Abstract
Vitamin B6 (VB6) is an important coenzyme factor which participates in many metabolic reactions, especially amino acid metabolism. There are few reports on how VB6 mediates weaned piglet intestinal health. This study purposed to investigate dietary VB6 effects on growth, diarrhea rates, and intestinal morphology and function in weaned piglets fed a high-crude protein (22% CP) diet. Eighteen 21-d-old weaned [(Yorkshire × Landrace) × Duroc] piglets with body weights of 7.03 ± 0.15 (means ± SEM) kg were randomly assigned into 3 VB6-containing dietary treatments. Vitamin B6 content was: 0, 4, and 7 mg/kg, respectively. The feeding period lasted 14 d. The results showed that no significant difference existed for the growth performance. The 7 mg/kg VB6 group had a tendency to decrease diarrhea rate (P = 0.065). Blood biochemical parameters analysis demonstrated that total protein, cholesterol, and high-density lipoprotein significantly increased in the 7 mg/kg VB6 group (P < 0.05). In the jejunum, no significant differences were detected for villus height, villus width, crypt depth, villus height and crypt depth ratios, and positive Ki67 counts and the mRNA expression of inflammatory cytokines. Vitamin B6 significantly increased the mRNA expression of SLC6A19 and SLC6A20 (P < 0.05) and decreased the mRNA expression of SLC36A1 (P < 0.05). In the ileum, VB6 significantly increased villus height and villus width (P < 0.05) while decreased positive Ki67 cell counts for 7 mg/kg VB6 group (P < 0.05). Vitamin B6 had significantly increased the mRNA expression of interleukin-1β, tumor necrosis factor-α,cyclo-oxygen-ase-2, and transforming growth factor-β (P < 0.05). Vitamin B6 also had significantly increased mRNA expression of SLC6A19, SLC7A6, SLC7A7, and SLC36A1 (P < 0.05). These findings suggest that dietary supplementation with VB6 may affect the intestinal morphology and absorption and metabolism of protein in weaned piglets fed a high-protein diet by altering the expression of intestinal inflammatory cytokines and amino acid transporters.
Keywords: amino acid transporters, crude protein, intestine, inflammation cytokines, vitamin B6, weaned piglets
Introduction
Crude protein (CP) is a necessary nutrient for piglet healthy growth. Dietary CP level is a main factor that affects growth, feed efficiency, postweaning diarrhea rates, and gastrointestinal health for weaned piglets(De Lange et al., 2010; Yin and Tan, 2010; Acciaioli et al., 2011; Gallo et al., 2014). Increasing CP amounts in the diet (Htoo et al., 2007) tend to increase gut protein fermentation. The fermentation of undigested dietary protein and proteins of endogenous origin entering the large intestine yields putatively toxic metabolites that can impair epithelial integrity. When gut function is destroyed, factors such as bacteria, toxins, and pathogenic microorganisms will invade through the intestinal epithelial cells and eventually causing intestinal inflammation, poor nutritional absorption, and diarrhea (Blecha and Charley, 1990; Montagne et al., 2007; Wijtten et al., 2011; Kang et al., 2012).
Vitamin B6 (VB6) is an indispensable water-soluble vitamin for animal health that functions as a coenzyme in intestinal amino acid metabolism and assists in the absorption and transportation of intestinal amino acids (Jacobs, 1962; Eliot and Kirsch, 2004). Low levels of plasma VB6 relates to inflammatory bowel disease (IBD). Vitamin B6 supplementation reduced the index of cell proliferation in mice colon while having no significant effects on colonic epithelium apoptosis (Komatsu et al., 2001). Whether VB6 supplement to a high-CP diet benefits intestinal health in piglet has not yet been reported. Therefore, we hypothesized that dietary supplementation with VB6 would improve intestinal health in weaned piglets fed a high-protein diet. This experiment intended to identify the effect of dietary VB6 on growth, diarrhea rate, intestinal morphology, inflammatory factors expression, and amino acid (AA) transporters expression in a high-protein diet fed to weaned piglets.
Materials and Methods
The experimental design and procedures in this study were reviewed and approved (Approval number 2016‐093) by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China (Yan et al., 2018).
Animals and Experimental Treatments
Eighteen crossbred piglets (Duroc × Landrace × Large White) with an initial body weight (IBW) of 7.03 ± 0.15 (means ± SEM) kg, weaned at 21 d, were randomly allotted to 3 dietary treatments and each treatment had 6 replicates. Corn and soya bean diets contained similar nutrient level but differed in their VB6 concentrations were provided to these piglets. The basal diet (Table 1) formulations met nutrient specifications for piglets (NRC, 2012). The extra VB6 content for each diet was 0, 4, and 7 mg/kg, respectively. The VB6 product used in our study was obtained from Royal DSM Vitamins Co., Ltd (Shanghai, China) and its purity was 99%. With zeolite powder as carrier, VB6 was added to VB6-free vitamin premix using step-by-step dilution. Then, the vitamin premixes with different VB6 contents were used for producing compound feed. All piglets were housed on plastic slotted floors (1.6 × 0.6 m per pen). Room temperatures were kept around 28 °C by heat lamps. The feeding period lasted 14 d. Any signs of sickness or abnormal behavior were noted during experimental period. Mash feed and water were provided ad libitum. They were fed 4 times a day at 08:00, 12:00, 16:00, and 2000 h.
Table 1.
