Abstract
Purpose
The present study aimed to examine association between inflammatory and endothelial function biomarkers and indices of cardiac autonomic control in T2DM patients.
Methods
50 T2DM patients were recruited for this study. For cardiac autonomic function, cardiovascular autonomic reflex tests (CARTs) and heart rate variability (HRV) analysis was performed. Blood samples were collected for evaluating inflammatory and endothelial function biomarkers. Multivariable linear regression analysis adjusted for diabetes duration, glycemic control, waist circumference, hypertension, dyslipidemia, metformin, and statins was performed to examine the association between the biomarkers and cardiac autonomic function parameters.
Results
Interleukin-6 was inversely related to total power (p = .009) and low frequency power (p = .04). Interleukin-18 and high sensitivity C-reactive protein inversely correlated with measures of cardiac vagal control (p < .05). Both nitric oxide and endothelial nitric oxide synthase were positively linked with cardiac vagal control indices (p < .05) whereas endothelin-1 did not show any independent association with cardiac autonomic function parameters.
Conclusions
Biomarkers of inflammation and endothelial function are associated with measures of cardiac vagal control and global HRV which suggest that there is some pathophysiological link between subclinical inflammation, endothelial dysfunction and cardiac autonomic dysfunction in T2DM.
Electronic supplementary material
The online version of this article (10.1007/s40200-019-00435-w) contains supplementary material, which is available to authorized users.
Keywords: Vascular endothelium, Biomarkers, Diabetes, Autonomic dysfunction, Interleukins
Introduction
In spite of the fact that cardiac autonomic neuropathy (CAN) is fairly prevalent in type 2 diabetes mellitus (T2DM), still, there seems to be lack of clarity on its pathophysiological links to other systems [1]. Subclinical inflammation has been found to accelerate the process of cardiovascular damage in T2DM [2] and devastating effects of T2DM on vascular endothelium remains to be the major cause behind various cardiovascular complications of diabetes [3]. However, the precise link of subclinical inflammation and endothelial dysfunction has not been understood in relation to the pathophysiology of CAN.
Literature [4] has indicated a clear relation between inflammation and development of sensorimotor polyneuropathy in individuals with diabetes, but their relevance for the development of CAN is not yet fully understood. The most common biomarkers of inflammation which are associated with cardiovascular dysfunction are interleukin-6 (IL-6) and high sensitivity C- reactive protein (hsCRP). Both IL-6 and hsCRP are found to be correlated with measures of cardiac autonomic function in healthy individuals [5, 6]. However, their association was found to be affected by the presence of cardiovascular risk factors in a recent study on T2DM patients [7]. In the same study [7], IL-18 was found to be inversely related with measures of cardiac vagal control. However, since the presence of CAN has the potential to alter the cardiovascular physiology [8] and possible inter-relationships between the systems, evaluation of the above mentioned associations are warranted in T2DM patients with CAN.
The endothelium is a complex organ with specific properties essential for control of vascular functions. The most widely used clinical endpoint for the assessment of endothelial function remains to be endothelium-dependent vasomotion. The primary vasodilator released by the endothelium is nitric oxide (NO) by the action of endothelial NO synthase (eNOS) [9]. NO produced by the vascular endothelium induces relaxation of the vascular smooth muscle by a sequence of enzymatic reactions. Endothelial dysfunction is associated with decreased NO availability [10] and is regarded as an important factor in the pathogenesis of diabetic micro and macroangiopathy [11]. Endothelin-1 (ET-1) is a potent vasoconstrictor peptide which promotes sympathetic innervations of the heart [12] and thus plays a crucial role in adverse cardiac remodeling [13]. Thus, autonomic and endothelial dysfunction usually co-exists in the development of cardiovascular diseases which suggests complex interactions between the two systems.
