Abstract
As an alternative to antimicrobial growth promoters, fermented feed (FF) has been continuously developed for two decades; however, its effects on feed, performance, digestibility, and meat quality of pigs have yet to be systematically and comprehensively evaluated. This study aimed to (i) quantitatively evaluate the effects of fermentation on nutritional components of feed stuffs; (ii) quantitatively evaluate the effects of FF on pig growth performance, digestibility, and meat quality; and (iii) explore the dose–effect relationship. From PubMed and Web of Science (searched range from January 1, 2000 to April 4, 2019), we collected 3,271 articles, of which 30 articles (3,562 pigs) were included in our meta-analysis. Our analysis revealed that fermentation significantly increased the CP content in feed (P < 0.05). For weaned piglets and growing pigs, FF significantly improved ADG, G:F, DM digestibility, N digestibility, and energy digestibility (P < 0.05). However, compared with the basal diet, FF had no significant effects on growth performance and nutrient digestibility in finishing pigs (P > 0.05). In the subgroup analyses, fermented ingredients increased the growth performance of weaned piglets and growing pigs, and fermented additives promoted the growth of pigs at all stages. The dose–effect analysis confirmed that the optimal doses of fermented ingredients and additives were 8% and 0.15%, respectively. Furthermore, FF had beneficial impacts on meat quality through increased lightness, redness, marbling and flavor and reduced drip loss (P < 0.05). In conclusions, FF improved growth performance and meat quality primarily due to its positive effects on nutritive value and utilization.
Keywords: dose–effect relationship, fermented feed, growth performance, meat quality, meta-analysis, pigs
Introduction
Antimicrobial growth promoters (AGPs) are widely used in animal husbandry due to their outstanding effects, such as bacterial pathogen inhibition and growth promotion (Cromwell, 2002; Van Boeckel et al., 2015). However, subtherapeutic AGP-treated animals have become a major contributor to antimicrobial-resistant bacterial strains, which are seriously endangering the public health globally (Marshall and Levy, 2011). Aimed at reversing or at least reducing the rising trend of antimicrobial resistance, governments, and world organizations have initiated a series of countermeasures and encouraged the research and development of AGP alternatives (Jensen and Hayes, 2014). Considering the beneficial impacts on feed safety, nutrient bioavailability, pig growth performance, and meat quality, fermented feed (FF) has been regarded as a novel alternative to AGPs in pigs. However, variability in the fermented products, bacterial strains, and experimental designs has restricted animal nutritionists from comprehensively evaluating the effects of FF on pigs. Thus, we performed a series of meta-analyses to (i) explore the effects of the fermentation process on feed nutrition components; (ii) quantitatively measure different FF factors on growth performance, digestibility, and meat quality in pigs at each growth stage; and (iii) investigate the dose–effect relationship between FF and growth performance to provide strategies for FF application in the pig industry. According to our knowledge, this is the first comprehensive and systematic overall assessment of FF in pigs.
Materials and Methods
This meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Moher et al., 2009).
Search Strategy
We searched for relevant studies published during the last 20 years (from January 1, 2000 to April 8, 2019) on PubMed (https://www.ncbi.nlm.nih.gov/pubmed; accessed April 8, 2019) and Web of Science (http://webofknowledge.com; accessed April 8, 2019). The search strategy involved a combination of the following free terms and keywords: “fermented” and “pig*”. The complete search method is shown in Supplementary Table S1. The search was restricted to articles published in English. In addition, a manual search was performed to obtain more studies.
Inclusion and Exclusion Criteria
Studies were considered eligible if they met the following inclusion criteria: (i) the breeding background was commercial pigs; (ii) the studies investigated changes in the composition of ingredients before and after fermentation; (iii) the studies investigated the effects of FF on pig growth performance; and (iv) the studies investigating the effects of FF on meat quality. The exclusion criteria were as follows: (i) the studies used a Latin Square design; (ii) the pigs were given a probiotic supplementation without a fermentation period for the feedstuff; (iii) the studies investigated the inhibitory effects of FF on pathogenic bacteria and infective viruses; (iv) the studies investigated distillers dried grains with soluble supplements; (v) the studies investigated fermented liquid feed and liquid feed; (vi) the studies lacked a control group; (vii) the studies did not assess pig growth in stages; and (viii) the studies investigated sows and litters. The summarized information of included studies was showed in Table 1.
Table 1.
Characteristics of the included studies1
| Study | Country | Treatment group | Bacteria | Add amount | Growth stage | Sample size | Crossbreed | Initial BW, kg | Trial duration or final BW at trial end |
|---|---|---|---|---|---|---|---|---|---|
| Ao et al. (2010) | Korea | Fermented soy protein | Aspergillus oryzae GB-107 | 5.00% | Weaned piglets | 60 | L × Y × D | 5.68 | 4 weeks |
