Abstract
Background
Many studies have reported that insulin resistance impairs the antioxidant defense system and causes male infertility. Moringa oleifera is a medicinal plant that has been employed for the medicament of many disorders. It controls the levels of glucose and manages male sexual disorders. However, its extracts can reverse insulin resistance-linked metabolic alterations remains unknown. Therefore, the current study investigated the potential of the aqueous leaves extract from Moringa oleifera to reverse insulin resistance and testicular disorders in rats.
Methods
Rats were fed either a chow (as a control group) or a high fructose diet (HFD, to persuade a state of insulin resistance), in addition to a group of rats fed HFD and treated with Moringa (300 mg/kg) for 4 weeks.
Results
Moringa reversed hepatic insulin insensitivity and this was linked to up-regulation of genes involved in insulin receptors and glucose uptake in the liver. These results were associated with amended the insulin level in serum and standardization of insulin sensitivity. In addition, it improved the serum testosterone level and the gene expression of the testicular steridogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD).
Conclusion
Taken together, our findings demonstrate that Moringa reversed HFD diet-induced insulin resistance and improved the testicular function.
Keywords: Insulin resistance, Moringa, Insulin receptor, Glucose transporter, StAR
Introduction
Modern human diets contain additional sugars, as fructose, which improves the taste of some products; seem not to be neutral for health. High fructose diet and corn syrup were confirmed to be linked with the increasing prevalence of metabolic syndrome and insulin resistance worldwide, causing function impairment in many tissues and organs [1, 2].
Insulin resistance is referred to the impaired ability of cells to respond to the insulin action, with consequences on carbohydrate, lipid and protein metabolism [3]. Insulin signaling pathway is triggered by the binding of insulin to the transmembrane insulin receptor (IR) followed by downstream events such as activation of insulin receptor substrates (IRSs) that trigger subsequent signal transduction leading finally to facilitate glucose, which is the basic substrate for the majority of cells, entering into cells by translocation of specific carriers called glucose transporters (GLUT). GLUT-4 is one of these transporters which, unlike other types, is dependent upon insulin [4, 5]. The main determinant of cellular response to insulin is the number of insulin receptors, any reduction significantly decreases insulin sensitivity [6].
Infertility is a persistent problem worldwide, with a percentage may reach to 30% in the developing countries [7, 8]. Obesity and associated metabolic abnormalities such as type 2 diabetes and insulin resistance are among the proposed causes of male infertility [9, 10]. Despite the importance of infertility problems, the therapeutic efficacy is still not in satisfaction level.
Insulin affects the male reproductive function through the hypothalamic-pituitary axis. It is well-known that testosterone, follicular stimulating hormone (FSH), and luteinizing hormone (LH) levels could be used as indicators of reproductive functions. So, the failure of the hypothalamic-pituitary-gonadal axis decreases the levels of these hormones and impairs the spermatogenic and sexual function, which in turn lead to infertility [11, 12].
Positive associations were proved between oxidative stress and insulin resistance [13]. Additionally, oxidative stress has been reported to affect the reproductive system of males by causing testicular dysfunction, reduced gonadotropin secretion and abnormal semen parameters that finally lead to infertility [14].
Moringa oleifera is one of the most important medicinal plants. It belongs to (family: Moringaceae) and has been used in the traditional medicine. The leaves of M. oleifera have been used as antiulcer, diuretic, anti-inflammatory and for wound healing [15–17]. Moreover, it can enhance the sexual functions in males including improvement of the sperm quality, libido and anti-erectile dysfunction [18]. Therefore, this study was designed to inspect the impact of the daily intake of the water extract of M. oleifera leaves to ameliorate the hyperinsulinemia and associated testicular disorders in the high fructose-fed rats.
Materials and methods
Animals
A total of 30 adult male Sprague Dawley rats weighing 140-320 g were used throughout this study. The rats were obtained from the Egyptian Organization for Biological Products and Vaccines (Helwan, Egypt). Rats were divided into three groups (10 rats each), housed in stainless steel cages (5/cage) at a constant environmental temperature (25 °C ± 5) and humidity (50% ± 10) with dark and light cycle (12 h). The rats were maintained for a week on a standard diet as an acclimatization period. Food and water were provided ad libitum. The experimental protocol was approved by the ethics committee of Faculty of Science, Al-Azhar University, Cairo, Egypt.
Preparation of diets
The standard and high fructose (60 g/100 g) diets were prepared as described previously [19].
Preparation of Moringa oleifera aqueous extract
Moringa oleifera was purchased from the local market of Marsa Matroh, Egypt. It was authenticated by the botanists (Botany Department, Faculty of Science, Al-Azhar University, Cairo, Egypt). Exactly 100 g dried leaves were mixed with 1 L boiling water for 5 min, filtered and stored at 4 °C for up to 7 days [20].
