Abstract
Fixation is the first step towards preservation of tissues and can impact downstream histological applications. Historically, formalin has been the fixative of choice in both research and clinical settings due to cost, accessibility, and broad applicability. Here, we describe a method for collection of porcine colon, and compare the usage of Carnoy’s solution (CS) to a 10% neutral buffered formalin (NBF) in tissue fixation. Consecutive colon samples were collected from 24 four-wk-old piglets and fixed in CS for 45 min or NBF for 24 h. We measured the thickness of the inner mucus layer using Alcian Blue stain and found thicker inner mucus layers in porcine colons fixed with CS as compared to NBF (P < 0.0001). Carnoy’s solution-fixed colon exhibited greater bacterial cell counts than NBF-fixed colon (P < 0.0022) after labeling with an eubacterial probe in fluorescent in situ hybridization (FISH). No difference was observed between the mucosal height (P = 0.42) and number of goblet cells (P = 0.66) between the 2 fixatives. From this, we concluded CS is more suitable than NBF for the preservation of the mucus layer and the associated mucosal bacteria in the porcine colon without compromising on overall tissue morphology. This study provides a useful sampling and fixation methodology for histology studies in the porcine gastrointestinal tract, and may be beneficial to microbiota, pathology, and nutrition studies.
Keywords: fluorescence in situ hybridization, gastrointestinal tract, histomorphometry, intestinal mucosa, mucus layer, swine
Introduction
Tissue fixation is the first essential step to observe morphologic features at the cellular level. Ideal fixatives preserve tissue morphology by maintaining chemical and structural integrity, preventing cell autolysis, and minimizing artifacts (Bancroft and Gamble, 2002). The fixation process depends both on rate of penetration and chemical interactions between the fixative and tissue (Stoward, 1973). Historically, formalin has been the most common fixative for the widest range of tissues and histology studies. Formalin is an aqueous derivative of formaldehyde stabilized by methanol or buffered solutions. Within tissue, formalin forms amino group crosslinks in cytoplasmic protein to create a macromolecular network (Stoward, 1973). In contrast, alcohol-based Carnoy’s solution (CS) fixes tissue through multiple chemical interactions occurring simultaneously as ethanol dehydrates tissue by removing water from protein groups, and chloroform and acetic acid interact through hydrogen bonding (Howat and Wilson, 2014). While formalin is often recommended for general use in clinical diagnostic and research settings due to low cost, accessibility, and adaptability, CS has been recommended for specific uses, including the preservation of nucleic acids and water-based substances such as the mucus in the gastrointestinal tract (Bancroft and Gamble, 2002; Johansson et al., 2008; Singhal et al., 2016).
Besides the tissue preservation, fixation can also impact the morphology and maintenance of associated structures, such as the mucus layers, which serve as a habitat for bacterial populations (Johansson et al., 2008). Previous studies have demonstrated that the intestinal mucus layers in pigs are influenced by the gut microbial composition and diet (Che et al. 2014; Frese et al., 2015). Disruption of the mucus layers has been associated with disease states including inflammatory bowel disease in humans (Swidsinski et al., 2007; Strugala et al. 2008) and swine dysentery (Quintana-Hayashi et al., 2015). Although metagenomic sequencing has identified bacterial populations of the pig gastrointestinal tract, these tools do not allow for localization of organisms in situ (Isaacson and Kim, 2012). The ability to locate both commensal and pathogenic bacteria as well as to preserve tissue morphology is essential for understanding the microbial roles in healthy and disease states. In order to study these bacterial populations in situ, it is crucial to utilize techniques that preserve the mucus layers. Here, we compare 2 fixatives, CS and 10% neutral-buffered formalin (NBF), in porcine colon samples. Fixed samples were stained with hematoxylin and eosin (H&E) and Alcian blue pH 2.5 (AB) stains to evaluate the tissue morphology and highlight the mucus layer, respectively. Fluorescent in situ hybridization (FISH) was performed to detect and visualize the superficial bacterial population.