| Ao et al. (2011) | Korea | Fermented red ginseng | Bifidobacterium H-1 | 0.10%, 0.20%, 0.40% | Finishing pigs | 48 | L × Y × D | 71.64 | 8 wk |
| Chamorro-Ramirez et al. (2017) | México | Solid-state fermented apple pomace | Yeast | 5.00%, 10.00%, 5.00%, 10.00% | Growing pigs | 8 | L × Y | 47.1 | 4 wk |
| Cho et al. (2007) | Korea | Fermented soy protein | A. oryzae GB-107 | 5.00%, 10.00%, 15.00% | Weaned piglets | 72 | NA | 8.09 | 4 wk |
| Cho et al. (2013) | Korea | Fermented oat | Bacillus subtilis 2-20cx | 5.00%, 10.00%, 15.00%, 20.00% | Growing pigs | 50 | L × Y × D | 20.5 | 2 wk |
| Fermented corn | B. subtilis 2-25cx | 5.00%, 10.00%, 15.00% | Growing pigs | 48 | L × Y × D | 24.4 | 6 wk | ||
| Fermented wheat | Aspergillus niger GB-125 | 5.00%, 10.00%, 15.00%, 20.00% | Growing pigs | 48 | L × Y × D | 29.6 | 6 wk | ||
| Feng et al. (2007) | China | Fermented soybean meal | A. Oryzae | 26.50% | Weaned piglets | 60 | L × Y × D | 8.6 | 17 kg |
| Hung et al. (2008) | China | Fermented soybean mealA/B | Streptococcus thermophilus, Lactobacillus acidophilus, Aspergillus awamori, Bifidobacterium thermophilus, Saccharomyces cerevisiae, Aspergillus niger and Trichoderma koningii | 22.90% | Growing pigs | 32 | Taoyuan×D | 30 | 80 kg |
| Finishing pigs | 32 | Taoyuan×D | 80 | 110 kg | |||||
| Jeong and Kim (2015) | Korea | Fermented medicinal plants | Lactobacillus plantarum, Saccharomyces cerevisiae, and Bacillus licheniformis | 0.05%, 0.10%, 0.20% | Growing pigs | 60 | L × Y | 25.5 | 6 wk |
| Jones et al. (2010) | American | Fermented soybean meal | NA | 3.75%, 6.00%, 7.50% | Weaned piglets | 84 | NA | 6.8 | 4 wk |
| Kim et al. (2006a) | Korea | Fermented persimmon shell | NA | 3.00%, 5.00%, 7.00% | Finishing pigs | 48 | Berkshire | 61 | 103 kg |
| Kim et al. (2006b) | Korea | Fermented persimmon shell | NA | 3.00%, 5.00%, 7.00% | Finishing pigs | 48 | Berkshire | 61 | 103 kg |
| Kim et al. (2006c) | Korea | Fermented soy protein | A. Oryzae | 3.00%, 6.00% | Weaned piglets | 120 | L × Y × D | 5.56 | 5 wk |
| Kraler et al. (2015) | Austria | Fermented wheat | Lactobacillus paracasei and Lactobacillus plantarum | 15.00% | Weaned piglets | 24 | L × Piétrain | 8.36 | 6 wk |
| Lee et al. (2009) | Korea | Fermented apple diet | NA | 2.00%, 4.00%, 6.00% | Finishing pigs | 60 | Berkshires | 81 | 5 wk |
| Li et al. (2011) | China | Fermented potato pulp | S. thermophilus (CGMCC No. 1.2471), B. subtilis (MA193), and S. cerevisae | 5.00% | Growing pigs | 222 | D × L × L | 25.6 | 4 wk |
| 5.00% | Finishing pigs | 191 | D × L × L | 86.4 | 109 kg | ||||
| Liu et al. (2017) | China | Fermented corn bran | B. subtilis MA139, Enterococcus faecium, and S. cerevisae | 10.00% | Finishing pigs | 40 | L × Y × D | 65.73 | 21 days |
| Park and Kim (2018) | Korea | High-density fermented corn | B. Subtilis | 11.60% | Growing pigs | 64 | L × Y × D | 29.59 | 6 wk |
| Low-density fermented corn | B. subtilis | 10.40% | Growing pigs | 64 | L × Y × D | 29.59 | 6 wk | ||
| Rafai et al. (2011) | Hungary | Fermented wheat germ extract | NA | 1.00%, 2.00% | Growing pigs | 16 | Hungarian L | 13.5 | 47 days |
| Shi et al. (2016) | China | Fermented rapeseed meal | A. niger | 10.00% | Growing pigs | 48 | NA | 40.8 | 6 wk |
| Wang et al. (2007) | China | Fermented soybean meal | Lactobacillus plantarum | 5.00%, 10.00% | Weaned piglets | 48 | L × Y × Beijing Black | 7.29 | 7 wk |
| Wang et al. (2014) | China | Fermented soybean meal | B. subtilis MA139 with S. thermophilus and S.cerevisiae | 6.00%, 12.00% | Weaned piglets | 72 | D × L × L | 8.8 | 4 wk |
| Tian et al. (2017); Xu et al. (2017) | China | Fermented biogas residue | Yeast | 5.00%, 10.00%, 15.00% | Growing pigs | 64 | D × L × Y | 40.24 | 60 days |
| Yan et al. (2011) | Korea | Fermented garlic powder | Weissella koreensis | 0.20%, 0.40% | Finishing pigs | 48 | (Y×L)×(Hampshire×D) | 55.8 | 12 wk |
| Yan et al. (2012b) | Korea | Fermented garlic powder | Weissella koreensis | 0.10%, 0.20%, 0.40% | Growing pigs | 40 | L × Y × D | 50.7 | 77 kg |
| Fermented garlic powder | W. koreensis | 0.10%, 0.20%, 0.40% | Finishing pigs | 40 | L × Y × D | 77 | 106 kg | ||
| Yan et al. (2012a) | Korea | Fermented chlorella | Baker’s yeast and lactic acid bacterium | 0.10%, 0.20% | Growing pigs | 48 | L × Y × D | 26.58 | 53.1 kg |
| Yan and Kim (2013) | Korea | Fermented garlic powder | W. koreensis | 0.05%, 0.10%, 0.20% | Weaned piglets | 72 | D × Y × L | 5.5 | 5 wk |
| Yuan et al. (2017) | China | Fermented soybean meal | B. subtilis, Hansenula anomala and Lactobacillus casei | 3.75%, 7.50% | Weaned piglets | 100 | NA | 10.8 | 23.3 kg |
| Zhang et al. (2018) | China | Fermented soybean meal | B. subtilis BS12 | 10.00% | Weaned piglets | 192 | D × L × Y | 9.35 | 24 days |
| Zhao et al. (2016) | Korea | Fermented medicinal plants | Lactobacillus plantarum, Yeasts and Bacillus licheniformis | 0.05%, 0.10%, 0.20% | Weaned piglets | 60 | L × Y × D | 6.08 | 6 wk |
| Zhou et al. (2015) | China | Fermented Ginkgo biloba L. residues | Candida tropicalis and A. oryzae | 5.00%, 10.00%, 15.00% | Weaned piglets | 48 | D × L × Y | 7.6 | 6 wk |
1D, Duroc; L, Landrace; Y, Yorkshire; NA, not applicable.
The Methodology of FF Preparation
The fermentation condition of FF from primary studies was summarized (Table 2). All methodologies of fermentation were solid-state fermentation. Fermentation temperature ranges from 25 to 40 ℃. In general, the fermentation time is longer than 2 d. The main strain starters included Aspergillus, Bacillus, and multiple strain starters.
Table 2.
