Abstract
Purpose
Diabetes mellitus type 2 with damaging effects on reproductive hormones and sperm quality parameters can often cause infertility in men. The aim of this study was to evaluate the effects of endurance, resistance and concurrent training on reproductive hormones, sperm parameter in the diabetic type 2 male rats.
Methods
In this experimental study 60 Wistar rats (200 ± 50 g) were randomly assigned into 5 groups: control; diabetic; diabetic endurance training; diabetic resistance training and diabetic concurrent training. For inducing diabetes, after 12 hours of food starvation nicotinamide (120 mg/kg) and STZ (65 mg/kg) were intraperitoneally injected. Twenty-four hours after the last training session, left epididymis of the rats was examined for studying sperm parameters and blood serum samples were examined for evaluating reproductive hormones. Data were analyzed using one-way ANOVA and Turkey’s Post Hoc test.
Results
Ten weeks of endurance and concurrent training induced significant decrease in the blood glucose in comparison to the diabetic group (P < 0.05). In addition, endurance, resistance and concurrent training induced significant increases in serum testosterone and LH levels in the comparison to the diabetic group (P < 0.005). In addition, sperm parameters revealed significant improvements in compared to the diabetic group (P = 0.002).
Conclusion
Endurance, resistance and combined training might improve sperm parameters, including viability and motility of sperms through increasing the serum testosterone and LH levels in rat model of diabetes mellitus type 2.
Keywords: Diabetes mellitus, Exercise, Male infertility, Testosterone
Introduction
Type 2 diabetes is one of the most common and developing metabolic disorder [1]. Due to uncontrolled high blood glucose, it can cause pathophysiological consequences [2]. Previous research studies in animal models and diabetic patient had been shown deficiency to the reproductive system [3–5]. Type 2 diabetes is a notable cause of cardiovascular, kidney and nerve diseases [6]. Data from animal studies strongly suggest that type 2 diabetes may impairs male fertility at multiple levels as a result of its impacts on endocrine control of spermatogenesis, spermatogenesis itself or by damaging penile erection and ejaculation [7, 8]. Recent studies also have indicated a significantly higher level of sperm nuclear DNA fragmentation in diabetic men [5, 9].
Exercise training is believed as important elements of the treatment strategy in adults with type 2 diabetes. Proper use of exercise could improve insulin sensitivity and decrease the need for insulin [10]. In addition, beneficial effects of exercise training on male reproductive system deficiency have been reported [11]. Although regular physical activity may hinder or delay diabetes, most people with type 2 diabetes are not active [12]. Exercise increases glucose disposal into the contracting muscles, leading to significant decrease in blood glucose concentrations [13]. The aim of this study was to investigate the effectiveness of endurance, resistance and combined training on reproductive hormones and sperm parameter of male rats with type 2 diabetes.
Materials and methods
Experimental animals and protocols
60 eight weeks old Sprague Dawley rats (200-250 g) were kept at controlled temperature (22 ± 2°c) and light/dark conditions (12/12 h) with free access to water and food. Rats were randomly assigned into five groups (1) control with normal blood glucose (2) diabetic animal (3) diabetic animal with endurance training (4) diabetic animal with resistance training (5) diabetic animal with combined training. For all groups n = 12. All research and animal care procedures were performed according to the Guide for the Care and Use of Laboratory Animals (8th edition; National Academies Press; 2011) and approved by the Review Board and Ethics Committee of Arak University of Medical Sciences (Agreement number of the ethics committee for this study is “IR.ARAKMU.REC.1394.329”).
Diabetic induction
Diabetes was induced after 12 h fasting. The rats were injected with nicotine amid (Sigma, USA) dissolved in normal saline at a dose of 120 mg/kg, and after 15 min streptozocin (STZ, Sigma, USA) dissolved in 0.1 M citrate buffer at a dose of 65 mg/kg was given in a single i.p. injection. 72 h after the injection, blood glucose was evaluated. Animals with blood glucose higher than 250 mg/dl were considered as diabetic [14].
