Klöting N, Berthold S, Kovacs P, Schön MR, Fasshauer M, Ruschke K, et al. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One. 2009;4(3):e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, et al. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23(11):1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao N, et al. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp Lung Res. 2015;41(5):261–269. doi: 10.3109/01902148.2015.1004206. [DOI] [PubMed] [Google Scholar]
- 63.Ortega FJ, Mercader JM, Catalán V, Moreno-Navarrete JM, Pueyo N, Sabater M et al. Targeting the circulating microRNA signature of obesity. Clinical chemistry. 2013:clinchem. 2012.195776. [DOI] [PubMed]
- 64.Wang Y, Liang J, Qin H, Ge Y, Du J, Lin J, et al. Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE. Arthritis Research & Therapy. 2016;18(1):263. doi: 10.1186/s13075-016-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Guan S-B, Lu Y, Wang F. MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed Pharmacother. 2017;96:208–214. doi: 10.1016/j.biopha.2017.09.079. [DOI] [PubMed] [Google Scholar]
- 66.Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59(11):2904–2915. doi: 10.2337/db10-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duroux-Richard I, Roubert C, Ammari M, Présumey J, Grün JR, Häupl T et al. miR-125b controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Blood. 2016:blood-2016-02-697003. [DOI] [PMC free article] [PubMed]
- 68.Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, et al. Dual anti-inflammatory and anti-angiogenic action of miR-15a in diabetic retinopathy. EBioMedicine. 2016;11:138–150. doi: 10.1016/j.ebiom.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Wang X, Liu X, Wang X, Xu J, Hou S, et al. miR-15a/16 are upreuglated in the serum of neonatal sepsis patients and inhibit the LPS-induced inflammatory pathway. Int J Clin Exp Med. 2015;8(4):5683. [PMC free article] [PubMed] [Google Scholar]
- 70.Peng J, Zhou Y, Deng Z, Zhang H, Wu Y, Song T, et al. miR-221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin-1 (SIRT1) J Cell Biochem. 2018;119(8):6418–6428. doi: 10.1002/jcb.26589. [DOI] [PubMed] [Google Scholar]
- 71.Chou W-W, Wang Y-T, Liao Y-C, Chuang S-C, Wang S-N, Juo S-HH. Decreased microRNA-221 is associated with high levels of TNF-α in human adipose tissue-derived mesenchymal stem cells from obese woman. Cell Physiol Biochem. 2013;32(1):127–137. doi: 10.1159/000350131. [DOI] [PubMed] [Google Scholar]
- 72.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25(8):2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 73.Teng G-g, Wang W-h, Dai Y, Wang S-j, Chu Y-x, Li J. Let-7b is involved in the inflammation and immune responses associated with helicobacter pylori infection by targeting toll-like receptor 4. PLoS One. 2013;8(2):e56709. doi: 10.1371/journal.pone.0056709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13(1):308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brennan E, Wang B, McClelland A, Mohan M, Marai M, Beuscart O et al. Protective effect of Let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes. 2017:db161405. [DOI] [PubMed]
- 76.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279(50):52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 78.Takanabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H, et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun. 2008;376(4):728–732. doi: 10.1016/j.bbrc.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 79.Zhu L, Shi C, Ji C, Xu G, Chen L, Yang L, et al. FFAs and adipokine-mediated regulation of hsa-miR-143 expression in human adipocytes. Mol Biol Rep. 2013;40(10):5669–5675. doi: 10.1007/s11033-013-2668-2. [DOI] [PubMed] [Google Scholar]
- 80.Brettfeld Caroline, Maver Ales, Aumuller Eva, Peterlin Borut, Haslberger Alexander G. MicroRNAs Responsible for Inflammation in Obesity. Journal of Endocrinology and Metabolism. 2017;7(3):77–85. [Google Scholar]
- 81.Brettfeld C, Maver A, Aumuller E, Peterlin B, Haslberger AG. MicroRNAs responsible for inflammation in obesity. Journal of Endocrinology and Metabolism. 2017;7(3):77–85. [Google Scholar]
