Abstract
Purpose
Glucose-6-phosphate dehydrogenase (G6PD) is the regulating enzyme in the pentose phosphate pathway. A link between the activity of G6PD and diabetes mellitus has previously been reported. The association of G6PD activity with the pathogenesis of gestational diabetes mellitus (GDM) has not yet been investigated. The aim of the present study was to investigate the association of erythrocyte G6PD activity with major characteristics of GDM.
Methods
This case-control study was conducted at Hafez Hospital, Shiraz University of Medical Sciences, Shiraz, Iran from March to November 2017. Eighty-four age-matched pregnant women including GDM (n = 33), impaired glucose tolerance (IGT; n = 7), and normal glucose tolerance (NGT; n = 44) subjects were enrolled in this study. The levels of erythrocyte G6PD activity, fasting plasma glucose (FPG), insulin, malondialdehyde (MDA), and ferric reducing power (FRAP) of serum were measured. The level of homeostasis model for the assessment of insulin resistance (HOMA-IR) was calculated. The data were analyzed using SPSS software. P < 0.05 was considered statistically significant.
Results
The values of FPG, insulin, HOMA-IR, G6PD activity, and FRAP were significantly higher in GDM patients compared to NGT subjects. G6PD activity was correlated with FPG ((r = 0.224; P = 0.041). Binary logistic regression analysis revealed independent association of body mass index >25.88 [OR = 3.23, 95% CI 1.071–9.75, P = 0.037], HOMA- IR >2.33 [OR = 7.15, 95% CI 2.26–22.56, P < 0.001], and G6PD activity>21.17 U/g Hb [OR = 4.63, 95% CI 1.49–14.38, P = 0.008] with an increased risk of GDM. No significant change was observed among serum MDA levels in the three groups.
Conclusion
The findings demonstrate that increased G6PD activity is positively associated with the risk of GDM.
Keywords: Glucose-6-phosphate dehydrogenase, Gestational diabetes, Insulin resistance, Oxidative stress
Introduction
Gestational diabetes mellitus (GDM) is a glucose intolerance that is developed or first diagnosed during pregnancy [1]. Genetic susceptibilities, obesity, insulin resistance, and oxidative stress are among the risk factors suggested to be involved in the development of GDM [2] . Oxidative stress is a consequence of imbalance between the cellular generation of oxidative factors and their neutralization by enzymatic and non-enzymatic antioxidant systems. Superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase are among the enzymes that participate in this phenomenon. The activities of these enzymes are dependent, directly or indirectly, on the presence of reduced nicotinamide adenine-dinucleotide phosphate (NADPH) that is mainly provided by pentose phosphate pathway [3] . In the erythrocytes and the insulin producing beta cells of pancreas, the pentose phosphate pathway is the only source of NADPH, hence a defense mechanism against oxidative damage in these cells is heavily dependent on this pathway. Glucose-6-phosphate dehydrogenase (G6PD) is the first and the regulating enzyme of pentose phosphate pathway. The gene encoding G6PD is a polymorphic X-linked gene (Xq28) with numerous variants [4]. G6PD variants are divided into five different classes based on their G6PD activity. Classes I to III of G6PD variants show less than 50% of residual G6PD activity and demonstrate various clinical phenotypes of G6PD deficiency. However, class IV of GP6D shows activities between 60 and 100% of the normal, while class V demonstrates more than 150% of the normal activity. Both of these two latter classes show no clinical manifestations [5]. G6PD deficiency is the most common genetic disorder with a prevalence rate of 8–15% [4]. This disease is observed more commonly in males due to its recessive X-linked inheritance. Deficiency of G6PD in females has been demonstrated in homozygous deficient individuals with defects in both G6PD alleles. In heterozygous females, a range of mild to severe G6PD deficiencies may occur due to random X inactivation phenomenon [6].
Hemolysis, reduced erythrocyte count, and hyperbilirubinemia after exposure to oxidizing substances, are the most prominent symptoms of G6PD deficiency [4]. Association of G6PD deficiency with several other disorders including diabetes types 1 [7] and 2 [8], and pre-eclampsia [9] have been reported in recent years. Increased prevalence of G6PD deficiency in the diabetic patients and its association with diabetes microvascular complications, have been shown in previous studies [10, 11]. In vitro studies have also revealed that high glucose levels impair G6PD activity and lead to decreased survival of endothelial, kidney, and pancreatic beta cells [12]. Nevertheless, in a recent study, it has been demonstrated that both acute and chronic hyperglycemia without a mutation in G6PD gene are not able to impair G6PD activity [13].
The pathogenesis and clinical features of GDM and type 2 diabetes are closely related and insulin resistance has been proposed as the cause of both [14]. To the best of our knowledge, the possible change in the G6PD activity of GDM patients has not been investigated so far. Therefore, the purpose of this preliminary study was to measure erythrocyte G6PD activity in women with or without GDM and evaluate the possible associations between G6PD activity, hyperglycemia, insulin resistance, and the risk of GDM.