Keeping in mind that CAN have devastating cardiovascular consequences, it becomes extremely important to identify the factors associated with its occurrence. Though subclinical inflammation and endothelial dysfunction are closely linked to cardiac autonomic function in both healthy [14, 15] and diseased conditions [16, 17], their association has been scantily investigated in T2DM patients. Therefore, the present study intended to investigate the association of biomarkers of subclinical inflammation and endothelial function with parameters of cardiac autonomic function in T2DM patients with CAN. We hypothesized that there will be a significant association between biomarkers of inflammation and endothelial function and parameters of cardiac autonomic function in these patients.
Methods
Study participants
The sample size for the present study was calculated using Software G. Power 3.1.9.2. Using the regression coefficient between IL-18 and high frequency (HF) power of HRV from a previous study [7], an effect size of .38 was obtained at an α value of .05 and power of .90. Based on these effect estimates, a sample size of 50 participants was found to be necessary to test the study hypothesis. Fifty T2DM patients were recruited from the medical centre of Jamia Millia Islamia after obtaining clearance from the Institutional Ethics Committee. Patients diagnosed with T2DM (≥ 1 year) and found to be positive for CAN based on standard clinical autonomic test battery [18] were enrolled into the study and those with any known cardiovascular disease (CVD), pulmonary disorder, uncontrolled hypertension, acute inflammatory disease, and morbid obesity were excluded. Written informed consent was obtained from each participant and study procedures were employed in accordance with the declaration of Helsinki, 1964.
Study protocol
After initial screening for eligibility by competent medical professionals, the tests for glycemic and lipid profile were performed in the pathology laboratory of the medical centre. CAN was evaluated using the standard clinical autonomic test battery and patients found to be positive for CAN were further assessed for heart rate variability (HRV). Venous blood samples of patients were collected on a separate day after overnight fasting in order to evaluate serum levels of inflammatory and endothelial function biomarkers.
Autonomic function testing
Cardiovascular autonomic reflex tests (CARTs)
CARTs comprised of 3 heart rate (HR) tests [deep breathing test (DBT), Valsalva maneuver (VM), head-up tilt (HUT) test] and two blood pressure (BP) tests [HUT, hand grip test (HGT)]. These tests were serially conducted to obtain measures of parasympathetic (30/15 ratio, ∆HR, Valsalva ratio) and sympathetic cardiac activity [∆ systolic BP (∆SBP), ∆ diastolic BP (∆DBP)]. SBP fall to postural stress and R-R responses at the 15th and 30th s of the HUT test were used to compute 30/15 ratio. DBT constituted of deep inspiration–expiration for six consecutive cycles, which was later analyzed to obtain change in HR (∆ HR) to deep breathing maneuver. ∆ HR shows the integrity of the vagal afferent and efferent pathways, whereas the 30/15 ratio examines the integrity of vagal-mediated baroreflex function. Furthermore, patients were asked to perform Valsalva maneuver for 15 s to raise the mercury level to 40 mmHg on a manual manometer. Valsalva ratio was calculated by dividing the largest and the smallest R-R values at the 2nd and 4th stages of the maneuver and is considered to represent both sympathetic and parasympathetic function. Finally, DBP response to HGT was assessed during a hold of hand grip dynamometer at 30% of the patient’s maximum voluntary isometric contraction (MVIC). SBP response to postural change and DBP response to HGT are considered as measures of sympathetic reactivity. Based on the findings of CARTs, patients were classified as no-CAN or with CAN (early, definite, or severe CAN) using the Ewing’s criteria (Ewing et al., 1985). During CARTs assessment, R-R intervals were recorded by Lab Chart software version 7.3.7. (Power Lab 8 SP, AD Instruments, New Zealand) while BP responses were assessed using a manual sphygmomanometer [18].