Basic diet composition and nutritional components
| Items | Dietary VB6, mg/kg | ||
|---|---|---|---|
| 0 | 4 | 7 | |
| Ingredient, % | |||
| Corn | 36.12 | 36.12 | 36.12 |
| Extruded Corn | 20.00 | 20.00 | 20.00 |
| Soybean Meal | 10.00 | 10.00 | 10.00 |
| Soy Protein Concentrate Powder | 8.93 | 8.93 | 8.93 |
| Whey Powder | 10.00 | 10.00 | 10.00 |
| Fish Meal | 5.00 | 5.00 | 5.00 |
| Spray-Dried Plasma Protein | 5.00 | 5.00 | 5.00 |
| Lys | 0.14 | 0.14 | 0.14 |
| Met | 0.05 | 0.05 | 0.05 |
| Soybean Oil | 2.30 | 2.30 | 2.30 |
| Limestone | 1.03 | 1.03 | 1.03 |
| Dicalcium Phosphate | 0.34 | 0.34 | 0.34 |
| Choline Chloride | 0.10 | 0.10 | 0.10 |
| Anti-oxidant | 0.05 | 0.05 | 0.05 |
| Citric acid | 0.30 | 0.30 | 0.30 |
| Vitamin and Mineral Premix | 0.65 | 0.65 | 0.65 |
| Calculated composition | |||
| CP, % | 22.00 | 22.00 | 22.00 |
| ME, MJ/kg | 14.23 | 14.23 | 14.23 |
| Ca,% | 0.80 | 0.80 | 0.80 |
| AP, % | 0.36 | 0.36 | 0.36 |
| Lys, % | 1.35 | 1.35 | 1.35 |
| Met, % | 0.39 | 0.39 | 0.39 |
| Met+Cys, % | 0.79 | 0.79 | 0.79 |
| Thr, % | 0.85 | 0.85 | 0.85 |
| Trp, % | 0.24 | 0.24 | 0.24 |
1Vitamin-mineral premix supplied per kilogram of feed: 2,200 IU of Vitamin A, 220 IU of Vitamin D3, 16 IU of Vitamin E, 0.5 mg of Vitamin K3, 0.0175 mg of Vitamin B12, 3.5 mg of riboflavin, 30 mg of niacin, 10 mg of d-pantothenic acid, 0.05 mg of biotin, 0.3 mg of folic acid, 1.0 mg of thiamine, 150 mg of Fe (FeSO4), 100 mg of Zn (ZnSO4), 30 mg of Mn (MnSO4), 25 mg of Cu (CuSO4), 0.5 mg of I (KIO3), 0.3 mg of Co (CoSO4), 0.3 mg of Se (Na2SeO3), and 4.0 mg of ethoxyquin.
Sample Collection
Blood samples (5 mL) were collected from each piglet after overnight fasting on day 14. Blood samples were collected through an anterior vena cava puncture into heparinized vacutainer tubes and then centrifuged at 4 °C, 3,000 × g for 10 min (Yin et al., 2010). Serum samples were stored at −80 °C for biochemistry analysis. Pigs were euthanized with an overdose of sodium pentobarbital solution (40 mg/kg BW) and then exsanguinated (Ren et al., 2014). The entire intestinal tract was removed. The small intestine was separated from the large intestine and the mesentery. The small intestine was divided into 3 segments: a proximate 10-cm segment to the pylorus as the duodenum; a middle portion as the jejunum; and a distal 5-cm section proximal to the ileocecal junction as the ileum. The middle portions of the jejunum and ileum (about 10 cm) segments were cut longitudinally to expose mucosa and washed 3 times with ice-cold PBS. Mucosa from the jejunum and ileum was scraped with glass slides, snap-frozen in liquid nitrogen, and stored at −80 °C until mRNA expression analysis. Other 2-cm sections from the middle portions of the jejunum and ileum, respectively, were fixed immediately in 10% neutral formalin for morphometric analysis.
Measurements
Growth Performance and Diarrhea Incidence
Piglets were weighed on days 1 and 14 after overnight fasting. Feed consumption was recorded accordingly. Average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratios (G/F) were calculated according to Yang et al. (2013a). Diarrhea incidence was defined as using Liu et al. method. Piglets diarrhea rates were recorded daily and calculated as follows: Diarrhea rate = total number of pigs with diarrhea/(total number of pigs × experimental days) × 100%, where “total number of pigs with diarrhea” was defined as the number of pigs with diarrhea observed on each day (Liu et al., 2008).
Serum Biochemical Parameters
For serum biochemical parameters, serum total protein (TP), urea nitrogen (BUN), ammonia (NH3L), total cholesterol (CHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), immunoglobulin G (IGG), immunoglobulin M (IGM), and glucose (GLU) levels were measured using spectrophotometric kits in accordance with manufacturer instructions (Nanjing Jiangcheng Biotechnology Institute, Jiangsu Province, China) and determined using an Automatic Biochemistry Radiometer (Hitachi Co., Tokyo, Japan).
Intestinal Morphology
Fixed tissues were dehydrated in a graded series of ethanol followed by xylol treatment. They were then embedded in paraffin wax. Sections 4-μm thick were cut from the paraffin blocks, dried at 37 °C, and then stained with hematoxylin and eosin using standard paraffin embedding procedures. Tissue sections were measured under a microscope using a 40 ×combined magnification, and an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, United Kingdom). At least 20 villi and corresponding crypts were randomly chosen from different well-oriented parts of the sections. Villus height (VH), villus width (VW), and crypt depth (CD) were measured using a light microscope fitted with an image analysis system (AxioScope A1, Carl Zeiss, Germany). The villus height to crypt depth ratio (VH/CD) was calculated (Zong et al., 2018).
Immunohistochemistry
A cell proliferation analysis of the crypt of jejunum and ileum consisting of Ki67 abundance was assessed by immunohistochemistry with anti-Ki67 antibodies (Abcam, Cambridge, dilution 1:800, United Kingdom). At least 20 crypts (200×) were observed for each section using Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA). The results were expressed as the number of Ki67 positive cells in each crypt. The detailed process was performed according to our previous studies (Yan et al., 2018).
Quantitative Real-Time PCR
Total RNA was isolated from liquid nitrogen-frozen jejunum and ileum using a TRIZOL regent (Invitrogen, USA). They were then treated with DNase I (Invitrogen, USA) according to the manufacturer instructions. Synthesis of the first strand (cDNA) was performed using oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen, USA). Primer information appears in Table 2. Target gene fold change was normalized to housekeeping gene (β-actin) and calculated using the 2−△△CT method. RNA extraction, reverse transcription, and real-time PCR were performed according to Yang et al. (2013b) methodologies.
Table 2.
