Abstract
The object of this study was to establish a new method to predict the content of DE and ME in sorghum fed to growing pigs by using near-infrared reflectance spectroscopy (NIRS). A total of 33 sorghum samples from all over China were used in this study. The samples were scanned for their spectra in the range of 12,000 to 4,000 cm−1. Based on principal components analysis of the spectra, the samples were split into a calibration set (n = 24) and a validation set (n = 9) according to the ratio of 3:1. With animal experiment values as calibration reference, the calibration models of DE and ME were established using partial least squares regression algorithm. Different spectral pretreatments were applied on the spectra to reduce the noise level. The best wavenumber ranges were also investigated. Results showed that DE and ME content in sorghum fed to growing pigs ranged from 14.57 to 16.70 MJ/kg DM and 14.31 to 16.35 MJ/kg DM, respectively. The optimal spectral preprocessing method for DE and ME was the combination of first derivative and multiplicative scatter correction. The most informative near-infrared spectral regions were 9,403.9 to 6,094.4 cm−1 and 4,605.5 to 4,242.9 cm−1 for both DE and ME. The best performance for DE and ME calibration models was the coefficient of determination of calibration (R2c) of 0.94 and 0.93, coefficient of determination of cross-external validation (R2cv) of 0.88 and 0.86, residual predictive deviation of cross-external validation (RPDcv) of 2.86 and 2.64, coefficient of determination of external validation (R2v) of 0.90 and 0.81, and residual predictive deviation of external validation (RPDv) of 3.15 and 2.35, respectively. There were no significant differences between the measured and NIRS predicted values for DE and ME (P = 0.895 for DE and P = 0.644 for ME). As the number of calibration samples increased from 24 to 33, the calibration performance of DE and ME models was improved, indicated by increased R2c, R2cv, and RPDcv values. In conclusion, NIRS quantitative models of the available energy in sorghum were established in this study. The results demonstrated that the content of DE and ME in sorghum could be predicted with relatively high accuracy based on NIRS and NIRS showed the superiority of speediness and practicality when compared with previous research methods including animal experiments, regression equations, and computer-controlled simulated digestion system.
Keywords: digestible energy; growing pigs; metabolizable energy; NIRS, sorghum
Introduction
Sorghum was first grown in tropical Africa and then gradually spread throughout the world (Bouchet et al., 2017). With the increased cost of conventional energetic feed ingredients, it is necessary to develop alternative energetic feed ingredients as renewable bioenergy feedstocks (Rooney et al., 2010). Sorghum is regarded as better substitute considering its advantage on price and availability over wheat, barley, and oats (Campher et al., 1984). In addition, sorghum is also one of the most important cereal crops rich in various phytochemicals compounds, contributing to health (Awika and Rooney, 2004). Therefore, the use of sorghum as a feed ingredient for growing pigs begins to increase in recent years (Pan et al., 2016, 2018a).
Many factors, including variety, planting year, climatic environment, planting area, rainfall, storage conditions, processing technology, and so forth, can lead to variations in nutrient composition and energetic values of feed ingredients. For example, the content of DE and ME in sorghum fed to growing pigs varied among different production areas and white sorghum contains more DE and ME than red sorghum (Pan et al., 2016, 2018a). Feed cost, a factor driving sustainable development of pork production, is influenced by accurate nutritional values during diet formulation (Stein et al., 2016). Feed energy usually accounts for a high proportion of the total feed cost (De Lange and Birkett, 2005). Therefore, it is important to estimate variation in the available energy for a specific sorghum before it is used for diet formulation (Barneveld et al., 2018).
Traditional animal experiments used to determine the available energy of a feedstuff is time consuming, expensive, and likely to have the risk of environmental pollution (Zhou et al., 2012). For these reasons, more and more methods such as prediction equations were developed to estimate the available energy in feed ingredients fed to growing pigs (Van Barneveld et al., 1999; Li et al., 2015, 2016). Pan et al. (2016) established DE and ME prediction equations for sorghum in pigs. However, prediction equations need chemical analysis before it can be used and the tannin content in sorghum is hard to determine.
Near-infrared reflectance spectroscopy (NIRS) is accepted widely for its advantage of fast, nondestructive, and accurate analysis with very little sample preparation required (Bart et al., 2007). Functional groups including −CH, −NH, and −OH are related to the main absorption bands (Xue et al., 2011). Nowadays, near-infrared technology has been increasingly used in quality control of feed ingredients (Montanhini Neto et al., 2017). Generation of reference data is indispensable to effective NIRS analysis. Moreover, DE and ME content of feed ingredients determined in digestion–metabolism experiments compared with values calculated by equations brought NIRS calibration models better predictive performance (Noblet and Perez, 1993; Li et al., 2016).
To our knowledge, no studies have been reported on the use of NIRS to predict the available energy of sorghum fed to growing pigs. The object of this study was to develop a fast method to predict the content of DE and ME in sorghum fed to growing pigs and lay the foundation for accurate diet formulation.
Materials and Methods
Sample Collection and Preparation
Sorghum samples (n = 33) used in this study included 24 samples used by Pan et al. (2016) and 9 additional samples collected from throughout China. Each sorghum sample was ground to flour using a universal grinder (Ever Bright Medical Instrument Co., company, Beijing, China) and then passed through a 40-mesh sieve before NIRS measurements.
Reference Data Determination
Digestible energy and ME content of 24 sorghum samples were previously reported by Pan et al. (2016). The DE and ME values of the remaining 9 samples were obtained as follows:
Twenty-seven healthy crossbred barrows [Yorkshire × Landrace × Duroc] with an initial body weight of 36.8 ± 3.5 kg for the first period and 41.3 ± 3.9 kg for the second period were assigned to 9 treatments according to a completely randomized block design. Each treatment contained 1 sorghum. The content of DE and ME of sorghum was determined using the total collection method. The experiment was conducted for 2 consecutive periods, with 3 replicates in each treatment per period and 1 pig in each replicate. In the 2 periods, a total of 6 replicates were used per treatment and each pig received different diet. For each period, pigs were allowed a 7-d acclimation period followed by a 5-d collection period.
On an as-fed basis, experimental diets contained sorghum (96.9%), dicalcium phosphate (1.7%), limestone (0.6%), salt (0.3%), mineral, and vitamin premix (0.5%). The only energy-supplying component of the diet was sorghum. The mineral and vitamin content of the diet exceeded nutritional needs of growing pigs published by NRC (2012).
The experiment was carried out in FengNing Swine Research Unit of China Agricultural University (Academician Workstation in Chengdejiuyun Agricultural and Livestock Co., Ltd). Pigs were housed in stainless steel metabolic crates individually. Pigs were fed at 4% of their initial body weight every day split into equally sized meals offered at 0800 and 1600 h. Pigs had free access to drinking water throughout the experiment. The pig houses were routinely disinfected and immunized. The metabolism crates were regularly cleaned to observe the health and feeding conditions of the experimental pigs. The experiment was conducted in accordance with the technical specifications for the evaluation of the nutritional value of pig feed issued by the Ministry of Agriculture and Rural Feed Efficacy and Safety Evaluation Center (Beijing).
During the collection period, orts were collected twice daily, dried, weighed, and recorded. Feces that appeared in the metabolism crates were collected immediately and placed in plastic bags. Fecal samples were stored at −20 °C. After each experimental period, all feces collected over the 5 d was mixed completely within pig. Fecal samples (700 g) were extracted from the mixture and added to 50 mL 6 mol/L HCl to fix nitrogen and then were dried at 65 °C for 72 h in a drying oven to a consistent weight. Fecal samples were ground to pass a 40-mesh sieve then sealed in bag for energy analysis.