Methods
Characteristics of study subjects
The present case-control study was approved by the Ethics Committee of Islamic Azad University, Shiraz Branch, Shiraz, Iran (Code: IR. IAU. SHIRAZ. REC.1397.007). Informed written consent was obtained from all participants. Eighty-four age-matched pregnant women were selected using simple random selection method and enrolled in this study. These subjects were referred to Hafez Hospital clinical laboratory (Shiraz, Iran) from March to November 2017 for the diagnosis of GDM using 3- h oral glucose tolerance test (OGTT) between 24 to 28 weeks of gestation. Carpenter and Coustan criteria based on OGTT results (fasting glucose level of 95 mg/dl; 1 h glucose level of 180 mg/dl; 2 h glucose level of 155 mg/dl; and 3 h glucose level of 140 mg/dl) was used for the diagnosis of GDM and IGT. The subjects were diagnosed as GDM if at least two of the four OGTT values were reached or exceeded the above-mentioned values [15]. If only one of four values was impaired, the subjects were classified as impaired glucose tolerance (IGT) [16, 17]. Subjects with normal OGTT values were considered in normal glucose tolerance (NGT) group. Weight and height of the subjects were measured. Body mass indices (BMI; kg/m2) for both pre- conception and during pregnancy were calculated. The clinical absence of any major disease and the lack of any medication use altering glucose tolerance were inclusion criteria for the NGT women. The inclusion criteria for the pregnant women with GDM were newly diagnosed cases with no previous use of oral hypoglycemic agents. The exclusion criteria of the study were the presence of type-1 or type-2 diabetes mellitus and other known major diseases.
Biochemical determinations
Fasting blood samples were obtained between 8:30 to 9:30 AM after a 12- h overnight- fast. The sera were prepared immediately and stored at −70 °C until used for biochemical analyses. Fasting plasma glucose (FPG), hematocrit (HTC), and hemoglobin (Hb) levels were measured using commercially available kits (Pars Azemoon Company, Iran). Serum insulin level was measured by Monobind ELISA quantitative kit (Monobind, USA) according to the manufacturer’s instructions. The activity of erythrocyte G6PD was determined using quantitative Baharafshan kit (Baharafshan Co., Iran) by measuring the rate of NADPH production. The lowest detectable level of G6PD activity was 0.5 U/g Hb and the coefficient of variation (CV %) was 2.7%. In this study, we calculated the QUICKI (quantitative insulin sensitivity check index) and HOMA-IR (homeostasis model of assessment for insulin resistance) for the evaluation of insulin sensitivity. The QUICKI was calculated by the following formula: 1/ (log [fasting insulin] + log [fasting glucose]). HOMA-IR is defined as ([fasting glucose] × [fasting insulin])/22.5 [18, 19]. Beta-cell function (HOMA-B) was calculated using the following formula [20 × fasting insulin (mU/L) / (fasting glucose (mmol/L) −3.5] [20]. Trapezoidal rule was used for the calculation of areas under the glucose curve (AUC glucose) during the OGTT [21].
Measurement of serum MDA and FRAP
The level of serum MDA was measured using thiobarbituric acid (TBA) reaction [22]. In brief, 200 μl of the serum was added to 1 ml of TBA (1% in 20% trichloroacetic acid) in a boiling water bath for 15 min followed by keeping the tubes in an ice-cold water bath for 10 min. After centrifugation at 4000 rpm, the supernatant solution was collected and its absorbance was read at 532 nm. The concentration of MDA was calculated using a standard curve of tetra-ethoxy propane. Ferric reducing power assay (FRAP) is a simple and reliable technique for the evaluation of the antioxidant capacity of serum or plasma. In this method the ability of serum to reduce ferric ion (FeIII) to FeII is measured [23]. Briefly, 100 μl of each serum sample was added to 5 ml of phosphate buffer solution (200 mM, pH 6.6) containing potassium ferricyanide(1%). After 20 min incubation at 50 °C, 2.5 ml of trichloroacetic acid (10%) was added and the tubes were centrifuged (10,000 rpm for 10 min). Five ml of distilled water and 1 ml of ferric chloride (0.1%) were then added to 5 ml of the supernatant solution and the absorbance of this solution was read at 700 nm. Reducing power of standard ascorbic acid solutions were also measured by the same method and a standard curve was prepared. The results were finally expressed as μmole/L equivalents of ascorbic acid.
Sample size determination and statistical analyses
Based on results of a pilot study, the mean erythrocyte G6PD activities were determined as 19.6 ± 3.05 for NGT and 24.08 ± 8.37 for GDM women (P = 0.018). By considering α = 0.05, β = 20%, d = 4.9, and ratio sample size of NGT/GDM = 1.5, the sample size was determined as 40 for NGT and 27 for GDM groups. MedCalc Statistical Software was used for the calculation of the sample size. Taking into account the probable attrition rate of 10%, 44 NGT subjects and 33 GDM patients were enrolled in this study. Although the aim of this study was to compare NGT and GDM subjects, however during OGTT diagnostic test, 7 subjects were diagnosed as IGT and were included in this study.
Statistical analyses were performed using SPSS software (version 21). Normal distribution of the variables was checked by the Shapiro–Wilk test. The differences across various normally distributed and not normally distributed variables were computed using one-way ANOVA and Kruskal-Wallis tests, respectively. The data were presented as mean ± standard deviation (SD) or median (1st Quartile – 3rd Quartile) for parametric and non-parametric variables, respectively. Pearson and Spearman correlation analyses were conducted to assess the correlation of G6PD activity with biochemical and demographic variables. Differences in G6PD activity between the NGT, IGT, and GDM groups were evaluated using Analysis of Covariance (ANCOVA). ANCOVA was conducted to adjust for maternal FPG. A receiver operating characteristic curve (ROC) analysis was performed to identify the cut-off point for HOMA-IR, BMI, and G6PD activity as indicative of GDM. The optimum cutoff for each of the above mentioned variables was calculated from ROC analysis [24]. This optimum cutoff was used to dichotomously classify each patient as positive or negative groups. Finally, a binary logistic regression was performed to examine the possible association of G6PD activity with the risk of GDM. Occurrence (GDM group) and non-occurrence of GDM (NGT group) were selected as dependent variables in the binary logistic regression model. P < 0.05 was considered statistically significant.
