Abstract
Intestinal challenges upon weaning would increase the needs of nucleotides for enterocyte proliferation, whereas de novo synthesis maybe insufficient. This study aimed to evaluate supplemental effects of dietary nucleotides on intestinal health and growth performance in newly weaned pigs. Fifty newly weaned pigs (19-d-old, 25 barrows and 25 gilts, 4.76 ± 0.42 kg BW) were individually housed and allotted to 5 treatments with increasing nucleotide supplementation (0, 50, 150, 250, and 500 mg/kg) based on a randomized complete block design with the initial BW and sex as blocks. Dietary nucleotides were provided from YT500 (Hinabiotech, Guangzhou, China). Pigs were fed for 21 d based on 2 phases (phase 1: 11 d and phase 2: 10 d) and experimental diets were formulated to meet or exceed nutrient requirements suggested by NRC (2012). Feed intake and BW were recorded. Titanium oxide (0.4%) was added as an indigestible marker from day 17. Plasma collected on day 18 was used to measure tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and malondialdehyde (MDA). Pigs were euthanized on day 21 to collect tissues to evaluate TNF-α, IL-6, MDA, morphology, and crypt cell proliferation rate in the jejunum. Ileal digesta were collected to measure ileal nutrient digestibility. Data were analyzed using contrasts in the MIXED procedure of SAS. Nucleotide supplementation increased (P < 0.05) ADFI in phase 1. Nucleotide supplementation at 50 and 150 mg/kg increased (P < 0.05) ADG in phase 1, whereas increased (P < 0.05) ADFI and tended to increase (P = 0.082) ADG in overall. Increasing nucleotide supplementation changed (quadratic, P < 0.05) villus height-crypt ratio (at 247 mg/kg) and decreased (linear, P < 0.05) crypt cell proliferation rate in the jejunum. Increasing nucleotide supplementation reduced (P < 0.05) jejunal IL-6 (at 50 and 150 mg/kg) and tended to change (quadratic, P = 0.074) plasma MDA (at 231 mg/kg). Nucleotide supplementation at 50 and 150 mg/kg increased (P < 0.05) ileal digestibility of energy and ether extract. In conclusion, nucleotide supplementation at a range of 50 to 250 mg/kg in the diets seems to be beneficial to newly weaned pigs by enhancing growth performance possibly due to reduced intestinal inflammation and oxidative stress as well as improved intestinal villi structure and energy digestibility.
Keywords: intestinal health, nucleotides, pigs
Introduction
Weaning is a critical events causing challenges to pigs for their growth and intestinal health (Pluske et al., 2002; Lallès, 2012). After weaning, nutrient digestibility is reduced by the collapse of the intestinal barriers due to intestinal inflammation (Pié et al., 2004; Moeser et al., 2017) and oxidative stress (Chen et al., 2017; Duarte et al., 2019). In addition, weaning can affect the metabolism and proliferation of enterocytes in nursery pigs (Yang et al., 2016).
Nucleotides in mammalian cells are continuously synthesized and degraded, whereas playing crucial roles in various biological processes (Zaharevitz et al., 1992; Sauer et al., 2011) for transferring energy (Carver and Allan Walker, 1995), RNA transcription (Grimble, 1994), and synthesis of coenzyme component (Cosgrove, 1998). Mateo et al. (2004) described that typical nursery diets severely lack nucleotides compared with those in colostrum and milk consumed before weaning. De novo synthesis of nucleotides in enterocytes and intestinal immune cells is insufficient and thus there is an elevated needs of nucleotides to support rapid turnover of enterocytes from damages after weaning (Uauy et al., 1990; Grimble, 1994; Sauer et al., 2011).
Weaver and Kim (2014) showed that supplementation of 5′inosine monophosphate (5′IMP) increased feed intake, whereas it reduced the intensity of the immune response and measures of oxidative stress in the plasma of newly weaned pigs ameliorating weaning stress. Waititu et al. (2017) also showed that supplementation of dietary nucleotides altered intestinal microbiome by decreasing proliferation of colonic Enterococcus spp. and improved proliferation of cecal Lactobbacillus spp. Moreover, previous studies found that supplementation of dietary nucleotides in nursery diets enhanced intestinal morphology (Li et al., 2016) and intestinal immunity (Waititu et al., 2017). However, dietary supplemental levels varied among studies with different extents of the results. There is limited information for the optimal supplemental levels of nucleotides in the nursery diets and exploring the sequential responses by dietary nucleotides to intestinal health including inflammatory status, oxidative stress, morphological changes, enterocyte proliferation, nutrient digestion, and finally growth performance as outcomes of these sequential responses. It is, therefore, hypothesized that supplementation of dietary nucleotides may enhance intestinal health and thus growth performance of newly weaned pigs as they have insufficient endogenous nucleotides upon weaning to overcome intestinal challenges upon weaning. To test the hypothesis, the objectives of this study was to evaluate effects of dietary nucleotides at various supplemental levels on intestinal health and growth performance of newly weaned pigs.
Materials and Methods
A protocol of this experiment was reviewed and approved by the Institutional Animal Care and Use Committee at North Carolina State University (Raleigh, NC). The experiment was conducted at the North Carolina State University Metabolism Educational Unit (Raleigh, NC).
Animals and Experimental Diets
A total of 50 newly weaned pigs (25 barrows and 25 gilts; initial BW 4.76 ± 0.42 kg) were randomly allotted to 5 treatments based on randomized complete block design with BW and sex as blocks. Each treatment had 10 replicates with 1 pig per pen. Pigs were provided with feed and water ad libitum during the experiment. The experimental period was divided into 2 phases; phase 1 (weaning to day 11) and phase 2 (day 12 to 21). The individual BW of pigs and feed intake were recorded for each phase. Duration of each phase was set based on average body weight of pigs according to NRC (2012).