Endurance training program
The endurance exercise was performed on a rodent motor-driven treadmill at a 0° slope. The rats exercised for 10 weeks and 5 days per week. The exercise training protocol was divided into 3 stages of familiarization, overload, and finally preservation and stabilization of exercise intensity. In the familiarity stage (first week), the rats walked on treadmill at a speed of 8 m/min for 10-15 min every day. In the overload stage (second to fourth weeks), the rats initially ran on treadmill at a speed of 27 m/min for 20 min, and then during 3 weeks the time of exercise increased (2 min in each session) gradually until reached 60 min. Finally, in the preservation and stabilization stage of exercise intensity, the rats did the endurance exercise for 3 weeks with a speed of 27 m/min for 60 min. In every exercise session, 5 min was allocated to warming up (16 m/min) and 5 min was allocated to cooling down (16 m/min and gradual decrease of intensity to the least amount) [15].
Resistance training program
The resistance training program consisted of climbing a 1 m long ladder, set at a 90-degree angle with a weight (resistance) attached to the animals’ tails. The length of the ladder required the animals to make 26 dynamic movements per climb. Each exercise session included 3 sets of 4 repetitions, at 2 min rest between sets and about 10s rest between repetitions. At the start of the program, the rats were familiarized how to climb the ladder for 1 week. Resistance applied to the rats was 30% of their total weights in the second week. Each week, some weights were added gradually so that in final week of the training, the total resistance applied to each animal was 200% of its total weight (Table 1) [16].
Table 1.
Resistance training in 3 sets of 4 repetitions Load (percent of body weight)
| Week | First | Second | Third | Fourth | Fifth | Sixth | Seventh | Eighth | Ninth | Tenth |
|---|---|---|---|---|---|---|---|---|---|---|
| Load | Familiarity | 30% | 80% | 100% | 120% | 140% | 160% | 180% | 190% | 200% |
Combined training program
The combined training program consisted of the aforementioned resistance and endurance training programs. Each training program was performed on alternate days. The whole program included 5 sessions per week with 2 days of rest in the middle and end of the week.
24 h after the last exercise session, all of the rats were anaesthetized by the injection of chloroform and sacrificed. Blood samples were collected by cardiac puncture and centrifuged at 3500 rpm for 10 min, and the serum samples were stored at −70°c for future analysis. Serum hormone levels of testosterone, LH, FSH were assayed using ELISA kits according to their manufacturer’s instructions. Testosterone (Rat ELISA Kit, Eastbiopharm, China); LH (Rat ELISA Kit, Eastbiopharm, China); FSH (Rat ELISA Kit, Eastbiopharm, China).
Sperm count
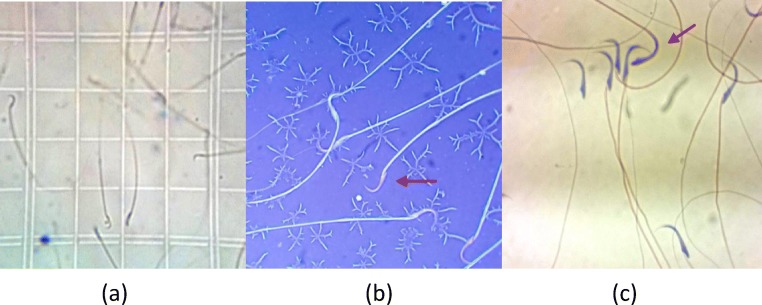
The excised left testis was weighed and the dissected epididymis was transferred into 5 cc (DMEM) medium and cut into small slices, in order to swim out the sperm into the medium. After 10 min of diffusion in 27°c temperature, 1 ml of the solution was diluted with 9 ml formaldehyde fixative. The diluted solution was transferred into each chamber of Neubauer hemocytometer (Fig. 1a) and sperm heads were manually counted under a microscope. Sperm count was carried out according to WHO guidelines and data was explained as the number of sperm per ml [17].