Results
Comparisons of demographic characteristics of women with GDM, IGT and NGT
The demographic and serum biochemical measurements of pregnant women with GDM, IGT, and NGT are summarized in Table 1. As illustrated in the table, there was no significant difference in maternal age of various groups. Pre-pregnancy weight, pregnancy weight, pre-pregnancy BMI, and pregnancy BMI were higher in GDM patients compared to NGT subjects (P < 0.001). However, no significant differences were observed between demographic characteristics of IGT and NGT, and or GDM and IGT subjects. FPG (P < 0.001), circulating insulin concentration (P = 0.007), and HOMA-IR (P < 0.001) were significantly higher and QUICKI (P < 0.001) and HOMA-B (P = 0.02) were significantly lower in GDM patients compared to NGT subjects. FPG was also significantly higher in the GDM group compared to IGT subjects. Significant differences were not observed in circulating insulin concentration, HOMA-IR, QUICKI, and HOMA-B between the NGT and IGT subjects (Table 1).
Table 1.
Clinical and biochemical characteristics of patients with gestational diabetes and healthy subjects
| NGT (n = 44) |
IGT (n = 7) |
GDM (n = 33) |
Pt | Pa | Pb | Pc | |
|---|---|---|---|---|---|---|---|
| Maternal age(y) | 29.0 ± 5.9 | 32.9 ± 7.8 | 31.2 ± 5.0 | 0.107 | 0. 1 | 0.09 | 0. 49 |
| Height(m) | 1.61 ± 0.06 | 1.64 ± 0.05 | 1.62 ± 0.07 | 0.515 | 0. 26 | 0. 61 | 0. 41 |
|
Pre-weight (kg) |
61.4 ± 11.0 | 64.7 ± 10.5 | 72.4 ± 11.7 | 0.00 | 0. 47 | <0.001* | 0. 11 |
|
P-weight (kg) |
70.4 ± 10.8 | 73.6 ± 12.1 | 79.2 ± 10.8 | 0.003 | 0.48 | 0.001* | 0. 48 |
|
Pre-BMI (Kg/m2) |
23.7 ± 4.2 | 24.1 ± 2.8 | 27.9 ± 4.9 | 0.00 | 0. 86 | <0.001* | 0. 04* |
|
P-BMI (Kg/m2) |
27.2 ± 3.9 | 27.3 ± 3.1 | 30.5 ± 4.5 | 0.003 | 0. 94 | 0.001* | 0. 07 |
| Hb(g/dl) | 12.6 ± 1.4 | 12.4 ± 1.3 | 12.1 ± 1.2 | 0.207 | 0. 56 | 0. 08 | 0. 59 |
| HCT(%) | 37.7 ± 4.1 | 37.6 ± 4.0 | 36.4 ± 3.1 | 0.306 | 0. 91 | 0. 13 | 0. 47 |
| FPG(mg/dl) # | 80(74–84) | 88(81–100) | 102(95–117) | 0.00 | 0.005* | <0.001* | 0.025* |
|
Insulin (μIU/ml) |
7.2 ± 5.5 | 9.2 ± 5.9 | 10.9 ± 6.5 | 0.027 | 0.41 | 0.007* | 0.47 |
| HOMA- IR | 1.4 ± 1.2 | 2.0 ± 1.3 | 3.1 ± 2.0 | 0.00 | 0.34 | <0.001* | 0.1 |
| QUICKI # | 0.38(0.34–0.42) | 0.34(0.32–0.44) | 0.32(0.31–0.37) | 0.00 | 0.2 | <0.001* | 0.21 |
| HOMA-B # | 138(80–237) | 109(56–201) | 87(40–138) | 0.06 | 0.49 | 0.02* | 0.46 |
Data are presented as mean ± SD and median (1st Quartile-3rd Quartile) for parametric and non- parametric variables (#), respectively. IGT, impaired glucose tolerance; NGT, normal glucose tolerance; GDM, gestational diabetes; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment index for insulin resistance; QUICKI, quantitative insulin sensitivity check index; BMI, body mass index; Hb, hemoglobin; HCT, hematocrit; Pre-weight, Pre pregnancy weight; P-weight, Pregnancy weight; Pre -BMI, Pre pregnancy BMI; P-BMI, pregnancy BMI. One-way ANOVA and Kruskal-Wallis tests were used to compare the differences across various normally and not normally distributed variables (#), respectively. Pt is total P value of one-way ANOVA and Kruskal-Wallis tests. Pa, Pb, and Pc show the P values for the differences between the NGT and IGT groups, the NGT and GDM groups, and the IGT and GDM groups, respectively. *indicates statistically significant difference between groups.
Comparisons of glucose tolerance curves in OGTT and AUC of glucose in pregnant women with NGT, IGT, and GDM
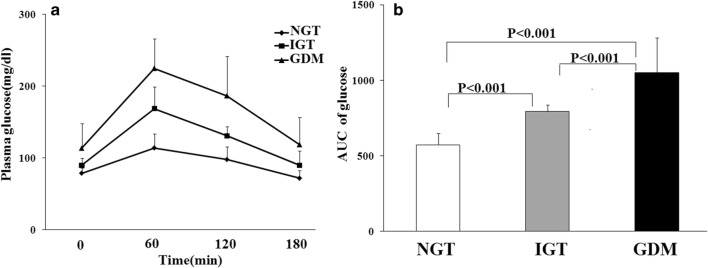
Figure 1a shows the results of OGTT in various groups. The GDM women had significantly higher fasting and 1-h,2-h, and 3-h blood glucose levels in OGTT compared to NGT and IGT women (P < 0.001). Statistically significant differences were also observed in plasma glucose levels of subjects with NGT and IGT subjects in four different time intervals (0, 1, 2, and 3 h) during OGTT (P < 0.001). Comparisons of the area under the curve (AUC) of OGTT among different groups are presented in Fig. 1b, which demonstrates significantly higher values of AUC of glucose in GDM patients compared to the IGT (P < 0.001) and NGT (P < 0.001) subjects. The women with IGT also had significantly higher value of AUC of glucose compared to NGT (P < 0.001).