Materials and Methods
The present study was performed in accordance with the United States Department of Agriculture (USDA) guidelines for animal welfare under USDA animal use protocol 2016003, and was approved by the Texas A&M University Institutional Animal Care and Use Committee under 2015-0372.
Sample Collection
A group of 24 four-wk-old, weaned barrows (Landrace x Large White; body weight of 18.3 ± 6.7 kg) was used in this study. The diet was a corn-soybean meal based pelleted diet formulated to meet or exceed recommended nutrition requirements of swine. A 12-h feed withdrawal was performed before euthanasia. A 6 cm longitudinal segment of descending colon was cut open and 2 consecutive 3 cm long samples were collected from each pig. Samples with adherent colonic contents were positioned mucosal side up on 5 × 5 cm cardboard pieces and tissue edges were secured on each edge with 3 cm binder clips to prevent tissue curling. One sample from each pig was immersed with the mucosal side exposed to CS (60% ethanol, 30% chloroform, and 10% glacial acetic acid) at a fixative to sample ratio of 20:1 for 30–45 min (Carnoy, 1887; Puchtler et al., 1968). The other consecutive sample was immersed with mucosal side exposed to NBF at a fixative to sample ratio of 10:1 for 24 h. Fixation for both CS and NBF occurred at room temperature (~22 °C). Both fixation processes were stopped with 70% ethanol, and remained in solution until routine processing (~7–14 d).
Each colonic sample was trimmed into 4 cross-sections of 5 mm width, processed and embedded into paraffin using standard procedures. Embedded tissues were cut into 4 μm consecutive sections and placed onto positively charged slides. These 4 μm sections were stained with H&E and AB as described previously (Luna, 1968). All histomorphometry measurements were performed on an Olympus BX43 microscope using cellSens Microscope Imaging (Olympus Corporation, Center Valley, PA). Objective lenses used in this study included 10×, 20×, and 40× UPLFLN/PLFLN lenses (Olympus Corporation) with 0.3, 0.5, and 0.75 apertures, respectively. The following measurements were performed for a CS slide and NBF slide from the 24 pigs.
Mucosal Height
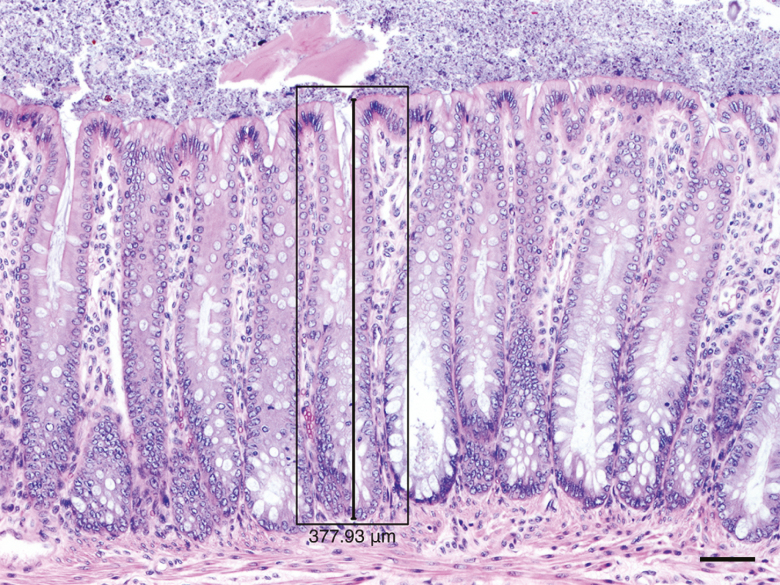
Thirteen mucosal height measurements were taken on H&E-stained sections at 100× magnification. All 4 cross-sections of colon in the slide were used for measurements. Mucosal height was defined as the length from the base of the colonocytes in the crypt to the apical membrane of the superficial colonocytes (Fig. 1). Qualifications for mucosal height measurement (qualifying crypts) included: 1) complete anchorage of the crypt in the submucosa (extending from the mucosal surface to its base in the deep lamina propria, adjacent to the muscularis mucosa), 2) intact luminal colonic epithelium at the mucosal surface, and 3) opening of the crypt between adjacent colonocytes on the mucosal surface.