The methodology of FF preparation for pigs diets
| Study | Time | Temperature | Starter cultures |
|---|---|---|---|
| Ao et al. (2010) | 48 h | NA 1 | Aspergillus oryzae GB-107 |
| Ao et al. (2011) | 5 d | NA | Bifidobacterium H-1 |
| Chamorro-Ramirez (2017) | 72 h | 28 to 32 ℃ | Yeast |
| Cho et al. (2007) | 48 h | NA | A. Oryzae GB-107 |
| Cho et al. (2013) | 36 h for oat and wheat; 42 h for corn | NA | Bacillus subtilis 2-19cx (oat and corn) or Aspergillus niger GB-124 (wheat) |
| Feng et al. (2007) | 48 h | NA | A. oryzae |
| Hung et al. (2008) | 72 h | 32 ± 2 ℃ | Probiotic |
| Jeong and Kim. (2015) | 5 d | 30 °C | Bacillus licheniformis species |
| Jones et al. (2010) | NA | NA | NA |
| Kim et al. (2006a) | 60 d | NA | NA |
| Kim et al. (2006b) | 60 d | NA | NA |
| Kim et al. (2006c) | NA | NA | Fungal and bacterial |
| Kim et al. (2010) | 48 h | NA | A. oryzae GB-107 |
| Kraler et al (2015) | NA | NA | Lactobacillus paracasei and Lactobacillus plantarum |
| Li et al. (2011) | 4 d | 25 °C | Streptococcus thermophilus (CGMCC No.1.2471), B. subtilis (MA 193), and Saccharomyces cerevisae (CGMCC No.2.1793). |
| Liu et al. (2017) | 14 d | 30 ◦C | B. subtilis MA139, Enterococcus faecium, and S. cerevisae |
| Park et al. (2018) | 42 h | NA | B. subtilis 2-19cx |
| Rafai et al. (2011) | 18 h | 30 ℃ | S. Cerevisiae |
| Shi et al. (2016) | 72 h | 32 °C | A. niger |
| Wang et al. (2007) | 72 h | 37 °C | Lactobacillus plantarum NF8 |
| Wang et al. (2014) | 5 d | 40 °C | B. subtilis MA139 with Streptococcus thermophilus and S. cerevisiae |
| Xu et al. (2017) | 48 h | NA | NA |
| Yan and Kim. (2013) | 2 d to 5 wk | 25 °C | Weissella koreensis |
| Yan et al. (2011) | 24 h | 25 °C | Weissella koreensis |
| Yan et al. (2012a) | 72 h | NA | Carlina vulgaris |
| Yan et al. (2012b) | 24 h | 25 °C | Weissella Koreensis |
| Yuan et al. (2017) | 48 h | 37 °C | Lactobacillus casei (CGMCC1.62), B. subtilis (CGMCC1.504), and Hansenula anomala (CGMCC2.881) |
| Zhang et al. (2018) | 24 h | 37 °C | B. Subtilis |
| Zhao et al. (2016) | 2 d | 30 °C | Bacillus licheniformis species |
| Zhou et al. (2015) | 96 h | 28 to 30 °C | Candida tropicalis and A. oryzae |
1 NA, not applicable.
Information Extraction
The following crucial information was retrieved from each included study: treatment supplement, amount of additive, growth stage, and outcome data. The outcome data included changes in the composition of ingredients before and after fermentation (DM, DE, CP, crude fat, crude ash, and crude fiber); growth performance (ADG, ADFI, and G:F); digestibility (DM, N, energy, essential amino acids [EAAs], nonessential amino acids [NEAAs]); meat quality (lightness, redness, yellowness, drip loss, marbling, and flavor).
Study Quality Assessment
We utilized a novel approach called the Study Quality Assessment on Nonruminants (SQANR) to assess the studies of nonruminants. The potential risk of bias derived from whether within-group differences were reported, whether experimental data were reported in multiple articles, whether the experimental information was complete, whether the sample size was appropriate, and whether the experimental design was rational. Two investigators (B.X. and L.Z.) performed independent study quality assessments.
Statistical Analyses
For outcomes with the same units, such as ADG, ADFI, and G:F, pooled estimates were expressed as weighted mean differences (WMDs). For outcomes that were measured with different units, the reported pooled estimate was presented as the standardized mean difference (SMD), and mean changes were calculated from the available data if the change means were not reported (Wan et al., 2014). Furthermore, pooled estimates for each outcome were presented as the SMD with 95% CI for visualization purposes (Noronha et al., 2019). The pooled estimates of the SMD with 95% CI for each outcome were evaluated by using a random-effects model. If the 95% CI contained a zero value, there was no difference. For all the analyses, heterogeneity was assessed with the I2 statistic (Higgins and Thompson, 2002) and Cochran’s Q test (Higgins et al., 2003); I2 > 50% and Pheterogeneity < 0.1 was regarded as a substantial heterogeneity. We used sensitivity analyses to exclude individual data with large deviations from the overall value. If 10 or more trials were available, we conducted subgroup analyses and meta-regression to explore potential sources of heterogeneity. The studies included in the meta-analysis were stratified into type of FF (fermented ingredients and fermented additives), growth stage (weaned piglets, growing pigs, and finishing pigs), dose (0.1–0.5%, 1–5%, 5–10%, and >10%), and strain starters (Aspergillus, Bacillus, multiple strain starters, and others). Publication bias was evaluated using Egger’s tests, for which the significance level was defined at P < 0.1 (Eng et al., 2014). To help establish an optimal amount of FF, we considered dose–effect curves for FF supplementation and the outcomes of pig growth performance and meat quality.
The statistical analyses were performed with Stata 14.0 (Stata Corp., USA).
Results
We identified 3,271 studies, of which 30 (with data for 3,562 pigs) were included in the meta-analyses (Kim et al., 2006a, 2006b, 2006c; Cho et al., 2007, 2013; Feng et al., 2007; Wang et al., 2007, 2014; Hung et al., 2008; Lee et al., 2009; Ao et al., 2010, 2011; Jones et al., 2010; Li et al., 2011; Rafai et al., 2011; Yan et al., 2011, 2012a, 2012b; Yan and Kim, 2013; Jeong and Kim, 2015; Kraler et al., 2015; Zhou et al., 2015; Shi et al., 2016; Zhao et al., 2016; Chamorro-Ramirez et al., 2017; Liu et al., 2017; Tian et al., 2017; Yuan et al., 2017; Park and Kim, 2018; Zhang et al., 2018). The selection process is shown in Fig. 1. The characteristics of the studies included in the meta-analyses are shown in Table 1. The data indicating health status are shown in Supplementary Table S6. The mean initial BWs of weaned piglets, growing pigs, and finishing pigs were 7.3, 30.8, 70.4 kg, respectively. FF was divided into 13 fermented ingredients (soy protein, apple pomace, oat, corn, wheat, soybean meal, persimmon shell, wheat, apple diet, potato pulp, rapeseed meal, biogas residue, and Ginkgo biloba L. residues) with concentrations greater than 1% and four fermented additives (red ginseng, Gynura procumbens, garlic powder, and Chlorella) with concentrations <0.5%. The study quality assessment based on SQANR is shown in Supplementary Table S2. The number of studies rated as “high,” “moderate,” and “low” were 7, 16, and 7, respectively. Unreported within-group standard deviation was the main reason for downgrading studies.
Figure 1.
Study selection process.