Preliminary phytochemical screening
Filtrates were subjected to preliminary phytochemical screening, to identify the chemical constituents [21–27].
Study design
Animals were allocated in 3 groups: Group I: Normal Control (NC): Animals fed standard diet and kept without any treatment. Group II: High Fructose Diet (HFD): Rats in this group fed HFD serving as the reference group for the corresponding treated group. Group III: (HFD/Moringa): The aqueous extract of Moringa oleifera was administered orally to high fructose-fed rats at a dose level of 300 mg/kg [28]. The animals were maintained for 4 weeks. Body weights of rats in all groups were recorded weekly throughout the experimental period and the body weight gain was calculated at the end of the feeding period.
Blood collection
At the end of the experiment, rats were weighed then anesthetized with Urethane (99%, Aldrich) at a dose of 1 g/kg body weight intraperitoneally. Blood samples were taken from the retro-orbital venous plexus after overnight fasting. Blood was centrifuged at 4000 rpm for 5 min then serum obtained was kept at −20 °C.
After dissection, the livers and testis were removed and perfused with phosphate buffer saline (PBS, pH 7.4), dried by filter paper, weighed and the relative weight was calculated.
Biochemical assay
Fasting glucose level in serum was determined by the enzymatic colorimetric method [29], while serum insulin was measured using the enzyme-linked immunoassay (Rat insulin ELISA kit, Glory science Co., USA) [30]. Homeostasis model assessment insulin resistance index (HOMA-IR) was calculated according to the formula of Pickavance et al. [31]. Serum level of testosterone was measured by electrochemiluminescence immunoassay according to the method of Rosner et al. [32], using the commercial kit (Roche Diagnostic, Germany), while the serum levels offollicle stimulating hormone (FSH) was determined by immunoradiometric assay according to the method of Clarke and Cummins [33], using the commercial kit (DIA source, Belgium).
Preparation of liver homogenate
About 0.5 g of liver was homogenized in 10 ml of ice-cold 0.05 mM potassium phosphate buffer solution (pH 7.4) to yield ultimately 5% (w/v) whole liver homogenate. The liver homogenates were centrifuged at 5000 rpm for 15 min at 4 °C then the supernatant was used for determination of malondialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT) by the methods of Ohkawa et al. [34], Nishikimi et al. [35] and Aebi [36], respectively.
Quantitative real time polymerase chain reaction (q- PCR)
Gene mRNA expression analysis of the hepatic insulin receptor (IR), insulin receptor substrate-1 (IRS-1), glucose transporters- 4 and 5 (GLUT-4 & GLUT-5) and superoxide dismutase in addition to the testicular steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD)were assessed by q-PCR as previously described. Primer sequences used are shown in the Table 1.
Table 1.
Primer sequences used for RT-PCR
| Gene | Primer Sequence | Reference | |
|---|---|---|---|
| StAR |
Forward Reverse |
5’-ATGCCTGAGCAAAGCGGTGTC-3′ 5’-CAAGTGGCTGGCGAACTCTATCTG-3’ |
Rizk et al. [37] |
| 3β-HSD |
Forward Reverse |
5’CCAGTGTATGTAGGCAATGTGGC-3′ 5’-CCATTCCTTGCTCAGGGTGC-3’ |
Rizk et al. [37] |
| IR |
Forward Reverse |
5’CTTCTCGCGGAGTATGTCCC3’ 5’CAGCACCGTTCCACAAACTG3’ |
Accession No. NM_017071.2 |
| IRS-1 |
Forward Reverse |
5’CTGCATAATCGGGCAAAGGC3’ 5’CATCGCTAGGAGAACCGGAC3’ |
Accession No. NM_012969.1 |
| GLUT-4 |
Forward Reverse |
5’GATTCTGCTGCCCTTCTGTC3’ 5’ATTGGACGCTCTCTCTCCAA3’ |
Accession No. |
| GLUT-5 |
Forward Reverse |
5’GTGTCTGTGACACTGGGAGG3’ 5’GTGACATGGCTGGGTCAGAA3’ |
Accession No. NM_031741.1 |
| SOD |
Forward Reverse |
5′GCAGAAGGCAAGCGGTGAAC3′ 5′TAGCAGGACAGCAGATGAGT3′ |
Limaye et al. [38] |
| GAPDH |
Forward Reverse |
5′- CTCCCATTCTTCCACCTTTG-3′ 5′- CTTGCTCTCAGTATCCTTGC-3′ |
Rizk et al. [37] |
Statistical analysis
All results are presented as mean ± SE. One-way analysis of variance (ANOVA) followed by post hoc–least significant difference analysis (LSD) was performed to compare all the treated groups using the statistical package for social sciences (SPSS) version 23 (Chicago, USA). Differences were considered statistically significant at p < 0.05.