Figure 1.
Qualifying crypt (black rectangle) for colonic mucosal height measurement (black line) in a 10% neutral-buffered formalin fixed section. H&E stain. Scale bar = 50 µm.
Goblet Cell Count
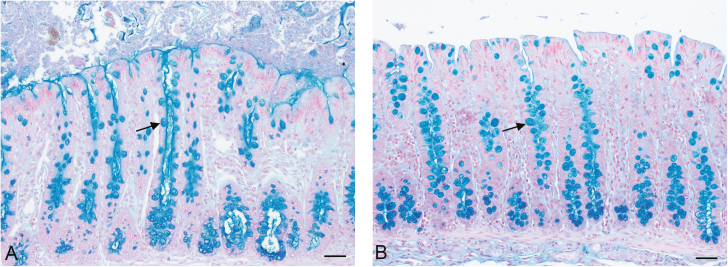
Goblet cells were manually counted from 13 qualifying crypts on sections stained with AB at 100× magnification (Fig. 2).
Figure 2.
Goblet cells (arrows) stained with Alcian Blue pH 2.5 in colonic sample fixed with (A) Carnoy’s solution and (B) 10% neutral-buffered formalin. Scale bar = 50 µm.
Inner Mucus Layer Thickness
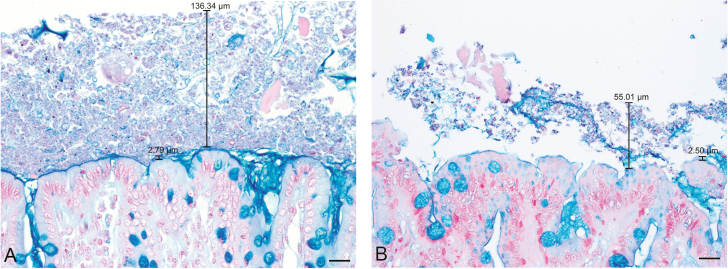
Fifteen measurements of the inner mucus layer were made from AB stained sections at 200× magnification (Fig. 3). The inner mucus layer was measured at sites where the apical membrane of the superficial colonocytes and the outer mucus layer were both intact, and the darker inner mucus layer was clearly visible.
Figure 3.
Inner mucus layer and glycocalyx (short black bar) and outer (long black bar) mucus layers in colonic samples from the same pig fixed in (A) Carnoy’s solution and (B) 10% neutral-buffered formalin stained with Alcian blue pH 2.5. The inner mucus layer and glycocalyx stained markedly darker than the looser outer layer. Mucus was also observed at the opening of crypts between adjacent colonocytes on the mucosal surface. These areas were not included in inner mucus layer measurements. Scale bar = 20 µm.