- 82.Park H, Huang X, Lu C, Cairo MS, Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015;290(5):2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L, et al. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interf Cytokine Res. 2014;34(5):342–348. doi: 10.1089/jir.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, et al. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS One. 2012;7(4):e34872. doi: 10.1371/journal.pone.0034872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1(4):402. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu D, Xi Q-Y, Cheng X, Dong T, Zhu X-T, Shu G et al. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. Journal of lipid research. 2016:jlr. M062497. [DOI] [PMC free article] [PubMed]
- 88.Roos J, Enlund E, Funcke J-B, Tews D, Holzmann K, Debatin K-M, et al. miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep. 2016;6:38339. doi: 10.1038/srep38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang X, Xue M, Fu Z, Ji C, Guo X, Zhu L, et al. Insight into the effects of adipose tissue inflammation factors on miR-378 expression and the underlying mechanism. Cell Physiol Biochem. 2014;33(6):1778–1788. doi: 10.1159/000362957. [DOI] [PubMed] [Google Scholar]
- 90.Xu L-l, Shi C-m, Xu G-f, Chen L, Zhu L-l, Zhu L, et al. TNF-α, IL-6, and leptin increase the expression of miR-378, an adipogenesis-related microRNA in human adipocytes. Cell Biochem Biophys. 2014;70(2):771–776. doi: 10.1007/s12013-014-9980-x. [DOI] [PubMed] [Google Scholar]
- 91.Ishida M, Shimabukuro M, Yagi S, Nishimoto S, Kozuka C, Fukuda D, et al. MicroRNA-378 regulates adiponectin expression in adipose tissue: a new plausible mechanism. PLoS One. 2014;9(11):e111537. doi: 10.1371/journal.pone.0111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li R, Shen Q, Wu N, He M, Liu N, Huang J, et al. MiR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine. 2018;60(1):73–82. doi: 10.1007/s12020-018-1521-8. [DOI] [PubMed] [Google Scholar]
- 93.Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11(5):276. doi: 10.1038/nrendo.2015.25. [DOI] [PubMed] [Google Scholar]
- 94.Fehlmann T, Ludwig N, Backes C, Meese E, Keller A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016;13(11):1084–1088. doi: 10.1080/15476286.2016.1234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinelli R, Nardelli C, Pilone V, Buonomo T, Liguori R, Castanò I, et al. miR-519d overexpression is associated with human obesity. Obesity. 2010;18(11):2170–2176. doi: 10.1038/oby.2009.474. [DOI] [PubMed] [Google Scholar]
- 96.Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010;5(2):e9022. doi: 10.1371/journal.pone.0009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012:DB_111508. [DOI] [PMC free article] [PubMed]
- 99.Lorente-Cebrián S, Mejhert N, Kulyté A, Laurencikiene J, Åström G, Hedén P, et al. MicroRNAs regulate human adipocyte lipolysis: effects of miR-145 are linked to TNF-α. PLoS One. 2014;9(1):e86800. doi: 10.1371/journal.pone.0086800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu L, Chen L, Shi C-M, Xu G-F, Xu L-L, Zhu L-L, et al. MiR-335, an adipogenesis-related microRNA, is involved in adipose tissue inflammation. Cell Biochem Biophys. 2014;68(2):283–290. doi: 10.1007/s12013-013-9708-3. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parra P, Serra F, Palou A. Expression of adipose microRNAs is sensitive to dietary conjugated linoleic acid treatment in mice. PLoS One. 2010;5(9):e13005. doi: 10.1371/journal.pone.0013005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meerson A, Traurig M, Ossowski V, Fleming J, Mullins M, Baier L. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia. 2013;56(9):1971–1979. doi: 10.1007/s00125-013-2950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sang W, Wang Y, Zhang C, Zhang D, Sun C, Niu M, et al. MiR-150 impairs inflammatory cytokine production by targeting ARRB-2 after blocking CD28/B7 costimulatory pathway. Immunol Lett. 2016;172:1–10. doi: 10.1016/j.imlet.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zou F, Mao R, Yang L, Lin S, Lei K, Zheng Y, et al. Targeted deletion of miR-139-5p activates MAPK, NF-kappaB and STAT3 signaling and promotes intestinal inflammation and colorectal cancer. FEBS J. 2016;283(8):1438–1452. doi: 10.1111/febs.13678. [DOI] [PubMed] [Google Scholar]