The experimental diets (Table 1) were mixed at North Carolina Feed Educational Unit (Raleigh, NC). Experimental diets contained low proportion of fish meal and poultry meal to reduce the amount of endogenous nucleotides coming from feed ingredients (Table 2) based on published data from Mateo (2005) and Suresh et al. (2011). The experimental diets were formulated to provide nutrients to meet or exceed requirements suggested by NRC (2012). Diets were supplemented with 0, 50, 150, 250, or 500 mg/kg of dietary nucleotides extracted from yeast cells (YT500; Hinabiotech, Guangzhou, China). The dietary nucleotides used for this study consisted of 33.2% adenosine 5′-monophosphate (5′AMP), 23.3% uridine 5′-monophosphate (5′UMP), 23.3% guanosine 5′-monophosphate (5′GMP), and 19.9% cytidine 5′monophosphate (5′CMP). Crude protein content in the supplement was 55% and replaced equal amounts of soybean meal as supplemented in the diets. On day 17, titanium dioxide (0.4%) was added as an indigestible marker in all diets and these diets were fed to pigs for 5 d to provide sufficient time before the sampling of ileal digesta.
Table 1.
Composition of experimental diets (as-fed basis)1
| Item | Phase 1 | Phase 2 |
|---|---|---|
| Ingredient, % | ||
| Corn, yellow | 42.96 | 54.58 |
| Soybean meal, 48% CP | 19.00 | 23.50 |
| Whey permeate | 20.00 | 10.00 |
| Blood plasma | 7.50 | 4.00 |
| Poultry meal | 3.20 | 2.00 |
| Fish meal | 2.00 | 0.00 |
| L-Lys HCl | 0.37 | 0.41 |
| DL-Met | 0.17 | 0.15 |
| L-Thr | 0.10 | 0.11 |
| Salt | 0.22 | 0.22 |
| Vitamin permix2 | 0.03 | 0.03 |
| Trace mineral permix3 | 0.15 | 0.15 |
| Dicalcium phosphate | 0.30 | 0.90 |
| Limestone | 1.00 | 0.95 |
| Poultry fat | 2.00 | 2.00 |
| Calculated composition | ||
| DM, % | 90.60 | 90.03 |
| ME, kcal/kg | 3,398 | 3,371 |
| CP, % | 23.40 | 21.52 |
| SID4 Lys, % | 1.50 | 1.35 |
| SID Cys + Met, % | 0.82 | 0.73 |
| SID Trp, % | 0.26 | 0.23 |
| SID Thr, % | 0.88 | 0.79 |
| Ca, % | 0.85 | 0.80 |
| STTD5 P, % | 0.44 | 0.40 |
| Total P, % | 0.64 | 0.63 |
| Nucleotides, mg/kg6 | 112.4 | 48.6 |
1Diets in each phase supplemented with increasing supplementation of 0, 0.005, 0.015, 0.025, and 0.05% nucleotides extracted from yeast cell (YT500, Hinabiotech, Guangzhou, China) for a total of 5 diets per phase.
2The vitamin premix provided per kilogram of complete diet: 6,614 IU of vitamin A as vitamin A acetate, 992 IU of vitamin D3, 19.8 IU of vitamin E, 2.64 mg of vitamin K as menadione sodium bisulfate, 0.03 mg of vitamin B12, 4.63 mg of riboflavin, 18.52 mg of D-pantothenic acid as calcium panthonate, 24.96 mg of niacin, and 0.07 mg of biotin.
3The trace mineral premix provided per kilogram of complete diet: 33 mg of Mn as manganous oxide, 110 mg of Fe as ferrous sulfate, 110 mg of Zn as zinc sulfate, 16.5 mg of Cu as copper sulfate, 0.30 mg of I as ethylenediamine dihydroiodide, and 0.30 mg of Se as sodium selenite.
4SID, standardized ileal digestible.
5STTD P, standardized total tract digestible phosphorus.
6Data from Mateo (2005) and Suresh et al. (2011).
Table 2.
Nucleotides composition in feed ingredients and experimental diets
| Item | Nucleotides, mg/kg | |||||
|---|---|---|---|---|---|---|
| AMP | CMP | GMP | IMP | UMP | Total | |
| Feed ingredients | ||||||
| Corn1 | 2 | 3 | 3 | 1 | 0 | 9 |
| Barley1 | 1 | 2 | 1 | 1 | 0 | 5 |
| Soybean meal1 | 8 | 16 | 3 | 2 | 0 | 29 |
| Fish meal (anchovy)2 | 312 | 28 | 83 | 1,150 | 32 | 1,605 |
| Poultry meal2 | 127 | 60 | 41 | 88 | 45 | 361 |
| Protein plasma spray dried1 | 2 | 2 | 2 | 1 | 0 | 7 |
| Whey powder1 | 19 | 270 | 0 | 4 | 1 | 294 |
| Calculated nucleotides composition | ||||||
| P1 diet | 17 | 61 | 5 | 27 | 2 | 112 |
| P2 diet | 8 | 34 | 3 | 3 | 1 | 49 |
1Data from Mateo (2005).
2Data from Suresh et al. (2011).
Sample Collection
Blood samples were collected (10 mL) using vacutainer tubes containing dipotassium EDTA (BD, Franklin Lakes, NJ) from the jugular vein of pigs in all pens on day 18. The tubes were centrifuged at 3,000 × g for 15 min and then plasma aliquoted to 1.5 mL tubes and stored at −80 °C until analysis. Blood sampling was done 5 d before the last day of the experiment to avoid impacts of potential stress from blood sampling on the final sampling. At the last day of experiment, all pigs were euthanized by the penetration of a captive bolt followed by exsanguination. After euthanasia, jejunal mucosa, jejunal tissues, and ileal digesta were collected. Jejunal tissues (15 cm) were obtained and flushed with saline solution to remove digesta. The first 10 cm was used to collect jejunal mucosa by scraping of the mucosal layer in jejunum using glass microscope slide and the remaining 5 cm was stored in 10% formalin buffer for histological evaluation. Plasma and jejunal mucosa samples were used to measure tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) as indicators of inflammatory status and malondialdehyde (MDA) as one of the indicators of oxidative stress status. Representing pro-inflammatory cytokines (TNF-α and IL-6) were selected to evaluate inflammatory status in the intestinal epithelium (Chen et al., 2017; Duarte et al., 2019) and MDA was selected as one of the biomarkers to indicate oxidative stress status in the intestinal epithelium (Shen et al., 2014; Park et al., 2018).