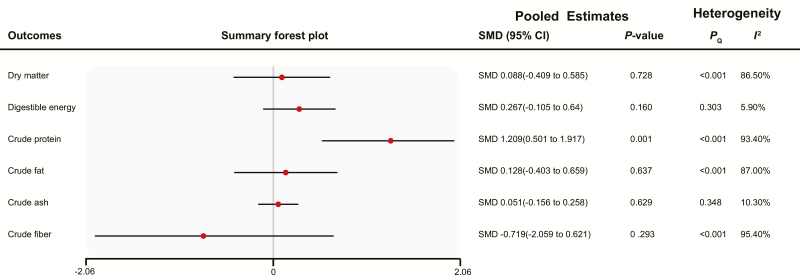
Effects of Fermentation on Nutritional Components of Feed Stuffs
As shown in Fig. 2, fermentation improved the CP of ingredients with substantial heterogeneity (SMD [95% CI] 1.209 [0.501, 1.917], I2 = 86.50%, PQ < 0.001), but fermentation had no effect on DM, crude fat, or crude fiber with substantial heterogeneity, DE, and crude ash with no evidence of heterogeneity.
Figure 2.
Summary forest plot of the effects of fermentation on ingredients. CI, confidence interval; SMD, standard mean difference; Pegger, P-value of Egger’s test. The red circle represents the point estimate for each individual trial, and the horizontal line extending from each solid diamond represents the upper and lower limits of the 95% CI. If the 95% CI contains a zero value, there was no difference.
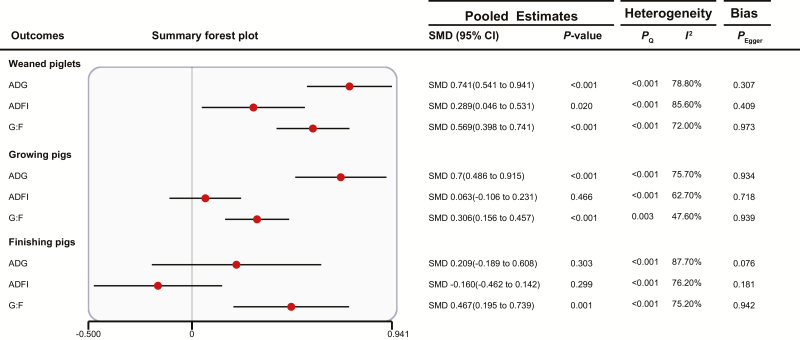
Effect of FF on Pig Growth Performance
As shown in Fig. 3, compared with the basal diet, the increments (i.e., the WMDs) of weaned piglets’ ADG, ADFI, and G:F with FF supplementation were 20.522 g/day, 9.716 g/day, and 0.026, respectively. The increments of growing pigs’ ADG and G:F with FF supplementation were 29.566 g/day and 0.013, respectively. The increment of finishing pigs’ G:F was 0.014. There was almost no publication bias observed. In subgroup analyses (Supplementary Table S3), fermented ingredients had no effect on weaned piglets’ ADFI (P > 0.05) and finishing pigs’ ADG and G:F (P > 0.05), but fermented additives improved weaned piglets’ ADFI (WMD 8.45 g/day [3.041 to 13.859]) and finishing pigs’ ADG and G:F (ADG: WMD 50.046 g/day [23.078 to 77.013]; G:F: WMD 0.024 (0.015 to 0.034)).
Figure 3.
Summary forest plot of the effects of FF on pig growth performance. CI, confidence interval; SMD, standard mean difference; Pegger, P-value of Egger’s test. The red circle represents the point estimate for each individual trial, and the horizontal line extending from each solid diamond represents the upper and lower limits of the 95% CI. If the 95% CI contains a zero value, there is no difference.
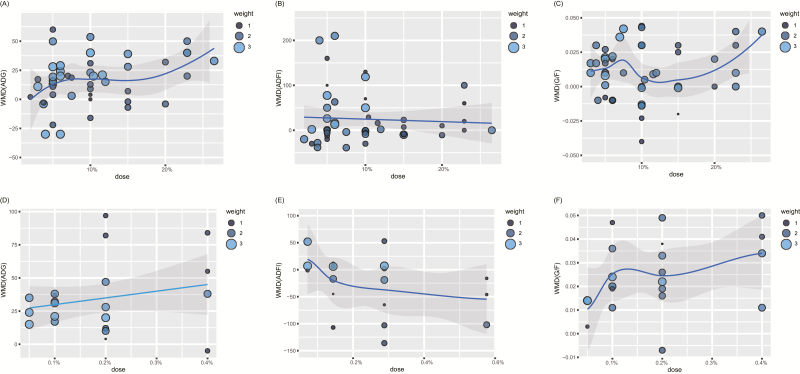
Dose–Effect Relationship between FF and Growth Performance
Figure 4 shows the dose–effect relationships between the dose of FF and growth performance of pigs, many of which are nonlinear with no sign of a plateau. When the supplementation dose of the fermented ingredients was ~8%, ADG and G:F showed a peak simultaneously, but ADFI remained in the plateau phase. Under the condition that the supplementation dose of the fermented additives decreased to 0.15%, G:F exhibited a climax and ADG maintained a gently rising trend, while ADFI slowly decreased without a significant difference compared with the basal diet treatment.
Figure 4.
Dose–effect relationship between FF and growth performance. (a) Fermented ingredients and ADG. (b) Fermented ingredients and ADFI. (c) Fermented ingredients and G:F. (d) Fermented additives and ADG. (e) Fermented additives and ADFI. (f) Fermented additives and G:F.
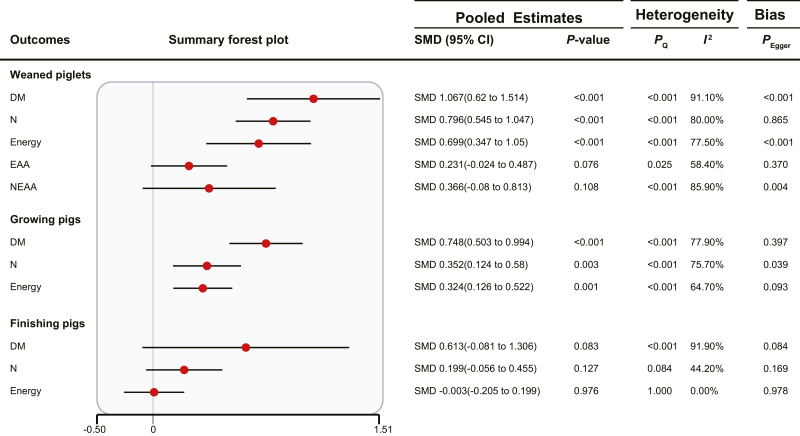
Effects of FF on Pig Nutrient Digestibility
Figure 5 shows that FF improved DM, N, and energy digestibility in weaned piglets and growing pigs (P < 0.05), but FF had no effect on EAA and NEAA digestibility in weaned piglets and DM, N, and energy digestibility in finishing pigs (P > 0.05). Three outcomes were detected to have publication bias. In the subgroup analyses (Supplementary Table S4), fermented additives had no effect on N and energy digestibility in growing pigs (P > 0.05), but fermented ingredients improved the above outcomes (P < 0.05).