Results
The preliminary phytochemical study confirmed the presence of phenols, tannins, and flavonoids in the Moringa oleifera leaves extract (Table 2).
Table 2.
Phytochemical screening of Moringa extract
| Chemical constituent | Moringa extract |
|---|---|
| Alkaloids | +ve |
| Glycosides | +ve |
| Cardiac glycosides | +ve |
| Saponins | +ve |
| Phenol | +ve |
| Sterol | +ve |
| Tannins | +ve |
| Flavonoids | +ve |
| Diterpene | -ve |
In the present study, the body weights of rats in all groups were increased progressively during the experimental period. However, the relative weights of testes in rats of the studied groups did not vary significantly, compared to the control rats (Table 3).On the other hand, the relative weights of livers in fructose-fed rats (HFD and HFD/Moringa) were significantly increased (p < 0.02 and p < 0.0001, respectively) as compared to the control rats.
Table 3.
Body weight gain, Relative weights of testis and liver as well as serum levels of glucose, insulin, HOMA-IR index, testosterone and FSH (Mean ± SE) in the experimental groups
| Groups | Control | HFD | HFD/Moringa |
|---|---|---|---|
| Parameters | |||
| Body weight gain (g) | 74.16 ± 6.11 | 49. 5 ± 6.94 | 83.38 ± 11.86 b |
| Relative weight of testes* | 0.010 ± 0.001 | 0.013 ± 0.001 | 0.011 ± 0.001 |
| Relative weight of liver* | 0.026 ± 0.005 | 0.031 ± 0.002a | 0.034 ± 0.002a |
| Glucose (mg/dl) | 95.17 ± 4.17 | 132.67 ± 6.45a | 128.83 ± 4.94a |
| Insulin (μIU/ml) | 2.26 ± 0.25 | 5.05 ± 0.33a | 2.64 ± 0.19b |
| HOMA-IR | 0.50 ± 0.05 | 1.65 ± 0.12a | 0.83 ± 0.07ab |
| Testosterone (ng/ml) | 2.12 ± 0.34 | 0.95 ± 0.09a | 1.65 ± 0.13b |
| FSH (mlU/ml) | 16.26 ± 0.79 | 14.56 ± 3.52 | 14.36 ± 1.60 |
The mean difference is significant at p < 0.05
Each group contains 10 rats
a Significance versus control, b Significance versus HFD
*: Relative weight of organ is the ratio of the organ weight to the whole body weight
Current results showed a state of moderate insulin resistance in fructose-fed rats demonstrated by hyperinsulinemia and increased HOMA-IR value as well as hyperglycemia. However, Moringa supplementation to fructose-fed rats caused a significant decrease in the levels of insulin and HOMA index (p < 0.001) as compared to HFD group. Additionally, Moringa administration returns the serum insulin level to the normal control level as shown in Table 3.
In parallel, rats fed HFD showed a significant decrease (p < 0.002) in the serum testosterone level as compared to the control group. Administration of Moringa improved the serum level of testosterone to be near the control level. With regard to serum FSH, non-significant differences were recorded in HFD-fed groups (HFD and HFD/Moringa) as compared to the control group (Table 3).
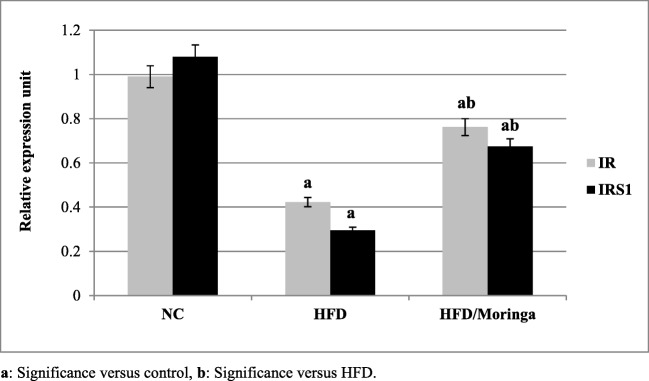
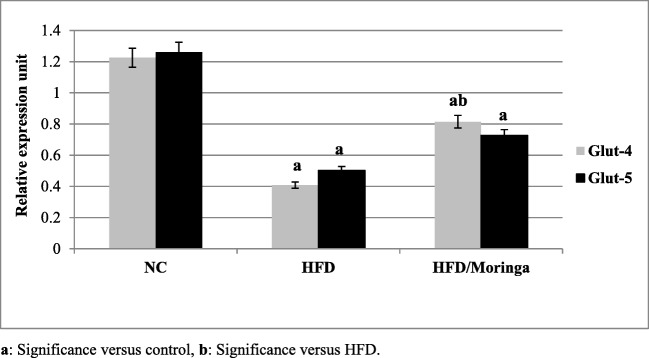
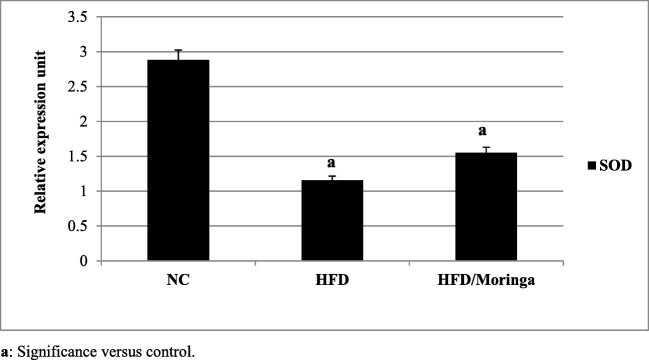
Significant down-regulation in hepatic IR, IRS1 and glucose transporters 4 and 5 (GLUT-4 and GLUT-5) as well as SOD genes expression (p < 0.001) were observed in HFD group as compared to the control group. However, administration of Moringa extract for one month was significantly improved the expression of IR, IRS1 (p < 0.01) and GLUT-4 (p < 0.05) genes as compared to the HFD group. As regards to GLUT-5 and SOD genes, non-significant increases were observed in HFD + Moringa group as compared to the HFD group (Figs. 1, 2 and 3).