All urine was collected daily and volume was recorded. Hydrochloric acid (50 mL, 6 mol/L) was added to collection buckets containing urine samples. A daily subsample (1/20) of total urine excreted was retained and stored at −20 °C. After each experimental period, all urine subsamples for 5 d collected from each pig were thawed and mixed completely within pig. The mixture was filtered through gauze into a 50-mL centrifuge tube and stored at −20 °C.
Gross energy of the diet, fecal, and urine samples was determined using PARR 1281 calorimeter (Instrument Company, Moline, IL). The content of DE and ME were calculated based on the following equations described by Adeola (2001):
| (1) |
| (2) |
where DE1 and DE2 represent the content of DE in each test diet and each test sorghum fed to growing pigs, respectively. Q1 and Q2 are the content of total energy in the test diet and fecal sample excreted by each experimental animal, respectively. M1 represents the weight of each test diet fed to growing pigs. X refers to the proportion of sorghum in each test diet.
| (3) |
| (4) |
where ME1 and ME2 represent the content of ME in each test diet and each sorghum fed to growing pigs, respectively. Q3 refers to the content of total energy in the urine sample excreted by each experimental animal.
NIRS Spectra Collection
NIRS spectra scanning was performed on the MATRIX I Fourier-Transform near-infrared instrument (Bruker Company, Germany) equipped with a cylindrical rotating sample cup with a diameter of 8.7 mm. The instrument was preheated for half an hour before use. Each sample was equilibrated to room temperature before NIRS measurements. The amount of sample in the sample cup was similar to ensure the same homogeneity and tightness of the sample every time samples were loaded. Shaking of the samples was avoided. For each sorghum sample, the average spectra for establishing calibration model was obtained by averaging 2 spectra from the same sample in the reflectance model to minimize noise in the spectral data. Background spectra were measured every half hour to eliminate background interference in the analysis. The parameters were set as follows: the wavenumber range was in the full spectra from 12,000 to 4,000 cm−1, the spectral resolution was 8 cm−1, and the scanning times were 64. The experiment has shown that 1,062 wavenumber points were recorded as absorbance of near-infrared light.
Sample Diversity
Principal components analysis (PCA) was used to reduce the dimension of the data set. After the NIRS spectra of 33 sorghum samples were obtained, sorghum samples were divided in a ratio of 3:1 into a calibration set comprised 24 sorghum samples and a validation set comprised 9 sorghum samples according to PCA analysis of 33 sorghum average spectra. The principal component distribution of the sorghum samples in the calibration set was similar to that of the validation set.
To investigate the effect of sample numbers on calibration performance of DE and ME, 33 sorghum samples were selected as the total set.
Calibration and Cross-external Validation
Calibration and cross-external validation processes were performed using OPUS software (Bruker Optics Inc., Billerica, MA). The calibration model for determination of the DE and ME content for growing pigs in sorghum were built based on partial least squares (PLS) regression algorithm, a mathematical optimization technique that finds the best function matching of a group of data by minimizing the error sums of squares.
Generally, if the analytical conditions have been optimized for maximum signal intensity, the increase in signal to noise ratio can be further achieved through spectral pretreatment which can reduce the noise level arising from random noise, baseline drift, wavelength shifts, or systematic measurement error, with this interference violating the assumptions upon which the calibration equations are based. Thus, spectral pretreatments including none, first derivative (1st D), multiplicative scatter correction (MSC), 1st D + MSC, standard normalized variate (SNV), 1st D + SNV, second derivative (2nd D), min max normalization (MMN), straight-line subtraction (SLS), 1st D + SLS, and constant offset elimination were applied to develop an optimal PLS regression model for determination of the content of DE and ME of sorghum for growing pigs.
The leave-one-out cross-external validation process removes the spectra of each sorghum from the calibration set one by one and leaves remnant spectra to establish new calibration models to predict the content of DE or ME of the removed sorghum until the spectra of each sorghum from the calibration set had been regarded as the object of prediction. The outliers in the sorghum calibration model were eliminated by calculating the Markov distance (MD).
Calibration models were assessed based on the coefficient of determination of calibration (R2c), root mean square error of calibration (RMSEC), coefficient of determination of cross-external validation (R2cv), root mean square error of cross-external validation (RMSECV), and residual predictive deviation of cross-external validation (RPDcv).
External Validation
External validation processes of DE and ME calibration models were performed on OPUS software (Bruker Optics Inc., Billerica, MA) using 9 sorghum samples from the validation set.
The external validation statistics output included the coefficient of determination of external validation (R2v), root mean square error of external validation (RMSEP), and residual predictive deviation of external validation (RPDv).
The accuracy and precision of the predicted values calculated by the best DE and ME models compared with the reference values were tested using the paired samples t-test procedure with SAS 9.4 (SAS Inst. Inc., Cary, NC) and statistical significance was declared at P < 0.05.
Results
Digestible Energy and ME Content of Sorghum Fed to Growing Pigs
Digestible energy and ME content of sorghum fed to growing pigs used in this study are shown in Table 1. As for the total set, the content of DE and ME ranged from 14.57 to 16.70 MJ/kg DM and 14.31 to 16.35 MJ/kg DM, respectively. The respective average content of DE and ME were 15.52 and 15.23 MJ/kg DM, respectively. The CV for DE and ME were 3.86% and 3.82%, respectively.
Table 1.