Fig. 1.
a. Sperms were counted through hemocytometer; b. Sperm viability were investigated through Eosin-Nigrosin staining; c. Sperm morphology were investigated through the papanicolao staining
Sperm motility
Measurement of sperm motility was performed according to WHO protocol. 10 μl of the sperm suspension was located on a microscope slide and coversliped. A minimum of five microscope fields were investigated to evaluate sperm motility on at least 200 sperm for each animal, then the percentage of sperm motility was computed [17].
Sperm viability
Eosin-nigrosin staining was used to evaluate sperm viability according to WHO protocol (Fig. 1). In this protocol, eosin (1%, Merk, Germany) and nigrosin (10%, Merk, Germany) were prepared in distilled water. At first, one volume of sperm suspension was blended with two volume of 1% eosin, then after 30 s, an equal volume of nigrosin was added to this prepared mixture. Finally, thin smears were assembled and observed under a light microscope with a magnification of 100X and the ratio of the live sperms percentage in different groups was computed. In this method, viable sperms appeared white while nonviable sperms stained red inclined to violet [17].
Sperm morphology
Before morphologic investigation of the sperm of each group, smears prepared from sperm suspension stained by the way of payanicolao (Fig. 1c), and then air dried and were utilized according to WHO. In each sample, 100 sperms with a magnification of 100X were investigated and existing abnormalities were stated as a percentage [17].
Statistical analysis
A Shapiro-Wilk test was applied to determine the normality of distribution of measures which were found to be normally distributed, and then a Leven test indicated that the variances were homogeneous. A one-way analysis of variance (ANOVA) was performed to determine the statistical differences among the groups. Significant differences were identified using a (Tukey) post hoc test. Data were expressed as means ±SD and significance was set as p < 0.05.
Findings
Body and testis weight
For each animal, body weight and left testis weight were recorded at the end of the period. No significant differences were found in the mean weight of all groups. But there were significant differences in the mean left testis weight of rats in control group compared to those in diabetic control group and diabetic endurance training group p = 0.046 (Table 2).
Table 2.
Body and left testis weight (Mean ± SD)
| Group | Body Weight (g) | Testis Weight (g) | |
|---|---|---|---|
| Pre test | Post test | ||
| Control | 219.4 ± 71 | 279.4 ± 38 | 1.58 ± 0.19 |
| Diabetic | 223.6 ± 36 | 250.6 ± 48 | 1.37 ± 0.25 |
| Diabetic Endurance Exercise | 224.3 ± 20 | 227.4 ± 38 | 1.26 ± 0.15 * |
| Diabetic Resistance Exercise | 219.2 ± 8 | 261.7 ± 37 | 1.40 ± 0.32 |
| Diabetic Combined Exercise | 215.2 ± 19 | 213.9 ± 33 * | 1.26 ± 0.23 * |
*Significant difference in comparision with control group (p < 0.05); Tukey post-test following the one-way ANOVA
Blood glucose
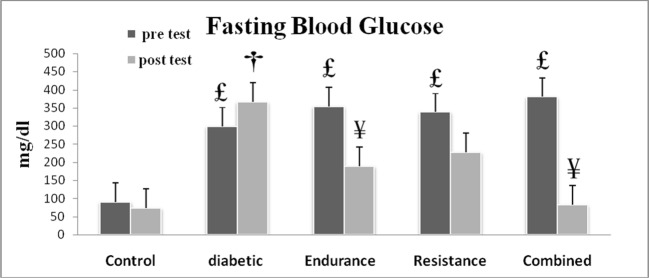
In investigating blood glucose levels of different groups, the results of the research indicated that there was no significant difference in fasting blood glucose levels of diabetic control group and diabetic endurance training group in the beginning of the period (p = 0.311), but after 10 weeks of endurance training, the results showed that there was a significant decrease in fasting blood glucose of diabetic endurance training group compared to diabetic control group (p = 0.13) (Fig. 2).