Fig. 1.
The graphs illustrate oral glucose tolerance test (OGTT) curves (a) and the area under the curve (AUC) of glucose levels OGTT (b) of normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and gestational diabetes mellitus (GDM) subjects during the 100-g OGTT. Statistical differences were observed among NGT, IGT, and GDM groups (One-Way ANOVA followed by LSD post hoc test). Data are presented as means ± SD
Bivariate correlation between erythrocyte G6PD activity and various clinical parameters
Pearson correlation analyses did not show any significant correlation between erythrocyte G6PD activity and GDM related risk factors including maternal age (r = −0.082; P = 0.457), BMI(r = 0.102; P = 0.357), and HOMA-IR(r = 0.121; P = 0.271), in the entire cohort. However, Spearman correlation analysis showed that erythrocyte G6PD activity was positively correlated with FPG (r = 0.224; P = 0.041).
Comparison of parameters related to oxidative status
Serum MDA level, FRAP, and erythrocyte G6PD activity were measured as markers of the oxidative stress of the subjects. G6PD deficiency (< 6.4 U/gHb) was not found in GDM patients, but it was observed in 1 (2.27%) NGT subject. The mean G6PD activity was significantly higher in GDM women compared to NGT group (P = 0.009). No significant differences were observed in G6PD activity between NGT and IGT (P = 0. 051) and between IGT and GDM women (P = 0.648) (Table 2). The results of ANCOVA (adjusted for FPG) revealed that the mean value of G6PD activity remained significantly higher (P = 0.013) in GDM group (23.7 ± 1.3, ranging from 21.2 to 26.3 U/g Hb) compared to NGT subjects (18.9 ± 1.1, ranging from 16.9 to 21.1 U/g Hb). Significantly higher G6PD activity (P = 0.038) was also observed in IGT group (24.4 ± 2.4, ranging from 19.7 to 29.1 U/g Hb) compared to NGT subjects after adjustment for FPG. The mean value of MDA was similar in the three groups and no significant difference was observed among them. The FRAP assay data showed a significant elevation in the reducing power of serum in GDM patients compared to that of NGT subjects (P = 0.025), however no significant difference was observed in the mean value of FRAP in IGT subjects compared to either NGT or GDM ones (Table 2).
Table 2.
Characteristics of oxidant status in various groups
| Markers | NGT (n = 45) |
IGT (n = 7) |
GDM (n = 33) |
Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| G6PD(U/gHb) | 19.4 ± 4.8 | 24.4 ± 6.6 | 23.2 ± 7.7 | 0.051 | 0.009* | 0.648 |
| MDA(μmole/L) | 3.56 ± 0.81 | 3.27 ± 0.67 | 3.27 ± 0.74 | 0.354 | 0.105 | 0.996 |
| FRAP(μmole/L) | 532.1 ± 140.8 | 613.9 ± 112.4 | 621.8 ± 143.4 | 0.245 | 0.025* | 1.000 |
Data are presented as mean ± SD. G6PD, glucose-6-phosphate dehyrogenase; MDA, malondialdehyde; FRAP, ferric reducing power; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; GDM, gestational diabetes. Pa, Pb, and Pc are P values for comparisons between NGT and IGT, NGT and GDM, and IGT and GDM, respectively. * indicates statistically significant differences between groups. Data of parametric (G6PD and MDA) and non-parametric variables (FRAP) were analyzed using one-way ANOVA followed by LSD post hoc test and Kruskal-Wallis test, respectively. *P < 0.05 indicates statistically significant differences between groups.
ROC curve analysis
ROC curve analyses were conducted to compare the performance of HOMA-IR, BMI, and G6PD activity for diagnosing GDM (Fig. 2). The findings indicated that the optimal cutoff point of HOMA-IR for the diagnosis of GDM was 2.33[AUC = 0.780; 95% CI, 0.676–0.883; P < 0.001]; sensitivity, 63.6%; specificity, 79.5%] (Fig. 2). The optimal cutoff point for BMI was 25.88 [AUC = 0.736; 95% CI, 0.624–0.847; P < 0.001]; sensitivity, 63.6%; specificity, 70.5%]. The AUC for G6PD activity was 0.676 (95% CI 0.547–0.804) with the optimum diagnostic cut off point of 21.17 U/g Hb and sensitivity of 60.6% and specificity of 79.5%.
Fig. 2.