Statistics on the available energy of the total set, calibration set, and validation set as DM basis
| Items | Total set (n = 33) | Calibration set (n = 24) | Validation set (n = 9) | |||
|---|---|---|---|---|---|---|
| DE | ME | DE | ME | DE | ME | |
| Mean, MJ/kg | 15.52 | 15.23 | 15.57 | 15.29 | 15.40 | 15.11 |
| Max, MJ/kg | 16.70 | 16.35 | 16.70 | 16.35 | 16.45 | 16.09 |
| Min, MJ/kg | 14.57 | 14.31 | 14.57 | 14.31 | 14.84 | 14.55 |
| SD | 0.60 | 0.58 | 0.63 | 0.61 | 0.54 | 0.51 |
| CV, % | 3.86 | 3.82 | 4.05 | 3.99 | 3.51 | 3.38 |
Spectral Pretreatment
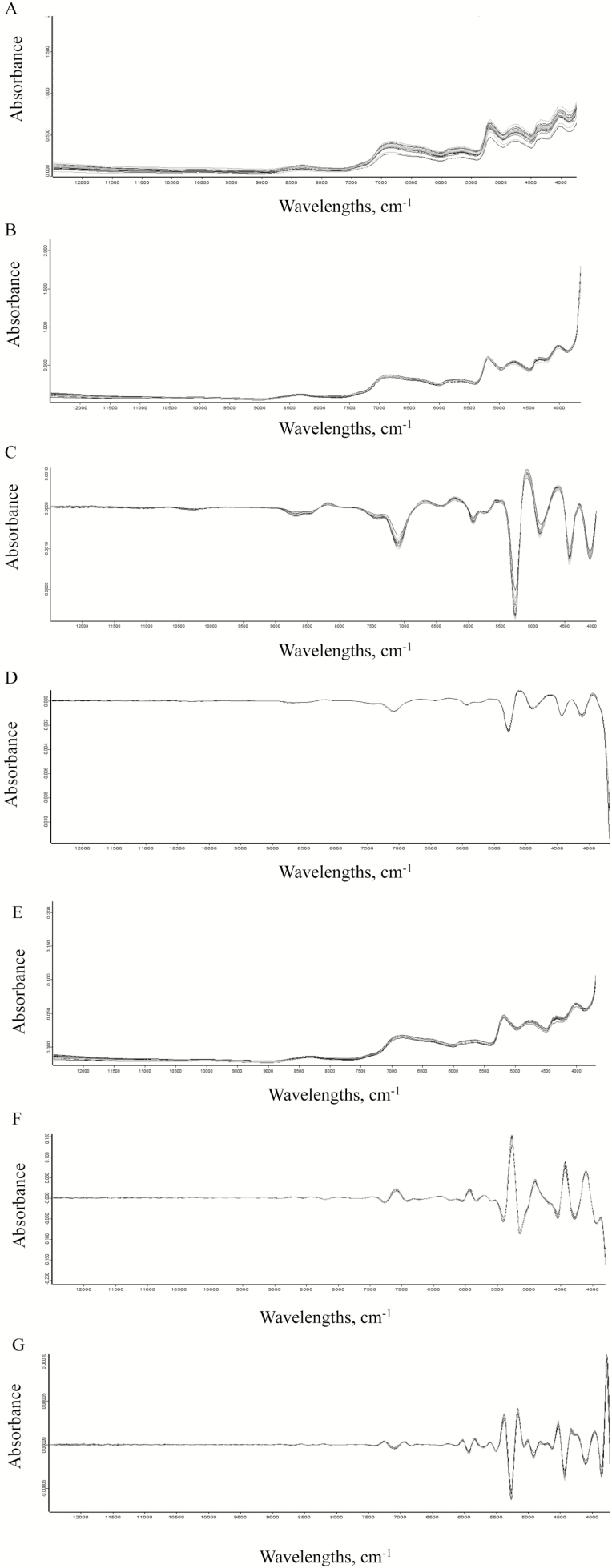
As shown in Fig. 1, the original and pretreated spectra of sorghum were recorded. The original spectra of 33 sorghum samples used in this study were Fig. 1A. Due to the influence of random noise and other factors, the original NIRS spectra showed some phenomena such as baseline drift as well as baseline rotation. Broad peaks caused by chemical groups in sorghum were also observed. The derivative pretreatments including first derivative and second derivative of NIRS spectra eliminated the influence of baseline drift and reduced the background interference, showing higher resolution and clearer spectral contour changes than the original NIRS spectra as shown in Fig. 1C and G. After spectral treatment by MSC in Fig. 1B, the effect of sample scattering on spectral changes such as baseline shift was clearly eliminated. Compositional differences of the samples could be highlighted when NIRS spectra were pretreated with SNV (Hui and Siesler, 2018).
Figure 1.
Reflectance spectra of sorghum tested in the study using different pretreatments, including none pretreatment (A), multiplicative scatter correction (B), first derivative (C), first derivative combined with multiplicative scatter correction (D), standard normalized variate (E), first derivative combined with standard normalized variate (F), and second derivative(G).
Calibration Process
The calibration models of DE and ME for sorghum fed to growing pigs built by NIRS are shown in Tables 2 and 3, respectively. As shown in Tables 2 and 3, the parameters for evaluating calibration models varied with the combination of different spectral preprocessing methods and different spectral ranges. No pretreatment had the greatest effect on the performance of calibration and cross-external validation for DE and ME. The model can be used for routine analysis when the RPDcv and RPD values of the NIRS calibration model were both greater than 2.5 (AACC, 2000). This study found that the RPDcv values of 11 models in the initially established DE models were greater than 2.5, whereas 6 of the ME calibration models met this condition. After comparison, 11 calibration models of DE and ME of sorghum fed to growing pigs with the greatest RPDcv values more than 2.50 for each spectral pretreatment method were selected and shown in Table 4. These 11 models with RPDcv values ranging from 2.52 to 3.35 were subject to external validation.
Table 2.
Comparison of DE calibration models for sorghum with different spectral ranges and pretreatment methods
| Processing spectra | Spectra region | Factors | R 2c1 | RMSEC2 | R 2cv3 | RMSECV4 | RPDcv5 |
|---|---|---|---|---|---|---|---|
| None6 | 6,102.1 to 5,446.4 | 10 | 0.99 | 0.10 | 0.91 | 0.18 | 3.35 |
| None | 6,102.1 to 5,770.4 | 7 | 0.95 | 0.18 | 0.86 | 0.23 | 2.67 |
| 1st D7 | 9,403.9 to 8,447.3; 6,102.1 to 5,446.4 | 9 | 0.98 | 0.13 | 0.87 | 0.22 | 2.83 |
| 1st D | 9,403.9 to 7,498.4; 6,102.1 to 5,446.4 | 9 | 0.98 | 0.13 | 0.86 | 0.23 | 2.65 |
| MSC8 | 6,102.1 to 5,446.4 | 7 | 0.97 | 0.13 | 0.84 | 0.24 | 2.53 |
| MSC | 9,403.9 to 6,094.4; 5,454.1 to 4,597.8 | 6 | 0.94 | 0.18 | 0.84 | 0.25 | 2.51 |
| MSC | 6,102.1 to 5,770.4 | 5 | 0.93 | 0.19 | 0.84 | 0.25 | 2.50 |
| 1st D + MSC9 | 9,403.9 to 6,094.4; 4,605.5 to 4,242.9 | 4 | 0.94 | 0.17 | 0.88 | 0.22 | 2.86 |
| 1st D + MSC | 9,403.9 to 5,446.4; 4,605.5 to 4,242.9 | 7 | 0.93 | 0.18 | 0.84 | 0.25 | 2.48 |
| SNV10 | 9,403.9 to 6,094.4; 4,605.5 to 4,242.9 | 5 | 0.82 | 0.30 | 0.70 | 0.34 | 1.83 |
| 1st D + SNV11 | 7,506.1 to 4,242.9 | 3 | 0.91 | 0.20 | 0.82 | 0.26 | 2.38 |
| 1st D + SNV | 6,102.1 to 4,242.9 | 3 | 0.90 | 0.21 | 0.82 | 0.26 | 2.36 |
| 2nd D12 | 9,403.9 to 7,498.4; 5,454.1 to 4,242.9 | 4 | 0.92 | 0.20 | 0.83 | 0.25 | 2.41 |
| 2nd D | 9,403.9 to 6,094.4; 5,454.1 to 4,242.9 | 4 | 0.92 | 0.20 | 0.83 | 0.26 | 2.40 |
| MMN13 | 7,506.1 to 4,597.8 | 5 | 0.92 | 0.20 | 0.82 | 0.26 | 2.39 |
| MMN | 7,506.1 to 5,446.4 | 7 | 0.94 | 0.18 | 0.80 | 0.27 | 2.26 |
| SLS14 | 6,102.1 to 5,770.4 | 6 | 0.95 | 0.16 | 0.87 | 0.22 | 2.82 |
| SLS | 6,102.1 to 5,446.4 | 8 | 0.97 | 0.14 | 0.87 | 0.22 | 2.79 |
| 1st D + SLS15 | 6,102.1 to 5,770.4 | 6 | 0.94 | 0.19 | 0.84 | 0.24 | 2.52 |
| 1st D + SLS | 6,102.1 to 5,446.4 | 7 | 0.95 | 0.18 | 0.84 | 0.25 | 2.47 |
| COE16 | 6,102.1 to 4,242.9 | 4 | 0.88 | 0.24 | 0.78 | 0.29 | 2.11 |
| COE | 6,102.1 to 5,168.6 | 5 | 0.90 | 0.22 | 0.77 | 0.29 | 2.10 |
1 R 2c, coefficient of determination of calibration.
2RMSEC, root mean square error of calibration.