HRV assessment
HRV testing was performed in a quiet, temperature-controlled room (24 °C) after a rest period of at least 15 min in the supine position. Lead II electrocardiogram (ECG) was recorded for 10 min for each patient. The last 5 min segment of the 10 min record was analyzed for time and frequency domain variables of HRV by locating peaks of R waves (Power Lab 8 SP, AD Instruments, New Zealand) while resting HR was derived from the resting ECG record. Data were visually inspected for ectopic beats and these were interpolated if found to be ≤10%, however, >10% ectopic beats in any data record were not considered acceptable for analysis. R-R interval time series was decomposed into its frequency components using Fast Fourier Transform (FFT) for spectral analysis. The power density in areas of low frequency (LF; 0.04 to 0.15 Hz) and HF (HF; 0.15 to 0.4 Hz) bands were calculated in absolute (ms2) and normalized units (nu). Standard time domain indices i.e. average of N-N intervals (mean NN), standard deviation of N-N intervals (SDNN), root mean square of successive differences between adjacent R-R intervals (RMSSD), percentage of consecutive N-N intervals that vary by more than 50 ms (pNN50) and frequency domain indices i.e. total power (TP), LF power, HF power and LF/HF ratio were obtained through analysis. Both data acquisition and post-acquisition analysis were carried out in accordance with the guidelines proposed by the Taskforce of European Society of Cardiology and North American Society of Pacing and Electrophysiology [19].
Biochemical analysis
Venous blood samples were collected after overnight fasting of 8–12 h for all participants. HbA1c was measured using high-performance liquid chromatography [20]. Fasting blood glucose was estimated by glucose oxidase-peroxidase method [21] and the lipid markers were measured by diagnostic kit method (Randox Labs Ltd., UK). Low density lipoprotein cholesterol and very low density lipoprotein cholesterol were calculated using Friedwald’s equation. Serum levels of endothelial function biomarkers [NO (sensitivity: 1.12 μmol/L, intra-assay coefficient of variability (CV) < 8%, inter-assay CV < 10%), eNOS (sensitivity: .25 U/ml, intra-assay CV < 8%, inter-assay CV < 10%)), ET-1(sensitivity: 1.01 ng/L, intra-assay CV < 8%, inter-assay CV < 10%)] and inflammatory biomarkers [IL-6 (sensitivity: 1.03 ng/L, intra-assay CV < 8%, inter-assay CV < 10%), IL-18 (sensitivity: .02 ng/L, intra-assay CV < 8%, inter-assay CV < 10%)] were evaluated using ELISA kit according to the manufacturer’s instructions (Bioassay Technology Laboratory, China). Intra- and inter-assay CV was <8% and < 10% respectively for these biochemical kits. hsCRP levels were measured by a highly sensitive ELISA kit (Elabscience, USA).
Statistical analysis
Data are presented as mean ± standard deviation, or as frequencies/percentages. Normality of the data was examined using Shapiro-Wilk test. Variables without Gaussian distribution were log-transformed prior to further analysis. First, the association between biomarkers and measures of cardiac autonomic function was assessed using Pearson’s correlation coefficient and those came out to be significant were further examined by multivariable linear regression analysis. Diabetes duration, HbA1c, waist circumference (WC), hypertension, dyslipidemia, medications such as metformin and statins (considering their influence on cardiac autonomic function) were used as co-variables in the regression model. Co-variables for adjustment in the multivariable linear regression model were selected based on their strong clinical association with measures of CAN. Previously, it has been shown that diabetes duration, HbA1c and WC are linearly correlated with cardiac autonomic dysfunction in diabetes [22, 23]. Presence of hypertension and dyslipidemia may also confound the relationship between biomarkers and measures of cardiac autonomic control as these conditions may independently cause cardiac autonomic dysfunction [24, 25]. Moreover, drugs such as metformin and statins may also modify cardiac autonomic control [26, 27]. Each biomarker which showed significant correlation with cardiac autonomic function was entered into the regression model along with the above-mentioned co-variables. Standardized regression coffiecient (β) and its confidence intervals (CI) were utilized to indicate the magnitude of association between the dependent (cardiac autonomic function) and independent variables (biomarkers) after adjusting for co-variables. Co-linearity between independent variables in multivariable regression models was assessed by calculating the variance inflation factor (VIF) which measures inflation in the variances of the parameter estimates due to multi-collinearity potentially caused by the correlated predictors. Although there are no universal cut-off values available for VIF, roughly a value of 3–5 is considered to be a cause of concern and a value ≥10 indicates serious co-linearity problems [28]. All statistical analysis was carried out using IBM SPSS Statistics ver 21.0., USA. P values <0.05 were considered to indicate statistically significant associations.