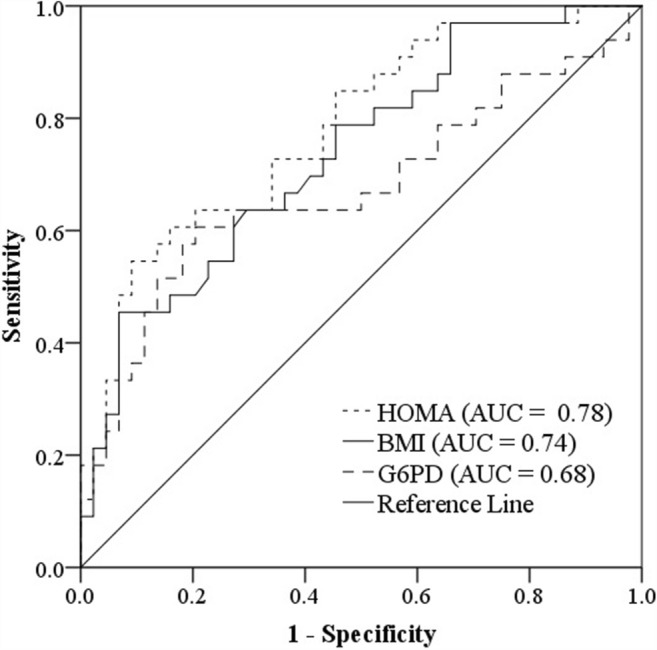
Receiver operator characteristic curves were used to compare the performance of HOMA-IR, BMI, and G6PD activity in the diagnosis of GDM, BMI, body mass index; HOMA-IR, homeostasis model assessment index for insulin resistance; G6PD, Glucose-6-phosphate dehydrogenase; AUC, area under the curve
Logistic regression analysis
Logistic regression analysis was used to estimate the probability and the OR of G6PD activity in comparison with other GDM characteristics including higher pre-pregnancy BMI and HOMA-IR that are well known as potential factors influencing the appearance of GDM. Due to age adjustment between cases and controls, maternal age was not included in this model. The results of logistic regression are shown in Table 3. The results demonstrate that HOMA-IR > 2.33, pre pregnancy BMI >25.88, and erythrocyte G6PD activity>21.17 U/g Hb were independent risk factors for GDM. As expected, HOMA-IR > 2.33 [OR 7.15(2.26–22.56), P < 0.001] was the most important factor increasing the risk of GDM about seven fold. BMI values higher than 25.88 [OR 3.23 (1.07–9.74), P = 0.037] elevate the risk of GDM about three fold. A significant independent association was also found between erythrocyte G6PD activity [OR 4.63(1.49–14.38), P = 0.008] and the increased significant risk of about four fold in developing GDM (Table 3).
Table 3.
Binary logistic regression analysis for prediction of gestational diabetes
| Variables | B | SE | Wald | P | OR | 95.0% C.I |
|---|---|---|---|---|---|---|
| HOMA-IR | 1.97 | 0.59 | 11.24 | <0.001 | 7.15 | 2.26–22.56 |
| BMI | 1.17 | 0.56 | 4.33 | 0.037 | 3.23 | 1.07–9.74 |
| G6PD | 1.53 | 0.58 | 7.03 | 0.008 | 4.63 | 1.49–14.38 |
GDM and NGT groups were considered as dependent variables in the binary logistic regression model. BMI, body mass index; HOMA-IR, homeostasis model assessment index for insulin resistance; G6PD, Glucose-6-phosphate dehydrogenase; SE, Standard error; OR, odds ratios; CI, confidence interval.
Discussion
Present study was conducted in order to evaluate the possible association between G6PD activity, insulin resistance, metabolic characteristics, and risk of developing GDM. This study had three main findings. The first was the presence of imbalance in the control of blood glucose in GDM patients shown by a significant increase in FPG, impairment of glucose tolerance test evidenced by an increase in AUC of glucose in OGTT, an increase in HOMA-IR, and a decrease in QUICKI. The second finding was the observation of the same levels of serum MDA among GDM and non-GDM subjects, while FRAP was significantly higher in GDM patients compared to NGT subjects. Finally, the activity of erythrocyte G6PD was significantly higher in the GDM patients compared to NGT subjects. Furthermore, increases in HOMA-IR, G6PD activity, and pre-pregnancy BMI were independent of the risk factors for GDM. To the best of our knowledge, this is the first study that has demonstrated the association of G6PD activity with GDM.
Insulin resistance and β-cell dysfunction are two well-known characteristics of GDM [20, 25]. In the present study, an increase in insulin resistance (HOMA-IR) and a decrease in insulin sensitivity (QUICKI) were observed between NGT and GDM subjects accompanied with an increase in FBS and impairment of glucose tolerance. These data are in accordance with previous studies [18, 25] and confirm the presence of a disturbance in blood glucose homeostasis due to insulin resistance in GDM patients enrolled in our investigation. Our data also revealed a lower value of HOMA-B, a marker of β-cell secretary function, in GDM women compared to NGT subjects, suggesting for the presence of β-cell dysfunction in GDM patients. Hyperglycemia-induced oxidative stress has been reported as a cause of GDM metabolic abnormalities [26]. In the present study, we used MDA and FRAP assays to compare the degree of oxidative stress between GDM and NGT pregnant women. Our findings revealed no statistically significant difference between serum MDA in the GDM group compared to the NGT control group; in agreement with a previous study [27]. Contradictory to our findings, some previous investigators have found an increase in serum MDA levels in GDM subjects, suggesting the presence of higher levels of oxidative stress in GDM patients compared to non-GDM control subjects. These discrepancies may have resulted from a variation in dietary habits of the patients such as inclusion of probiotics and omega 3 fatty acids in their diets [28, 29], and also the gestational age at which blood samples were taken. Arribas et al. [30] have shown higher levels of MDA in the first and second trimesters and no significant difference in the third trimester of pregnancy in GDM patients compared to the normal control. Also such contradictions may be due to different sample sizes used in these investigations by different authors. Serum contains a mixture of antioxidant compounds including vitamins, bilirubin, albumin, and uric acid (UA) which act cumulatively to reduce and neutralize reactive oxygen and nitrogen species. UA is the most important reducing compound of the serum and provides more than half of the reducing ability of serum [31]. In the FRAP assay, total reducing power or total antioxidant capacity of serum is measured [23]. Our findings revealed a significant elevation in reducing power of serum in the GDM patients compared to that of NGT subjects. This finding is in agreement with the results of previous studies which have reported increased antioxidant capacity of saliva in GDM patients compared to non-diabetic pregnant women [32, 33]. Although the exact underlying mechanism is unknown, increase in FRAP may relate to elevating serum UA level that occurs in GDM patients. In support of this theory, an increase in serum UA level at 24–28 weeks of gestation was reported in women with GDM compared to normal pregnant women [34]. Insulin resistance which occurred during pregnancy [35] and possibly adaptive response to protect against oxidative stress were enumerated as the mechanisms responsible for the increase in UA level in diabetes [36].