- 106.Kumar A, Bhatia HS, de Oliveira AC, Fiebich BL. microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia. J Neurochem. 2015;135(6):1189–1202. doi: 10.1111/jnc.13364. [DOI] [PubMed] [Google Scholar]
- 107.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286(2):1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tili E, Michaille J-J, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 109.O'connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33(4):607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, et al. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep. 2015;5:18648. doi: 10.1038/srep18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bao M-h, Li J-M, Luo H-q, Tang L, Lv, Q-l, Li G-y et al. NF-κB-regulated miR-99a modulates endothelial cell inflammation. Mediators of inflammation. 2016;2016. [DOI] [PMC free article] [PubMed]
- 112.Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288(49):35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deiuliis JA, Syed R, Duggineni D, Rutsky J, Rengasamy P, Zhang J, et al. Visceral adipose microRNA 223 is upregulated in human and murine obesity and modulates the inflammatory phenotype of macrophages. PLoS One. 2016;11(11):e0165962. doi: 10.1371/journal.pone.0165962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 115.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 116.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey A-A, Pich D, McInnes IB, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. 2012;189(8):3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 117.Chen L, Dai Y-M, Ji C-B, Yang L, Shi C-M, Xu G-F, et al. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol Cell Endocrinol. 2014;393(1–2):65–74. doi: 10.1016/j.mce.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 118.Munetsuna E, Yamada H, Ando Y, Yamazaki M, Tsuboi Y, Kondo M et al. Association of subcutaneous and visceral fat with circulating microRNAs in a middle-aged Japanese population. Ann Clin Biochem 2017:0004563217735124. [DOI] [PubMed]
- 119.Xie W, Li Z, Li M, Xu N, Zhang Y. miR-181a and inflammation: miRNA homeostasis response to inflammatory stimuli in vivo. Biochem Biophys Res Commun. 2013;430(2):647–652. doi: 10.1016/j.bbrc.2012.11.097. [DOI] [PubMed] [Google Scholar]
- 120.Yu J, Kong X, Liu J, Lv Y, Sheng Y, Lv S, et al. Expression profiling of PPARγ-regulated microRNAs in human subcutaneous and visceral adipogenesis in both genders. Endocrinology. 2014;155(6):2155–2165. doi: 10.1210/en.2013-2105. [DOI] [PubMed] [Google Scholar]
- 121.Estep M, Armistead D, Hossain N, Elarainy H, Goodman Z, Baranova A, et al. Differential expression of miRNAs in the visceral adipose tissue of patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;32(3):487–497. doi: 10.1111/j.1365-2036.2010.04366.x. [DOI] [PubMed] [Google Scholar]
- 122.Gerin I, Clerbaux L-A, Haumont O, Lanthier N, Das AK, Burant CF, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285(44):33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramírez CM, et al. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33(11):2339–2352. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McGregor A. R, S Choi M. microRNAs in the regulation of adipogenesis and obesity. Curr Mol Med. 2011;11(4):304–316. doi: 10.2174/156652411795677990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakanishi N, Nakagawa Y, Tokushige N, Aoki N, Matsuzaka T, Ishii K, et al. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem Biophys Res Commun. 2009;385(4):492–496. doi: 10.1016/j.bbrc.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 127.Xu J, Hu G, Lu M, Xiong Y, Li Q, Chang CC, et al. MiR-9 reduces human acyl-coenzyme a: cholesterol acyltransferase-1 to decrease THP-1 macrophage-derived foam cell formation. Acta Biochim Biophys Sin. 2013;45(11):953–962. doi: 10.1093/abbs/gmt096. [DOI] [PubMed] [Google Scholar]