Figure 5.
Summary forest plot of the effects of FF on pig nutrient digestibility. CI, confidence interval; SMD, standard mean difference; Pegger, P-value of Egger’s test. The red circle represents the point estimate for each individual trial, and the horizontal line extending from each solid diamond represents the upper and lower limits of the 95% CI. If the 95% CI contains a zero value, there is no difference.
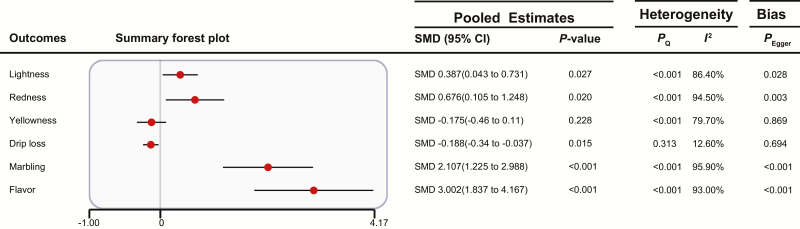
Effects of FF on Meat Quality
As shown in Fig. 6, compared with the basal diet, FF improved lightness, redness, marbling, and flavor and reduced drip loss in finishing pigs (P < 0.05). Four outcomes were detected to have publication bias. In the subgroup analyses (Supplementary Table S5), compared with the basal diet, fermented ingredients significantly increased lightness, redness, and flavor and reduced drip loss and yellowness (P < 0.05), and fermented additives significantly reduced redness and increased marbling (P < 0.05).
Figure 6.
Summary forest plot of the effects of FF on meat quality. CI, confidence interval; SMD, standard mean difference; Pegger, P-value of Egger’s test. The red circle represents the point estimate for each individual trial, and the horizontal line extending from each solid diamond represents the upper and lower limits of the 95% CI. If the 95% CI contains a zero value, there is no difference.
Meta-Regression
We used sensitivity analyses to exclude individual data with large deviation from the overall value. As shown in Table 3, the different types of FF had a significant impact on ADG and redness (P < 0.05), the different growth stages had a significant impact on G:F and N digestibility (P < 0.05), and the association of the dose of FF supplement with a range of outcomes had no observed significance (P > 0.05).
Table 3.
Regression analyses of the covariates
| Outcomes | Type of FF P-value1 | Growth stages P-value | Dose P-value |
|---|---|---|---|
| ADG | 0.017 | 0.628 | 0.088 |
| ADFI | 0.704 | 0.328 | 0.269 |
| G:F | 0.084 | 0.015 | 0.648 |
| DM digestibility | 0.234 | 0.189 | 0.241 |
| N digestibility | 0.957 | 0.040 | 0.742 |
| Energy digestibility | 0.137 | 0.436 | 0.359 |
| Lightness | 0.377 | NA | 0.736 |
| Redness | <0.001 | NA | 0.800 |
| Yellowness | 0.148 | NA | 0.827 |
| Drip loss | 0.749 | NA | 0.630 |
1 P-value of regression, P < 0.05 indicates a significant difference.
Discussion
The meta-analyses suggest that CP significantly improved after fermentation. Overall, FF improved nutrient digestibility and growth performance in weaned piglets and growing pigs but had little or no effect on these outcomes in finishing pigs. Moreover, FF improved meat quality in finishing pigs. Findings from the subgroup analyses showed that fermented ingredients had no effect on ADFI and had significant effects on weaner and grower pigs. Fermented additives positively promoted growth at all stages. The result of the dose–effect relationship suggested that the optimal amount of fermented ingredients was ~8% and that of fermented additives was ~0.15%.
Producers and animal nutritionists regard growth performance as a very important economic trait. Consumers regard meat quality as an important quality indicator to decide whether to purchase the meat. Growth performance and meat quality depend on the nutritional value of feed and nutrient digestibility.
In terms of the nutritional value of feed (Fig. 2), we only observed some nutritional components of feed stuffs, such as DM and CP, due to the lack of studies reporting changes in nutritional components after fermentation. If a sufficient number of studies had reported changes in multiple antinutritional factors, we might have detected changes in phytates and protease inhibitors. Furthermore, substantial heterogeneity in many of the outcomes suggests that different ingredients, fermentation technologies, and fermentation parameters can cause considerable changes in nutritional components of feed stuffs. However, changes in CP and crude ash did not exhibit heterogeneity, resulting in convincing evidence. We speculated that fermentation increases the ratio of small to large peptides and reduces antinutritional factors. Therefore, we suggest that further studies should pay attention to changes in multiple antinutritional factors after fermentation.
With regard to nutrient digestibility (Fig. 5 and Table 4), we observed positive effects of FF on main nutrient digestibility (DM, N, and energy digestibility) in weaned piglets and growing pigs, but FF had no effect on this parameter in finishing pigs. We speculate that the developed digestive system of finishing pigs determines the upper limit of nutrient digestibility, as nutrition uptake ability is related to the intestinal villi height and the ratio of villi height to crypt depth (Wang et al., 2007). These findings show that fermentation promotes an increase in protein and energy utilization, which may be due to the elimination of trypsin inhibitors and the degradation of large-size protein (Feng et al., 2007). We observed that FF had no influence on EAA and NEAA digestibility with substantial heterogeneity in weaned piglets. However, the association of FF with certain AA digestibility is not yet clear and should be considered in further studies.
Table 4.
The findings of the effects of FF on pig nutrient digestibility
| Type of FF | Dose | Outcomes | Growth stage of commercial pigs | Health status | Findings |
|---|---|---|---|---|---|
| Fermented ingredients | 2% to 15% | DM, N, energy | Weaned piglets and growing pigs | There were no differences in RBC, WBC concentrations, and lymphocyte percentage among different groups | The fermented ingredients significantly improved DM, N, and energy digestibility of weaned piglets and growing pigs |
| EAA, NEAA | Weaned piglets | Compared with the basal diet, fermented ingredients significantly increased the levels of serum IgG, IgA, and IgM of weaned pigs. Fermented ingredients significantly increased intestinal Lactobacillus number and decreased intestinal Escherichia coli number of weaned piglets compared with the basal diet group. The villous height to crypt depth ratio of weaned piglets was not affected by fermented ingredients | The fermented ingredients have no effect on EAA and NEAA digestibility of weaned piglets | ||
| Fermented additives | 0.05% to 0.40% | DM, N, energy | Weaned piglets | Compared with the basal diet, fermented ingredients significantly increased the RBC, WBC concentrations, and significantly decreased intestinal Escherichia coli number of weaned piglets | The fermented additives significantly improved DM digestibility and notably increased N and energy digestibility of weaned piglets |
| DM, N, energy | growing pigs | Compared with the basal diet, fermented ingredients significantly increased the WBC concentrations of growing pigs. Fermented ingredients significantly increased intestinal Lactic acid bacteria number and decreased intestinal E. coli number of growing pigs compared with the basal diet group | The fermented additives significantly improved DM digestibility and had no effect on N and energy digestibility of growing pigs | ||
| FF | DM, N, energy | Finishing pigs | The fermented additives and fermented ingredients have no effect on health status of finishing pigs | The fermented additives and fermented ingredients have no effect on DM, N, and energy digestibility of finishing pigs |
N, nitrogen; EAA, essential amino acids; NEAA, non-essential amino acids; RBC, red blood cell; WBC, white blood cell.