Primers used for real-time PCR analysis
| Gene | Sequence, 5′-3′ | Product length, bp |
|---|---|---|
| β-actin | F: AGTTGAAGGTGGTCTCGTGG R: TGCGGGACATCAAGGAGAAG |
216 |
| IL-1β | F: CCTGGACCTTGGTTCTCT | 123 |
| R: GGATTCTTCATCGGCTTCT | ||
| TNF-α | F: ACAGGCCAGCTCCCTCTTAT | 102 |
| R: CCTCGCCCTCCTGAATAAAT | ||
| COX2 | F: CCAGGTTTAAGATCTGATGTGGGGA | 165 |
| R: TGCCCTTCCATCATTACGAATCCTT | ||
| TGF-β | F: CGAGCCCTGGATACCAACTA | 164 |
| R: AGGCTCCAGATGTAGGGACA | ||
| SLC1A5 | F: GGAGATGGAGGATGTGGGGA | 140 |
| R: GGAAGCGGTAGGGGTTTTTG | ||
| SLC6A19 | F: GCAACGTGACGCAGGAGAAC | 234 |
| R: ACGACAGGCCCAGACAGAAG | ||
| SLC6A20 | F: CCAATGGCCTGGTGTACATG | 216 |
| R: CGACACTGGCAAAGATGGAG | ||
| SLC7A6 | F: CAGGACGCCTTTGAGGGTTC | 126 |
| R: TTCTGGGTTTTTGATTTCTTCTGTT | ||
| SLC7A7 | F: GCATTATCCTGCTTTGGTGG | 227 |
| R: AAGAACCAGTAGCTGAAGCTGTAGT | ||
| SLC36A1 | F: CAACTGCCACAACAACGAGA | 173 |
| R: AGATCATGACCAGGCTGACC |
SLC1A5, Solute carrier family 1 member 5; SLC6A19, Solute carrier family 6 member 19; SLC6A20, Solute carrier family 6 member 20; SLC7A6, Solute carrier family 7 member 6; SLC7A7, Solute carrier family 7 member 7; SLC36A1, Solute carrier family 36 member 1.
Statistical Analysis
Datas analysis including growth performance, diarrhea rate, blood biochemical parameters, intestinal morphology, immunohistochemistry, expression of inflammatory cytokines, and AA transporters was examined by one-way ANOVA using the GLM procedure of SAS (Version 9.3; SAS Institute, Inc., Cary, NC). Data were presented as means ± SEM. P < 0.05 indicated that the difference was statistically significant. Towards significance is indicated by 0.05 ≤ P < 0.1.
Results
Growth Performance, Diarrhea Incidence, and Biochemical Parameters
No significant differences in growth performance were observed among the 3 treatments. The 7 mg/kg VB6 group had the greated ADG, ADFI, and G/F. They improved by 23.02%, 10.21%, and 10.17%, respectively, compared with 0 mg/kg VB6 group. The 7 mg/kg VB6 group had a tendency to decrease diarrhea rate (P = 0.065; Table 3). Blood biochemical parameters demonstrated that 7 mg/kg VB6 group TP, CHOL, and LDL were significantly higher compared with 4 mg/kgVB6 group(P < 0.05; Table 4).
Table 3.
Effects of vitamin B6 on growth performance and diarrhea rate1
| Items | VB6, mg/kg | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 4 | 7 | |||
| IBW, kg | 7.03 | 7.02 | 7.03 | 0.15 | 0.999 |
| FBW, kg | 8.92 | 8.97 | 9.35 | 0.21 | 0.680 |
| ADG, g | 134.52 | 139.29 | 165.48 | 9.63 | 0.392 |
| ADFI, g | 250.99 | 248.65 | 276.61 | 11.54 | 0.576 |
| G:F, g/g | 0.53 | 0.56 | 0.59 | 0.02 | 0.317 |
| DR,% | 3.57ab | 14.29a | 2.38b | 2.34 | 0.065 |
1IBW, Initial body weight; FBW, Final body weight. DR (%) = total number of pigs with diarrhea/(total number of pigs × experimental days) × 100%.
a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM. n = 6.
Table 4.
Effects of vitamin B6 on serum biochemical parameters1
| Items | VB6, mg/kg | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 4 | 7 | |||
| TP, g/L | 46.88ab | 44.40b | 51.53a | 1.21 | 0.039 |
| BUN, mmol/L | 4.12 | 3.63 | 4.05 | 0.16 | 0.433 |
| NH3L, μmol/L | 349.57 | 303.17 | 334.92 | 15.55 | 0.488 |
| CHOL, mmol/L | 1.95a | 1.49b | 2.04a | 0.10 | 0.037 |
| HDL, mmol/L | 0.76 | 0.58 | 0.80 | 0.05 | 0.129 |
| LDL, mmol/L | 0.98ab | 0.85b | 1.16a | 0.05 | 0.044 |
| IGG, g/L | 1.25 | 1.19 | 1.28 | 0.05 | 0.791 |
| IGM, g/L | 0.43 | 0.45 | 0.41 | 0.02 | 0.744 |
| GLU, mmol/L | 4.58 | 4.25 | 4.48 | 0.12 | 0.539 |
1BUN, Blood Urea Nitrogen; NH3L, Ammonia; HDL, High-density Lipoprotein; IGG, Immunoglobulin G; IGM, Immunoglobulin M; GLU, Glucose.
a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM. n = 6.