- 128.Wen J, Friedman JR. miR-122 regulates hepatic lipid metabolism and tumor suppression. J Clin Invest. 2012;122(8):2773–2776. doi: 10.1172/JCI63966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 130.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem Biophys Res Commun. 2009;390(2):247–251. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 131.Vickers KC, Shoucri BM, Levin MG, Wu H, Pearson DS, Osei-Hwedieh D, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57(2):533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Aguiar Vallim TQ, Tarling EJ, Kim T, Civelek M, Baldán Á, Esau C, et al. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor Farnesoid X ReceptorNovelty and significance. Circ Res. 2013;112(12):1602–12. doi: 10.1161/CIRCRESAHA.112.300648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramírez CM, Rotllan N, Vlassov AV, Dávalos A, Li M, Goedeke L, et al. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. 2013;112(12):1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramírez CM et al. A regulatory role for miRNA-33* in controlling lipid metabolism gene expression. Molecular and cellular biology. 2013:MCB. 01714–12. [DOI] [PMC free article] [PubMed]
- 135.Gerin I, Clerbaux L-A, Haumont O, Lanthier N, Das AK, Burant CF et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. Journal of Biological Chemistry. 2010:jbc. M110. 152090. [DOI] [PMC free article] [PubMed]
- 136.Fernández-Hernando C, Suárez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011;22(2):86. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. American Journal of Physiology-Endocrinology and Metabolism. 2010;299(2):E198–E206. doi: 10.1152/ajpendo.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, et al. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378. Proc Natl Acad Sci. 2012;201207605. [DOI] [PMC free article] [PubMed]
- 139.Chen T, Yan H, Li Z, Jing T, Zhu W, Ge J, et al. MicroRNA-155 regulates lipid uptake, adhesion/chemokine marker secretion and SCG2 expression in oxLDL-stimulated dendritic cells/macrophages. Int J Cardiol. 2011;147(3):446–447. doi: 10.1016/j.ijcard.2010.10.133. [DOI] [PubMed] [Google Scholar]
- 140.Novák Jan, Bienertová-Vašků Julie, Kára Tomáš, Novák Miroslav. MicroRNAs Involved in the Lipid Metabolism and Their Possible Implications for Atherosclerosis Development and Treatment. Mediators of Inflammation. 2014;2014:1–14. doi: 10.1155/2014/275867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, et al. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 2009;83(1):131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 142.Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci. 2014;111(40):14518–14523. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jones S, Watkins G, Le Good N, Roberts S, Murphy C, Brockbank S, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthr Cartil. 2009;17(4):464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 144.Sonkoly E, Pivarcsi A. microRNAs in inflammation. Int Rev Immunol. 2009;28(6):535–561. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- 145.S-h H, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122(8):2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, Wang C, Liu Y, Tang L, Zheng M, Xu C, et al. miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn’s disease. Biochem Biophys Res Commun. 2013;438(1):133–139. doi: 10.1016/j.bbrc.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 147.Lee J-J, Drakaki A, Iliopoulos D, Struhl K. MiR-27b targets PPARγ to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31(33):3818. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu H. Down-regulation of miR-144 after mycobacterium tuberculosis infection promotes inflammatory factor secretion from macrophages through the Tpl2/ERK pathway. Cell Mol Biol. 2016;62(2):87–93. [PubMed] [Google Scholar]
- 149.Baba O, Horie T, Nakao T, Hakuno D, Nakashima Y, Nishi H et al. MicroRNA-33 regulates the population of peripheral inflammatory Ly6Chigh monocytes through dual pathways. Molecular and cellular biology. 2018:MCB. 00604–17. [DOI] [PMC free article] [PubMed]
- 150.Xie Q, Wei M, Zhang B, Kang X, Liu D, Zheng W, et al. MicroRNA-33 regulates the NLRP3 inflammasome signaling pathway in macrophages. Mol Med Rep. 2018;17(2):3318–3327. doi: 10.3892/mmr.2017.8224. [DOI] [PubMed] [Google Scholar]