Figure 3, Table 5, and Fig. 4 show the effects of FF on pig growth performance and their dose–effect relationship. Together with the findings on the effect of FF on nutrient digestibility, the improvement in nutrient availability and utilization contributes to promoting the growth performance of weaner and grower pigs. The findings of the subgroup analyses suggest that fermented ingredients are appropriate for weaned piglets and growing pigs, and fermented additives are applicable to all growth stages of pigs. Fermented additives are usually fermented medicinal plants added at low levels; these additives can increase oxidation activity and other properties. Furthermore, considering the cost of FF together with the lack of significant effects on ADFI, the optimal amount of fermented ingredients and fermented additives was ~8% and 0.15%, respectively.
Table 5.
The findings of the effects of FF on pig growth performance
| Type of FF | Dose | Outcomes | Growth stage of commercial pigs | Findings |
|---|---|---|---|---|
| Fermented ingredients | 2% to to 15% | ADG, G:F | Weaned piglets and growing pigs | The fermented ingredients significantly improved ADG and G:F of weaned piglets and growing pigs. The optimal dose of fermented ingredients is 8% |
| ADFI | Overall growth stage | The fermented ingredients have no effect on ADFI of pigs | ||
| ADG, G:F, ADFI | Finishing pigs | The fermented ingredients have no effect on the growth performance of finishing pigs | ||
| Fermented additives | 0.05% to to 0.40% | ADG, ADFI, G:F | Overall growth stage | Overall, the fermented additives notably increased growth performance of pigs. The optimal dose of fermented ingredients is 0.15% |
Figure 6 and Table 6 show the effects of FF on meat quality. FF improved meat color, such as lightness and redness, the content of intermuscular fat, and meat flavor, which are important appearance parameters to attract customers. Moreover, FF reduced drip loss, possibly because of its antioxidant activity (Ao et al., 2011). FF has a low pH environment and relatively high organic acid content that promotes the secretion of gastric juices and improves the absorption of iron combined with myoglobin, enhancing the red color of the muscles (Lee et al., 2009; Plumed-Ferrer and Von Wright, 2009). The intramuscular fat content also influences tenderness, succulence, and flavor (Lee et al., 2009; Tian et al., 2017). Flavor-associated AAs such as Ala, Gly, Glu, Asp, and Ser, which are precursor AAs for flavor development, added to feed might influence meat favor. However, in the present meta-analyses, we did not analyze changes in flavor-associated AAs in feed or meat or their digestibility due to the limited number of articles. Further studies should focus on what components of FF affect the flavor-associated AAs in meat and their efficiency in conversion and deposition.
Table 6.
The findings of the effects of FF on meat quality
| Type of FF | Dose | Carcass weight of commercial finishing pigs | Meat quality (treatment group) | Meat quality (control group) | Outcomes | Findings |
|---|---|---|---|---|---|---|
| Fermented ingredients | 2% to to 15% | 103 to 110 kg | Mean of lightness, redness, yellowness, drip loss (%), marbling, and flavor were 46.41, 7.25, 4.85, 4.50, 6.17, and 6.30, respectively | Mean of lightness, redness, yellowness, drip loss (%), marbling, and flavor were 45.93, 6.70, 5.11, 5.14, 5.80, and 5.72, respectively | Lightness, redness, yellowness, drip loss, marbling, and flavor | The fermented ingredients significantly improved meat color, marbling, and flavor, and decreased drip loss |
| Fermented additives | 0.05% to to 0.40% | 106 to –108 kg | Mean of lightness, redness, yellowness, drip loss (%), and marbling were 52.72, 16.00, 7.31, 7.47, and 1.69, respectively | Mean of lightness, redness, yellowness, drip loss (%), and marbling were 45.93, 16.84, 7.04, 7.83, and 1.56, respectively | Redness and marbling | The fermented additives significantly improved marbling, and decreased redness of meat |
| Lightness, yellowness, drip loss, and flavor | The fermented additives have no effect on lightness, yellowness, drip loss, and flavor |
A major strength of the present study is that we performed a comprehensive overview of FF ingredients and their effects on pig growth performance and meat quality; moreover, we demonstrated the logical relevance and consistency of the results and provided an optimal supplement dosage and use strategy. One of our important innovations is SQANR, which is used to assess the quality of primary studies on nonruminants. Compared with Cochrane collaboration’s tool (Higgins et al., 2011) and other measurement tools targeted for medical animal trials (STAIR [Fisher et al., 2009], CAMARADES [Crossley et al., 2008], and ARRIV [Schulz et al., 2011] methodologies), SQANR was more suitable for studies on nonruminants because we considered the differences among humans, medical animals, and pigs. We also considered experimental design, information exhibition, error reporting, and sample size.
The main limitation in the present study is that there were many outcomes with substantial and unexplainable heterogeneity. The results of meta-regression suggest that factors not used as a subgroup category were not relevant to a range of outcomes and covariates, such as the type of FF and growth stages of pigs, in the analysis of heterogeneity sources, significance and direction. Therefore, we tentatively believe that the heterogeneity resulted from uncontrollable factors such as environmental conditions and experimental targets. Publication bias might have resulted from missing negative reports and samples, which are inevitable and unexplainable. We failed to explore effects of fermentation of different strains on nutritional components of feed stuffs because of the low number of articles. Further studies should focus on the effects of different strains on antinutrients and organic acids in ingredients.
In conclusion, our findings indicate that FF is capable of improving the ADG and G:F of weaning piglets and growers; this improvement is related to the beneficial effects of the fermentation process on the nutritive value and utilization of feed. Given the cost of substrate feed, the optimal doses of fermented ingredients and fermented additives in the basal diet are 8% and 0.15%, respectively. Moreover, FF can improve meat quality (lightness, flavor, and intramuscular marbling) and reduce drip loss. The current analysis, which is comprehensive and systematic, favors FF supplementation in pig feed.