G6PD is a critical enzyme in protecting cells especially erythrocytes and beta cell insulin producing cells against oxidative stress damage [6]. Experimental data have revealed a decrease in G6PD gene expression and enzyme activity in beta cells exposed to high levels of glucose which may be considered as a cause for the gradual loss of beta cell function in diabetic patients [12]. Furthermore, a decrease in erythrocyte G6PD activity in diabetic patients has been demonstrated in some studies [8]. Epidemiological studies have also demonstrated the association of G6PD deficiency with an increased risk of diabetes in several populations [26, 37]. Contradictory to such findings, Park et al. have reported an association of G6PD overexpression with insulin resistance in adipocytes [38]. Due to similarities between the pathogenesis of type 2 diabetes and GDM, it is anticipated that the activity of G6PD is reduced in GDM patients compared to NGT subjects. Unexpectedly, the results of the present study showed a higher erythrocyte G6PD activity in GDM patients compared to NGT subjects. After adjustment for confounding factors, G6PD activity still remained significantly higher in GDM patients compared to NGT subjects. Logistic multivariate regression analysis also revealed that G6PD activity was independently associated with the increased risk of GDM. These finding suggest that further investigation is needed to clarify the association of G6PD with diabetes.
The exact molecular mechanisms that would be responsible for an increase in G6PD activity in patients with GDM was not investigated in the present study. Given the fact that G6PD gene is a very polymorphic one with mutations that affect its activity [4], a possible description for the difference between G6PD activity of GDM and NGT subjects may be related to the variation in the mutations of G6PD gene in the two groups. Furthermore, previous studies have shown that the activity of G6PD is influenced by several hormones including thyroid hormones, cortisol, and insulin. Therefore, the difference in hormonal status of GDM patients compared to NGT pregnant women may affect the activity of this enzyme. Finally, several biological conditions including the proportion of young erythrocytes in the blood circulation of each subject that has higher G6PD activity compared to the older ones, and a variation in reticulocyte and leukocyte counts of the studied groups may be responsible for the higher G6PD activity observed in GDM patients [39].
In agreement with the previous studies [2, 20], the results of logistic regression analyses in the present study revealed a strong association between insulin resistance (OR = 7.15) and BMI (OR = 3.23) with increased risk of GDM. Our data also revealed for the first time a significant association between G6PD activity and the increased risk of GDM (OR = 4.63), although further studies are needed to explain how an increase in G6PD activity is related to GDM. Several possible mechanisms may involve in this association. G6PD is a ubiquitous enzyme, which is expressed in the erythrocytes as well as other tissues most notably liver and the adipose tissue, therefore the association that we observed in the activity of G6PD and the risk of GDM may occur in other tissues. In support of this theory, previous studies have shown that G6PD deficiency which causes impairment in the function of erythrocytes also led to dysfunction in the lipid metabolism in the liver and adipose tissue [40, 41]. Therefore, we can expect that increase in the erythrocytes G6PD activity that we have observed in the GDM patients, may also exist in the adipose tissue of the patients. Previous studies have shown that an increase in the activity of G6PD in adipocytes can lead to insulin resistance through disturbances in insulin signaling [38, 42]. Insulin resistance is a well-known characteristic of GDM [2, 20]; hence the association between G6PD activity and GDM may be explained through insulin resistance. Obesity is highly accepted as a risk factor of GDM [43]. Animal studies on both genetically and diet-induced obesity have suggested an association between obesity and increased G6PD activity in adipose tissue [38]. Therefore, the second possible mechanism linking G6PD activity to GDM is its association with obesity. Finally, Park et al. [38] have demonstrated that the expression of adipokines including tumor necrosis factor alpha (TNF-α) and resistin, was elevated, while that of adiponectin was reduced following the up-regulation of G6PD in adipocytes. It is well documented that elevation in TNF-α and resistin and a reduction in the expression of adiponectin, increase the risk of GDM [19]. Therefore, a modification in the expression of adipokines is another possible mechanism linking increased G6PD activity with the risk of developing GDM. Finally, our data revealed a positive correlation between FPG levels and the activity of G6PD. Therefore, increases in erythrocyte G6PD that was observed in GDM patients may reflect the degree of hyperglycemia in GDM patients.
Our study had several limitations. The main limitation was the relatively small sample size of the present study especially in the IGT subjects. Furthermore, the difference in G6PD activity between subjects that we observed in the present study may be related to differences in mutations of G6PD gene that influence enzyme activity. Thus, determination of G6PD activity alone and in the absence of gene mutation analyses does not explain the exact cause of differences in G6PD activities among these subjects. Therefore, further investigation with a larger sample size and the evaluation of the genotypes of the subjects are required to clarify the changes in G6PD activity in GDM patients and its impact on the risk of developing GDM.