Bacterial Count
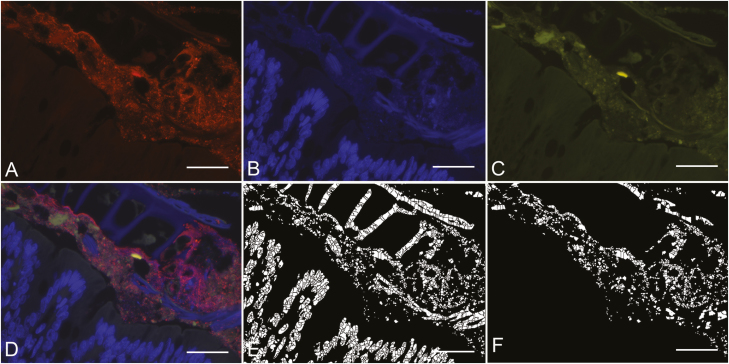
Fluorescence in situ hybridization was performed using a universal bacteria probe EUB338 (Amann et al., 1990) labeled with a 5′ Cy3 fluorophore (Invitrogen, cat #10336022), targeting bases 338 to 355 of the eubacterial 16S rRNA (5′-GCTGCCTCCCGTAGGAGC-3′). Paraffin-embedded tissue sections of 3 μm thickness were placed onto positively charged slides (Leica Biosystems, cat #3800202). Slides were warmed to 46 °C in a hybridization oven for 10 min, followed by deparaffinization in xylene and rehydration in ethanol gradients. Probes were diluted to a final working solution of 300 ng/μL with hybridization buffer pH 7.2 (20 mM Tris, 0.876 g NaCl, 0.1 g SDS, 10 g dextran sulfate, 20 mL formamide per liter). Hybridization was performed overnight at 46 °C in a humid chamber within a hybridization oven. Subsequently, slides were washed in 50 mL of washing buffer (20 mM Tris, 0.9% NaCl) at 46 °C, and incubated with washing buffer at 48 °C for 30 min. Slides were washed in 50 mL of washing buffer at 46 °C followed by 50 mL of Millipore water. Slides were air dried and mounted with 1–2 drops of ProLong Gold antifade with DAPI (Invitrogen, cat #P36930). Bacterial cell counting was performed and total counts averaged across 10 fields with labeled bacteria at 400×. Counting was performed with CellC software, using watershed segmentation to divide cells by intensity (Fig. 4) (Selinummi et al., 2005).
Figure 4.
Porcine colon sample fixed in Carnoy’s solution. (A) Bacteria labelled with EUB338 probe under TRITC filter. (B) Nuclei of colonocytes under DAPI filter. (C) Autofluorescent tissue and intestinal contents under FITC filter. (D) Layered images taken under TRITC, DAPI, and FITC filters. (E) Segmented view of all fluorescing structures in the layered image using CELLC software. (F) Segmented view of fluorescing cells in layered image also fluorescent under the TRITC filter. Scale bar = 20 µm.
Statistics