- 151.Liu S, Yang Y, Wu J. TNFα-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem Biophys Res Commun. 2011;414(3):618–624. doi: 10.1016/j.bbrc.2011.09.131. [DOI] [PubMed] [Google Scholar]
- 152.Skårn M, Namløs HM, Noordhuis P, Wang M-Y, Meza-Zepeda LA, Myklebost O. Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev. 2011;21(6):873–883. doi: 10.1089/scd.2010.0503. [DOI] [PubMed] [Google Scholar]
- 153.Karkeni E, Astier J, Tourniaire F, El Abed M, Romier B, Gouranton E, et al. Obesity-associated inflammation induces microRNA-155 expression in adipocytes and adipose tissue: outcome on adipocyte function. J Clin Endocrinol Metab. 2016;101(4):1615–1626. doi: 10.1210/jc.2015-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(8):2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 155.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, et al. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci. 2008;105(8):2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ando Y, Yang G-X, Kenny TP, Kawata K, Zhang W, Huang W, et al. Overexpression of microRNA-21 is associated with elevated pro-inflammatory cytokines in dominant-negative TGF-β receptor type II mouse. J Autoimmun. 2013;41:111–119. doi: 10.1016/j.jaut.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem cells (Dayton, Ohio) 2009;27(12):3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 159.Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol. 2009;23(6):925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276(8):2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C et al. Pro-inflammatory role for let-7 microRNAs in experimental asthma. Journal of Biological Chemistry. 2010:jbc. M110. 145698. [DOI] [PMC free article] [PubMed]
- 162.Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, et al. Let-7 microRNA–mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol. 2011;128(5):1077–1085. doi: 10.1016/j.jaci.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 163.Lin Z, Ge J, Wang Z, Ren J, Wang X, Xiong H, et al. Let-7e modulates the inflammatory response in vascular endothelial cells through ceRNA crosstalk. Sci Rep. 2017;7:42498. doi: 10.1038/srep42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, et al. MicroRNA let-7 modulates the immune response to mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-κB pathway. Cell Host Microbe. 2015;17(3):345–356. doi: 10.1016/j.chom.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 166.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 167.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu D, Xi Q-Y, Cheng X, Dong T, Zhu X-T, Shu G, et al. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J Lipid Res. 2016;57(8):1360–1372. doi: 10.1194/jlr.M062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rockstroh D, Löffler D, Kiess W, Landgraf K, Körner A. Regulation of human adipogenesis by miR125b-5p. Adipocyte. 2016;5(3):283–297. doi: 10.1080/21623945.2016.1195044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, et al. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol Physiol. 2011;38(4):239–246. doi: 10.1111/j.1440-1681.2011.05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garikipati VN, Verma SK, Jolardarashi D, Cheng Z, Ibetti J, Cimini M, et al. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovasc Res. 2017;113(8):938–949. doi: 10.1093/cvr/cvx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lei L, Zhou C, Yang X, Li L. Down-regulation of micro RNA-375 regulates adipokines and inhibits inflammatory cytokines by targeting AdipoR2 in non-alcoholic fatty liver disease. Clinical and Experimental Pharmacology and Physiology. 2018. [DOI] [PubMed]