Fig. 2.
Fasting blood glucose of the training groups in the first week of training period (pre test) and after 10 weeks of the training (post test). £Significant difference when compared with pre test of control group; †significant difference when compared with post test of control group; ¥significant difference when compared with post test of diabetic group. Data reported as Mean ± SD (p < 0.05)
Sperm count
The results showed a highly significant decrease in epididymal sperm number of diabetic control group compared to healthy control group (p < 0.000). The results also showed that the average sperm number of diabetic endurance training group (26 ± 13.2) was higher than that of diabetic control group (11.75 ± 5.7), but this difference was not statistically significant. In other words endurance training could not statistically compensate destructive effect of type 2 diabetes on the sperm number.
Sperm viability
The mean percentage of viable sperms in diabetic control group was significantly lower than healthy control group (p = 0.000). On the other hand, there was a significant decrease in sperm viability in diabetic endurance training group compared to diabetic control group (p = 0.000). In other words, endurance training could significantly compensate destructive effect of type 2 diabetes on the sperm viability.
Sperm morphology
There was a significant difference in the mean percentage of morphologic natural sperms between the rats of healthy control group and diabetic control group (p < 0.000), but the difference between diabetic control group and diabetic endurance training group was not significant (p = 0.067) indicating the endurance training was not able to have positive impact in reducing morphologic effects of type 2 diabetes.
Sperm motility
The mean percentage of sperm motility in diabetic control group was significantly lower than that in control healthy group (p = 0.000), while there was a significant increase in sperm motility in diabetic endurance training group compared to diabetic control group (p = 0.000). In other words, endurance training was able to significantly compensate destructive effects of diabetes type 2 on sperm motility (Table 3).
Table 3.
Data for epididymal sperm number, motility, viability and morphology demonstrated in the experimental groups. (Means±SD)
| Group | Sperm Count (106) | Sperm Viability (%) | Sperm Motility (%) | Sperm Morphology (%) |
|---|---|---|---|---|
| Control | 39.3 ± 13 | 77.5 ± 4.6 | 60.8 ± 6.5 | 95.4 ± 1.3 |
| Diabetic | 11.7 ± 5.7 a | 29.7 ± 16.2 a | 32.5 ± 1.1 a | 85.2 ± 7.5 a |
| Diabetic Endurance Exercise | 26 ± 13.2 | 41.7 ± 7.2 a | 40 ± 6.5 a | 88 ± 8.8 |
| Diabetic Resistance Exercise | 31.7 ± 10 b | 60.6 ± 8 abc | 41.4 ± 4.5 ab | 89.1 ± 6.9 |
| Diabetic Combined Exercise | 31 ± 5.7 b | 59.2 ± 8.5 abc | 34.4 ± 2.6 ab | 93.4 ± 2.7 |
One-way ANOVA and Tukey post-test were used for data analysis. asignificant difference between control and diabetic groups (p < 0.05); bsignificant difference between control and diabetic endurance training group (p < 0.05); csignificant difference between diabetic group and diabetic endurance training group (p < 0.05).
Hormonal levels
The results of the study showed that the means of serum testosterone and LH concentrations decreased significantly in diabetic control group when compared with healthy control group (p = 0.006) & (p = 0.000). The data also revealed that the means of serum testosterone and LH concentrations of diabetic endurance training group were significantly greater than those of diabetic control group (p = 0.000) & (p = 0.002). In the other words, it is thought that endurance training was able to compensate the destructive effects of diabetes on sperm parameters through increases in serum levels of testosterone and LH (Table 4).
Table 4.