All analyses were performed using a commercial software package (JMP Pro 10, SAS Institute, Cary, NC). Mucosal height measurements, inner mucus layer thickness, and bacterial cell count were compared between the 2 fixative groups using Mann-Whitney U test for non-parametric conditions. The data for goblet cell count were normally distributed and therefore compared between the 2 groups using a Student’s t-test. Statistical significance was defined as a P < 0.01.
Results
Histomorphometry
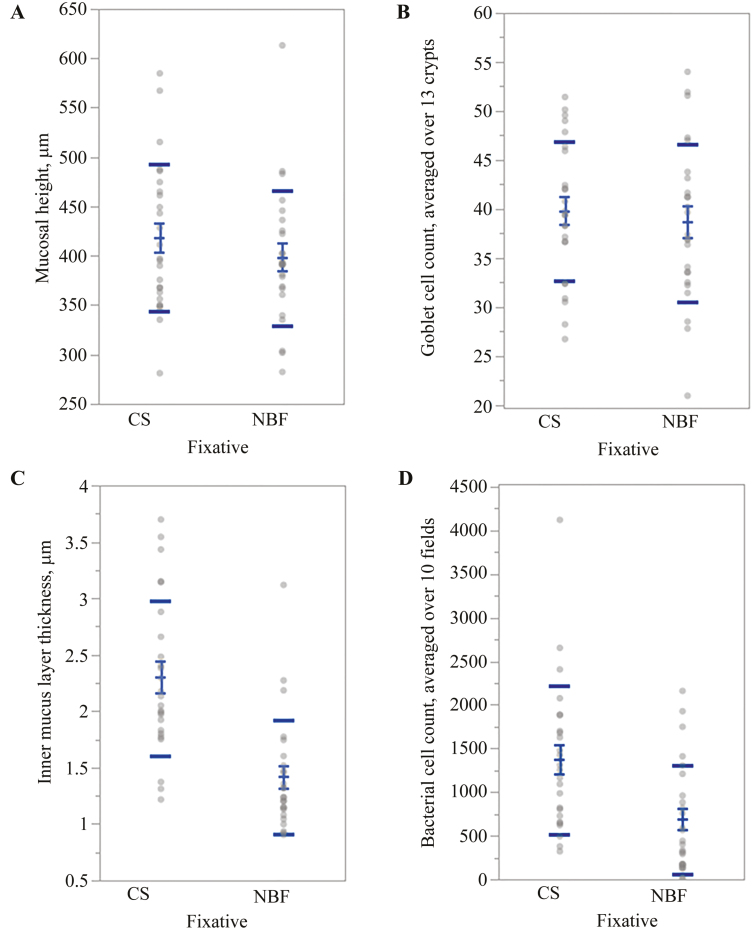
The mucosal height in CS-fixed colon samples was approximately 5% greater than NBF-fixed samples (418±14.9 µm vs. 398±13.7 µm per 13 qualifying crypts; P = 0.42; Fig. 5A), although this was not statistically different. The number of goblet cells was not significantly different between the 2 fixatives (39 ± 1.4 goblet cells vs. 38 ± 1.6 goblet cells per 13 crypts; P = 0.66; Fig. 5B). The inner mucus layer was significantly thicker in CS-fixed tissues as compared to NBF-fixed (2.29 ± 0.14 µm vs. 1.41 ± 0.10 µm, respectively; P < 0.0001; Fig. 5C).
Figure 5.
Distribution and comparison of mean values of tissues fixed with Carnoy’s solution and 10% neutral-buffered formalin from each pig. Standard error of the mean (short bars) and standard deviations (long bars) for (A) mucosal height, (B) goblet cell count, (C) inner mucus layer thickness, and (D) bacterial cell count. Significant statistical differences between fixatives were identified for inner mucus layer thickness and bacterial cell count.
Bacterial Count
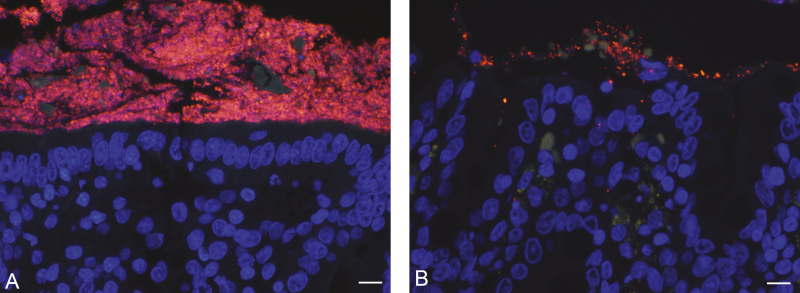
Both CS- and NBF-fixed samples contained bacteria above the colonic mucosa. Carnoy’s solution-fixed colon had significantly higher bacterial cell counts as compared to NBF-fixed samples (1,369.12 ± 170.14 cells vs. 683.69 ± 124.85 cells per 10 fields; P < 0.0022) (Figs 5D and 6).
Figure 6.
Bacteria labeled with EUB338 probe and 5’ Cy3 fluorophore (red), nuclei of colonocytes labeled with DAPI (blue), and tissue auto-fluorescence (green) in tissue fixed with (A) Carnoy’s solution and (B) 10% neutral-buffered formalin. Scale bar = 50 µm.