Supplementary Material
Acknowledgments
This study was supported by the Key Program of the National Natural Science Foundation of China (grant no. 3163000269), National Special Fund for Modern Industrial Technology System (grant no. CARS-35), the Fundamental Research Funds for the Central Universities (2018QNA6031), and Major Science and Technology Special Fund of Zhejiang Province (grant no. 2015C02022).
Conflict of interest statement
None declared.
Literature Cited
- Ao X., Kim H. J., Meng Q. W., Yan L., Cho J. H., and Kim I. H.. . 2010. Effects of diet complexity and fermented soy protein on growth performance and apparent ileal amino acid digestibility in weanling pigs. Asian-Australas. J. Anim. Sci. 23:1496–1502. doi: 10.5713/ajas.2010.10109 [DOI] [Google Scholar]
- Ao X., Meng Q. W., and Kim I. H.. . 2011. Effects of fermented red ginseng supplementation on growth performance, apparent nutrient digestibility, blood hematology and meat quality in finishing pigs. Asian-Australas. J. Anim. Sci. 24:525–531. doi: 10.5713/ajas.2011.10397 [DOI] [Google Scholar]
- Chamorro-Ramirez F. H., Duran-Melendez L. A., Garcia-Macias J. A., Pena-Gonzalez E. M., and Gonzalez-Sanchez J. F.. . 2017. Productivity of pigs fed with solid-state fermented apple pomace and an enzymatic complex. Ecosistemas Y Recur. Agropecu. 4:453–463. doi: 10.19136/era.a4n12.1081 [DOI] [Google Scholar]
- Cho J. H., Min B. J., Chen Y. J., Yoo J. S., Wang Q., Kimi J. D., and Kim I. H.. . 2007. Evaluation of FSP (fermented soy protein) to replace soybean meal in weaned pigs: growth performance, blood urea nitrogen and total protein concentrations in serum and nutrient digestibility. Asian-Australas. J. Anim. Sci. 20:1874–1879. doi: 10.5713/ajas.2007.1874 [DOI] [Google Scholar]
- Cho J. H., Zhang Z. F., and Kim I. H.. . 2013. Effects of fermented grains as raw cereal substitutes on growth performance, nutrient digestibility, blood profiles, and fecal noxious gas emission in growing pigs. Livest. Sci. 154:131–136. doi: 10.1016/j.livsci.2013.03.011 [DOI] [Google Scholar]
- Cromwell G. L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7–27. doi: 10.1081/ABIO-120005767 [DOI] [PubMed] [Google Scholar]
- Crossley N. A., Sena E., Goehler J., Horn J., van der Worp B., Bath P. M., Macleod M., and Dirnagl U.. . 2008. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 39:929–934. doi: 10.1161/STROKEAHA.107.498725 [DOI] [PubMed] [Google Scholar]
- Eng C., Kramer C. K., Zinman B., and Retnakaran R.. . 2014. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384:2228–2234. doi: 10.1016/S0140-6736(14)61335-0 [DOI] [PubMed] [Google Scholar]
- Feng J., Liu X., Xu Z. R., Lu Y. P., and Liu Y. Y.. . 2007. The effect of Aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim. Feed Sci. Technol. 134:295–303. doi: 10.1016/j.anifeedsci.2006.10.004 [DOI] [Google Scholar]
- Fisher M., Feuerstein G., Howells D. W., Hurn P. D., Kent T. A., Savitz S. I., and Lo E. H.. . 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 40:2244–2250. doi: 10.1161/strokeaha.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., Savovic J., Schulz K. F., Weeks L., and Sterne J. A.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group 2011. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., and Thompson S. G.. . 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21:1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., and Altman D. G.. . 2003. Measuring inconsistency in meta-analyses. Br. Med. J. 327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung A. T. Y., Su T. M., Liao C. W., and Lu J. J.. . 2008. Effect of probiotic combination fermented soybean meal on growth performance, lipid metabolism and immunological response of growing-finishing pigs. Asian J. Anim. Vet. Adv. 3:431–436. doi: 10.3923/ajava.2008.431.436 [DOI] [Google Scholar]
- Jensen H. H., and Hayes D. J.. . 2014. Impact of Denmark’s ban on antimicrobials for growth promotion. Curr. Opin. Microbiol. 19:30–36. doi: 10.1016/j.mib.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Jeong J. S., and Kim I. H.. . 2015. Effect of probiotic bacteria-fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, Scutellaria baicalensis) as performance enhancers in growing pigs. Anim. Sci. J. 86:603–609. doi: 10.1111/asj.12331 [DOI] [PubMed] [Google Scholar]
- Jones C. K., DeRouchey J. M., Nelssen J. L., Tokach M. D., Dritz S. S., and Goodband R. D.. . 2010. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci. 88:1725–1732. doi: 10.2527/jas.2009–2110 [DOI] [PubMed] [Google Scholar]
- Kim Y. G., Lohakare J. D., and Chae B. J.. . 2006c. Growth performance, nutrient digestibility and intestinal morphology in weaned piglets fed fungal and bacterial fermented soya proteins. J. Anim. Feed Sci. 15:213–224. doi: 10.22358/jafs/66894/2006 [DOI] [Google Scholar]
- Kim H. Y., Song Y. M., Jin S. K., Kim I. S., Kang Y. S., Lee S. D., Chowdappa R., Ha A. H., and Kang S. M.. . 2006a. The effect of change in meat quality parameters on pig longissimus dorsi muscle by the addition of fermented persimmon shell diet. Asian-Australas. J. Anim. Sci. 19:286–291. doi: 10.5713/ajas.2006.286 [DOI] [Google Scholar]
- Kim H. Y., Song Y. M., Kang Y. S., Kim C. H., Lee S. D., Chowdappa R., Ha J. H., and Kang S. M.. . 2006b. The effect of fermented persimmon shell diet supplementation on the growth performance and blood parameters in finishing pigs. Anim. Sci. J. 77:314–319. doi: 10.1111/j.1740-0929.2006.00354.x [DOI] [Google Scholar]
- Kim S. W., van Heugten E., Ji F., Lee C. H., and Mateo R. D.. . 2010. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88:214–224. [DOI] [PubMed] [Google Scholar]
- Kraler M., Schedle K., Schwarz C., Domig K. J., Pichler M., Oppeneder A., Wetscherek W., Prückler M., Pignitter M., Pirker K. F., . et al. 2015. Fermented and extruded wheat bran in piglet diets: impact on performance, intestinal morphology, microbial metabolites in chyme and blood lipid radicals. Arch. Anim. Nutr. 69:378–398. doi: 10.1080/1745039X.2015.1075671 [DOI] [PubMed] [Google Scholar]
- Lee S. D., Kim H. Y., Jung H. J., Ji S. Y., Chowdappa R., Ha J. H., Song Y. M., Park J. C., Moon H. K., and Kim I. C.. . 2009. The effect of fermented apple diet supplementation on the growth performance and meat quality in finishing pigs. Anim. Sci. J. 80:79–84. doi: 10.1111/j.1740-0929.2008.00598.x [DOI] [PubMed] [Google Scholar]