3 R 2cv, coefficient of determination of cross-external validation.
4RMSECV, root mean square error of cross-external validation.
5RPDcv, residual predictive deviation of cross-external validation.
6None, without spectral pretreatment method.
71st D, first derivative.
8MSC, multiplicative scatter correction.
91st D + MSC, first derivative combined with multiplicative scatter correction.
10SNV, standard normalized variate.
111st D + SNV, first derivative combined with standard normalized variate.
122nd D, second derivative.
13MMN, min max normalization.
14SLS, straight-line subduction.
151st D + SLS, first derivative combined with straight-line subduction.
16COE, constant offset elimination.
Table 3.
Comparison of ME calibration models for sorghum with different spectral ranges and pretreatment methods
| Processing spectra | Spectra region | Factors | R 2c1 | RMSEC2 | R 2cv3 | RMSECV4 | RPDcv5 |
|---|---|---|---|---|---|---|---|
| None6 | 6,102.1 to 5,446.4 | 10 | 0.98 | 0.11 | 0.89 | 0.20 | 3.03 |
| None | 6,102.1 to 5,770.4 | 7 | 0.94 | 0.17 | 0.85 | 0.23 | 2.62 |
| 1st D7 | 9,403.9 to 8,447.3; 6,102.1 to 5,446.4 | 9 | 0.97 | 0.14 | 0.84 | 0.24 | 2.54 |
| 1st D | 9,403.9 to 7,498.4; 6,102.1 to 5,446.4 | 9 | 0.97 | 0.14 | 0.82 | 0.25 | 2.40 |
| MSC8 | 6,102.1 to 5,446.4 | 7 | 0.96 | 0.14 | 0.82 | 0.25 | 2.34 |
| MSC | 9,403.9 to 6,094.4; 5,454.1 to 4,597.8 | 6 | 0.93 | 0.19 | 0.82 | 0.26 | 2.34 |
| 1st D + MSC9 | 9,403.9 to 6,094.4; 4,605.5 to 4,242.9 | 4 | 0.93 | 0.18 | 0.86 | 0.23 | 2.64 |
| 1st D + MSC | 9,403.9 to 7,498.4; 6,102.1 to 5,446.4; 4,605.5 to 4,242.9 | 4 | 0.92 | 0.19 | 0.81 | 0.26 | 2.31 |
| SNV10 | 7,506.1 to 6,094.4; 5,029.8 to 4,597.8 | 9 | 0.96 | 0.15 | 0.88 | 0.21 | 2.85 |
| SNV | 7,506.1 to 6,094.4; 5,454.1 to 4,597.8 | 10 | 0.97 | 0.14 | 0.83 | 0.24 | 2.46 |
| 1st D + SNV11 | 7,506.1 to 4,242.9 | 3 | 0.90 | 0.21 | 0.81 | 0.26 | 2.28 |
| 1st D + SNV | 6,102.1 to 4,242.9 | 3 | 0.90 | 0.21 | 0.81 | 0.26 | 2.27 |
| 2nd D12 | 9,403.9 to 5,446.4; 4,605.5 to 4,242.9 | 4 | 0.91 | 0.20 | 0.79 | 0.27 | 2.18 |
| 2nd D | 7,506.1 to 5,446.4; 4,605.5 to 4,242.9 | 4 | 0.90 | 0.21 | 0.79 | 0.27 | 2.17 |
| MMN13 | 7,506.1 to 4,597.8 | 5 | 0.91 | 0.20 | 0.80 | 0.27 | 2.24 |
| MMN | 7,506.1 to 5,446.4 | 7 | 0.93 | 0.19 | 0.79 | 0.28 | 2.16 |
| SLS14 | 6,102.1 to 5,770.4 | 6 | 0.95 | 0.16 | 0.86 | 0.22 | 2.72 |
| SLS | 6,102.1 to 5,446.4 | 8 | 0.96 | 0.15 | 0.84 | 0.24 | 2.48 |
| 1st D + SLS15 | 4,428.1 to 4,242.9 | 5 | 0.90 | 0.22 | 0.83 | 0.24 | 2.45 |
| 1st D + SLS | 4,605.5 to 4,242.9 | 6 | 0.92 | 0.20 | 0.82 | 0.25 | 2.38 |
| COE16 | 6,102.1 to 4,242.9 | 4 | 0.87 | 0.24 | 0.76 | 0.29 | 2.04 |
| COE | 6,102.1 to 4,597.8 | 4 | 0.86 | 0.25 | 0.75 | 0.30 | 1.99 |
1 R 2c, coefficient of determination of calibration.
2RMSEC, root mean square error of calibration.
3 R 2cv, coefficient of determination of cross-external validation.
4RMSECV, root mean square error of cross-external validation.
5RPDcv, residual predictive deviation of cross-external validation.
6None, without spectral pretreatment method.
71st D, first derivative.
8MSC, multiplicative scatter correction.
91st D + MSC, first derivative combined with multiplicative scatter correction.
10SNV, standard normalized variate.
111st D + SNV, first derivative combined with standard normalized variate.
122nd D, second derivative.
13MMN, min max normalization.
14SLS, straight-line subduction.
151st D + SLS, first derivative combined with straight-line subduction.
16COE, constant offset elimination.
Table 4.
Calibration models of DE and ME of sorghum fed to growing pigs with the greatest RPDcv values more than 2.50 for each spectral pretreatment method
| Items | Processing spectra | Spectra region | Factors | R 2c1 | RMSEC2 | R 2cv3 | RMSECV4 | RPDcv5 |
|---|---|---|---|---|---|---|---|---|
| DE | None6 | 6,102.1 to 5,446.4 | 10 | 0.99 | 0.10 | 0.91 | 0.18 | 3.35 |
| DE | 1st D7 | 9,403.9 to 8,447.3; 6,102.1 to 5,446.4 | 9 | 0.98 | 0.13 | 0.87 | 0.22 | 2.83 |
| DE | MSC8 | 6,102.1 to 5,446.4 | 7 | 0.97 | 0.13 | 0.84 | 0.24 | 2.53 |
| DE | 1st D + MSC9 | 9,403.9 to 6,094.4; 4,605.5 to 4,242.9 | 4 | 0.94 | 0.17 | 0.88 | 0.22 | 2.86 |
| DE | SLS10 | 6,102.1 to 5,770.4 | 6 | 0.95 | 0.16 | 0.87 | 0.22 | 2.82 |
| DE | 1st D + SLS11 | 6,102.1 to 5,770.4 | 6 | 0.94 | 0.19 | 0.84 | 0.24 | 2.52 |
| ME | None | 6,102.1 to 5,446.4 | 10 | 0.98 | 0.11 | 0.89 | 0.20 | 3.03 |
| ME | 1st D | 9,403.9 to 8,447.3; 6,102.1 to 5,446.4 | 9 | 0.97 | 0.14 | 0.84 | 0.24 | 2.54 |
| ME | 1st D + MSC | 9,403.9 to 6,094.4; 4,605.5 to 4,242.9 | 4 | 0.93 | 0.18 | 0.86 | 0.23 | 2.64 |
| ME | SNV12 | 7,506.1 to 6,094.4; 5,029.8 to 4,597.8 | 9 | 0.96 | 0.15 | 0.88 | 0.21 | 2.85 |
| ME | SLS | 6,102.1 to 5,770.4 | 6 | 0.95 | 0.16 | 0.86 | 0.22 | 2.72 |
1 R 2c, coefficient of determination of calibration.
2RMSEC, root mean square error of calibration.