Discussion
In this study, we demonstrated the ability of CS compared to NBF fixative to preserve colonic mucus and the associated mucosal microbiota. The mucosa of the colon is lined by a single layer of epithelial cells known as colonocytes, and goblet cells which secrete mucus to form inner and outer mucus layers (Johansson et al., 2008). Although other studies describe tissue shrinkage in the gastrointestinal tract using CS fixative for 6–30 h (Rieger et al., 2013; Singhal et al., 2016), no significant shrinkage was observed in the CS-fixed colon samples in this study, likely due to the shorter CS-fixation time of 30–45 min. While tissue shrinkage is expected with any fixative and process for embedding, the shorter CS-fixation period used in this study could also explain the lack of difference of mucosal height between CS- and NBF-fixed colon (Puchtler et al., 1968). No significant difference of goblet cell counts of CS-fixed and NBF-fixed tissues indicated the acidophilic mucin glycoprotein detection was preserved similarly between the fixatives.
The inner mucus layer can be defined as a dense layer of mucus that lacks bacteria that is firmly adhered to epithelial cells in the large intestine (Johansson et al., 2008). Studies have described an inner/sterile mucus layer of 116 µm thickness in fresh colon of rats (Atuma et al., 2001), 20–50 µm thickness in CS-fixed colon of mice (Johansson et al., 2008; Kamphuis et al., 2017), and 56 µm thickness in CS-fixed colon of humans (Swidsinski et al., 2007). The average inner mucus layer thickness of the porcine colon in this study is quite smaller than the ~30 µm previously reported in samples cryopreserved by liquid nitrogen (Röhe et al., 2018). The discrepancy in thickness is likely due to the difference between chemical fixation and cryopreservation. Studies have demonstrated cryopreservation as a superior technique for the preservation of the structural integrity of inner mucus layer (Röhe et al., 2018; Cohen et al., 2012) and demonstrated shrinkage of the inner mucus layer during CS fixation (Matsuo et al., 1997). Further studies to improve the preservation of the inner mucus layer in swine will require modifications of methods used in laboratory animals. The larger circumference of porcine colon does not allow the same sampling technique of mice and rats, where circumferential sections of the intestine allow the retention of the fecal pellet within the intestinal lumen (Kamphuis et al., 2017). In our study, longitudinal sections of the colon allowed the fixatives to fully penetrate the tissue and intestinal contents and retained the outer mucus layer on the mucosal surface in CS-fixed samples. Additionally, alternative methods of embedding have successfully preserved the inner mucus layer of mice (Hasegawa et al., 2017) and should be tested for efficacy in porcine tissues.
CS-fixed tissues had increased bacterial cell counts, indicating the outer mucus layer, which inhabited by commensal bacteria, was better preserved as compared to NBF-fixed tissues. Since water makes up 70 to 95% of pig intestinal mucus by weight and CS largely acts by dehydration, it is likely CS acted by dehydrating the mucus layer, allowing mucus and the bacteria encased within to remain adhered to the surface of the colon (Larhed et al., 1998). CS dehydrates tissue by removing water from free hydroxyl, carboxyl, amino, amindo, and imino groups within proteins (Baker, 1958; Howat and Wilson, 2014). As bacteria were labeled with a eubacteria probe, this study demonstrates that the dehydration process of CS fixation occurs without significant damage to or masking of bacterial nucleic acids. This technique could also be employed using species-specific probes to locate and quantify individual bacterial species within the mucus layers and/or mucosa-associated content (Burrough et al., 2017). Another benefit to employing a FISH-based technique is the ability to colocalize with multiple target probes and distinct fluorophores. This could be applied to explore the role of probiotic bacteria in the gut (Li et al., 2008), and colocalize secretory IgA with bacteria (Rogier et al., 2014).
Modern techniques such as metagenomic sequencing have shown bacterial species diversity and richness vary across gastrointestinal tract segments (Zhao et al., 2015). However, preparation of the gut bacteria for sequencing homogenizes the mucus layers and gut microhabitats, making distinction of microbial communities within each mucus layer unattainable. Since bacterial populations play a large role in the physiology, metabolism, and immunology of the host (Fouhse et al., 2016), understanding the biogeography of bacterial populations in the gastrointestinal tract of pigs is of particular importance due to their role in food animal production and as models of gastrointestinal disease (Zhang et al., 2013).