Measured level of luteinizing hormone (LH; mIU/ml), follicle stimulating hormone (FSH; mIU/ml), testosterone (nmol/l), adiponectin (mg/l) and Insulin (mIU/L) in experimental groups
| Group | LH (mIU/ml) | FSH (mIU/ml) | Testosterone (nmol/L) |
|---|---|---|---|
| Control | 5.6 ± 1.6 | 4.1 ± 1.5 | 4.5 ± 0.9 |
| Diabetic | 3.3 ± 0.4 a | 2.7 ± 0.33 | 3.3 ± 0.2 a |
| Diabetic Endurance Exercise | 6.7 ± 1.9 b | 5.8 ± 4.01 b | 4.8 ± 0.9 b |
| Diabetic Resistance Exercise | 6.3 ± 1.6 b | 4.4 ± 0.75 | 5.2 ± 1.5 b |
| Diabetic Combined Exercise | 6.3 ± 1.8 b | 5 ± 4.27 | 5 ± 1.8 b |
One-way ANOVA and Tukey post-test were used for data analysis. asignificant difference between control and diabetic groups (p < 0.05), bsignificant difference between control group and diabetic endurance training group (p < 0.05).
Discussion
In the present study we examined the effects of 10 weeks of endurance, resistance and combined training on sperm parameters, serum levels of sex hormones in rats model of type 2 diabetes induced by streptozocin-nicotinamid. Our data revealed significant decrease in sperm parameters and serum level of sex hormones of diabetic rats. While training protocols were able to repair these negative effects.
Previous reports indicating negative consequences of diabetic situation on reproductive hormones in different animal models of diabetes [18, 19]. In accordance with the previous studies, our data confirmed decreased level of testosterone, LH and FSH following streptozotocin-nicotinamide induced diabetic conditions [20, 21]. In addition and probably as consequences of this hormonal deficiency, sperm count, their motility and morphology negatively were affected in diabetic animals.
We investigated the effects of endurance, resistance and combined training on serum levels of sex hormones in diabetic rats and our data indicates that endurance, resistance and concurrent training in diabetic animals induced significant increases in serum level of testosterone and LH in the comparison with the non-trained diabetic animals. This finding is in agreement with those studies which had been shown the increase in sexual hormones in response to exercise and training [22].
The results also revealed that the serum levels of testosterone and LH in diabetic rats decreased significantly, but it was not significant for FSH levels. The decrease has caused a significant reduction in all cases of sperm parameters (count, motility, morphology, and viability) in diabetic group. Also, 10 weeks of endurance exercise caused a significant reduction in blood glucose of experimental group. This resulted in the return of testosterone and LH hormones to natural levels and no significant difference between healthy control group and experimental group was observed. There was also no difference between two groups in respect of sperm parameters (sperm count and sperm morphology). This shows that endurance, resistance and combined training improves these factors of fertility.
Diabetes causes oxidative stress and free radicals that reduce different cells of testis and serum testosterone levels [4]. Further, the increase of oxidative stress damage to sperm DNA and causes the death of sperm cells, which is responsible for poor reproductive outcomes in diabetic men, While it has been clearly shown that regular endurance exercise reduces the effects of oxidative stress resulted from diabetes [11]. Additionally, Gordon et al. (2008) examined the effects of exercise therapy on oxidative stress in patients with type 2 diabetes in trained group compared to untrained one, they found that 24 weeks of endurance exercise (4 sessions per week and 2 h per session) reduces MDA and oxidative stress index of lipids, but increases SOD, which is an antioxidant capacity index [23]. In addition, Botezelli et al. (2011) examined the effect of concurrent training on the antioxidant status in rats and found that concurrent training significantly reduced lipid peroxidation markers (TBARS) and increased serum superoxide dismutase activity (SOD) [24].
Therefore, one of the suggested mechanisms in improving fertility in diabetic people following a regular endurance exercise is: reducing oxidative stress, increasing antioxidant capacity, and protecting different types of testicular cells, which are responsible for spermatogenesis and secreting testosterone [10, 25].