Fig. 1.
Effect of Moringa oleifera on hepatic IR and IRS-1 gene expressions in HFD-fed rats. Data are presented as mean ± SE and were analyzed using ANOVA followed by LSD. The mean difference is significant at p < 0.05. Each group contained 10 rats
Fig. 2.
Effect of Moringa oleifera on hepatic GLUT-4 and 5 gene expressions in HFD-fed rats. Data are presented as mean ± SE and were analyzed using ANOVA followed by LSD. The mean difference is significant at p < 0.05. Each group contained 10 rats
Fig. 3.
Effect of Moringa oleifera on hepatic SOD gene expression in HFD-fed rats. Data are presented as mean ± SE and were analyzed using ANOVA followed by LSD. The mean difference is significant at p < 0.05. Each group contained 10 rats
Data in Table 4, revealed a significant increase (p < 0.001) in the hepatic concentration of MDA in rats fed HFD, compared to the control rats. However, treatment with of Moringa extract reduced this elevation significantly (p < 0.001), compared to the HFD and control groups. In contrast, the antioxidant enzymes (SOD and CAT) were significantly reduced in the HFD-fed (p < 0.001) and HFD-fed rats treated with Moringa (p < 0.002) as compared to the control. Additionally, Moringa improved the hepatic level of SOD and CAT (p < 0.02 and 0.009, respectively) as compared to the non-treated HFD group.
Table 4.
Hepatic levels of MDA, SOD and CAT presented as (Mean ± SE) in the experimental groups
| Groups | Control | HFD | HFD/Moringa |
|---|---|---|---|
| Parameters | |||
| MDA (nmol/g tissue) | 54.49 ± 3.86 | 80.30 ± 2.28a | 34.05 ± 2.89ab |
| SOD (U/g tissue) | 3.65 ± 0.41 | 0.61 ± 0.11a | 1.74 ± 0.18ab |
| CAT (U/g tissue) | 2.17 ± 0.18 | 0.67 ± 0.14a | 1.28 ± 0.18ab |
The mean difference is significant at p < 0.05
Each group contains 10 rats
a Significance versus control, b Significance versus HFD
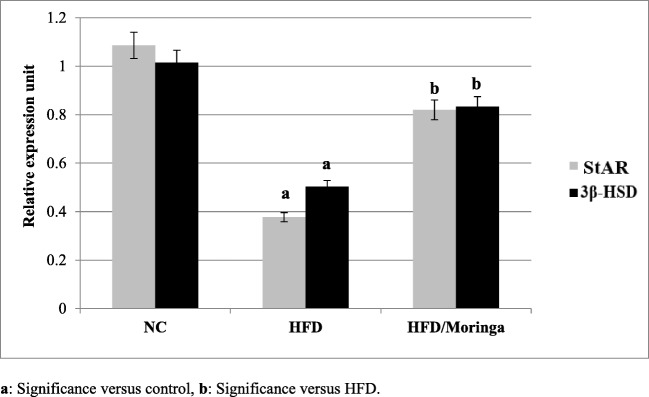
Figure 4 revealed significant reductions (p < 0.001) in the testicular StAR and 3β-HSD mRNA levels in the HFD group, compared to the control group. Distinctively, the addition of Moringa was significantly improved the testicular StAR (p < 0.006) expression and 3β-HSD mRNA (p < 0.02) levels, compared to the HFD group, but still non significantly lower than the control levels.
Fig. 4.