ELISA for TNF-α, IL-6, and MDA
Jejunal mucosa was weighed to 0.5 g and homogenized (Tissuemiser, Thermo Fisher Scientific Inc., Rockford, IL) on ice in 2 mL PBS. Sample preparation for analysis was followed as previously described by Chen et al. (2017). Homogenized samples were centrifuged at 15,000 × g for 30 min and then the supernatant was collected. The protein amount in supernatant was measured using Pierce BCA Protein Assay Kit (23225#, Thermo Fisher Scientific Inc., Rockford, IL) to determine concentrations of TNF-α, IL-6, and MDA per mg of protein in the jejunal mucosa.
Concentration of TNF-α, IL-6, and MDA in plasma and jejunal mucosa were measured using ELISA kits for TNF-α and IL-6 (R&D Systems, Minneapolis, MN) and Thiobarbituric Acid Reactive Substance (TBARS) Assay Kit for MDA (Cell Biolabs, Inc., San Diego, CA) following the manufacturer’s protocols. These kit analysis was followed as previously described by Chen et al. (2017).
Histology and Immunohistochemistry
Jejunal tissue samples were fixed in 10% formalin buffer for 3 wk and sent to the Histology Laboratory of North Carolina State University (Raleigh, NC) for hematoxylin and eosin staining as well as immunohistochemistry for detecting Ki67+ antibody as a biological maker for measuring the proliferation of enterocytes. A total of 15 villus and 15 crypt in each slide were selected to measure villus height (VH), villus width (VW), crypt depth (CD), and percent of Ki67+ enterocyte using a microscope (Olympus CX31 microscope). The ratio of villus height to crypt depth (VH:CD) was calculated. The measuring histomorphology was followed as previously described by Duarte et al. (2019).
Chemical Analysis
Ileal digesta were stored at −20 °C and freeze-dried (24D 48, Virtis, Gardiner, NY). The diets and freeze-dried digesta samples were ground and analyzed for dry matter concentration (method 930.15; AOAC Int., 2007), titanium dioxide concentration following the methodology described by Short et al. (1996), nitrogen using TruSpec N Nitrogen Determinator (LECO Corp., St. Joseph, MI) to calculate crude protein (6.25*N), ether extract (method 2003.06; AOAC Int., 2007), and gross energy using a calorimeter (Model 6200, Parr Instrument Company). Apparent ileal digestibility of gross energy (GE), ether extract (EE), and crude protein (CP) were calculated using titanium concentration in the diets and digesta followed as previously described by Chen et al. (2017). Measuring AID of nutrients using T-cannulation could be an effective method (Fuller, 1988; Donkoh et al., 1994) compared with the current method (Li et al., 2007; He et al., 2016) but due to the complication of multiple samplings, the current method was used.
Statistical Analysis Data
A randomized complete block design was used in this study with sex and initial BW as blocking criteria. In the model, a fixed effect was the nucleotide supplementation and random effects were sex and initial BW. The number of replications was determined based on a power test (Martin et al., 1987) to determine the effects of increasing supplemental levels of dietary nucleotides. The data were analyzed using Mixed procedure of SAS (version 9.4, SAS Inst., Inc., Cary, NC). A pen was the experimental unit. Contrasts were preplanned to determine the effects of dietary supplemental nucleotides for the 1) linear responses, 2) quadratic responses, and 3) responses at 50 + 150 mg/kg which was determined based on previous studies (Shen et al., 2009; Sauer et al., 2011). Preplanned contrasts were determined using the Interactive Matrix Language (IML) procedure of SAS to generate coefficients for the unevenly spaced orthogonal contrasts. These coefficients generated by the IML procedure were then used in the Mixed procedure for contrasts. Contrasts were for linear effects of the P values less than 0.05 were considered statistically significance and between 0.05 and 0.10 were considered tendency.
Results
Growth Performance
Dietary nucleotide supplementation at 50 and 150 mg/kg tended to increase BW on day 11 (P = 0.063) and on day 21 (P = 0.075) compared with no nucleotide supplementation (Table 3). During the phase 1, dietary nucleotide supplementation at 50 and 150 mg/kg tended to increase (P = 0.059) ADG and increased (P < 0.05) ADFI compared with no nucleotide supplementation. Dietary nucleotide supplementation increased (P < 0.05) ADFI compared with no nucleotide supplementation. During the phase 2, dietary nucleotide supplementation at 50 and 150 mg/kg tended to increase (P = 0.059) ADFI compared with no nucleotide supplementation. During the overall period, dietary nucleotide supplementation at 50 and 150 mg/kg tended to increase (P = 0.067) ADG and increased (P < 0.05) ADFI compared with no nucleotide supplementation. The G:F was not influenced by dietary nucleotide supplementation.
Table 3.