Therefore, the use of an optimal fixative in the gastrointestinal tract is critical for defining biologically meaningful spatial relationships of microbiota in the gut. Mapping the intestinal distribution of bacteria in different intestinal segments will generate further understanding of the microbiome–host relationship, with the potential to inform disease pathogenesis and nutrition studies. This will be especially useful in the current era of phasing out broad-spectrum antibiotics and promoting probiotic administration in food-producing animals, and for studies that use pigs as models of gastrointestinal disease.
In conclusion, we described a method for sampling the porcine colon. CS fixative is an accessible and convenient chemical fixative that allows for downstream applications including in situ identification of bacterial nucleic acids while preserving intestinal tissue morphology. Although CS fixative may have caused shrinkage of the inner mucus layer during fixation and paraffin embedding, it was extremely useful preserving the outer mucus layer on the colonic surface.
Acknowledgments
The authors would like to thank Abby M. Korn, Ken Genovese, Jeann Leal, and Caitlin Older for their assistance during necropsy. This work was funded by the Bill and Melinda Gates Foundation (OPP1139800) and Texas A&M AgriLife Research. C.B.-V. had a fellowship from Texas A&M University Global One Health Office.
Literature Cited
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., and Stahl D. A.. . 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atuma C., Strugala V., Allen A., and Holm L.. . 2001. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- Baker J. R. 1958. Fixation in cytochemistry and electron-microscopy. J. Histochem. Cytochem. 6:303–308. doi: 10.1177/6.5.303. [DOI] [PubMed] [Google Scholar]
- Bancroft J. and Gamble M.. . 2002. Theory and practice of histological techniques. 5th ed Edinburgh, Scotland: Churchill Livingstone. [Google Scholar]
- Burrough E. R., Arruda B. L., and Plummer P. J.. . 2017. Comparison of the luminal and mucosa-associated microbiota in the colon of pigs with and without swine dysentery. Front. Vet. Sci. 4:139. doi: 10.3389/fvets.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnoy J. 1887. Les globules polaries de l’Ascaris clavata. Cellule 3:247–324. [Google Scholar]
- Che L., Chen H., Yu B., He J., Zheng P., Mao X., Yu J., Huang Z., and Chen D.. . 2014. Long-term intake of pea fiber affects colonic barrier function, bacterial and transcriptional profile in pig model. Nutr. Cancer 66:388–399. doi: 10.1080/01635581.2014.884229. [DOI] [PubMed] [Google Scholar]
- Cohen M., Varki N. M., Jankowski M. D., and Gagneux P.. . 2012. Using unfixed, frozen tissues to study Natural Mucin distribution. J. Vis. Exp. 67:e3928. doi: 10.3791/3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouhse J. M., Zijlstra R. T., and Willing B. P.. . 2016. The role of gut microbiota in the health and disease of pigs. Animal Frontiers. 6:30–36. doi: 10.2527/af.2016-0031. [DOI] [Google Scholar]
- Frese S. A., Parker K., Calvert C. C., and Mills D. A.. . 2015. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Mark Welch J. L., Rossetti B. J., and Borisy G. G.. . 2017. Preservation of three-dimensional spatial structure in the gut microbiome. Plos One 12:e0188257. doi: 10.1371/journal.pone.0188257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howat W. J., and Wilson B. A.. . 2014. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods 70:12–19. doi: 10.1016/j.ymeth.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R., and Kim H. B.. . 2012. The intestinal microbiome of the pig. Anim. Health Res. Rev. 13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Phillipson M., Petersson J., Velcich A., Holm L., and Hansson G. C.. . 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis J. B. J., Mercier-Bonin M., Eutamène H., and Theodorou V.. . 2017. Mucus organisation is shaped by colonic content; a new view. Sci. Rep. 7:8527. doi: 10.1038/s41598-017-08938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhed A. W., Artursson P., and Björk E.. . 1998. The influence of intestinal mucus components on the diffusion of drugs. Pharm. Res. 15:66–71. doi:10.1023/a:1011948703571. [DOI] [PubMed] [Google Scholar]