Effect of Moringa oleifera on the testicular StAR and 3β-HSD gene expression in HFD-fed rats. Data are presented as mean ± SE and were analyzed using ANOVA followed by Post hoc test. The mean difference is significant at p < 0.05. Each group contained 10 rats
Discussion
Plants are an important source of new drugs. Many reports on the nutritional value of Moringa exist in the scientific literature as well as in the popular one. For many years, M. oleifera was used in the traditional treatment of diabetes and infertility [39, 40]. Hence this study was undertaken to determine the effects of leaves extract of M. oleifera to reverse insulin resistance and associated testicular disorders in the high fructose-fed rats.
In order to assess the concomitant effects of the administered dose level of Moringa extract, all animals were weighed weekly to record the body weight changes at each week point, compared to their initial body weight. In comparison to the normal control, statistically non-significant changes were chronicled in the body weight gain. Organ weight can also serve as a sensitive index of the effect of a tested compound, since the differences in organ weight may occur without any morphological changes between treated and untreated animals [41]. Thus, the calculation of organ weight to the whole-body weight ratio justifies the usefulness of the data obtained. No significant changes were recorded in the relative weight of testis in all studied groups. With regard to the relative liver weight, fructose-fed rats (HFD and HFD/Moringa groups) showed an increase in the relative liver weight. For the HFD group, this may be attributed to increase liver fats as previously reported [42–44]. However, in the case of HFD+ Moringa group, this increase may be related to the increase in the body weight, since this group has the highest body weight gain percent. These results indicate that the plant extract has nontoxic effects on the body weight.
Feeding rats with HFD for one month reduced the insulin sensitivity as indicated by hyperglycemia and the increased levels of serum insulin as well as calculated HOMA-IR. Additionally, HFD down-regulates the hepatic insulin receptor and its substrate (IR and IRS-1) in addition to the glucose transporters (GLUT4 and GLUT5). These results agree with previous studies which reported impaired insulin action and a decrease in GLUT4 expression in insulin resistance [44–48].
In normal physiology, the transduction of insulin signal occurs through the phosphatidyl inositol-3-kinase (PI3K) pathway that induces the uptake of insulin-dependent glucose in muscle and fat. However, in the pathophysiological case of insulin resistance, this pathway is selectively impaired [49]. Thus, the noticed hyperglycemia may be a result of the impaired glucose uptake by tissues and/or due to elevated levels of hepatic glucose-6-phosphatase that catalyzes the reaction of both glycogenolysis and gluconeogenesis [50].
It was reported that the aqueous extract or even the tablet form of M. oleifera leaves had significant hypoglycemic and antidiabetic potential in diabetic rats and human, respectively [51–53]. However, Tende et al. [54] reported that the ethanolic extract of M. oleifera leaves reduced blood glucose levels only in diabetic rats but not in normal animals. However, in this study the administration of Moringa reduced the fasting blood insulin level and HOMA-IR value along with a non-significant reduction in the fasting blood glucose level as compared to the HFD group. Moreover, Moringa extract not only up-regulated the expression of hepatic IR and IRS-1 but also increased the expression of GLUT4 in rats’ liver as compared to the HFD group. These molecular changes exhibiting the protective effect of Moringa against high-fructose evoked down-regulation of the insulin signaling pathway. Thus, the aqueous extract of leaves has to some extent an unambiguous effect on tissues by enhancing their glucose uptake, by inhibiting gluconeogenesis in liver or entrance of glucose into the muscles and adipose tissues. Hence, the weight gain after administration of the extract in Moringa treated HFD-fed rats is simply attributed to the ability of the extract to improve the hepatic insulin resistant state.
Hyperinsulinemia, hyperglycemia in addition to fructose itself create a state of oxidative stress as a result of free radical production. In the current study, the oxidative stress was indicated by a marked elevation of the hepatic MDA, the marker of lipid peroxidation. Moreover, insulin-resistant rats (HFD group) displayed impairment in the antioxidant defense system indicated by the highly significant reduction in hepatic SOD and CAT as compared to the control group. These results agree with previous studies [44, 46, 55].
Importantly, lower hepatic levels of MDA indicated that lipid peroxidation is decreased in hyperinsulinemic rats treated with Moringa extract. This is possibly explained by the lower availability of the reactive oxygen species (ROS)-induced lipid peroxidation in the liver since Moringa extract possesses many types of free radical scavengers, as flavonoids and phenols [56]. Additionally, the water extract of Moringa leaves has some direct effect on the antioxidant enzymes at both protein and gene levels.