Growth performance of nursery pigs fed diets with increasing levels of nucleotides (NUC) on day 21
| P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotides1, mg/kg | NUC | 0 vs. | ||||||||
| Item | 0 | 50 | 150 | 250 | 500 | SEM | Linear | Quadratic | NUC | 50 + 150 |
| BW, kg | ||||||||||
| Day 0 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 0.2 | 0.778 | 0.676 | 0.963 | 0.720 |
| Day 11 | 6.9 | 7.4 | 7.4 | 6.8 | 7.1 | 0.4 | 0.919 | 0.894 | 0.195 | 0.063 |
| Day 21 | 10.6 | 11.6 | 11.6 | 10.8 | 10.5 | 0.7 | 0.230 | 0.310 | 0.336 | 0.075 |
| ADG, g/d | ||||||||||
| Phase 1 | 190 | 233 | 239 | 189 | 215 | 25 | 0.968 | 0.815 | 0.182 | 0.059 |
| Phase 2 | 378 | 424 | 425 | 397 | 350 | 32 | 0.208 | 0.248 | 0.589 | 0.206 |
| Overall | 280 | 324 | 323 | 288 | 272 | 25 | 0.244 | 0.290 | 0.340 | 0.067 |
| ADFI, g/d | ||||||||||
| Phase 1 | 218 | 276 | 283 | 231 | 250 | 24 | 0.997 | 0.418 | 0.049 | 0.015 |
| Phase 2 | 464 | 532 | 538 | 486 | 450 | 36 | 0.259 | 0.195 | 0.313 | 0.059 |
| Overall | 335 | 398 | 404 | 359 | 346 | 28 | 0.449 | 0.165 | 0.124 | 0.023 |
| G:F | ||||||||||
| Phase 1 | 0.85 | 0.84 | 0.87 | 0.82 | 0.84 | 0.05 | 0.778 | 0.908 | 0.873 | 0.949 |
| Phase 2 | 0.82 | 0.79 | 0.78 | 0.82 | 0.78 | 0.03 | 0.620 | 0.888 | 0.453 | 0.386 |
| Overall | 0.83 | 0.81 | 0.81 | 0.80 | 0.79 | 0.03 | 0.350 | 0.864 | 0.390 | 0.565 |
1Nucleotides were extracted from yeast cell and provided from Hinabiotech (Guangzhou, China).
Jejunal Morphology and Crypt Cell Proliferation
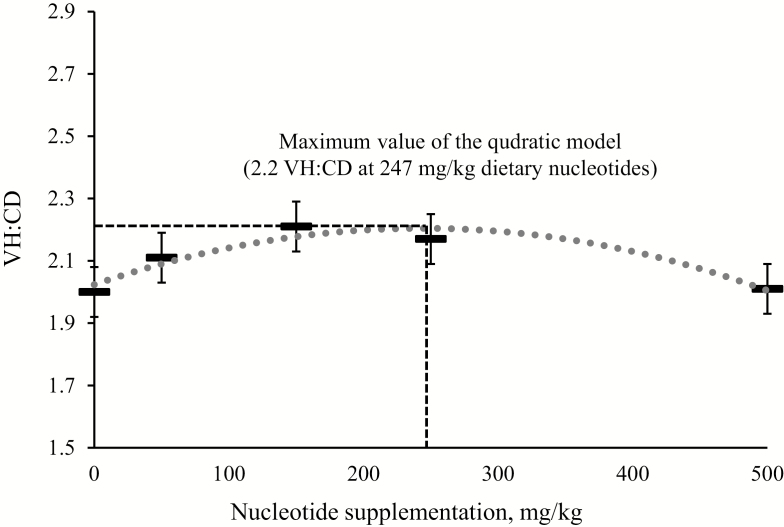
Villus height and crypt depth were not influenced by dietary nucleotide supplementation (Table 4). Villus height to crypt depth ratio showed a quadratic change (P < 0.05) as dietary nucleotide supplementation increased. A maximum villus height to crypt depth ratio (2.2) was observed when dietary nucleotides were supplemented at 247 mg/kg (Fig. 1). The proportion of newly proliferating enterocytes to mature enterocytes in the crypts was linearly decreased (P < 0.05) as dietary nucleotide supplementation increased.
Table 4.
Jejunal morphology and crypt cells proliferation of weaned pigs fed diets with increasing levels of nucleotides (NUC) on day 21
| Item | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotides1, mg/kg | NUC | 0 vs. | ||||||||
| 0 | 50 | 150 | 250 | 500 | SEM | Linear | Quadratic | NUC | 50 + 150 | |
| Jejunum | ||||||||||
| Villus height, μm | 474 | 462 | 525 | 511 | 469 | 36 | 0.993 | 0.137 | 0.622 | 0.602 |
| Villus width, μm | 98 | 100 | 97 | 101 | 103 | 4 | 0.298 | 0.874 | 0.532 | 0.882 |
| Crypt depth, μm | 263 | 245 | 262 | 261 | 258 | 13 | 0.830 | 0.816 | 0.628 | 0.494 |
| VH:CD2 | 2.00 | 2.11 | 2.21 | 2.17 | 2.01 | 0.09 | 0.712 | 0.024 | 0.144 | 0.140 |
| Crypt proliferation, % | 20.0 | 19.8 | 18.7 | 19.6 | 17.1 | 1.1 | 0.032 | 0.602 | 0.265 | 0.476 |
1Nucleotides were extracted from yeast cell and provided from Hinabiotech (Guangzhou, China).
2Villus height to crypt depth ratio.
Figure 1.
VH:CD of nursery pigs fed diets with increasing levels of nucleotide on day 21. The nucleotide supplementation that maximized VH:CD was calculated to be 247 mg/kg; the quadratic model: Eq. Y = −3·10−6·X2 − 15·10−4·X + 2.022: X = nucleotide supplementation (mg/kg); Y = VH:CD; P < 0.05; R2 = 0.91.