- 173.Li Y, Zu L, Wang Y, Wang M, Chen P, Zhou Q. miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. Journal of Thoracic Disease. 2015;7(9):1563. doi: 10.3978/j.issn.2072-1439.2015.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.He L, Qu L, Wei L, Chen Y, Suo J. Reduction of miR-132-3p contributes to gastric cancer proliferation by targeting MUC13. Mol Med Rep. 2017;15(5):3055–3061. doi: 10.3892/mmr.2017.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zheng Y-B, Luo H-P, Shi Q, Hao Z-N, Ding Y, Wang Q-S, et al. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J Gastroenterol: WJG. 2014;20(21):6515. doi: 10.3748/wjg.v20.i21.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Z-G, Chen W-X, Wu Y-H, Liang H-F, Zhang B-X. MiR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochem Biophys Res Commun. 2014;454(1):109–114. doi: 10.1016/j.bbrc.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 177.Park J-K, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, et al. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406(4):518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Laios A, O'Toole S, Flavin R, Martin C, Kelly L, Ring M, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7(1):35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, et al. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2008;214(1):17–24. doi: 10.1002/path.2251. [DOI] [PubMed] [Google Scholar]
- 180.Tsai K-W, Liao Y-L, Wu C-W, Hu L-Y, Li S-C, Chan W-C, et al. Aberrant hypermethylation of miR-9 genes in gastric cancer. Epigenetics. 2011;6(10):1189–1197. doi: 10.4161/epi.6.10.16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Selcuklu SD, Donoghue MT, Rehmet K, de Souza Gomes M, Fort A, Kovvuru P et al. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. Journal of Biological Chemistry. 2012:jbc. M111. 335943. [DOI] [PMC free article] [PubMed]
- 182.Cekaite L, Rantala JK, Bruun J, Guriby M, Ågesen TH, Danielsen SA, et al. MiR-9,-31, and-182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14(9):IN20–IIN1. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Park S-Y, Lee JH, Ha M, Nam J-W, Kim VN. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol. 2009;16(1):23. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 185.Jiang H, Zhang G, Wu J-H, Jiang C-P. Diverse roles of miR-29 in cancer. Oncol Rep. 2014;31(4):1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 186.Spizzo R, Nicoloso M, Lupini L, Lu Y, Fogarty J, Rossi S, et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ. 2010;17(2):246. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci. 2009;106(9):3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, et al. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34(5):1461–1466. [PubMed] [Google Scholar]
- 189.Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102(5):883. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72(5–6):397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 191.Xu Q, Liu L-Z, Qian X, Chen Q, Jiang Y, Li D, et al. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2011;40(2):761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Arndt GM, Dossey L, Cullen LM, Lai A, Druker R, Eisbacher M, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9(1):374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huang S, Chen Y, Wu W, Ouyang N, Chen J, Li H, et al. miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PLoS One. 2013;8(12):e80707. doi: 10.1371/journal.pone.0080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang N, Wei X, Xu L. miR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett. 2013;587(15):2346–2351. doi: 10.1016/j.febslet.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 195.Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K, et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 2010;392(3):340–345. doi: 10.1016/j.bbrc.2009.12.182. [DOI] [PubMed] [Google Scholar]
- 196.Farhana L, Dawson MI, Murshed F, Das JK, Rishi AK, Fontana JA. Upregulation of miR-150* and miR-630 induces apoptosis in pancreatic cancer cells by targeting IGF-1R. PLoS One. 2013;8(5):e61015. doi: 10.1371/journal.pone.0061015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma Y, Zhang P, Wang F, Zhang H, Yang J, Peng J, et al. miR-150 as a potential biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Gut. 2012;61(10):1447–1453. doi: 10.1136/gutjnl-2011-301122. [DOI] [PubMed] [Google Scholar]
- 198.Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One. 2014;9(3):e92099. doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang H-G, Luo X, Wu S, Jian B. MiR-99a inhibits cell proliferation and tumorigenesis through targeting mTOR in human anaplastic thyroid cancer. Asian Pac J Cancer Prev. 2015;16(12):4937–4944. doi: 10.7314/apjcp.2015.16.12.4937. [DOI] [PubMed] [Google Scholar]