Previous studies proposed that ROS prohibit the StAR protein function in the steroidogenic cells [57]. The steroidogenic acute regulatory protein (StAR) is responsible for the cholesterol transport into the mitochondria, which is the rate-limiting step in the steroid hormones biosynthesis in the testis [58, 59]. Additionally, the testicular steroid synthesis changes may be attributed to the changes in steroidogenic enzymes. One of the key enzymes in androgens biosynthesis and other active steroids is 3β-HSD. Therefore, enhanced 3β-HSD activity in the testes is crucial for normal steroidogenesis and reproduction [60]. In the present study, these two steroidogenic enzymes were down-regulated in HFD-fed rats resulting in the reduction of serum testosterone level.
The primary function of testosterone is to stimulate spermatogenesis and support the maturation of immature spermatozoa [61, 62]. It has been affirmed that insulin signaling is important for spermatogenesis, sperm maturation and quality, and steroidogenesis [63, 64]. Insulin resistance may cause the instigation of high oxidative stress which could affect the normal functioning of the hypothalamus and pituitary gland which directly suppress the release of gonadotrophin-releasing hormone (GnRH) and FSH/LH, respectively [65]. This could be the reason for the decrease of testosterone and FSH hormones in HFD-fed rats.
In the present experiment, insulin resistance induced disturbances in spermatogenesis in rats have been improved with Moringa application which modulates insulin signaling. Moreover, the preliminary phytochemical examination of the leaves extract of M. oleifera revealed the existence of alkaloids, saponins, phenols, tannins, and flavonoids. It was reported that plant steroid and saponin reign fertility potentiating properties. Saponin may increase the level of testosterone in the body, as observed in this study [66]. Moreover, alkaloids and flavonoids alter the androgen levels [67]. So, the improvement in the sexual function illustrated in the present investigation might be attributed to the presence of such phytochemicals in M. oleifera.
Conclusions
Our findings shed light on the mechanism by which Moringa improves metabolic health. Moringa modulates key hepatic genes involved in the modulation of the insulin signaling, thus markedly improving the insulin resistance state. Additionally, Moringa improves the testicular function. Further investigations are required to recognize the active constitutes responsible for these improvement activities.
Acknowledgments
This study was done in Faculty of Science, Al-Azhar University, Cairo, Egypt. Authors thank the laboratory technical staff in making specimens available for processing. The authors gratefully acknowledge staff of Botany, Zoology and Chemistry Departments, for their organizational support throughout the experimental period.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interests in this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zagrodzki P, Joniec A, Gawlik M, Gawlik M, Krośniak M, Fołta M, Bartoń H, Paśko P, Chłopicka J, Zachwieja Z. High fructose model of oxidative stress and metabolic disturbances in rats. part I. antioxidant status of rats’ tissues. Bull Vet Inst Pulawy. 2007;51:407–412. [Google Scholar]
- 2.Zhang D, Jiao R, Kong L. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients. 2017;9:335. doi: 10.3390/nu9040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Consensus Development Conference on Insulin Resistance. Diabetes Care. 1997;21:310–314. doi: 10.2337/diacare.21.2.310. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE. Guyton and Hall textbook of medical physiology e-Book. Amsterdam: Elsevier Health Sciences; 2015. [Google Scholar]
- 5.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 6.Olatunbosun ST, Schade D. Insulin resistance. eMedicine. Accessed February 26, 2018.
- 7.Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14:605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 9.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 11.Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo-pituitary-adrenal axis in streptozotocin-induced diabetes: effects of insulin treatment. Endocrinol. 2001;142:4872–4879. doi: 10.1210/endo.142.11.8474. [DOI] [PubMed] [Google Scholar]
- 12.Shi G-J, Zheng J, Wu J, et al. Protective effects of Lycium barbarum polysaccharide on male sexual dysfunction and fertility impairments by activating hypothalamic pituitary gonadal axis in streptozotocin-induced type-1 diabetic malemice. Endocr J. 2017;64:907–922. doi: 10.1507/endocrj.EJ16-0430. [DOI] [PubMed] [Google Scholar]
- 13.Park K, Gross M, Lee D, Holvoet P, Himes JH, Shikany JM, Jacobs DR. Oxidative Stress and Insulin Resistance The Coronary Artery Risk Development in Young Adults study. Diabetes Care. 2009;32:1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:1–11. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caceres A, Saravia A, Rizzo S, Zabala L, Leon ED, Nave F. Pharmacology properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J Ethnopharmacol. 1992;36:233–237. doi: 10.1016/0378-8741(92)90049-w. [DOI] [PubMed] [Google Scholar]
- 16.Udupa SL, Udupa AL, Kulkarni DR. Studies on anti-inflammatory and wound healing properties of Moringa oleifera and Aegle marmelos. Fitoterapia. 1994;65:119–123. [Google Scholar]
- 17.Pal SK, Mukherjee PK, Saha BP. Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother Res. 1995;9:463–465. [Google Scholar]
- 18.Prabsattroo T, Wattanathorn J, Iamsa-ard S, Muchimapura S, Thukhammee W. Moringa Oleifera Leaves Extract Attenuates Male Sexual Dysfunction. Am J Neurosci. 2012;3:17–24. [Google Scholar]
- 19.Rajasekar P, Kaviarasan S, Anuradha CV. L-carnitine administration prevents oxidative stress in high fructose-fed insulin resistant rats. Diabetol Croat. 2005;34:21–28. [Google Scholar]
- 20.Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brieskorn CZ, Klinger H, Polonius W. Triterpenes and sterols in leaves of Salvia trioloba and Pyrus malus. Arch Pharm. 1961;294:389–391. [Google Scholar]
- 22.Geissmann TA. The Chemistry of Flavonoids Compounds. New York: Pergamon Press; 1962. [Google Scholar]
- 23.Woo WS, Chi HJ, Yun HS, Hye S. Alkaloid screening of some Saudi Arabian plants. Kor J Pharmacog. 1977;8:109–113. [Google Scholar]
- 24.Treare GE, Evans WC. Pharmacognosy. 17. London: Bahiv Tinal; 1985. [Google Scholar]
- 25.Kokate CK, Purohit AP, Gokhale SB. Carbohydrate and derived products, drugs containing glycosides, drugs containing tannins, lipids and protein alkaloids. Textbook of Pharmacognosy. 2001.