TNF-α, IL-6, and MDA
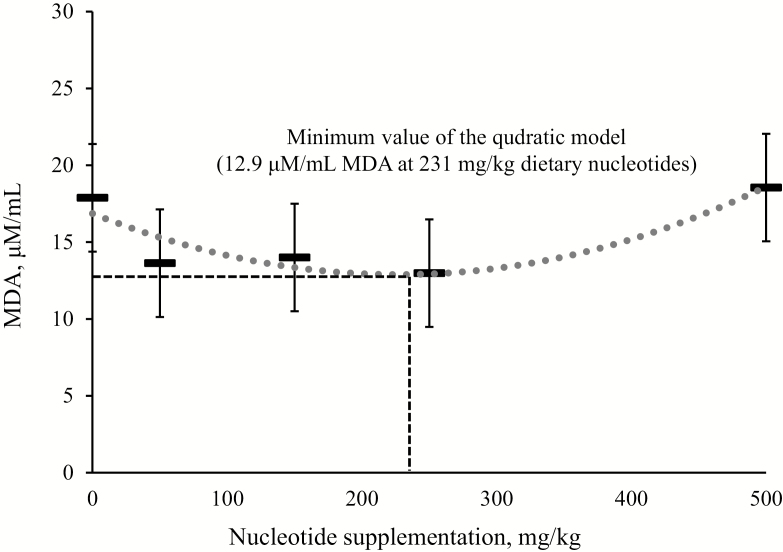
In the jejunal mucosa, dietary nucleotide supplementation at 50 and 150 mg/kg decreased (P < 0.05) IL-6 compared with no nucleotide supplementation, whereas plasma inflammatory cytokines (TNF-α and IL-6) was not influenced by dietary nucleotide supplementation (Table 5). Plasma MDA tended to show a quadratic change (P = 0.074) as dietary nucleotide supplementation increased (Fig. 2). A minimum plasma MDA concentration (12.9 μM/mL) was observed when dietary nucleotides were supplemented at 231 mg/kg. The jejunal mucosa MDA was not influenced by dietary nucleotide supplementation.
Table 5.
Inflammatory cytokines and MDA of weaned pigs fed diets with increasing levels of nucleotides (NUC) on day 21
| Item2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotides1, mg/kg | NUC | 0 vs. | ||||||||
| 0 | 50 | 150 | 250 | 500 | SEM | Linear | Quadratic | NUC | 50 + 150 | |
| Plasma | ||||||||||
| TNF-α, pg/mL | 186.1 | 144.7 | 174.6 | 187.8 | 165.5 | 47.1 | 0.996 | 0.640 | 0.418 | 0.226 |
| IL-6, pg/mL | 29.3 | 30.1 | 34.6 | 31.9 | 33.7 | 2.5 | 0.232 | 0.453 | 0.237 | 0.212 |
| MDA, μM/mL | 17.9 | 13.6 | 14.0 | 13.0 | 18.6 | 3.5 | 0.486 | 0.074 | 0.271 | 0.181 |
| Jejunal mucosa | ||||||||||
| TNF-α, pg/g of protein | 3.84 | 3.48 | 3.17 | 3.20 | 5.47 | 0.85 | 0.124 | 0.132 | 0.998 | 0.324 |
| IL-6, pg/g of protein | 3.08 | 2.63 | 2.34 | 3.35 | 2.76 | 0.33 | 0.935 | 0.921 | 0.394 | 0.040 |
| MDA, μM/g of protein | 0.40 | 0.42 | 0.37 | 0.43 | 0.39 | 0.09 | 0.982 | 0.946 | 0.912 | 0.993 |
1Nucleotides were extracted from yeast cell and provided from Hinabiotech (Guangzhou, China).
2TNF-α, tumor necrosis factor- α; IL-6 interleukin-6; MDA, malondialdehyde.
Figure 2.
Malondialdehyde (MDA) in the plasma of nursery pigs fed diets with increasing levels of nucleotides on day 21. The nucleotide supplementation that minimized MDA was calculated to be 231 mg/kg; the quadratic model: Eq. Y = 7·10−5·X2 − 0.034X + 16.842: X = nucleotide supplementation (mg/kg); Y = amount of MDA in the plasma (μM/mL); P = 0.074; R2 = 0.84.
Apparent Ileal Digestibility
Dietary nucleotide supplementation tended to increase (P = 0.072) energy digestibility (Table 6). Dietary nucleotide supplementation at 50 and 150 mg/kg increased (P < 0.05) energy digestibility and tended to increase (P = 0.083) ether extract digestibility compared with no nucleotide supplementation. The crude protein digestibility was not influenced by dietary nucleotide supplementation.
Table 6.
Apparent ileal digestibility of weaned pigs fed diets with increasing levels of nucleotides (NUC) on day 21
| P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotides1, mg/kg | NUC | 0 vs. | ||||||||
| Item | 0 | 50 | 150 | 250 | 500 | SEM | Linear | Quadratic | NUC | 50 + 150 |
| GE, % | 70.9 | 76.1 | 76.2 | 75.5 | 73.6 | 2.9 | 0.923 | 0.179 | 0.072 | 0.040 |
| EE, % | 73.5 | 78.9 | 77.4 | 73.8 | 74.9 | 2.6 | 0.640 | 0.779 | 0.247 | 0.083 |
| CP, % | 77.6 | 78.6 | 79.4 | 74.3 | 79.4 | 2.1 | 0.849 | 0.297 | 0.854 | 0.458 |
1Nucleotides were extracted from yeast cell and provided from Hinabiotech (Guangzhou, China).
Discussion
In this study, the results indicate that the supplementation of dietary nucleotides can mitigate the negative effects induced by weaning stress in pigs. Previous studies described that weaning stress causes BW loss and reduced feed intake with reduced nutrient digestibility as well as diarrhea (Campbell et al., 2013). In terms of intestinal health, nursery pigs with weaning challenge also shows increased pro-inflammatory cytokines (Pié et al., 2004), increased oxidative stress (Shen et al., 2015; Xiong et al., 2019), and increased proliferation of enterocytes (Duarte et al., 2019) with impaired intestinal histomorphology. Interestingly, this study showed that the supplementation of dietary nucleotides reduced negative responses induced by weaning on growth performance and intestinal histomorphology with alleviating inflammatory response and oxidative stress.