Conclusion
This study showed an increase in erythrocyte G6PD activity in GDM patients which was positively associated with fasting plasm glucose. G6PD activity was also an independent risk factor for the development of GDM that may be used as a potential indicator of this disease. Further studies using a larger sample size and doing genetic analyses are needed to confirm the present findings in order to explore the potential mechanisms by which G6PD activity modulates susceptibility to GDM.
Acknowledgments
This research was financially supported by a research grant from Shiraz Azad University.
Compliance with ethical standards
Conflict of interest
None declared.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parvaneh Asadi, Email: farfala1349@yahoo.com.
Mahmood Vessal, Email: mahmoodv@yahoo.com.
Marjan Khorsand, Email: ma.kh58@yahoo.com.
Mohammad Ali Takhshid, Email: takhshidma@sums.ac.ir.
References
- 1.Coustan DR. Gestational diabetes mellitus. Clin Chem. 2013;59:1310–1321. doi: 10.1373/clinchem.2013.203331. [DOI] [PubMed] [Google Scholar]
- 2.Farahvar S, Walfisch A, Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab. 2019;14:63–74. doi: 10.1080/17446651.2018.1476135. [DOI] [PubMed] [Google Scholar]
- 3.Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, Ling ZQ, Hu FJ, Sun YP, Zhang JY, Yang C, Yang Y, Xiong Y, Guan KL, Ye D. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014;33:1304–1320. doi: 10.1002/embj.201387224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-Manzo Saúl, Marcial-Quino Jaime, Vanoye-Carlo America, Serrano-Posada Hugo, Ortega-Cuellar Daniel, González-Valdez Abigail, Castillo-Rodríguez Rosa, Hernández-Ochoa Beatriz, Sierra-Palacios Edgar, Rodríguez-Bustamante Eduardo, Arreguin-Espinosa Roberto. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. International Journal of Molecular Sciences. 2016;17(12):2069. doi: 10.3390/ijms17122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67:601–11. [PMC free article] [PubMed]
- 6.Wang J, Xiao Q-Z, Chen Y-M, Yi S, Liu D, Liu Y-H, et al. DNA hypermethylation and X chromosome inactivation are major determinants of phenotypic variation in women heterozygous for G6PD mutations. Blood Cell Mol Dis. 2014;53:241–245. doi: 10.1016/j.bcmd.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Cappai G, Songini M, Doria A, Cavallerano J, Lorenzi M. Increased prevalence of proliferative retinopathy in patients with type 1 diabetes who are deficient in glucose-6-phosphate dehydrogenase. Diabetologia. 2011;54:1539–1542. doi: 10.1007/s00125-011-2099-3. [DOI] [PubMed] [Google Scholar]
- 8.Khanam A, Akter Q, Karim F, Zannat M. Erythrocyte Glucose-6-phosphate dehydrogenase level in type 2 diabetes male. Mymensingh Med J. 2018;27:103–107. [PubMed] [Google Scholar]
- 9.Popovic J. Glucose-6-phosphate Dehydrogenase Deficiency and Pre-eclampsia: Possibility of Treatment. Iran J Med Sci. 2011;36:324. [PMC free article] [PubMed] [Google Scholar]
- 10.Santana MS, Monteiro WM, Costa MR, Sampaio VS, Brito MA, Lacerda MV, Alecrim MG. High frequency of diabetes and impaired fasting glucose in patients with glucose-6-phosphate dehydrogenase deficiency in the Western brazilian Amazon. Am J Trop Med Hyg. 2014;91:74–76. doi: 10.4269/ajtmh.13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai YK, Lai NM, Lee SWH. Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: a systematic review and meta-analysis. Ann Hematol. 2017;96:839–845. doi: 10.1007/s00277-017-2945-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J. 2010;24:1497–1505. doi: 10.1096/fj.09-136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choukem SP, Sobngwi E, Garnier JP, Letellier S, Mauvais-Jarvis F, Calvo F, Gautier JF. Hyperglycaemia per se does not affect erythrocyte glucose-6-phosphate dehydrogenase activity in ketosis-prone diabetes. Diabetes Metab. 2015;41:326–330. doi: 10.1016/j.diabet.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Plows Jasmine, Stanley Joanna, Baker Philip, Reynolds Clare, Vickers Mark. The Pathophysiology of Gestational Diabetes Mellitus. International Journal of Molecular Sciences. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2017;8:CD007122. doi: 10.1002/14651858.CD007122.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SJ, Kim TN, Baik SH, Kim TS, Lee KW, Nam M, Park YS, Woo JT, Kim YS, Kim SH. Insulin secretion and insulin resistance in Korean women with gestational diabetes mellitus and impaired glucose tolerance. Korean J Intern Med. 2013;28:306–313. doi: 10.3904/kjim.2013.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun IO, Ko YM, Kim EY, Park KS, Jung HS, Ko SH, Chung BH, Choi BS, Park CW, Kim YS, Yang CW. Clinical characteristics and outcomes in renal transplant recipients with renal cell carcinoma in the native kidney. Korean J Intern Med. 2013;28:347–351. doi: 10.3904/kjim.2013.28.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takhshid MA, Haem Z, Aboualizadeh F. The association of circulating adiponectin and + 45 T/G polymorphism of adiponectin gene with gestational diabetes mellitus in Iranian population. J Diabetes Metab Disord. 2015;14:30. doi: 10.1186/s40200-015-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takhshid MA, Zare Z. Resistin - 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus. J Diabetes Metab Disord. 2015;14:37. doi: 10.1186/s40200-015-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Leng J, Li W, Zhang C, Feng L, Wang P, et al. Roles of insulin resistance and beta cell dysfunction in macrosomia among Chinese women with gestational diabetes mellitus. Prim Care Diabetes. 2018;12:565–573. doi: 10.1016/j.pcd.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 22.Bhutia Y, Ghosh A, Sherpa ML, Pal R, Mohanta PK. Serum malondialdehyde level: surrogate stress marker in the Sikkimese diabetics. J Nat Sci Biol Med. 2011;2:107–112. doi: 10.4103/0976-9668.82309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Fan W, Dong J, Lu J, Chen J, Shan L, et al. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008;107:296–304. doi: 10.1016/j.foodchem.2007.08.018. [DOI] [Google Scholar]