Intestinal Morphology and Immunohistochemistry
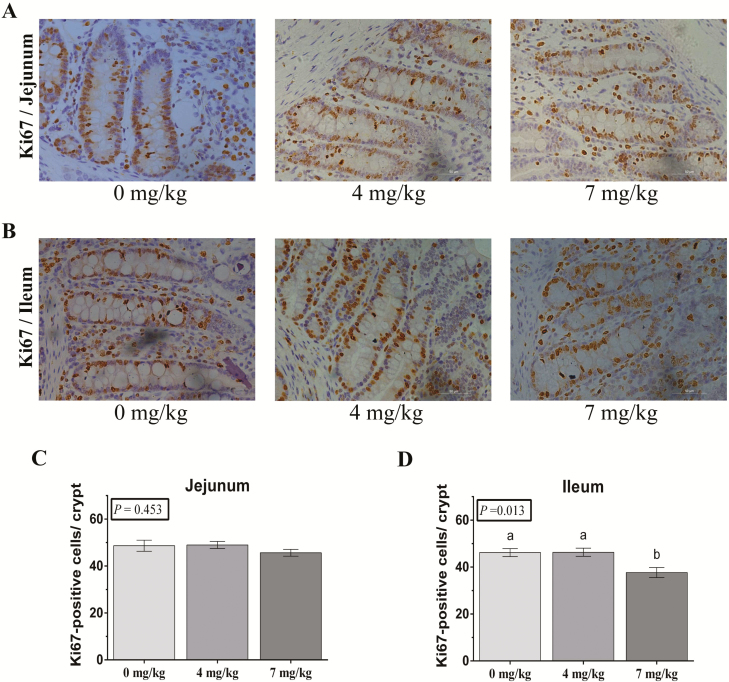
Histological evaluation indicated that VB6 did not affect duodenal and jejunal VH, VW, CD, and VH:CD. Ileal VH and VW significantly increased for 7 mg/kg VB6 group (P < 0.05; Table 5). Intestinal cell proliferation was evaluated using immunohistochemistry for Ki67. The Ki67 positive cells per crypt in the jejunum and ileum were counted. Vitamin B6 did not affect Ki67 positive cells in the jejunum (Figure 1A). The 7 mg/kg VB6 group had notably decreased the Ki67 positive cells in the ileum (P < 0.05; Figure 1B).
Table 5.
Effects of vitamin B6 on small intestine morphological indicators
| Items | VB6, mg/kg | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 4 | 7 | |||
| Duodenum | |||||
| VH, μm | 270.22 | 287.79 | 282.99 | 8.62 | 0.779 |
| VW, μm | 137.30 | 152.58 | 138.84 | 2.75 | 0.123 |
| CD, μm | 339.44 | 393.82 | 395.49 | 15.95 | 0.458 |
| VH/CD, μm/μm | 0.85 | 0.75 | 0.75 | 0.04 | 0.662 |
| Jejunum | |||||
| VH, μm | 360.79 | 356.93 | 319.08 | 11.34 | 0.259 |
| VW, μm | 119.51 | 130.70 | 117.32 | 2.92 | 0.171 |
| CD, μm | 226.83 | 247.00 | 248.05 | 7.43 | 0.475 |
| VH/CD, μm/μm | 1.63 | 1.45 | 1.29 | 0.07 | 0.111 |
| Ileum | |||||
| VH, μm | 241.71b | 270.89ab | 286.45a | 7.78 | 0.047 |
| VW, μm | 114.74b | 122.23ab | 129.62a | 2.39 | 0.028 |
| CD, μm | 220.80 | 257.99 | 239.08 | 8.75 | 0.232 |
| VH/CD, μm/μm | 1.12 | 1.07 | 1.22 | 0.05 | 0.434 |
a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM. n = 6.
Figure 1.
Post-B6 supplementation weaned piglet intestinal cell proliferation. (A and B) The representative images of immunohistochemistry (IHC) staining with Ki67 antibody in weaned piglet jejunum and ileum shown (×200; n = 6). (C and D) The statistical analysis of Ki67-positive cells in each crypt from images shown on the (A and B). The data were analyzed by one-way ANOVA using the GLM procedure of SAS. a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM; SEM = standard error of the mean. n = 6.
The mRNA Expression of Inflammatory Cytokines in Intestinal Mucosa
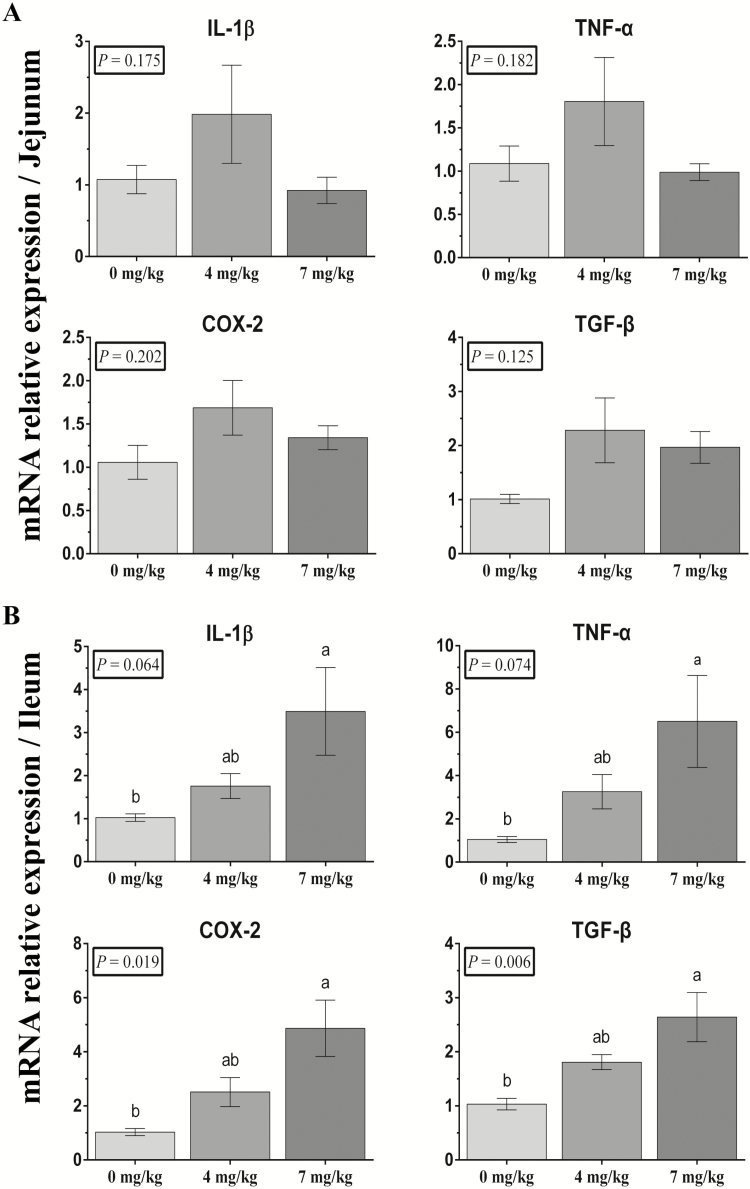
The mRNA expression of inflammatory cytokines in intestinal mucosa was measured. Vitamin B6 did not affect jejunal relative mRNA expression of Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), cyclo-oxygen-ase-2 (COX-2), and transforming growth factor-β (TGF-β) (Figure 2A). Relative ileal mRNA expression of IL-1β, TNF-α, COX-2, and TGF-β in 7 mg/kg VB6 group significantly increased compared with 0 mg/kg VB6 group (P < 0.05; Figure 2B).