3 R 2cv, coefficient of determination of cross-external validation.
4RMSECV, root mean square error of cross-external validation.
5RPDcv, residual predictive deviation of cross-external validation.
6None, without spectral pretreatment method.
71st D, first derivative.
8MSC, multiplicative scatter correction.
91st D + MSC, first derivative combined with multiplicative scatter correction.
10SLS, straight-line subduction.
111st D + SLS, first derivative combined with straight-line subduction.
12SNV, standard normalized variate.
External Validation Process
External validation results are shown in Table 5. The calibration models of RPDcv above 2.5 had different external validation results. The R2v of DE and ME calibration models were from −0.11 to 0.90 and −0.38 to 0.81, respectively, and the RPDv ranged from 1.22 to 3.15 and 1.08 to 2.35, respectively. Compared with other spectral pretreatments, no pretreatment weakened predictive ability of DE and ME models to the greatest extent with the lowest RPDv (1.22 for DE and 1.08 for ME) and R2v values (−0.11 for DE and −0.38 for ME).
Table 5.
Results of external validation of DE and ME of sorghum fed to growing pigs
| n 1 | Items | Processing spectra | R2v2 | Bias | RMSEP3 | RPDv4 | Intercept | Slope |
|---|---|---|---|---|---|---|---|---|
| 9 | DE | None5 | −0.11 | −0.34 | 0.53 | 1.22 | −3.41 | 1.24 |
| DE | 1st D6 | 0.27 | −0.25 | 0.43 | 1.42 | −0.06 | 1.02 | |
| DE | MSC7 | 0.64 | −0.16 | 0.30 | 1.96 | −1.43 | 1.10 | |
| DE | 1st D + MSC8 | 0.90 | −0.01 | 0.16 | 3.15 | 1.18 | 0.92 | |
| DE | SLS9 | 0.21 | −0.24 | 0.45 | 1.33 | −3.44 | 1.24 | |
| DE | 1st D + SLS10 | 0.32 | −0.18 | 0.42 | 1.34 | 0.57 | 0.98 | |
| ME | None | −0.38 | −0.35 | 0.57 | 1.08 | −3.48 | 1.25 | |
| ME | 1st D | 0.04 | −0.26 | 0.47 | 1.23 | 0.49 | 0.99 | |
| ME | 1st D + MSC | 0.81 | −0.03 | 0.21 | 2.35 | 1.97 | 0.87 | |
| ME | SNV11 | 0.76 | 0.07 | 0.24 | 2.11 | 3.86 | 0.74 | |
| ME | SLS | 0.03 | −0.25 | 0.47 | 1.19 | −3.27 | 1.23 |
1 n, number of samples.
2R2v, coefficient of determination of external validation.
3RMSEP, root mean square error of external validation.
4RPDv, residual predictive deviation of external validation.
5None, without spectral pretreatment method.
61st D, first derivative.
7MSC, multiplicative scatter correction.
81st D + MSC, first derivative combined with multiplicative scatter correction.
9SLS, straight-line subduction.
101st D + SLS, first derivative combined with straight-line subduction.
11SNV, standard normalized variate.
The Best DE and ME Calibration Models
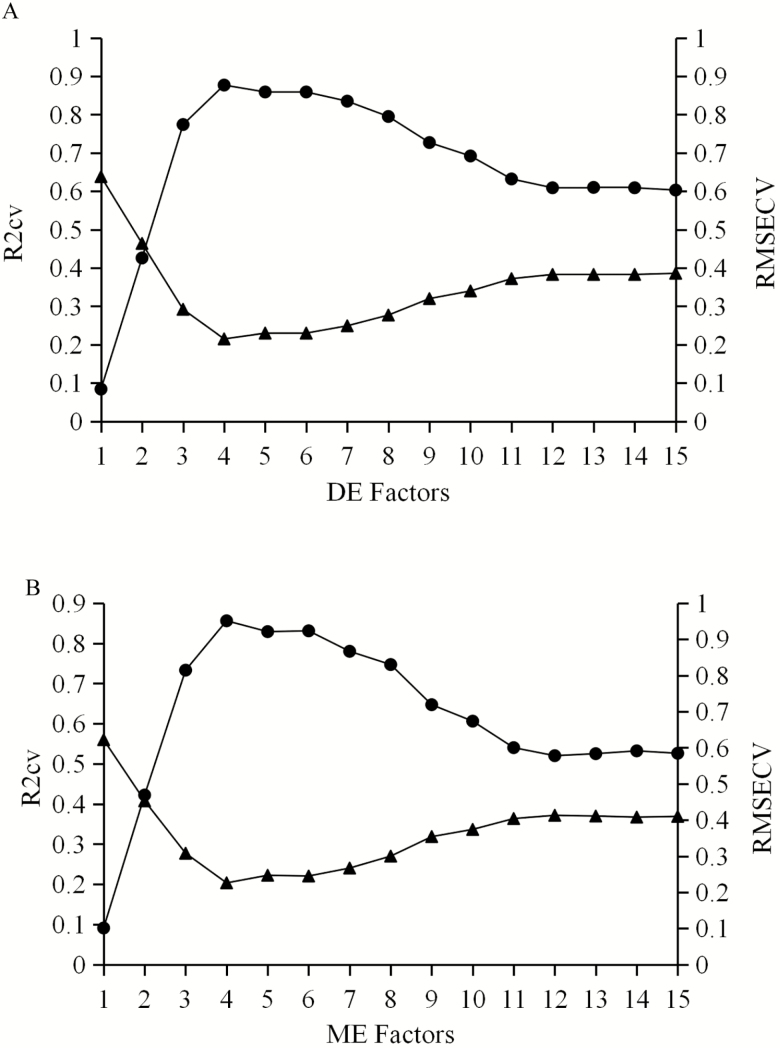
After comparison, the best calibration models are presented in Table 6. Good calibration models were obtained for both DE and ME, with R2c values greater than 0.90, R2cv values greater than 0.85, and RPDcv values greater than 2.50. The 1st D + MSC was the most suitable spectral preprocessing method for DE and ME. The most informative near-infrared spectral regions were 9,403.9 to 6,094.4 cm−1 and 4,605.5 to 4,242.9 cm−1 for both DE and ME. The R2v for the best DE and ME calibration models were 0.90 and 0.81, respectively. Figure 2 shows the changes of R2cv and RMSECV in different factors for DE and ME models of sorghum, the optimal factors showing the dimensionality of spectral data were 4 for both DE and ME due to the greatest R2cv and the lowest RMSECV value at a factor level of 4.
Table 6.
The optimal DE and ME calibration models of sorghum fed to growing pigs
| Items | Processing spectra | Spectra region | Factors | R 2c1 | R 2cv2 | RPDcv3 | R 2v4 | RMSEP5 | RPDv6 |
|---|---|---|---|---|---|---|---|---|---|
| DE | 1st D + MSC7 | 9,403.9 to 6,094.4 | 4 | 0.94 | 0.88 | 2.86 | 0.90 | 0.16 | 3.15 |
| 4,605.5 to 4,242.9 | |||||||||
| ME | 1st D + MSC | 9,403.9 to 6,094.4 | 4 | 0.93 | 0.86 | 2.64 | 0.81 | 0.21 | 2.35 |
| 4,605.5 to 4,242.9 |
1 R 2c, coefficient of determination of calibration.
2 R 2cv, coefficient of determination of cross-external validation.
3RPDcv, residual predictive deviation of cross-external validation.
4 R 2v, coefficient of determination of external validation.
5RMSEP, root mean square error of external validation.
6RPDv, residual predictive deviation of external validation.