- 200.Yin H, Ma J, Chen L, Piao S, Zhang Y, Zhang S, et al. MiR-99a enhances the radiation sensitivity of non-small cell lung Cancer by targeting mTOR. Cell Physiol Biochem. 2018;46(2):471–481. doi: 10.1159/000488615. [DOI] [PubMed] [Google Scholar]
- 201.Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, et al. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015;457(1):58–64. doi: 10.1016/j.bbrc.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 202.Zhang Q, Feng Y, Liu P, Yang J. MiR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer. Tumor Biol. 2017;39(7):1010428317711312. doi: 10.1177/1010428317711312. [DOI] [PubMed] [Google Scholar]
- 203.He Z, Yi J, Liu X, Chen J, Han S, Jin L, et al. MiR-143-3p functions as a tumor suppressor by regulating cell proliferation, invasion and epithelial–mesenchymal transition by targeting QKI-5 in esophageal squamous cell carcinoma. Mol Cancer. 2016;15(1):51. doi: 10.1186/s12943-016-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Peng J, Zhou Y, Deng Z, Zhang H, Wu Y, Song T et al. miR-221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin-1 (SIRT1). Journal of cellular biochemistry. 2017. [DOI] [PubMed]
- 205.Garofalo M, Quintavalle C, Romano G, Croce MC, Condorelli G. miR221/222 in cancer: their role in tumor progression and response to therapy. Curr Mol Med. 2012;12(1):27–33. doi: 10.2174/156652412798376170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang C, Tabatabaei SN, Ruan X, Hardy P. The dual regulatory role of MiR-181a in breast Cancer. Cell Physiol Biochem. 2017;44(3):843–856. doi: 10.1159/000485351. [DOI] [PubMed] [Google Scholar]
- 207.Parikh A, Lee C, Joseph P, Marchini S, Baccarini A, Kolev V, et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial–mesenchymal transition. Nat Commun. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin Y, Zhao J, Wang H, Cao J, Nie Y. miR-181a modulates proliferation, migration and autophagy in AGS gastric cancer cells and downregulates MTMR3. Mol Med Rep. 2017;15(5):2451–2456. doi: 10.3892/mmr.2017.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shin K-H, Bae SD, Hong HS, Kim RH, Kang MK, Park N-H. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;404(4):896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 210.Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends in Cardiovascular Medicine. 2014;24(3):105–112. doi: 10.1016/j.tcm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu J, Shi W, Wu C, Ju J, Jiang J. miR-181b as a key regulator of the oncogenic process and its clinical implications in cancer. Biomedical Reports. 2014;2(1):7–11. doi: 10.3892/br.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sun X, Liu J, Xu C, Tang SC, Ren H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J Cell Mol Med. 2016;20(9):1779–1788. doi: 10.1111/jcmm.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boyerinas B, Park S-M, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17(1):F19–F36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- 214.Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, et al. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7(7):e40037. doi: 10.1371/journal.pone.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gao Y, Zeng F, Wu J-Y, Li H-Y, Fan J-J, Mai L, et al. MiR-335 inhibits migration of breast cancer cells through targeting oncoprotein c-met. Tumor Biol. 2015;36(4):2875–2883. doi: 10.1007/s13277-014-2917-6. [DOI] [PubMed] [Google Scholar]
- 216.Liu J, Bian T, Feng J, Qian L, Zhang J, Jiang D, et al. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci. 2018;109(2):289–296. doi: 10.1111/cas.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen C-YA, Chang JT, Ho Y-F, Shyu A-B. MiR-26 down-regulates TNF-α/NF-κB signalling and IL-6 expression by silencing HMGA1 and MALT1. Nucleic Acids Res. 2016;44(8):3772–3787. doi: 10.1093/nar/gkw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu X-X, Li X-J, Zhang B, Liang Y-J, Zhou C-X, Cao D-X, et al. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011;585(9):1363–1367. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 219.Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol. 2013;231(3):388–399. doi: 10.1002/path.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H et al. A novel regulator of macrophage activation: miR-223 in obesity associated adipose tissue inflammation. Circulation. 2012:CIRCULATIONAHA. 111.087817. [DOI] [PubMed]