Results
All 50 participants completed cardiac autonomic and biochemical testing. Demographics, clinical characteristics and medications used by the patients are presented in Table 1. Descriptive statistics for biomarkers of inflammation and endothelial function along with parameters of cardiac autonomic function are presented in Table 2. VIF came out to be <2 for all co-variables (Supplementary material 1) which indicates that the present analysis was not affected by co-linearity among independent variables in the multivariable linear regression analysis.
Table 1.
Demographic and clinical characteristics of type 2 diabetes mellitus patients
| Variables | Mean ± SD (n = 50) |
|---|---|
| Demographics | |
| Age (years) | 53.1 ± 7.42 |
| Sex (M/F), n | 28/22 |
| Weight (kg) | 72.3 ± 12.25 |
| Height (cm) | 161.6 ± 8.76 |
| BMI (kg/m2) | 27.7 ± 4.46 |
| DM duration (years) | 8.5 ± 6.32 |
| Physical activity levels (MET-min/week) | 533.1 ± 436.69 |
| Glycemic control | |
| FBG (mg/dl) | 158.2 ± 57.80 |
| PPBG (mg/dl) | 223.7 ± 87.67 |
| HbA1c (%) | 8.2 ± 1.60 |
| Cardiovascular risk profile | |
| TC (mg/dl) | 179.3 ± 34.89 |
| TG (mg/dl) | 159.7 ± 88.02 |
| LDL (mg/dl) | 104.8 ± 32.74 |
| HDL (mg/dl) | 43.9 ± 7.66 |
| VLDL (mg/dl) | 27.5 ± 10.77 |
| HR (bpm) | 81.6 ± 13.06 |
| SBP (mmHg) | 125.5 ± 14.05 |
| DBP (mmHg) | 75.8 ± 7.97 |
| WC (cm) | 94.7 ± 10.71 |
| WHR | 1.0 ± 0.12 |
| BF (%) | 28.3 ± 10.35 |
| DM complications (n, n%) | |
| Peripheral neuropathy | 6 (12) |
| Retinopathy | 3 (6) |
| Microalbuminuria | 1 (2) |
| Co-morbidities (n, n%) | |
| Hypertension | 20 (40) |
| Dyslipidemia | 28 (56) |
| Thyroid dysfunction | 12 (24) |
| Musculoskeletal issues | 5 (10) |
| Medications (n, n%) | |
| Metformin | 29 (58) |
| Sulphonylureas | 13 (26) |
| DPP4 inhibitors | 4 (8) |
| β blockers | 6 (12) |
| ACE inhibitors | 6 (12) |
| Calcium channel blockers | 1 (2) |
| Insulin | 4 (8) |
| Statins | 9 (18) |
| Levo-thyroxine | 6 (12) |
| Thiazolidinedione | 1 (2) |
| Dapagliflozin | 1 (2) |
| PPI inhibitors | 1 (2) |
| Glucosidase inhibitor | 1 (2) |
| Gliclazide | 1 (2) |
M: males; F: females; BMI: body mass index; DM: diabetes mellitus; MET: metabolic equivalent; FBG: fasting blood glucose; PPBG: post-prandial blood glucose; HbA1c: glycosylated hemoglobin; TC: total cholesterol; TG: triglycerides; LDL: low density lipoproteins; HDL: high density lipoproteins; VLDL: very low density lipoproteins; WC: waist circumference; WHR: waist-hip ratio; BF: body fat; HR: heart rate; DPP4: dipeptidyl peptidase-4; ACE: angiotensin converting enzyme; PPI: proton pump inhibitor; n: number; %: percentage
Table 2.