Previous studies described that growth retardation induced by weaning stress is related to intestinal inflammatory responses (Pié et al., 2004; Moeser et al., 2017). This study shows that pigs fed with dietary nucleotides had lower concentration of IL-6 in the jejunum, which is a mediator for the intestinal inflammatory response (McCracken et al., 1999; Zhang and An, 2007). Previous studies showed that an increase in local and circulatory IL-6 concentrations is associated with local and systemic inflammation (Dienz et al., 2009; Tanaka et al., 2014). According to in vitro studies, supplemental nucleotide showed anti-inflammatory effects by downregulating pro-inflammatory cytokines involved in macrophages and T-cell functions (Deguchi et al., 1998; Haskó et al., 2000). Moreover, dietary nucleotides can also be involved in enhancing immune cell proliferation and differentiation as de novo synthesis of nucleotides is insufficient under stressful conditions (Elías and Fló, 2002; Hess and Greenberg, 2012). This study indicates that supplementation of dietary nucleotides helped the recovery of small intestine from weaning stress and modulated immune cells to attenuate inflammatory response and oxidative stress response (Hess and Greenberg, 2012).
Weaning stress can also affect the intestinal histomorphology with increased inflammatory response (Pluske et al., 1997; Shen et al., 2009) and reduces the energy utilization for growth (Chen et al., 2017; Huntley et al., 2018). In this study, supplementation of dietary nucleotides in nursery diets increased villus height to crypt depth ratio with increased ADG, indicating that dietary nucleotides reduced mucosal inflammatory response induced by weaning stress with improved growth performance and intestinal histomorphology. In contrast, enterocytes proliferation was decreased by the addition of dietary nucleotides, indicating that nucleotides may induce increased enterocyte turnover rate faster for recovery from weaning stress (Kuhn et al., 2014). With maintaining healthy intestinal histomorphology, the nutrients and energy would be more absorbed and directly used for growth instead of enterocyte proliferation to recover from intestinal damages (Buccigrossi et al., 2010; Wang et al., 2018). This study showed that supplementation of dietary nucleotides reduced oxidative stress of newly weaned pigs. Reduction in oxidative stress by dietary nucleotides can be associated with improved health status and growth performance in nursery pigs (Lykkesfeldt and Svendsen, 2007; Shen et al., 2014). The concentration of MDA could have been broadly used as indicator of lipid peroxidation (Shen et al., 2012; Weaver et al., 2014). Salobir et al. (2005) suggested that dietary nucleotides could improve synthesis of RNA involved in the enzymes related to reduced oxidative stress. Some studies showed that dietary nucleotides could reduce cellular damage in newly weaned pigs (Godlewski et al., 2009; Weaver and Kim, 2014). Moreover, this study indicates that specific supplemental levels of dietary nucleotides at 247 and 231 mg/kg showed improved histomorphology in the jejunum and minimized concentration of MDA in the plasma, respectively.
Ohyanagi et al. (1989) found that excessive addition of dietary nucleotides at 3,350 mg/L reduced the synthesis of their DNA and RNA in liver cells and lower level of dietary nucleotides at 335 mg/L enhanced the growth of liver cells of rats under in vitro experiments. Dose–response of nucleotides has been shown in fish production (Li and Gatlin, 2006) and overdose of nucleotides was related to reduced growth, impaired immune function, and reduced health in channel catfish (Welker et al., 2011), rainbow trout (Tacon and Cooke, 1980), and malabar grouper (Lin et al., 2009). Causes of negative impact of overdosing nucleotides could be related to unbalance among nucleotides in the body (Lane and Fan, 2015) which warrant further investigation of proper mechanisms. This study indicates that the beneficial effects of dietary nucleotides are dose dependent and thus evaluation of an optimal supplemental level of dietary nucleotides seems to be important in the application.
Previous studies described that reduction of feed intake is one of the reasons for growth retardation upon weaning (Campbell et al., 2013). This study shows that dietary nucleotides improved feed intake of nursery pigs immediately after weaning. Supplementation of dietary nucleotides could be related to increased feed intake due to reduction in intestinal inflammation upon weaning. Weaver and Kim (2014) also demonstrated increased feed intake and reduced systemic inflammation by dietary supplementation of nucleotides. Reduced inflammatory response may potentially affect the secretion of cholecystokinin (CCK) and intestinal peptides enhancing the appetite in pigs fed with dietary nucleotides (Moeser et al., 2017; Rehfeld, 2017).
However, some of previous nucleotide studies showed that dietary nucleotides did not affect the growth performance of nursery pigs (Andrés-Elias et al., 2007; Superchi et al., 2012; Waititu et al., 2017). These variable outcomes in the growth performance were related to the type of source (either purified or yeast extraction) and composition of dietary nucleotides. In this study, pigs were fed diets with dietary nucleotides having 33.2% 5′AMP, 23.3% 5′UMP, 23.3% 5′GMP, and 19.9% 5′CMP. These nucleotides were extracted from yeast cells. The source, composition, and level effects might imply a complex mechanism due to the change in needs of nucleotides for enterocytes depending on the specific conditions.
In conclusion, nucleotide supplementation seems to be beneficial at a range of 50 to 250 mg/kg to newly weaned pigs by enhancing growth performance possibly due to reducing intestinal inflammation and oxidative stress as well as enhancing intestinal villi structure and energy digestibility.