- Li X. J., Yue L. Y., Guan X. F., and Qiao S. Y.. . 2008. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 104:1082–1091. doi: 10.1111/j.1365-2672.2007.03636.x. [DOI] [PubMed] [Google Scholar]
- Luna L. G. 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology. New York, NY: McGraw-Hill. [Google Scholar]
- Matsuo K, Akamatsu T, and Katsuyama T. . 1997. Histochemistry of the surface mucous gel layer of the human colon. Gut 40(January): 782–89. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchtler H., Waldrop F. S., Conner H. M., and Terry M. S.. . 1968. Carnoy fixation: Practical and theoretical considerations. Histochemie. 16:361–371. doi: 10.1007/bf00306359. [DOI] [PubMed] [Google Scholar]
- Quintana-Hayashi M. P., Mahu M., De Pauw N., Boyen F., Pasmans F., Martel A., Premaratne P., Fernandez H. R., Teymournejad O., Vande Maele L., . et al. 2015. The levels of Brachyspira hyodysenteriae binding to porcine colonic mucins differ between individuals, and binding is increased to mucins from infected pigs with de novo MUC5AC synthesis. Infect. Immun. 83:1610–1619. doi: 10.1128/IAI.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger J., Twardziok S., Huenigen H., Hirschberg R. M., and Plendl J.. . 2013. Porcine intestinal mast cells. Evaluation of different fixatives for histochemical staining techniques considering tissue shrinkage. Eur. J. Histochem. 57:e21. doi: 10.4081/ejh.2013.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogier E. W., Frantz A. L., Bruno M. E., and Kaetzel C. S.. . 2014. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 29:390–403. doi: 10.3390/pathogens3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhe I., Hüttner F. J., Plendl J., Drewes B., and Zentek J.. . 2018. Comparison of different histological protocols for the preservation and quantification of the intestinal mucus layer in pigs. Eur. J. Histochem. 62:2874. doi: 10.4081/ejh.2018.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinummi J., Seppälä J., Yli-Harja O., and Puhakka J. A.. . 2005. Software for quantification of labeled bacteria from digital microscope images by automated image analysis. Biotechniques 39:859–863. doi: 10.2144/000112018. [DOI] [PubMed] [Google Scholar]
- Singhal P., Singh N. N., Sreedhar G., Banerjee S., Batra M., and Garg A.. . 2016. Evaluation of histomorphometric changes in tissue architecture in relation to alteration in fixation protocol - an invitro study. J. Clin. Diagn. Res. 10:ZC28–ZC32. doi: 10.7860/JCDR/2016/19007.8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoward P. J. 1973. Fixation in histochemistry. Chapman and Hall, London, UK. doi: 10.1007/978-1-4899-3260-0. [DOI] [Google Scholar]
- Strugala V., Dettmar P. W., and Pearson J. P.. . 2008. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int. J. Clin. Pract. 62:762–769. doi: 10.1111/j.1742-1241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Loening-Baucke V., Theissig F., Engelhardt H., Bengmark S., Koch S., Lochs H., and Dörffel Y.. . 2007. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Widmer G., and Tzipori S.. . 2013. A pig model of the human gastrointestinal tract. Gut Microbes 4:193–200. doi: 10.4161/gmic.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Wang Y., Liu S., Huang J., Zhai Z., He C., Ding J., Wang J., Wang H., Fan W., Zhao J., and Meng H.. . 2015. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One. 10:e0117441. doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]