71st D + MSC, first derivative combined with multiplicative scatter correction.
Figure 2.
The coefficient of determination of cross-external validation (R2cv) and root mean square error of cross-external validation (RMSECV) in different factors for DE (A) and ME (B) models of sorghum.
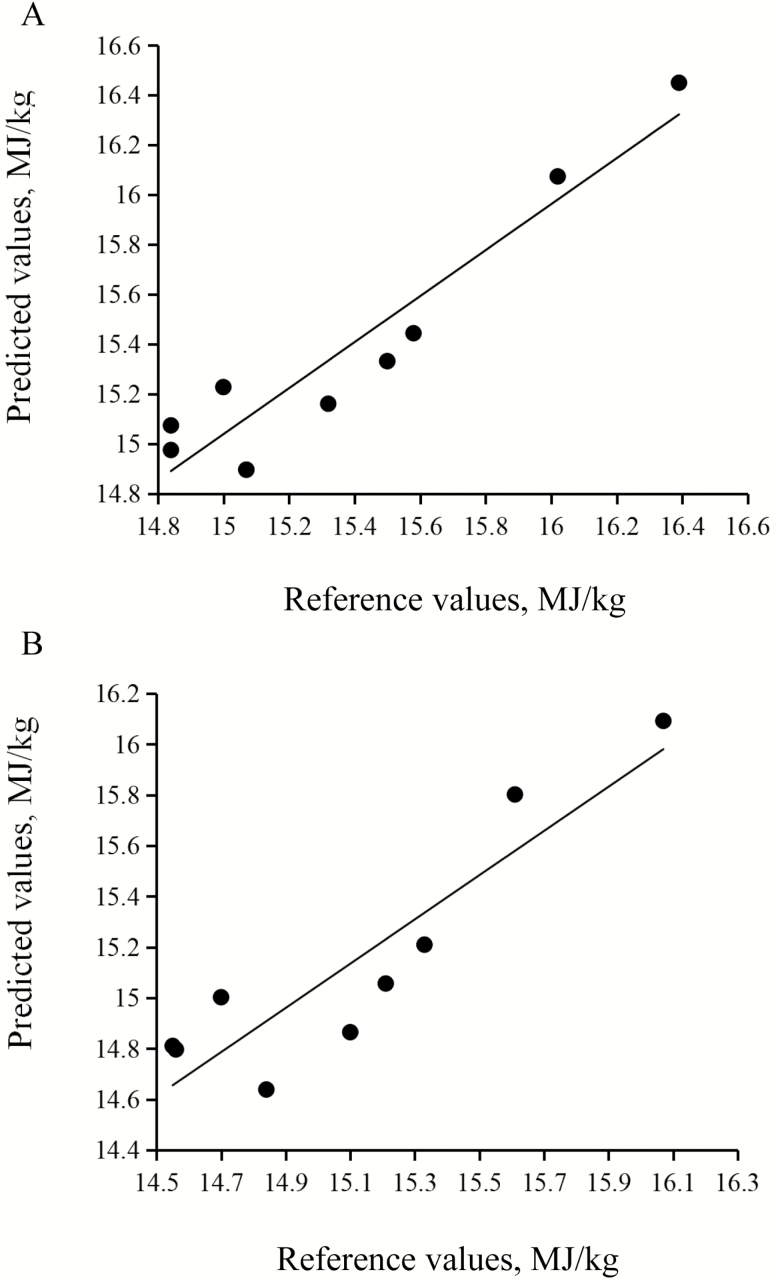
Comparison of the reference and predicted values of DE and ME in sorghum is shown in Table 7. The absolute deviations of the predicted values of DE and ME in sorghum from the reference values ranged from 0.06 to 0.23 MJ/kg and 0.02 to 0.30 MJ/kg, respectively. In addition, no samples from DE and ME validation set were identified as outliers. From a plot of analyzed values through digestion–metabolism experiments vs. predicted values using NIRS in Fig. 3, it was expected that NIRS could be a feasible and reliable method to predict the content of DE and ME in sorghum fed to growing pigs, although the ME plot was more scattered around the regression line than for DE.
Table 7.
Comparison of the reference and predicted values of DE and ME of sorghum as DM basis
| DE content, MJ/kg | ME content, MJ/kg | |||||
|---|---|---|---|---|---|---|
| Sample | Reference values1 | Predicted values2 | Absolute deviation | Reference values | Predicted values | Absolute deviation |
| 1 | 16.39 | 16.45 | −0.06 | 16.07 | 16.09 | −0.02 |
| 2 | 14.84 | 14.98 | −0.14 | 14.56 | 14.80 | −0.24 |
| 3 | 15.00 | 15.23 | −0.23 | 14.70 | 15.00 | −0.30 |
| 4 | 14.84 | 15.07 | −0.23 | 14.55 | 14.81 | −0.26 |
| 5 | 15.07 | 14.90 | 0.17 | 14.84 | 14.64 | 0.20 |
| 6 | 15.50 | 15.33 | 0.17 | 15.21 | 15.06 | 0.15 |
| 7 | 15.32 | 15.16 | 0.16 | 15.10 | 14.87 | 0.23 |
| 8 | 15.58 | 15.44 | 0.14 | 15.33 | 15.21 | 0.12 |
| 9 | 16.02 | 16.07 | −0.05 | 15.61 | 15.80 | −0.19 |
1Values are means of total 6 pigs for each treatment.
2Values are predicted by near-infrared reflectance spectroscopy.
Figure 3.
Scatter plot of reference values vs. predicted values of DE (A) and ME (B) content of sorghum fed to growing pigs.
The results of paired samples t-test of the predicted values calculated by the best DE and ME models compared with the reference values are shown in Table 8. There were no significant differences between the measured and NIRS predicted values for DE and ME (P = 0.895 for DE and P = 0.644 for ME).
Table 8.
T-test of the predicted values calculated by the best DE and ME models compared with the reference values
| Items | Paired differences | Significance (2-tailed) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | SEM | 95% confidence interval of the difference | T | |||
| Lower | Upper | ||||||
| DE | 0.008 | 0.171 | 0.057 | −0.124 | 0.139 | 0.136 | 0.895 |
| ME | 0.034 | 0.215 | 0.072 | −0.131 | 0.120 | 0.480 | 0.644 |
Sample Numbers for NIRS Modeling
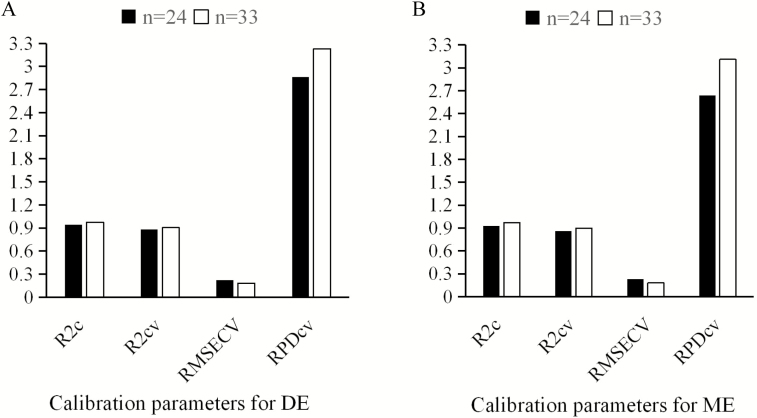
Effect of sample numbers of NIRS model on calibration performance of DE and ME is shown in Fig. 4. As the number of calibration samples increased from 24 to 33, the calibration performance of DE and ME models was improved, indicated by increased R2c, R2cv, and RPDcv values.