Literature Cited
- Andrés-Elias N., Pujols J., Badiola I., and Torrallardona D.. 2007. Effect of nucleotides and carob pulp on gut health and performance of weanling piglets. Livest. Sci. 108:280–283. doi: 10.1016/j.livsci.2007.01.080 [DOI] [Google Scholar]
- AOAC Int. 2007. Official methods of analysis of AOAC Int. 18th ed. Rev. 2. Gaithersburg, MD: AOAC Int. [Google Scholar]
- Buccigrossi V., Giannattasio A., Armellino C., Lo Vecchio A., Caiazzo M. A., and Guarino A.. 2010. The functional effects of nutrients on enterocyte proliferation and intestinal ion transport in early infancy. Early Hum. Dev. 86(Suppl. 1):55–57. doi: 10.1016/j.earlhumdev.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Campbell J. M., Crenshaw J. D., and Polo J.. 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi: 10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver J. D., and Allan Walker W.. 1995. The role of nucleotides in human nutrition. J. Nutr. Biochem. 6:58–72. doi: 10.1016/0955-2863(94)00019-I [DOI] [Google Scholar]
- Chen H., Zhang S., Park I., and Kim S. W.. 2017. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim. Nutr. 3:359–365. doi: 10.1016/j.aninu.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove M. 1998. Nucleotides. Nutrition 14:748–751. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Takeya H., Urano H., Gabazza E. C., Zhou H., and Suzuki K.. 1998. Adenosine regulates tissue factor expression on endothelial cells. Thromb. Res. 91:57–64. doi: 10.1016/s0049-3848(98)00045-0 [DOI] [PubMed] [Google Scholar]
- Dienz O., Eaton S. M., Bond J. P., Neveu W., Moquin D., Noubade R., Briso E. M., Charland C., Leonard W. J., Ciliberto G., . et al. 2009. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 206:69–78. doi: 10.1084/jem.20081571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkoh A., Moughan P. J., and Smith W. C.. 1994 Comparison of the slaughter method and simple T-piece cannulation of the terminal ileum for determining ileal amino acid digestibility in meat and bone meal for the growing pig. Anim. Feed Sci. Technol. 49:43–56. doi: 10.1016/0377-8401(94)90080-9 [DOI] [Google Scholar]
- Duarte M. E., Zhou F. X., Dutra W. M., and Kim S. W.. 2019. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. In press. doi: 10.1016/j.aninu.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elías F., and Fló J.. 2002. Modulation of the immune response mediated by oral transgene administration of IL-10. Cell. Immunol. 216:73–81. doi: 10.1016/s0008-8749(02)00507-5 [DOI] [PubMed] [Google Scholar]
- Fuller M. F. 1988. Methods of protein evaluation for non-ruminants. In: Orskov E. R., editor, Feed science. Elsevier Science Publishers, New York, NY: p. 81–101. [Google Scholar]
- Godlewski M. M., Bierła J. B., Strzałkowski A., Martinez-Puig D., Pajak B., Kotunia A., Chetrit C., and Zabielski R.. 2009. A novel cytometric approach to study intestinal mucosa rebuilding in weaned pigs fed with dietary nucleotides. Livest. Sci. 123:215–220. doi: 10.1016/j.livsci.2008.11.012 [DOI] [Google Scholar]
- Grimble G. K. 1994. Dietary nucleotides and gut mucosal defence. Gut 35(1 Suppl): S46–S51. doi: 10.1136/gut.35.1_suppl.s46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G., Kuhel D. G., Chen J. F., Schwarzschild M. A., Deitch E. A., Mabley J. G., Marton A., and Szabó C.. 2000. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14:2065–2074. doi: 10.1096/fj.99-0508com [DOI] [PubMed] [Google Scholar]
- He L., Wu L., Xu Z., Li T., Yao K., Cui Z., Yin Y., and Wu G.. 2016. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 48:21–30. doi: 10.1007/s00726-015-2059-1 [DOI] [PubMed] [Google Scholar]
- Hess J. R., and Greenberg N. A.. 2012. The role of nucleotides in the immune and gastrointestinal systems: potential clinical applications. Nutr. Clin. Pract. 27:281–294. doi: 10.1177/0884533611434933 [DOI] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn K. A., Manieri N. A., Liu T. C., and Stappenbeck T. S.. 2014. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One 9:e114195. doi: 10.1371/journal.pone.0114195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallès J. 2012. Long term effects of pre-and early postnatal nutrition and environment on the gut. J. Anim. Sci. 90:421–429. [DOI] [PubMed] [Google Scholar]
- Lane A. N., and Fan T. W.. 2015. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 43:2466–2485. doi: 10.1093/nar/gkv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., and Gatlin D. M.. 2006. Nucleotide nutrition in fish: current knowledge and future applications. Aquaculture 251:141–152. [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., and Wu G.. 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Li B., Zhou H., Wu X., Chen Z., Yao J., and Yin Y.. 2016. Effects of dietary supplementation with uridine monophosphate on performance and intestinal morphology of weanling piglets. J. Anim. Sci. 94:82–86. doi: 10.2527/jas.2015-9440 [DOI] [Google Scholar]
- Lin Y. H., Wang H., and Shiau S. Y.. 2009. Dietary nucleotide supplementation enhances growth and immune responses of grouper, Epinephelus malabaricus. Aquac. Nutr. 15:117–122. [Google Scholar]
- Lykkesfeldt J., and Svendsen O.. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 173:502–511. doi: 10.1016/j.tvjl.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., and Willeberg P.. 1987. Veterinary epidemiology – principles and methods. Iowa State Univ. Press, Ames, IA. [Google Scholar]
- Mateo C. D. 2005. Aspects of nucleotide nutrition in pigs. Doctoral dissertation. Brookings. SD: South Dakota State University. [Google Scholar]
- Mateo C. D., Peters D. N., and Stein H. H.. 2004. Nucleotides in sow colostrum and milk at different stages of lactation. J. Anim. Sci. 82:1339–1342. doi: 10.2527/2004.8251339x [DOI] [PubMed] [Google Scholar]
- McCracken B. A., Spurlock M. E., Roos M. A., Zuckermann F. A., and Gaskins H. R.. 1999. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 129:613–619. doi: 10.1093/jn/129.3.613 [DOI] [PubMed] [Google Scholar]
- Moeser A. J., Pohl C. S., and Rajput M.. 2017. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim. Nutr. 3:313–321. doi: 10.1016/j.aninu.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. The National Academies Press, Washington, DC. [Google Scholar]