Endurance, resistance and concurrent training can also influence sexual hormones of testosterone, LH, and FSH independently. In a study, Tremblay et al. (2005) found that after endurance training for 40 min and 80 min, serum levels of testosterone increase and serum levels of LH decrease [26]. Also Cadore et al. (2012) had been reported that resistance training resulted in increased levels of total testosterone and free testosterone [27]. In contrast, some studies indicate different findings, Hackney et al. (2008), for example, found that long distance endurance training causes the reduction of rest testosterone concentrations, while LH remains unchanged [25]. Whereas it seems that few studies have been conducted concerning the effects of endurance and resistance exercise on serum levels of sexual hormones and sexual hormones of testosterone, LH, FSH, and sperm parameters in diabetic men. Therefore, it is suggested that this research be conducted on diabetic men and other kinds of exercises, which decline diabetes effects following changes in serum levels of sexual hormones, in order to find suitable strategies in improving fertility in diabetic men.
Conclusion
The results demonstrated that diabetes is associated with dysfunction in sperm parameters in the rat model. These dysfunctions might be due to the high blood glucose and also low levels of sex hormones. On the other hand, it seems that endurance, resistance and combined training could be able to improve this fertility status of diabetic rats through reducing the blood glucose, increasing the serum concentrations of testosterone and LH, and also its positive effects on sperm parameters.
Acknowledgments
This research was funded by Bu-Ali Sina University, Hamadan, Iran. Authors thanks Arak University of Medical Sciences for technical support.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coppari R. Diabetes present and future. Int J Biochem Cell Biol. 2017;88:197. doi: 10.1016/j.biocel.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13(1):3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 3.Agbaje I.M., Rogers D.A., McVicar C.M., McClure N., Atkinson A.B., Mallidis C., Lewis S.E.M. Insulin dependant diabetes mellitus: implications for male reproductive function. Human Reproduction. 2007;22(7):1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 4.Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod BioMed Online. 2012;25(3):292–299. doi: 10.1016/j.rbmo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Casado L, Juncos-Tobarra MA, Chafer-Rudilla M, de Onzono LI, Blazquez-Cabrera JA, Miralles-Garcia JM. Effect of experimental diabetes and STZ on male fertility capacity. Study in rats. J Androl. 2010;31(6):584–592. doi: 10.2164/jandrol.108.007260. [DOI] [PubMed] [Google Scholar]
- 6.Bate KL. And G Jerums, 3: Preventing complications of diabetes. Med J Aust. 2003;179(9):498–503. doi: 10.5694/j.1326-5377.2003.tb05655.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Malini T, Rengarajan S, Balasubramanian K. Impact of experimental diabetes and insulin replacement on epididymal secretory products and sperm maturation in albino rats. J Cell Biochem. 2009;108(5):1094–1101. doi: 10.1002/jcb.22337. [DOI] [PubMed] [Google Scholar]
- 8.Shi Guang-Jiang, Zheng Jie, Wu Jing, Qiao Hai-Qi, Chang Qing, Niu Yang, Sun Tao, Li Yu-Xiang, Yu Jian-Qiang. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food & Function. 2017;8(3):1215–1226. doi: 10.1039/C6FO01575A. [DOI] [PubMed] [Google Scholar]
- 9.Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev. 2014;10(3):147–157. doi: 10.2174/1573399810666140606111745. [DOI] [PubMed] [Google Scholar]
- 10.Colberg S. R., Sigal R. J., Fernhall B., Regensteiner J. G., Blissmer B. J., Rubin R. R., Chasan-Taber L., Albright A. L., Braun B. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos M, Rodríguez-González GL, Ibáñez C, Vega CC, Nathanielsz PW, Zambrano E. Adult exercise effects on oxidative stress and reproductive programming in male offspring of obese rats. Am J Physiol Regul Integr Comp Physiol. 2015;308(3):R219–R225. doi: 10.1152/ajpregu.00398.2014. [DOI] [PubMed] [Google Scholar]