Figure 2.
Effects of vitamin B6 dietary supplementation on weaned piglet jejunal (A) and ileal (B) mRNA expression. IL-1β, TNF-α, COX-2, and TGF-β abundance were normalized using β-actin as an internal control. The data were analyzed by one-way ANOVA using the GLM procedure of SAS. a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM; SEM = standard error of the mean. n = 6.
The mRNA Expression of AA Transporters in Intestinal Mucosa
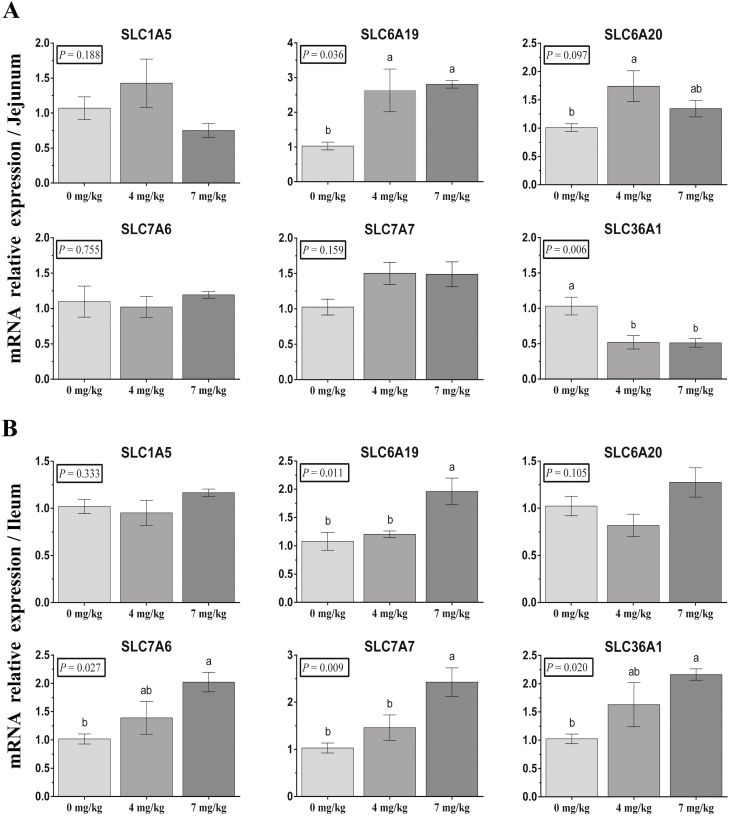
The mRNA expression of AA transporters was detected in the intestinal mucosa. In the jejunum, there were no significant differences in the relative mRNA expression of SLC1A2, SLC7A6, and SLC7A7. Vitamin B6 significantly increased SLC6A19 and SLC6A20 (P < 0.05) mRNA expression and decreased SLC36A1(P < 0.05) mRNA expression (Figure 3A). In the ileum, there were no significant differences in the relative mRNA expression of SLC1A5 or SLC6A20. Vitamin B6 significantly increased the mRNA expression of SLC6A19, SLC7A6, SLC7A7, and SLC36A1 (P < 0.05; Figure 3B).
Figure 3.
Effects of dietary supplementation with vitamin B6 on mRNA expression of jejunal (A) and ileal (B) AA transporters in weaned piglets. The mRNA expression abundance of SLC1A5, SLC6A19, SLC6A20, SLC7A6, SLC7A7, and SLC36A1 were normalized using β-actin as an internal control. The data were analyzed by one-way ANOVA using the GLM procedure of SAS. a,bWithin a variable, values with different superscripts differ (P < 0.05). Data are expressed as means ± SEM; SEM = standard error of the mean. n = 6.
Discussion
Little research on the precise need for VB6 in piglets has been done. Historical requirements estimates for pigs in the 10 to 20 kg range have been from 1.2 to 1.8 mg of VB6/kg in the diet. The dietary requirement is 1 to 2 mg/kg diet for a pig weighing initially about 2 kg and fed to 10-kg body weight (Sewell et al., 1964; Kosters and Kirchgessner, 1976a, b). The concentration amounts for vitamins in NRC (2012) come directly from the NRC (1998). The VB6 requirement for piglets is 7 mg/kg. Usually no shortage occurs from a corn-soybean diet as corn and soybeans are rich in VB6. In this study, growth performance was not obviously affected by VB6. The ADG (165.48 g/d) and ADFI (276.61 g/d) of 7 mg/kg group have improved 23.02% and 10.21%, respectively, compared with 0 mg/kg group (ADG: 134.52g/d; ADFI: 250.99 g/d). Many studies had shown that there existed a strong association between the VB6 requirement and CP content in mixed feed for livestock and poultry (Canham et al., 1969; Matte et al., 2001). Vitamin B6 has been reported to be a coenzyme for over 100 enzymatic reactions and nearly half involve transamination-type reactions (Schneider et al., 2000). It could partly account for the better growth performance in 7 mg/kg group. However, as only a few piglets (less than 10 per treatment) were used in the present experiments, more studies on a greater number of animals should be conducted to confirm growth performance. The 4 mg/kg group tended to increase diarrhea rate, whereas 7 mg/kg group tended to decrease diarrhea rate. This is consistent with the results for growth performance. This may be related to intestinal function alterations and amino acid absorption and metabolism. It requires further study to measure nutrient digestibility. Serum biochemical parameters analysis showed that VB6 supplementation had a different impact on the TP, CHOL, and LDL between 4 and 7 mg/kg group. Adding VB6 had an effect on some piglet blood biochemical parameters and there was a transitional dose between 4 and 7 mg/kg groups. No difference existed among the 3 treatments for IgG and IgM. This is was not consistent with Kumar’s rat study (Kumar and Axelrod, 1968). It may be caused by species differences.