- Ohyanagi H., Nishimatsu S., Kanbara Y., Usami M., and Saitoh Y.. 1989. Effects of nucleosides and a nucleotide on DNA and RNA syntheses by the salvage and de novo pathway in primary monolayer cultures of hepatocytes and hepatoma cells. JPEN. J. Parenter. Enteral Nutr. 13:51–58. doi: 10.1177/014860718901300151 [DOI] [PubMed] [Google Scholar]
- Park I., Pasquetti T., Malheiros R. D., Ferket P. R., and Kim S. W.. 2018. Effects of supplemental L-methionine on growth performance and redox status of Turkey poults compared with the use of DL-methionine. Poult. Sci. 97:102–109. doi: 10.3382/ps/pex259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J. P., Blazy F., Laffitte J., Sève B., and Oswald I. P.. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134:641–647. doi: 10.1093/jn/134.3.641 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Pluske J. R., Pethick D. W., Hopwood D. E., and Hampson D. J.. 2002. Nutritional influences on some major enteric bacterial diseases of pig. Nutr. Res. Rev. 15:333–371. doi: 10.1079/NRR200242 [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. 2017. Cholecystokinin-from local gut hormone to ubiquitous messenger. Front. Endocrinol. (Lausanne) 8:47. doi: 10.3389/fendo.2017.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salobir J., Rezar V., Pajk T., and Levart A.. 2005. Effect of nucleotide supplementation on lymphocyte DNA damage induced by dietary oxidative stress in pigs. Anim. Sci. 82:2259–2273. [Google Scholar]
- Sauer N., Mosenthin R., and Bauer E.. 2011. The role of dietary nucleotides in single-stomached animals. Nutr. Res. Rev. 24:46–59. doi: 10.1017/S0954422410000326 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Ferket P., Park I., Malheiros R. D., and Kim S. W.. 2015. Effects of feed grade L-methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. J. Anim. Sci. 93:2977–2986. doi: 10.2527/jas.2015-8898 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Piao X. S., Kim S. W., Wang L., Liu P., Yoon I., and Zhen Y. G.. 2009. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 87:2614–2624. doi: 10.2527/jas.2008-1512 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Voilqué G., Kim J. D., Odle J., and Kim S. W.. 2012. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 90:2264–2275. doi: 10.2527/jas.2011-4203 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Weaver A. C., and Kim S. W.. 2014. Effect of feed grade L-methionine on growth performance and gut health in nursery pigs compared with conventional DL-methionine. J. Anim. Sci. 92:5530–5539. doi: 10.2527/jas.2014-7830 [DOI] [PubMed] [Google Scholar]
- Short F. J., Gorton P., Wiseman J., and Boorman K. N.. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 59:215–221. doi: 10.1016/0377-8401(95)00916-7 [DOI] [Google Scholar]
- Superchi P., Saleri R., Borghetti P., De Angelis E., Ferrari L., Cavalli V., Amicucci P., Ossiprandi M. C., and Sabbioni A.. 2012. Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Animal 6:902–908. doi: 10.1017/S1751731111002473 [DOI] [PubMed] [Google Scholar]
- Suresh A. V., Kumaraguru Vasagam K. P., and Nates S.. 2011. Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture 319:132–140. doi: 10.1016/j.aquaculture.2011.06.039 [DOI] [Google Scholar]
- Tacon A. G. J., and Cooke D. J.. 1980. Nutritional value of dietary nucleic acids to trout. Nutr. Rep. Int. 22:631–640. [Google Scholar]
- Tanaka T., Narazaki M., and Kishimoto T.. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6:a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R., Stringel G., Thomas R., and Quan R.. 1990. Effect of dietary nucleosides on growth and maturation of the developing gut in the rat. J. Pediatr. Gastroenterol. Nutr. 10:497–503. doi: 10.1097/00005176-199005000-00014 [DOI] [PubMed] [Google Scholar]
- Waititu S. M., Yin F., Patterson R., Yitbarek A., Rodriguez-Lecompte J. C., and Nyachoti C. M.. 2017. Dietary supplementation with a nucleotide-rich yeast extract modulates gut immune response and microflora in weaned pigs in response to a sanitary challenge. Animal 11:2156–2164. doi: 10.1017/S1751731117001276 [DOI] [PubMed] [Google Scholar]
- Wang Q., Xiong X., Li J., Tu Q., Yang H., and Yin Y.. 2018. Energy metabolism in the intestinal crypt epithelial cells of piglets during the suckling period. Sci. Rep. 8:12948. doi: 10.1038/s41598-018-31068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. C., Campbell J. M., Crenshaw J. D., Polo J., and Kim S. W.. 2014. Efficacy of dietary spray dried plasma protein to mitigate the negative effects on performance of pigs fed diets with corn naturally contaminated with multiple mycotoxins. J. Anim. Sci. 92:3878–3886. doi: 10.2527/jas.2013-6939 [DOI] [PubMed] [Google Scholar]
- Weaver A. C., and Kim S. W.. 2014. Supplemental nucleotides high in inosine 5’-monophosphate to improve the growth and health of nursery pigs. J. Anim. Sci. 92:645–651. doi: 10.2527/jas.2013-6564 [DOI] [PubMed] [Google Scholar]
- Welker T. L., Lim C., Yildirim-Aksoy M., and Klesius P. H.. 2011. Effects of dietary supplementation of a purified nucleotide mixture on immune function and disease and stress resistance in channel catfish, Ictalurus punctatus. Aquac. Res. 42:1878–1889. doi: 10.1111/j.1365-2109.2010.02794.x [DOI] [Google Scholar]
- Xiong X., Tan B., Song M., Ji P., Kim K., Yin Y., and Liu Y.. 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xiong X., Wang X., Li T., and Yin Y.. 2016. Effects of weaning on intestinal crypt epithelial cells in piglets. Sci. Rep. 6:1–11. doi: 10.1038/srep36939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharevitz D. W., Anderson L. W., Malinowski N. M., Hyman R., Strong J. M., and Cysyk R. L.. 1992. Contribution of de-novo and salvage synthesis to the uracil nucleotide pool in mouse tissues and tumors in vivo. Eur. J. Biochem. 210:293–296. doi: 10.1111/j.1432-1033.1992.tb17420.x [DOI] [PubMed] [Google Scholar]
- Zhang J. M., and An J.. 2007. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 45:27–37. doi: 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]