- 221.Taïbi F, Metzinger-Le Meuth V, Massy ZA, Metzinger L. miR-223: an inflammatory oncomiR enters the cardiovascular field. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2014;1842(7):1001–1009. doi: 10.1016/j.bbadis.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 222.Li X-Q, Yu Q, Tan W-F, Zhang Z-L, Ma H. MicroRNA-125b mimic inhibits ischemia reperfusion-induced neuroinflammation and aberrant p53 apoptotic signalling activation through targeting TP53INP1. Brain Behav Immun. 2018;74:154–165. doi: 10.1016/j.bbi.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 223.Le MT, Shyh-Chang N, Khaw SL, Chin L, Teh C, Tay J, et al. Conserved regulation of p53 network dosage by microRNA–125b occurs through evolving miRNA–target gene pairs. PLoS Genet. 2011;7(9):e1002242. doi: 10.1371/journal.pgen.1002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Diao W, Lu L, Li S, Chen J, Zen K, Li L. MicroRNA-125b-5p modulates the inflammatory state of macrophages via targeting B7-H4. Biochem Biophys Res Commun. 2017;491(4):912–918. doi: 10.1016/j.bbrc.2017.07.135. [DOI] [PubMed] [Google Scholar]
- 225.Zhang B, Wang L-S, Zhou Y-H. Elevated microRNA-125b promotes inflammation in rheumatoid arthritis by activation of NF-κB pathway. Biomed Pharmacother. 2017;93:1151–1157. doi: 10.1016/j.biopha.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 226.Zhang L, Ge Y, Fuchs E. miR-125b can enhance skin tumor initiation and promote malignant progression by repressing differentiation and prolonging cell survival. Genes Dev. 2014;28(22):2532–2546. doi: 10.1101/gad.248377.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Huang K, Dong S, Li W, Xie Z. The expression and regulation of microRNA-125b in cancers. Acta Biochim Biophys Sin. 2013;45(10):803–805. doi: 10.1093/abbs/gmt073. [DOI] [PubMed] [Google Scholar]
- 228.Budd WT, Seashols-Williams SJ, Clark GC, Weaver D, Calvert V, Petricoin E, et al. Dual action of miR-125b as a tumor suppressor and oncomiR-22 promotes prostate cancer tumorigenesis. PLoS One. 2015;10(11):e0142373. doi: 10.1371/journal.pone.0142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang CH, Pfeffer SR, Sims M, Yue J, Wang Y, Linga VG, et al. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem. 2015;290(10):6037–6046. doi: 10.1074/jbc.M114.632125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yan L-X, Huang X-F, Shao Q, Huang M-Y, Deng L, Wu Q-L, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. Rna. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li X, Kong D, Chen H, Liu S, Hu H, Wu T, et al. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci Rep. 2016;6:21789. doi: 10.1038/srep21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ye J, Guo R, Shi Y, Qi F, Guo C, Yang L. miR-155 regulated inflammation response by the SOCS1-STAT3-PDCD4 axis in atherogenesis. Mediators of inflammation. 2016;2016. [DOI] [PMC free article] [PubMed]
- 233.Liu J, Chen Z, Xiang J, Gu X. MicroRNA-155 acts as a tumor suppressor in colorectal cancer by targeting CTHRC1 in vitro. Oncol Lett. 2018;15(4):5561–5568. doi: 10.3892/ol.2018.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiology and Prevention Biomarkers. 2012;21(8):1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 235.Bleau A-M, Redrado M, Nistal-Villan E, Villalba M, Exposito F, Redin E, et al. miR-146a targets c-met and abolishes colorectal cancer liver metastasis. Cancer Lett. 2018;414:257–267. doi: 10.1016/j.canlet.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 236.Li Y, Xu Y, Yu C, Zuo W. Associations of miR-146a and miR-146b expression and breast cancer in very young women. Cancer Biomarkers. 2015;15(6):881–887. doi: 10.3233/CBM-150532. [DOI] [PubMed] [Google Scholar]
- 237.Pfeiffer D, Roßmanith E, Lang I, Falkenhagen D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an in vitro sepsis model. PLoS One. 2017;12(6):e0179850. doi: 10.1371/journal.pone.0179850. [DOI] [PMC free article] [PubMed] [Google Scholar]