Intestinal morphology reflected by villus height, villus width, and crypt depth plays essential roles in nutrient absorption as well as providing a protective barrier. Nutrient transport is closely related to villi height and width as well as crypt depth (Hedemann et al., 2003). Diarrhea usually leads to intestinal dysfunction including crypt depth increase and villus height reduction (Boudry et al., 2004; Verdonk et al., 2007). Vitamin B6 plays a primary role catalyzing more than 160 distinct enzymatic reactions and the intestinal tract is one of the major sites (Albersen et al., 2013). In the present study, VB6 supplement in the diet had insignificant effects on the duodenum or jejunum morphology. It did alter ileum morphology. More microbes are implanted in ileum than that in the duodenum and jejunum. We speculated that VB6 or one of its metabolites made a complex interaction with the ileal microbiome, which caused the alteration of ileal development and morphology. Further studies are needed to detect the specific mechanism between microbes and VB6’s metabolism. Intestinal epithelial cell proliferation has a positive connection with weaning piglet VH and CD (Wang et al., 2018). Epithelial cells positive for Ki67 were mostly present in crypts and cell proliferation of the gastrointestinal tract epithelium were evaluated by counting Ki67 positive cells (De et al., 2010). It was found that dietary VB6 inhibited ileal epithelial cells proliferation, but not jejunal. This suggests that jejunal and ileal cells have different phenotypes and functions. However, the underlying mechanism how does VB6 inhibit proliferation of ileal cells remains to be revealed.
Intestinal inflammation reflects the degree of intestinal health and associates with diarrhea. Vitamin B6 supplementation alters mRNA inflammatory cytokine expression in the gut. Previous reports have shown that low VB6 values are observed in inflammatory bowel disease (Selhub et al., 2013). Pro-inflammatory cytokines IL-1β and TNF-α are necessary to initiate an inflammatory response during infection. Overexpression of these cytokines can affect pathological responses (Clark, 2007; Zelnickova et al., 2008). Anti-inflammatory cytokines inhibit pro-inflammatory cytokines and other mediator overexpression that may lead to hyper-activation of immune responses in weaned piglets (King et al., 2003; Schiepers et al., 2005). Low levels of VB6 due to inflammation might be caused by plasma VB6 acting as a coenzyme for the production of cytokines and other polypeptide mediators during the inflammatory response (Galloway et al., 2000; Friso et al., 2001). There is a growing body of evidence that VB6 has anti-inflammatory activity. Patients with inflammation have significantly lower VB6 blood levels (Saibeni et al., 2003; Sakakeeny et al., 2012). Low-dose VB6 supplementation (50 mg/d) for 30 d did not suppress pro-inflammatory cytokine (TNF-α) production in patients with rheumatoid arthritis. High-dose VB6 supplementation (100 mg/d) suppressed pro-inflammatory cytokines (Chiang et al., 2005). This study showed that there were no significant differences for the mRNA expression of pro-inflammatory cytokines IL-1β, TNF-α, COX-2, and anti-inflammatory cytokines TGF-β in the jejunum, whereas their expression increased with the VB6 supplementation level in the ileum. TGF-β is related to cell growth control, cell proliferation, and cell differentiation (Kropf et al., 1997). It may be why there are changes in ileal proliferation and morphology.
AAs derived from dietary protein in the intestine are important for animal growth and health. They are essential substrates for protein synthesis in other tissues (Broer, 2008). They also play important roles in cellular growth, proliferation, and inflammatory response in mammalian intestines (Naomoto et al., 2005; He et al., 2018). AAs are mainly transported into the enterocyte in free form by specific AA transporters (Palacin and Kanai, 2004). Prior reports suggested that VB6 participated in many phases of protein and amino acid metabolism and played roles in the active process of cellular uptake of amino acids (None 1950; Christensen et al., 1954). In the present study, VB6 supplementation increased mRNA abundance of SLC6A19 and SLC6A20 and decreased mRNA abundance of SLC36A1 in jejunal mucous. Vitamin B6 supplementation also increased mRNA abundance of SLC6A19, SLC7A6, SLC7A7, and SLC36A1 in ileal mucous. It is suggested that VB6 may mediate the metabolism of protein metabolism and absorption of amino acids by altering the transportion of AAs in weaned piglets. Considering with the previous results of this study, it is speculated that change of AA transporters may be associated with intestinal cellular proliferation and inflammatory status. Further study of the regulatory mechanism of molecular and signal pathways among them needs to be done.
In summary, VB6 supplementation in weaned piglets increases villus height and villus width, decreases Ki67 positive cells counts, promotes mRNA expression of inflammatory cytokines in the ileum, and alters the mRNA expression of AA transporters in the jejunum and ileum. These findings suggest that VB6 dietary supplementation may affect intestinal morphology and absorption and metabolism of protein in weaned piglets fed a high-protein diet by altering the expression of inflammatory cytokines and AA transporters in the intestine.
Conflict of Interest
None declared.