- 12.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 13.Musi N., Fujii N., Hirshman M. F., Ekberg I., Froberg S., Ljungqvist O., Thorell A., Goodyear L. J. AMP-Activated Protein Kinase (AMPK) Is Activated in Muscle of Subjects With Type 2 Diabetes During Exercise. Diabetes. 2001;50(5):921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 14.Punitha IS, et al. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2(3):375–381. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afzalpour ME, Chadorneshin HT, Foadoddini M, Eivari HA. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol Behav. 2015;147:78–83. doi: 10.1016/j.physbeh.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Kim Hee-Jae, So Byunghun, Son Jun Seok, Song Han Sol, Oh Seung Lyul, Seong Je Kyung, Lee Hoyoung, Song Wook. Resistance training inhibits the elevation of skeletal muscle derived-BDNF level concomitant with improvement of muscle strength in zucker diabetic rat. Journal of Exercise Nutrition & Biochemistry. 2015;19(4):281–288. doi: 10.5717/jenb.2015.15112402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Yongxin, Yang Jiali, Jia Yanping, Xiong Chengliang, Meng Tianqing, Guan Huangtao, Xia Wei, Ding Mingyue, Yuchi Ming. Variability in the morphologic assessment of human sperm: use of the strict criteria recommended by the World Health Organization in 2010. Fertility and Sterility. 2014;101(4):945–949. doi: 10.1016/j.fertnstert.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25(5):706–719. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 19.Olivares Aleida, Méndez Juan Pablo, Cárdenas Mario, Oviedo Norma, Palomino Miguel Ángel, Santos Isis, Perera-Marín Gerardo, Gutiérrez-Sagal Rubén, Ulloa-Aguirre Alfredo. Pituitary–testicular axis function, biological to immunological ratio and charge isoform distribution of pituitary LH in male rats with experimental diabetes. General and Comparative Endocrinology. 2009;161(3):304–312. doi: 10.1016/j.ygcen.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Ahangarpour A, Oroojan AA, Heidari H, Ghaedi E, Taherkhani R. Effects of hydro-alcoholic extract from Arctium lappa L. (burdock) root on gonadotropins, testosterone, and sperm count and viability in male mice with nicotinamide/ Streptozotocin-induced type 2 diabetes. Malays J Med Sci. 2015;22(2):25–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaei N, Mardanshahi T, Shafaroudi MM, Abedian S, Mohammadi H, Zare Z. Effects of l-carnitine on the follicle-stimulating hormone, luteinizing hormone, testosterone, and testicular tissue oxidative stress levels in Streptozotocin-induced diabetic rats. J Evid Based Integr Med. 2018;23:2515690X18796053. doi: 10.1177/2515690X18796053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges MC, Lawlor DA, de Oliveira C, White J, Horta BL, Barros AJD. Role of adiponectin in coronary heart disease risk: a Mendelian randomization study. Circ Res. 2016;119(3):491–499. doi: 10.1161/CIRCRESAHA.116.308716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon LA, Morrison EY, McGrowder DA, Young R, Fraser YTP, Zamora EM, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med. 2008;8:21. [DOI] [PMC free article] [PubMed]
- 24.Botezelli JD, Cambri LT, Ghezzi AC, Dalia RA, M Scariot PP, Ribeiro C, et al. Different exercise protocols improve metabolic syndrome markers, tissue triglycerides content and antioxidant status in rats. Diabetol Metab Syndr. 2011;3:35. [DOI] [PMC free article] [PubMed]
- 25.Hackney AC. Effects of endurance exercise on the reproductive system of men: the "exercise-hypogonadal male condition". J Endocrinol Investig. 2008;31(10):932–938. doi: 10.1007/BF03346444. [DOI] [PubMed] [Google Scholar]
- 26.Tremblay MS, Copeland JL, Van Helder W. Influence of exercise duration on post-exercise steroid hormone responses in trained males. Eur J Appl Physiol. 2005;94(5–6):505–513. doi: 10.1007/s00421-005-1380-x. [DOI] [PubMed] [Google Scholar]
- 27.Cadore EL and LFM Kruel, Acute and chronic testosterone responses to physical exercise and training, in Sex hormones. 2012, InTech.