Biomarkers and parameters of cardiac autonomic function in Type 2 diabetes mellitus patients
| Variables | Mean ± SD (n = 50) |
|---|---|
| Inflammatory and endothelial function | |
| IL-6 (ng/L) | 21.8 ± 13.98 |
| IL-18 (ng/L) | 183.2 ± 136.89 |
| hs CRP (mg/L) | 2.3 ± 1.39 |
| NO (μmol/L) | 107.1 ± 92.54 |
| ENOS (U/ml) | 77.0 ± 59.62 |
| Endothelin 1 (ng/L) | 120.5 ± 76.59 |
| Heart rate variability | |
| mean NN (ms) | 742.7 ± 118.01 |
| SDNN (ms) | 29.6 ± 19.28 |
| RMSSD (ms) | 33.8 ± 17.03 |
| pNN50 (%) | 1.75 ± 2.67 |
| TP (ms2) | 750.9 ± 527.40 |
| LF power (ms2) | 160.9 ± 126.13 |
| LFnu | 43.0 ± 21.71 |
| HF power (ms2) | 223.8 ± 206.38 |
| HFnu | 49.9 ± 21.36 |
| LF/HF ratio | 1.5 ± 2.67 |
| CARTs | |
| ∆ HR (bpm) | 12.2 ± 5.26 |
| VR | 1.3 ± 0.45 |
| 30/15 ratio | 1.1 ± 0.18 |
| ∆ DBP (mmHg) | 11.6 ± 8.12 |
| ∆ SBP (mmHg) | 4.7 ± 12.39 |
IL: interleukin; hsCRP: high-sensitivity C-reactive protein; NO: nitric oxide; ENOS: endothelial nitric oxide synthase; Mean NN: average of N-N intervals; SDNN: standard deviation of N-N intervals; RMSSD: root mean square of successive differences between adjacent R-R intervals; pNN50: percentage of consecutive N-N intervals that differ by more than 50 ms; TP: total power; LF: low frequency power; HF: high frequency power; LF/HF ratio: ratio of low and high frequency power; CARTs: cardiovascular autonomic reflex tests; ∆: delta; VR: valsalva ratio; 30/15 ratio: ratio of R-R interval during 30th and 15th second of the head-up tilt test; DBP: diastolic blood pressure; SBP: systolic blood pressure; HGT: hand grip test; ms: milliseconds; %: precent; nu: normalized units; ng: nanogram; L: liters; ml: milliliters; ms: milliseconds; bpm: beats per minute;
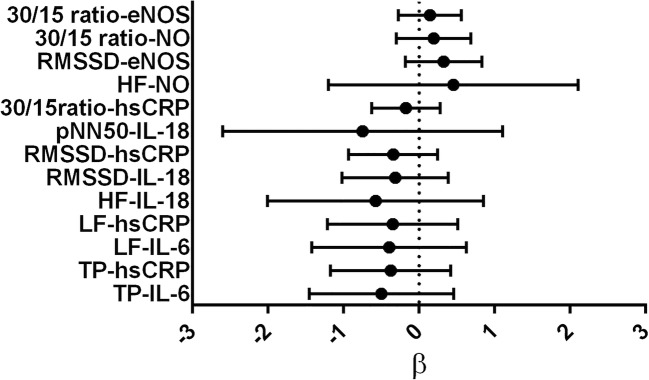
Initially, significant inverse correlation was observed between IL-6 and TP (r = −.34; p = .01) and LF power (r = −.36; p = .009) which was later confirmed to be significant and independent in the regression analysis. Significant inverse associations were also found between IL-18 and measures of cardiac vagal control (RMSSD, pNN50 and HF power) (p < .05) which remained robust after adjusting various clinical confounders. hsCRP demonstrated significant inverse association with CARTs such as ∆HR (r = −.40; p < 0.01) and 30/15 ratio (r = −.32; p = .02) and also with HRV parameters such as RMSSD (r = −.40; p = .003), TP (r = −.35; p = .01), LF (r = −.31; p = .02) and HF power (r = −.42; p = .002). However, association of hsCRP with ∆HR and HF power were confounded by WC (∆HR, p = .01; HF power, p = .04) when assessed by multivariable regression analysis (Tables 3 and 4; Fig. 1).