Literature Cited
- Acciaioli A., Sirtori F., Pianaccioli L., Campodoni G., Pugliese C., Bozzi R.. and Franci O.. 2011. Comparison of total tract digestibility and nitrogen balance between cinta senese and large white pigs fed on different levels of dietary crude protein. Anim. Feed Sci. Tech. 169:134–139. doi: 10.1016/j.anifeedsci.2011.05.009 [DOI] [Google Scholar]
- Albersen M., Bosma M., Knoers N. V., de Ruiter B. H., Diekman E. F., de Ruijter J., Visser W. F., de Koning T. J., and Verhoeven-Duif N. M.. 2013. The intestine plays a substantial role in human vitamin B6 metabolism: a Caco-2 cell model. PLoS ONE. 8:e54113. doi: 10.1371/journal.pone.0054113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecha F., and Charley B.. 1990. Rationale for using immunopotentiators in domestic food animals. Adv. Vet. Sci. Comp. Med. 35:3–19. doi: 10.1016/b978-0-12-039235-3.50007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry G., Péron V., Le Huërou-Luron I., Lallès J. P., and Sève B.. 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256 [DOI] [PubMed] [Google Scholar]
- Bröer S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88:249–286. doi: 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- Canham J. E., Baker E. M., Harding R. S., Sauberlich H. E., and Plough I. C.. 1969. Dietary protein–its relationship to vitamin B6 requirements and function. Ann. N. Y. Acad. Sci. 166:16–29. doi: 10.1111/j.1749-6632.1969.tb54252.x [DOI] [PubMed] [Google Scholar]
- Chiang E. P., Selhub J., Bagley P. J., Dallal G., and Roubenoff R.. 2005. Pyridoxine supplementation corrects vitamin B6 deficiency but does not improve inflammation in patients with rheumatoid arthritis. Arthritis Res. Ther. 7:R1404–R1411. doi: 10.1186/ar1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N., Riggs T. R., and Coyne B. A.. 1954. Effects of pyridoxal and indoleacetate on cell uptake of amino acids and potassium. J. Biol. Chem. 209:413–427. doi: 10.2307/2485221 [DOI] [PubMed] [Google Scholar]
- Clark I. A. 2007. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 18:335–343. doi: 10.1016/j.cytogfr.2007.04.002 [DOI] [PubMed] [Google Scholar]
- De Conto C., Oevermann A., Burgener I. A., Doherr M. G., and Blum J. W.. 2010. Gastrointestinal tract mucosal histomorphometry and epithelial cell proliferation and apoptosis in neonatal and adult dogs. J. Anim. Sci. 88:2255–2264. doi: 10.2527/jas.2009-2511 [DOI] [PubMed] [Google Scholar]
- De Lange C. F. M., Pluske J., Gong J., and Nyachoti C. M.. 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 134:124–134. doi: 10.1016/j.livsci.2010.06.117 [DOI] [Google Scholar]
- Eliot A. C., and Kirsch J. F.. 2004. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu. Rev. Biochem. 73:383–415. doi: 10.1146/annurev.biochem.73.011303.074021 [DOI] [PubMed] [Google Scholar]
- Friso S., Jacques P. F., Wilson P. W., Rosenberg I. H., and Selhub J.. 2001. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 103:2788–2791. doi: 10.1161/01.cir.103.23.2788 [DOI] [PubMed] [Google Scholar]
- Gallo L., Dalla Montà G., Carraro L., Cecchinato A., Carnier P., and Schiavon S.. 2014. Growth performance of heavy pigs fed restrictively diets with decreasing crude protein and indispensable amino acids content. Livest. Sci. 161:130–138. doi: 10.1016/j.livsci.2013.12.027 [DOI] [Google Scholar]
- Galloway P., McMillan D. C., and Sattar N.. 2000. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 37 (Pt 3):289–297. doi: 10.1258/0004563001899429 [DOI] [PubMed] [Google Scholar]
- He F., Wu C., Li P., Li N., Zhang D., Zhu Q., Ren W., and Peng Y.. 2018. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed. Res. Int. 2018:9171905. doi: 10.1155/2018/9171905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedemann M. S., Højsgaard S., and Jensen B. B.. 2003. Small intestinal morphology and activity of intestinal peptidases in piglets around weaning. J. Anim. Physiol. Anim. Nutr. (Berl). 87:32–41. doi:10.1046/j.1439-0396.2003.00405.x [DOI] [PubMed] [Google Scholar]
- Htoo J. K., Araiza B. A., Sauer W. C., Rademacher M., Zhang Y., Cervantes M., and Zijlstra R. T.. 2007. Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs. J. Anim. Sci. 85(12):3303–3312. doi:10.2527/jas.2007-0105 [DOI] [PubMed] [Google Scholar]
- Jacobs F. A. 1962. Role of vitamin B6 in intestinal absorption of amino acids in situ. JAMA. 179:523–525. doi: 10.1001/jama.1962.03050070000009 [DOI] [PubMed] [Google Scholar]
- Kang P., Wang M., Hou Y. Q., Yin Y. L., Ding B. Y., Zhu H. L., Liu Y. L., Qiu Y. S., Yi D., Wang L., and Gong J.. 2012. Effects of oral administration of spermine on the development of small intestine and growth performance of weaned pigs. J. Anim. Vet. Adv. 11:2782–2787. doi: 10.3923/javaa.2012.2782.2787 [DOI] [Google Scholar]
- King M., Kelly D., Morel P., and Pluske J.. 2003. Aspects of intestinal immunity in the pig around weaning. Weaning the pig: concepts and consequences. p. 219. doi: 10.3920/978-90-8686-513-0 [DOI] [Google Scholar]
- Komatsu S. I., Watanabe H., Oka T., Tsuge H., Nii H., and Kato N.. 2001. Vitamin B-6-supplemented diets compared with a low vitamin B-6 diet suppress azoxymethane-induced colon tumorigenesis in mice by reducing cell proliferation. J. Nutr. 131:2204–2207. doi: 10.1093/jn/131.8.2204 [DOI] [PubMed] [Google Scholar]
- Kösters W. W., and Kirchgessner M.. 1976a. [Changes in feed intake of early-weaned swine in response to varying vitamin B6 supply]. Z. Tierphysiol. Tierernahr. Futtermittelkd. 37:247–254. https://www.ncbi.nlm.nih.gov/pubmed/1070212. [PubMed] [Google Scholar]
- Kösters W. W., and Kirchgessner M.. 1976b. Growth rate and feed efficiency of early-weaned swine with varying vitamin B6 supply. Z. Tierphysiol. Tierernahr. Futtermittelkd. 37:235–246. https://www.ncbi.nlm.nih.gov/pubmed/1007644. [PubMed] [Google Scholar]