Figure 4.
Effect of sample numbers of NIRS model on calibration performance of DE (A) and ME (B). R2c = coefficient of determination of calibration, R2cv = coefficient of determination of cross-external validation, RMSECV = root mean square error of cross-external validation, RPDcv = residual predictive deviation of cross-external validation.
Discussion
The large CV of more than 3.80% in sorghum DE and ME offered an advantage to establish reliable and practical calibration models (Zijlstra et al., 2011). The range of DE and ME in the calibration set covered the range of DE and ME in the validation set, respectively. The similar sample distributions with similar mean values, SD, and CV were also observed for the total set, calibration set, and validation set, which is essential for excellent NIRS models (Shenk and Westerhaus, 1991). Due to possible regional differences, the average DE and ME values of the sorghum in this study was lower when compared with the data published by the NRC and Canadian varieties (Yin et al., 2002; NRC, 2012).
Spectral data pretreatment played an important role in developing NIRS models (Chu et al., 2004). Generally, noise information contained in the near-infrared spectra can be eliminated by different mathematical pretreatment methods or by combining methods. Thus, a number of signal processing techniques were applied to data on the NIRS spectra. When 1st D was combined with MSC, functional absorption peaks related to the available energy in sorghum was greatly enhanced in specific spectral regions. Thereby, the model became more robust for routine analysis of the available energy in sorghum.
Models for both DE and ME without spectral preprocessing had the best calibration results, but the external validation results of these models were not ideal. Some studies regarded the greatest RPDcv and the lowest RMSECV values as parameters to select the best calibration model, and the external validation statistics showed satisfactory validation performance for DE and ME, with R2v (0.88 to 0.91) and RPDv (2.75 to 3.06) (Zhou et al., 2012; Hu et al., 2018). However, the results of this study were not consistent with that. It was difficult to select a good calibration model based on the principle described above. Our study emphasizes the importance of external validation in the process of NIRS modeling. Both the calibration and external validation performance were essential indicators for evaluating NIRS prediction equations.
Near-infrared calibration models with an optimal number of factors had better predictive ability than models with an unsuitable number of factors which may lead to the problem of underfitting or overfitting (Xue et al., 2018). With the increase of factors, the value of R2c increased while the RMSEC decreased, and this indicated that the models gradually became robust (Xue et al., 2018). The sorghum DE and ME models tend to be stable until factor numbers reached 4. Moreover, no samples from DE and ME validation set identified as outliers proved that 4 factors were suitable for building applicable NIRS models using 24 sorghum.
Zijlstra et al. (2011) showed that NIRS was a respectable method with an R2v value of 0.74 for predicting the content of DE in barley fed to growing pigs. Xiccato et al. (1999) who used NIRS to predict the DE content in compound rabbit feeds also found estimate precision was good. Li et al. (2016) reported the calibration model of DE was better than ME. Similarly, in the work presented herein, the calibration model of DE with greater R2v, and RPDv values and lower RMSEP values showed better predictive performance compared with model for ME. This may be caused by the steps of collecting urine and measuring energy in urine, which could introduce more chances for error in the metabolic experiment. The DE model with both RPDcv and RPDv values greater than 2.50 established by this study showed excellent performance for predicting the content of DE in sorghum fed to growing pigs. However, the ME model still needs further optimization due to a slightly lower RPDv value of 2.35.
Despite the relatively small number of sorghum samples for NIRS modeling used in this study, the calibration models still exhibited good calibration performance, especially for DE. A DE calibration model for pigs was also established using 33 wheat brans (CV = 2.53%), and the calibration results were not satisfactory with the SEC value of 0.33 and the R2c value of 0.17 (Garnsworthy et al., 2000). Compared with Li et al. (2016), the DE model in this study was similar to that built with 88 corn samples for pigs, but the ME model was poor with relatively low RPDv (2.35 vs 2.64). These observations indicate that feed variety is a potential factor affecting the calibration model and the variation of samples is related to modeling performance. In addition, when the NIRS model was built with fewer samples, the influence of the sample numbers on the model performance should not be ignored.
Near-infrared technology showed practical prospects for predicting the available energy in sorghum fed to growing pigs in this work, and the t-test results showed that there was no significant difference between the animal experiment and the near-infrared method. NIRS could also be used to determine the energy value of compound feeds for swine and ruminants (Jocelyne Aufrère et al., 1996). In some situations, the indirect methods for rapid and practical determination of DE and ME in feed are accepted widely. Although the available energy prediction equations based on sorghum’s chemical composition established by Pan et al. (2016) showed great accuracy with R2 values of more than 0.90, the prediction equation is limited to the accuracy of the chemical composition determination. Determination of chemical composition is also time consuming. A computer-controlled simulated digestion system was used to predict digestive energy of sorghum for growing pigs (Pan et al., 2018b). Differences between predicted DE values and determined DE values in that study were within 90 kcal/kg of each other, which is still greater than the DE maximum absolute deviation in the present study. Losada et al. (2010) reported that in vitro organic matter digestibility was an inaccurate method with R2c only of 0.756 to predict the AME content of oil seeds and oil seed byproducts for poultry. However, NIRS showed the best prediction performance compared with the other regression methods in that work. Furthermore, near-infrared technology has been more favored by producers because of its fast and nondestructive advantages.
Conclusion
In this study, NIRS quantitative models of the available energy in sorghum were established. The results demonstrate that the content of DE and ME in sorghum could be predicted with relatively high accuracy based on NIRS. NIRS showed the superiority of speediness and practicality when compared with previous research methods including animal experiments, regression equations, and computer-controlled simulated digestion system. In addition, we speculate that the large sample variation was important for the development of robust calibration performance, especially for NIRS models established with a low number of samples.