Table 3.
Correlation coefficients between biomarkers and parameters of cardiac autonomic function
| Variable | ∆ HR | VR | ∆DBP | 30/15 ratio | ∆SBP | mean NN | SDNN | RMSSD | pNN50 | TP | LF power | HF power | LF/HF ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | −.03 | −.03 | −.011 | −.011 | .11 | −.16 | −.20 | −.10 | −.002 | −.34* | −.36* | −.17 | .009 |
| IL-18 | −.18 | −.16 | −.17 | −.04 | .007 | −.03 | −.18 | −.28 | −.53** | −.25 | −.21 | −.34* | .21 |
| hsCRP | −.4** | −.008 | −.23 | −.32* | −.03 | .03 | −.24 | −.40** | −.36 | −.35* | −.31* | −42** | .22 |
| NO | .55** | 0.05 | .10 | .46** | .09 | .05 | .26 | .65** | .01 | .30* | .13 | .32* | −.25 |
| eNOS | .50** | −.006 | .10 | .46** | .14 | −.14 | −.06 | .43** | −.07 | .07 | −.002 | .17 | −.20 |
| ET-1 | .55** | −.05 | −.09 | −.18 | −.07 | −.02 | −.001 | −.22* | .03 | −.03 | −.02 | −.18 | .22 |
IL: interleukin; hsCRP: high-sensitivity C-reactive protein; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; ET-1: endothelin-1; mean NN: average of N-N intervals; SDNN: standard deviation of N-N intervals; RMSSD: root mean square of successive differences between adjacent R-R intervals; pNN50: percentage of consecutive N-N intervals that differ by more than 50 ms; TP: total power; LF: low frequency; HF: high frequency; LF/HF ratio: ratio of low and high frequency power; ∆HR: change in heart rate during deep breathing test; VR: valsalva ratio; 30/15 ratio: ratio of R-R interval during 30th and 15th second of the head-up tilt test; ∆DBP: change in diastolic blood pressure during hand grip test; ∆SBP: change in systolic blood pressure during head-up tilt test; *p < .05; **p < .01
Table 4.
Multivariable linear regression models explaining association between biomarkers and parameters of cardiac autonomic function
| Cardiac autonomic function measures | Biomarkers | β | CI | p value |
|---|---|---|---|---|
| TP | IL-6 | −.46 | −.90, −.13 | .009* |
| LF power | IL-6 | −.35 | −.83, −.01 | .04* |
| RMSSD | IL-18 | −.37 | −.57, −.01 | .03* |
| pNN50 | IL-18 | −.52 | −1.58, −.14 | .02* |
| HF power | IL-18 | −.40 | −1.22, −.11 | .01* |
| ∆ HR | hsCRP | −.37 | −.41, −.05 | .01# |
| RMSSD | hsCRP | −.41 | −.54, −.08 | .009* |
| 30/15 ratio | hsCRP | −.37 | −.14, −.01 | .01* |
| TP | hsCRP | −.37 | −.70, −.06 | .01* |
| LF power | hsCRP | −.34 | −.70, −.004 | .02* |
| HF power | hsCRP | .49 | −1.21, −.33 | .001# |
| ∆ HR | NO | .60 | .23, .54 | < .001# |
| 30/15 ratio | NO | .41 | .02, .15 | .006* |
| RMSSD | NO | .74 | .55, 4.20 | < .001* |
| TP | NO | .43 | .15, .79 | .005# |
| HF power | NO | .41 | −.19, 1.14 | .007* |
| ∆ HR | eNOS | .47 | .10, .37 | .001# |
| 30/15 ratio | eNOS | .33 | .005, .10 | .03* |
| RMSSD | eNOS | .44 | .09, .45 | .004* |
| ∆ HR | ET-1 | −.56 | −.64, −.24 | <.001# |
β: beta; CI: confidence intervals; ∆HR: change in heart rate during deep breathing test; 30/15 ratio: ratio of R-R interval during 30th and 15th second of the head-up tilt test; RMSSD: root mean square of successive differences between adjacent R-R intervals; pNN50: percentage of consecutive N-N intervals that differ by more than 50 ms TP: total power; LF: high frequency; HF: high frequency; IL: interleukin; hsCRP: high-sensitivity C-reactive protein; NO: nitric oxide; ENOS: endothelial nitric oxide synthase; *significant independent association; #association explained by co-variables; refer to supplementary material 2 and 3 for more details
Fig. 1.