- 26.Ahmad B, Naeem AK, Ghufran A, Innamudin I. Pharmacological Investigation of Cassia sophera, Linn. Var. purpura, Roxb. Med J Islam World Academy Sci. 2005;15:105–109. [Google Scholar]
- 27.Nikhal SB, Dambe PA, Ghongade DB, Goupale DC. Hydroalcoholic extraction of Mangifera indica (leaves) by Soxhletion. Inter J Pharm Sci. 2010;2:30–32. [Google Scholar]
- 28.Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, Sharma G, Sharma B, Khair S, Watal G. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;2013:426–432. doi: 10.1016/S1995-7645(13)60068-1. [DOI] [PubMed] [Google Scholar]
- 29.Sharp P. Interference in glucose oxidase-peroxidase blood glucose methods. Clin Chem Acta. 1972;40:115–120. doi: 10.1016/0009-8981(72)90257-4. [DOI] [PubMed] [Google Scholar]
- 30.Dhahir FJ, Cook DB, Self CH. Amplified enzyme-linked immunoassay of human pro-insulin in serum. Clin Chem. 1992;38:227–232. [PubMed] [Google Scholar]
- 31.Pickavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JP. Therapeutic index for rosiglitazone in dietary obese rats. Separation of efficacy and haemodilution. Br J Pharmacol. 1999;128:1570–1576. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosner W, Auchus RJ, Azzis R. Position statement: utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society Positions Statement. J Clin Endocrinol Metab. 2007;92:404–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 33.Clarke JJ, Cummins JT. Pulsatility of reproductive hormones: physiological basis and clinical implications. Baillieres Clin Endocrinol Metab. 1987;1:1–21. doi: 10.1016/s0950-351x(87)80050-2. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi W, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Nishikimi M, Roa NA. Yogi k. Measurement of superoxide dismutase. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. In: Packer L, editor. Methods in Enzymology. Oxygen Radicals in Biological systems. Orlando, FL: Academic Press; 1984. pp. 121–126. [Google Scholar]
- 37.Rizk SM, Zaki HF, Mina MA. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem Toxicol. 2014;67:176–186. doi: 10.1016/j.fct.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003;243:147–152. doi: 10.1023/a:1021620414979. [DOI] [PubMed] [Google Scholar]
- 39.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 40.Zade VS, Dabhadkar DK, Thakare VG, Pare SR. Effect of Aqueous Extract of Moringa oleifera Seed on Sexual Activity of Male Albino Rats. Biological Forum – An International Journal. 2013;5:129–140. [Google Scholar]
- 41.Bailey SA, Zidell RH, Perry RW. Relationships Between Organ Weight and Body/Brain Weight in the Rat: What Is the Best Analytical Endpoint? Toxicol Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 42.Kumamoto R, Uto H, Oda K, Ibusuki R, Tanoue S, Arima S, Mawatari S, Kumagai K, Numata M, Tamai T, Moriuchi A, Fujita H, Oketani M, Ido A, Tsubouchi H. Dietary fructose enhances the incidence of precancerous hepatocytes induced by administration of diethylnitrosamine in rat. Eur J Med Res. 2013;18:54. doi: 10.1186/2047-783X-18-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz J, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, Bergeron N, Bersot TP, Rao MN, Schambelan M, Mulligan K. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J Clin Endocrinol Metab. 2015;100:2434–2442. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abd El-Wahab HMF, Mohamed MA, El Sayed HH, Bauomy AE. Modulatory effects of rice bran and its oil on lipid metabolism in insulin resistance rats. J Food Biochem. 2017;41:e12318. [Google Scholar]
- 45.Nieto-Vazquez I, Fernández-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Mahfouz MH, Ghanem HM, Mohamed MA. Modulation of insulin receptor substrate-1 and some inflammatory variables in hyperinsulinemic rats treated with cinnamon extract. Am J Biochem Biotech. 2010;6:11–18. [Google Scholar]