- Kropf J., Schurek J. O., Wollner A., and Gressner A. M.. 1997. Immunological measurement of transforming growth factor-beta 1 (TGF-beta1) in blood; assay development and comparison. Clin. Chem. 43:1965–1974. doi: 10.1016/j.ejrad.2007.03.033 [DOI] [PubMed] [Google Scholar]
- Kumar M., and Axelrod A.. 1968. Cellular antibody synthesis in vitamin B6-deficient rats. J. Nutr. 96:53–59. doi: 10.1093/jn/96.1.53 [DOI] [Google Scholar]
- Liu P., Piao X. S., Kim S. W., Wang L., Shen Y. B., Lee H. S., and Li S. Y.. 2008. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 86:2609–2618. doi: 10.2527/jas.2007-0668 [DOI] [PubMed] [Google Scholar]
- Matte J. J., Girard C. L., and Sève B.. 2001. Effects of long-term parenteral administration of vitamin B6 on B6 status and some aspects of the glucose and protein metabolism of early-weaned piglets. Br. J. Nutr. 85:11–21. doi: 10.1079/bjn2000221. [DOI] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huërou-Luron I., Lallès J. P., and Sève B.. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi: 10.1017/S000711450720580X [DOI] [PubMed] [Google Scholar]
- Naomoto Y., Yamatsuji T., Shigemitsu K., Ban H., Nakajo T., Shirakawa Y., Motok T., Kobayashi M., Gunduz M., and Tanaka N.. 2005. Rational role of amino acids in intestinal epithelial cells (Review). Int. J. Mol. Med. 16:201–204. [PubMed] [Google Scholar]
- None. 1950. VITAMIN B6 and metabolism of d-amino acids. Nutr. Rev. 8: 202–203. doi: 10.1111/j.1753-4887.1950.tb02437.x [DOI] [PubMed] [Google Scholar]
- NRC. 1998. Nutrient requirements of swine, 10th revised ed Washington, D.C.: National Academy Press. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine, 11th revised ed Washington, D.C.: National Academy Press. [Google Scholar]
- Palacín M., and Kanai Y.. 2004. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch. 447:490–494. doi: 10.1007/s00424-003-1062-7 [DOI] [PubMed] [Google Scholar]
- Ren W., Yin J., Wu M. M., Liu G., Yang G., Yan X., Su D. D., Wu L., Li T. J., and Chen S.. 2014. Serum Amino Acids profile and the beneficial effects of L-Arginine or L-Glutamine SUPPLEMENTATION in Dextran Sulfate Sodium Colitis. PLoS ONE. 9:e88335. doi: 10.1371/journal.pone.0088335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibeni S., Cattaneo M., Vecchi M., Zighetti M. L., Lecchi A., Lombardi R., Meucci G., Spina L., and de Franchis R.. 2003. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am. J. Gastroenterol. 98:112–117. doi: 10.1111/j.1572-0241.2003.07160.x [DOI] [PubMed] [Google Scholar]
- Sakakeeny L., Roubenoff R., Obin M., Fontes J. D., Benjamin E. J., Bujanover Y., Jacques P. F., and Selhub J.. 2012. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J. Nutr. 142:1280–1285. doi: 10.3945/jn.111.153056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers O. J., Wichers M. C., and Maes M.. 2005. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 29:201–217. doi: 10.1016/j.pnpbp.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Schneider G., Käck H., and Lindqvist Y.. 2000. The manifold of vitamin B6 dependent enzymes. Structure 8:R1–R6. doi: 10.1016/s0969-2126(00)00085-x [DOI] [PubMed] [Google Scholar]
- Selhub J., Byun A., Liu Z., Mason J. B., Bronson R. T., and Crott J. W.. 2013. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J. Nutr. Biochem. 24:2138–2143. doi: 10.1016/j.jnutbio.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R. F., Nugara D., Hill R., and Knapp W.. 1964. Vitamin B 6 Requirement of Early-Weaned Pigs. J. Anim. Sci. 23:694–699. doi: 10.2527/jas1964.233694x [DOI] [Google Scholar]
- Verdonk J., Bruininx E., Van Der Meulen J., and Verstegen M.. 2007. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest. Sci. 108:146–149. doi: 10.1016/j.livsci.2007.01.093 [DOI] [Google Scholar]
- Wang L. X., Yan S. L., Li J. Z., Li Y. L., Ding X. Q., Yin J., Xiong X., Yang H. S., and Yin Y. L.. 2018. Rapid communication: the relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 97(1):353–358. doi:10.1093/jas/sky388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtten P. J., van der Meulen J., and Verstegen M. W.. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi: 10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Yan S. L., Long L. N., Zong E. Y., Huang P. F., Yang H. S., Li J. Z., Li Y. L., Ding X. Q., Xiong X., and Yin Y. L.. 2018. Dietary sulfur amino acids affect jejunal cell proliferation and functions by affecting antioxidant capacity, Wnt/β-Catenin and the mechanistic target of rapamycin signaling pathways in weaning piglets. J. Anim. Sci. doi: 10.1093/jas/sky349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. S., Fu D. Z., Kong X. F., Wang W. C., Yang X. J., Nyachoti C. M., and Yin Y. L.. 2013a. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012-5795 [DOI] [PubMed] [Google Scholar]
- Yang H. S., Li F. N., Xiong X., Kong X. F., Zhang B., Yuan X. X., Fan J. X., Duan Y. F., Geng M. M., and Li L. L.. 2013b. Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol. Cell. Endocrinol. 365:44–51. doi: 10.1016/j.mce.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Yin Y., Yao K., Liu Z., Gong M., Ruan Z., Deng D., Tan B., Liu Z., and Wu G.. 2010. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486. doi: 10.1007/s00726-010-0612-5 [DOI] [PubMed] [Google Scholar]
- Zelnickova P., Leva L., Stepanova H., Kovaru F., and Faldyna M.. 2008. Age-dependent changes of proinflammatory cytokine production by porcine peripheral blood phagocytes. Vet. Immunol. Immunopathol. 124:367–378. doi: 10.1016/j.vetimm.2008.04.016 [DOI] [PubMed] [Google Scholar]
- Zong E. Y., Huang P. F., Yang H. S., Zhang W., Li J. Z., Li Y. L., Ding X. Q., Xiong X., and Yin Y. L.. 2018. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J Anim Sci. 96:1130–1139. doi: 10.1093/jas/skx003 [DOI] [PMC free article] [PubMed] [Google Scholar]