Literature Cited
- AACC 2000. Approved methods of the American Association of Cereal Chemists. 10th ed.AACC, St. Paul. [Google Scholar]
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis J. and Southern L. L., editors, Swine nutrition. CRC Press, Washington, DC: p. 903–916. [Google Scholar]
- Awika J. M., and Rooney L. W.. 2004. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 65:1199–1221. doi: 10.1016/j.phytochem.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Barneveld R. J. V., Graham H., and Diffey S.. 2018. Predicting the nutritional quality of feed ingredients for pigs using near-infrared spectroscopy (NIRS) and chemical analysis. Anim Prod Sci. 58:709. doi: 10.1071/an17144 [DOI] [Google Scholar]
- Bart M. N., Katrien B., Els B., Ann P., Wouter S., Karen I. T., and Jeroen L.. 2007. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 46:99–118. doi: 10.1016/j.postharvbio.2007.06.024 [DOI] [Google Scholar]
- Bouchet S., Olatoye M. O., Marla S. R., Perumal R., Tesso T., Yu J., Tuinstra M., and Morris G. P.. 2017. Increased power to dissect adaptive traits in global sorghum diversity using a nested association mapping population. Genetics 206:573–585. doi: 10.1534/genetics.116.198499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campher J. P., Meissner H. H., Shelby T., and Rensburg N. J. V.. 1984. Paraformaldehyde-treated bird-resistant sorghum grain as energy source in fattening diets for beef steers. S. Afr. J. Anim. Sci. 14:52–54. [Google Scholar]
- Chu X. L., Yuan H. F., and Lu W. Z.. 2004. Progress and application of spectral data pretreatment and wavelength selection method in NIR analytical technique. Progr. Chem. Beijing 16:528–542. [Google Scholar]
- De Lange C. F. M., and Birkett S. H.. 2005. Characterization of useful energy content in swine and poultry feed ingredients. Can. J. Anim. Sci. 85:269–80. doi: 10.4141/a04-057 [DOI] [Google Scholar]
- Garnsworthy P. C., Wiseman J., and Fegeros K.. 2000. Prediction of chemical, nutritive and agronomic characteristics of wheat by near infrared spectroscopy. J. Agric. Sci. (Belihuloya) 135:409–417. doi: 10.1017/s0021859699008382 [DOI] [Google Scholar]
- Hu J. Q., Wang Z., Wu Y. W., Liu Y. G., and Ouyang J.. 2018. Rapid determination of the texture properties of cooked cereals using near-infrared reflectance spectroscopy. Infrared Phys. Technol. 94:165–172. doi: 10.1016/j.infrared.2018.09.023 [DOI] [Google Scholar]
- Hui Y., and Siesler H. W.. 2018. Quantitative analysis of a pharmaceutical formulation: Performance comparison of different handheld near-infrared spectrometers. J. Pharm. Biomed. Anal. 160:179–186. doi: 10.1016/j.jpba.2018.07.048 [DOI] [PubMed] [Google Scholar]
- Aufrère Jocelyne, Graviou D., Demarquilly C., Perez J. M., and Andrieu J.. 1996. Near infrared reflectance spectroscopy to predict energy value of compound feeds for swine and ruminants. Anim. Feed Sci. Technol. 62:77–90. doi: 10.1016/s0377-8401(96)00995-9 [DOI] [Google Scholar]
- Li J. T., Li Q. F., Li D. F., Chen Y. Q., Wang X. X., Yang W. J., and Zhang L. Y.. 2016. Use of near-infrared reflectance spectroscopy for the rapid determination of the digestible energy and metabolizable energy content of corn fed to growing pigs. J. Anim. Sci. Biotechnol. 7:1–9. doi: 10.1186/s40104-016-0105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. C., Wang X. X., Guo P. P., Liu L., Piao X. S., Hans H. Stein D. F. Li, and Lai C. H.. 2015. Prediction of digestible and metabolisable energy in soybean meals produced from soybeans of different origins fed to growing pigs. Arch. Anim. Nutr. 69:473–486. doi: 10.1080/1745039x.2015.1095461 [DOI] [PubMed] [Google Scholar]
- Losada B., García-Rebollar P. C., álvarez P., Cachaldora M. A. Ibáez, and Méndez J.. 2010. The prediction of apparent metabolisable energy content of oil seeds and oil seed by-products for poultry from its chemical components, in vitro analysis or near-infrared reflectance spectroscopy. Anim. Feed Sci. Technol. 160:62–72. doi: 10.1016/j.anifeedsci.2010.06.012 [DOI] [Google Scholar]
- Montanhini Neto R., N’Guetta E., Gady C., Francesch M., and Preynat A.. 2017. Combined effect of using near-infrared spectroscopy for nutritional evaluation of feed ingredients and non-starch polysaccharide carbohydrase complex on performance of broiler chickens. Anim. Sci. J. 88:1979–1986. doi: 10.1111/asj.12822 [DOI] [PubMed] [Google Scholar]
- Noblet J., and Perez J. M.. 1993. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 71:3389–3398. doi: 10.2527/1993.71123389x. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pan L., Li P., Ma X. K., Xu Y. T., Tian Q. Y., Liu L., Li D. F., and Piao X. S.. 2016. Tannin is a key factor in the determination and prediction of energy content in sorghum grains fed to growing pigs. J. Anim. Sci. 94:2879–2889. doi: 10.2527/jas.2016-0457. [DOI] [PubMed] [Google Scholar]
- Pan L., Ma X. K., Hu J. X., Liu L., Li D. F., and Piao X. S.. 2018a. Low-tannin white sorghum contains more digestible and metabolisable energy than high-tanin red sorghum if fed to growing pigs. Anim Prod Sci. 59:524–530. doi: 10.1071/an17245 [DOI] [Google Scholar]
- Pan L., Piao X. S., Wu Y., Ma H., and Li D. F.. 2018b. Digestible energy of sorghum grain for pigs could be predicted using a computer-controlled simulated digestion system. Anim. Feed Sci. Technol. 240:31–39. doi: 10.1016/j.anifeedsci.2018.03.007 [DOI] [Google Scholar]
- Rooney W. L., Blumenthal J., Bean B., and Mullet J. E.. 2010. Designing sorghum as a dedicated bioenergy feedstock. Biofuel Bioprod. Bioref. 1:147–157. doi: 10.1002/bbb.15 [DOI] [Google Scholar]
- Shenk J. S., and Westerhaus M. O.. 1991. Population definition, sample selection and calibration procedures for near infrared reflectance spectroscopy. Crop Sci. 31:469–474. doi: 10.2135/cropsci1991.0011183x003100020049x [DOI] [Google Scholar]
- Stein H. H., Lagos L. V., and Casas G. A.. 2016. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 218:33–69. doi: 10.1016/j.anifeedsci.2016.05.003 [DOI] [Google Scholar]
- Van Barneveld R., Nuttall J., Flinn P., and Osborne B.. 1999. Near infrared reflectance measurement of the digestible energy content of cereals for growing pigs. J. Near Infrared Spectrosc. 7:1–7. doi: 10.1255/jnirs.228 [DOI] [Google Scholar]
- Xiccato G., Trocino A., Carazzolo A., Meurens M., Maertens L., and Carabano R.. 1999. Nutritive evaluation and ingredient prediction of compound feeds for rabbits by near-infrared reflectance spectroscopy (NIRS). Anim. Feed Sci. Technol. 77:201–212. doi: 10.1016/s0377-8401(98)00253-3 [DOI] [Google Scholar]
- Xue J. T., Liu Y. F., Ye L. M., Li C. Y., Yang Q. W., Wang W. Y., Jing Y., Zhang M. X., and Li P.. 2018. Rapid and simultaneous analysis of five alkaloids in four parts of coptidis rhizoma by near-infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 188:611–618. doi: 10.1016/j.saa.2017.07.053 [DOI] [PubMed] [Google Scholar]
- Xue J. T., Wu C. J., Wang L. L., Jiang S., Huang G., Zhang J. L., Wen S. L., and Ye L. M.. 2011. Dynamic prediction models for alkaloid content using NIR technology for the study and online analysis of parching in areca seed. Food Chem. 126:725–730. doi: 10.1016/j.foodchem.2010.11.036 [DOI] [Google Scholar]
- Yin Y. L., Gurung Nar K., Jeaurond E. A., Sharpe P. H., and de Lange C. F. M.. 2002. Digestible energy and amino acid contents in Canadian varieties of sorghum, pearl millet, high-oil corn, high-oil–high-protein corn and regular corn samples for growing pigs. Can. J. Anim. Sci. 82:385–391. doi: 10.4141/a01-086 [DOI] [Google Scholar]
- Zhou L. J., Zhang L. Y., Zhang E. X., Li J. T., Yang W. J., and Wang Z. Y.. 2012. Rapid determination of swine available energy and amino acids in corn distillers dried grains with solubles by near-infrared reflectance spectroscopy. Anim. Feed Sci. Technol. 175:198–202. doi: 10.1016/j.anifeedsci.2012.06.001 [DOI] [Google Scholar]
- Zijlstra R. T., Swift M. L., Wang L. F., Scott T. A., and Edney M. J.. 2011. Short communication: Near infrared reflectance spectroscopy accurately predicts the digestible energy content of barley for pigs. Can. J. Anim. Sci. 91:301–304. doi: 10.4141/cjas10063 [DOI] [Google Scholar]