- 24.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb) 2016;26:297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takhshid MA, Zahediannejad Z, Aboualizadeh F, Moezzi L, Ranjbaran R. G22A polymorphism of adenosine Deaminase and its association with biochemical characteristics of gestational diabetes mellitus in an Iranian population. Iran J Med Sci. 2015;40:170–174. [PMC free article] [PubMed] [Google Scholar]
- 26.Mule NK, Singh JN. Diabetes mellitus to neurodegenerative disorders: is oxidative stress fueling the flame? CNS Neurol Disord Drug Targets. 2018;17:644–653. doi: 10.2174/1871527317666180809092359. [DOI] [PubMed] [Google Scholar]
- 27.Araujo JR, Ramalho C, Correia-Branco A, Faria A, Ferraz T, Keating E, et al. A parallel increase in placental oxidative stress and antioxidant defenses occurs in pre-gestational type 1 but not gestational diabetes. Placenta. 2013;34:1095–1098. doi: 10.1016/j.placenta.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J Clin Nutr. 2018;27:581–591. doi: 10.6133/apjcn.082017.03. [DOI] [PubMed] [Google Scholar]
- 29.Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, Akbari M, Asemi Z. A randomized controlled clinical trial investigating the effects of Omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can J Diabetes. 2017;41:143–149. doi: 10.1016/j.jcjd.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Arribas L, Almansa I, Miranda M, Muriach M, Romero FJ, Villar VM. Serum malondialdehyde concentration and glutathione peroxidase activity in a longitudinal study of gestational diabetes. PLoS One. 2016;11:e0155353. doi: 10.1371/journal.pone.0155353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabbrini E, Serafini M, Baric IC, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63:976–981. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamani-Ahari U, Zamani-Ahari S, Fardi-Azar Z, Falsafi P, Ghanizadeh M. Comparison of total antioxidant capacity of saliva in women with gestational diabetes mellitus and non-diabetic pregnant women. J Clin Exp Dent. 2017;9:e1282. doi: 10.4317/jced.53845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surdacka A, Ciężka E, Pioruńska-Stolzmann M, Wender-Ożegowska E, Korybalska K, Kawka E, et al. Relation of salivary antioxidant status and cytokine levels to clinical parameters of oral health in pregnant women with diabetes. Arch Oral Biol. 2011;56:428–436. doi: 10.1016/j.archoralbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Gungor ES, Danisman N, Mollamahmutoglu L. Relationship between serum uric acid, creatinine, albumin and gestational diabetes mellitus. Clin Chem Lab Med. 2006;44:974–977. doi: 10.1515/CCLM.2006.173. [DOI] [PubMed] [Google Scholar]
- 35.Laughon SK, Catov J, Roberts JM. Uric acid concentrations are associated with insulin resistance and birthweight in normotensive pregnant women. Am J Obstet Gynecol. 2009;201:582 e1–582 e6. doi: 10.1016/j.ajog.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–28. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- 37.Carette C, Dubois-Laforgue D, Gautier JF, Timsit J. Diabetes mellitus and glucose-6-phosphate dehydrogenase deficiency: from one crisis to another. Diabetes Metab. 2011;37:79–82. doi: 10.1016/j.diabet.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Park J, Rho HK, Kim KH, Choe SS, Lee YS, Kim JB. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol. 2005;25:5146–5157. doi: 10.1128/MCB.25.12.5146-5157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minucci A, Giardina B, Zuppi C, Capoluongo E. Glucose-6-phosphate dehydrogenase laboratory assay: how, when, and why? IUBMB Life. 2009;61:27–34. doi: 10.1002/iub.137. [DOI] [PubMed] [Google Scholar]
- 40.Dessi S, Batetta B, Spano O, Pulisci D, Mulas MF, Muntoni S, et al. Serum lipoprotein pattern as modified in G6PD-deficient children during haemolytic anaemia induced by fava bean ingestion. Int J Exp Pathol. 1992;73:157–160. [PMC free article] [PubMed] [Google Scholar]
- 41.Dessi S, Chiodino C, Batetta B, Fadda AM, Anchisi C, Pani P. Hepatic glucose-6-phosphate dehydrogenase, cholesterogenesis, and serum lipoproteins in liver regeneration after partial hepatectomy. Exp Mol Pathol. 1986;44:169–176. doi: 10.1016/0014-4800(86)90067-5. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Choe SS, Choi AH, Kim KH, Yoon MJ, Suganami T, Ogawa Y, Kim JB. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes. 2006;55:2939–2949. doi: 10.2337/db05-1570. [DOI] [PubMed] [Google Scholar]
- 43.Torloni M, Betran A, Horta B, Nakamura M, Atallah A, Moron A, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]