- Li P. F., Xue L. F., Zhang R. F., Piao X. S., Zeng Z. K., and Zhan J. S.. . 2011. Effects of fermented potato pulp on performance, nutrient digestibility, carcass traits and plasma parameters of growing-finishing pigs. Asian-Australas. J. Anim. Sci. 24:1456–1463. doi: 10.5713/ajas.2011.11169 [DOI] [Google Scholar]
- Liu P., Zhao J., Guo P., Lu W., Geng Z., Levesque C. L., Johnston L. J., Wang C., Liu L., Zhang J., . et al. 2017. Dietary corn bran fermented by Bacillus subtilis MA139 decreased gut cellulolytic bacteria and microbiota diversity in finishing pigs. Front. Cell. Infect. Microbiol. 7:526. doi: 10.3389/fcimb.2017.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. M., and Levy S. B.. . 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24:718–733. doi: 10.1128/CMR.00002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., and Altman D. G.; PRISMA Group 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha J. C., Nishi S. K., Braunstein C. R., Khan T. A., Blanco Mejia S., Kendall C. W. C., Kahleová H., Rahelić D., Salas-Salvadó J., Leiter L. A., . et al. 2019. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 42:767–776. doi: 10.2337/dc18-2270 [DOI] [PubMed] [Google Scholar]
- Park J. W., and Kim I. H.. . 2018. Effect of dietary fermented corn in different energy dense diets on growth performance, nutrient digestibility, ileal microorganisms, and fecal noxious gas emission of growing pigs. Can. J. Anim. Sci. 98:325–332. doi: 10.1139/cjas-2016-0075 [DOI] [Google Scholar]
- Plumed-Ferrer C., and von Wright A.. . 2009. Fermented pig liquid feed: nutritional, safety and regulatory aspects. J. Appl. Microbiol. 106:351–368. doi: 10.1111/j.1365-2672.2008.03938.x [DOI] [PubMed] [Google Scholar]
- Rafai P., Papp Z., Jakab L., Tuboly T., Jurkovich V., Brydl E., Ozsvari L., and Kosa E.. . 2011. The effect of fermented wheat germ extract on production parameters and immune status of growing pigs. J. Anim. Feed Sci. 20:36–46. doi: 10.22358/jafs/66156/2011 [DOI] [Google Scholar]
- Schulz K. F., Altman D. G., and Moher D.; CONSORT Group 2011. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 9:672–677. doi: 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Shi C., He J., Wang J., Yu J., Yu B., Mao X., Zheng P., Huang Z., and Chen D.. . 2016. Effects of Aspergillus niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim. Sci. J. 87:557–563. doi: 10.1111/asj.12457 [DOI] [PubMed] [Google Scholar]
- Tian S., Xu Q., Jiang R., Han T., Sun C., and Na L.. . 2017. dietary protein consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Nutrients 9:982. doi: 10.3390/nu9090982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., Robinson T. P., Teillant A., and Laxminarayan R.. . 2015. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 112:5649–5654. doi: 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., Wang W., Liu J., and Tong T.. . 2014. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. F., Chen Q., Le G. W., Shi Y. H., and Sun J.. . 2007. Effect of lactic acid fermented soyabean meal on the growth performance, intestinal microflora and morphology of weaned piglets. J. Anim. Feed Sci. 16:75–85. doi: 10.22358/jafs/66728/2007 [DOI] [Google Scholar]
- Wang Y., Lu W. Q., Li D. F., Liu X. T., Wang H. L., Niu S., and Piao X. S.. . 2014. Energy and ileal digestible amino Acid concentrations for growing pigs and performance of weanling pigs fed fermented or conventional soybean meal. Asian-Australas. J. Anim. Sci. 27:706–716. doi: 10.5713/ajas.2013.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li L. M., Li B., Guo W. J., Ding X. L., and Xu F. Z.. . 2017. Effect of fermented biogas residue on growth performance, serum biochemical parameters, and meat quality in pigs. Asian-Australas. J. Anim. Sci. 30:1464–1470. doi: 10.5713/ajas.16.0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., and Kim I. H.. . 2013. Effects of dietary supplementation of fermented garlic powder on growth performance, apparent total tract digestibility, blood characteristics and faecal microbial concentration in weanling pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 97:457–464. doi: 10.1111/j.1439-0396.2012.01286.x [DOI] [PubMed] [Google Scholar]
- Yan L., Lim S. U., and Kim I. H.. . 2012a. Effect of fermented chlorella supplementation on growth performance, nutrient digestibility, blood characteristics, fecal microbial and fecal noxious gas content in growing pigs. Asian-Australas. J. Anim. Sci. 25:1742–1747. doi: 10.5713/ajas.2012.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Meng Q. W., Ao X., Zhou T. X., Yoo J. S., Kim H. J., and Kim I. H.. . 2011. Effects of fermented garlic powder supplementation on growth performance, blood characteristics and meat quality in finishing pigs fed low-nutrient-density diets. Livest. Sci. 137:255–259. doi: 10.1016/j.livsci.2010.09.024 [DOI] [Google Scholar]
- Yan L., Meng Q. W., and Kim I. H.. . 2012b. Effects of fermented garlic powder supplementation on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Anim. Sci. J. 83:411–417. doi: 10.1111/j.1740-0929.2011.00973.x [DOI] [PubMed] [Google Scholar]
- Yuan L., Chang J., Yin Q., Lu M., Di Y., Wang P., Wang Z., Wang E., and Lu F.. . 2017. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 3:19–24. doi: 10.1016/j.aninu.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shi C., Wang C., Lu Z., Wang F., Feng J., and Wang Y.. . 2018. Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol. 29:133–146. doi: 10.1080/09540105.2017.1360258 [DOI] [Google Scholar]
- Zhao P., Li H., Lei Y., Li T., Kim S., and Kim I.. . 2016. Effect of fermented medicinal plants on growth performance, nutrient digestibility, fecal noxious gas emissions, and diarrhea score in weanling pigs. J. Sci. Food Agric. 96:1269–1274. doi: 10.1002/jsfa.7217 [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang C., Ye J., Chen H., and Tao R.. . 2015. Effects of dietary supplementation of fermented Ginkgo biloba L. residues on growth performance, nutrient digestibility, serum biochemical parameters and immune function in weaned piglets. Anim. Sci. J. 86:790–799. doi: 10.1111/asj.12361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.