- 47.Hu Y, Hou Z, Yi R, Wang Z, Sun P, Li G, Zhao X, Wang Q. Tartary buckwheat flavonoids ameliorate high fructose-induced insulin resistance and oxidative stress associated with the insulin signaling and Nrf2/HO-1 pathways in mice. Food Funct. 2017;8:2803–2816. doi: 10.1039/c7fo00359e. [DOI] [PubMed] [Google Scholar]
- 48.López M, Rios-Silva M, Huerta M, Cárdenas Y, Bricio-Barrios JA, Diaz-Reval MI, Urzúa Z, Huerta-Trujillo M, López-Quezada K, Trujillo X. Effects of Moringa oleifera leaf powder on metabolic syndrome induced in male Wistar rats: a preliminary study. J Inter Med Res. 2018;46:3327–3336. doi: 10.1177/0300060518781726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munir KM, Chandrasekaran S, Gao F, Quon MJ. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: therapeutic implications for diabetes and its cardiovascular complications. Am J Physiol Endocrinol Metab. 2013;305:E679–E686. doi: 10.1152/ajpendo.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nandhini AT, Anuradha CV. Taurine modulates kallikrein activity and glucose metabolism in insulin-resistant rats. Amino Acids. 2002;22:27–38. doi: 10.1007/s726-002-8199-3. [DOI] [PubMed] [Google Scholar]
- 51.Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123:392–396. doi: 10.1016/j.jep.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Kumari DJ. Hypoglycemic effect of Moringa oleifera and Azadirachta indica in type-2 diabetes. Bioscan. 2010;5:211–214. [Google Scholar]
- 53.Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats. Acta Histochem. 2014;116:844–854. doi: 10.1016/j.acthis.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Tende JA, Ezekiel I, Dikko AAU, Goji ADT. Effect of Ethanolic Leaves Extract of Moringa oleifera on Blood Glucose Levels of Streptozocin-Induced Diabetics and Normoglycemic Wistar Rats. Br J Pharmacol Toxicol. 2011;2:1–4. [Google Scholar]
- 55.Abdel-Kawi SH, Hassanin KM, Hashem KS. The effect of high dietary fructose on the kidney of adult albino rats and the role of curcumin supplementation: A biochemical and histological study. Beni-Suef Univ J Basic App Sci. 2016;5:52–60. [Google Scholar]
- 56.Akunna GG, Ogunmodede OS, Saalu CL, Ogunlade B, Bello AJ, Salawu EO. Ameliorative Effect of Moringa oleifera (drumstick) Leaf Extracts on Chromium-Induced Testicular Toxicity in Rat Testes. World J Life Sci and Med Res. 2012;2:20. [Google Scholar]
- 57.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- 58.Christenson LK, Strauss I, Jerome F. Steroidogenic acute regulatory protein; an update on its regulation and mechanism of action. Arch Med Res. 2001;32:576–586. doi: 10.1016/s0188-4409(01)00338-1. [DOI] [PubMed] [Google Scholar]
- 59.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen MK, Ekstrand B, Zamaratskaia G. Regulation of 3β-Hydroxysteroid dehydrogenase/ Δ5-Δ4 isomerase: A Review. Int J Mol Sci. 2013;14:17926–17942. doi: 10.3390/ijms140917926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen C, McLachlan R. Testosterone deficiency in men: diagnosis and management. Aust Family Phys. 2003;32:422–429. [PubMed] [Google Scholar]
- 62.Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36:353–368. doi: 10.3109/01480545.2012.710631. [DOI] [PubMed] [Google Scholar]
- 63.Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction. 2008;136:313–322. doi: 10.1530/REP-08-0167. [DOI] [PubMed] [Google Scholar]
- 64.Priyadarshani N, Varma MC. Effect of Moringa oleifera leaf powder on sperm count, histology of testis and epididymis of hyperglycaemic mice Mus musculus. Am Inter J Res For Appl Nat Sci. 2014;7:7–13. [Google Scholar]
- 65.Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, Capelli M, Bortoluzzi L. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–104. doi: 10.1016/0026-0495(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 66.Shukla VN, Khanuja SP. Chemical, Pharmacological and botanical studies on Pedalium murex. J Med Aromatic Plant Sci. 2004;26:64–96. [Google Scholar]
- 67.Padashetty SA, Mishra SH. Aphrodisiac studies of Tricholepis glaberrima with supportive action from antioxidant enzyme. Pharm Biol. 2007;45:580–586. [Google Scholar]