Forest plots demonstrating regression coefficients (β) and corresponding 95% confidence intervals for significant associations between biomarkers and measures of cardiac autonomic function in type 2 diabetes mellitus patients. Associations which were independent of the influence of any co-variable are presented. IL-6: interleukin-6; IL-18: interleukin-18; hsCRP: high sensitivity C-reactive protein; TP: total power; LF: low frequency power; NO: nitric oxide; eNOS: nitric oxide synthase; HF: high frequency power; RMSSD: root mean square of successive differences between adjacent R-R intervals; pNN50: percentage of N-N intervals that vary by more than 50 ms; 30/15 ratio: ratio of 30th and 15th R-R interval during head-up tilt test
NO showed significant positive association with ∆HR (r = .55, p < 0.001), 30/15 ratio (r = .46; p = .001), RMSSD (r = .65, p < .001), TP (r = .30; p = .03) and HF power (r = .32, p = .02). Except for ∆HR and TP which were explained by WC (p = .003) and the presence of hypertension (p = .002), association with other measures of cardiac autonomic function remained intact when NO was entered into multivariable regression model. There were significant positive correlations between eNOS and ∆HR (r = .50; p < .001), 30/15 ratio (r = .46; p = .001) and RMSSD (r = .43; p = .002) which remained robust with 30/15 ratio [β (CI) = .33 (.005, .04; p = .03] and RMSSD [β (CI) = .44 (.09, .45); p = .004] in the regression analysis. ET-1 demonstrated significant inverse associations only with ∆HR (r = −.55; p = .001) which was confounded by WC (p = .004) in the regression analysis (Tables 3 and 4; Fig. 1). Detailed results of the multi-variable regression analysis are presented in supplementary material 2 and 3.
Discussion
Findings of the present study suggest that biomarkers of subclinical inflammation (IL-6, IL-18 and hsCRP) were inversely associated with parameters of cardiac vagal control (RMSSD, pNN50, HF power) and global variability (TP and LF power) in T2DM patients. Biomarkers of endothelial function such as NO and eNOS were independently and positively associated with parameters of cardiac vagal control (30/15 ratio, RMSSD, HF power) whereas ET-1 is not independently associated with any measure of cardiac autonomic control.
In the present study, IL-6 was inversely related to TP and LF power of HRV. IL-6 is a cytokine that acts both in the innate and adaptive immune response [29]. Although it is known as a chief regulator of acute phase inflammatory response [30], however, its critical role in the transformation from acute to chronic inflammation has also been established [31]. A significant independent inverse association of IL-6 with TP and LF power clearly indicates that increased IL-6 levels may compromise HRV in T2DM patients. Although in the present study, IL-6 was not associated with any specific parameter of either sympathetic or vagal cardiac control which indicates the imprecision of its relationship with cardiac autonomic function in T2DM. Recently, Herder et al. [7], in a study on T2DM patients showed that IL-6 was inversely related to LF and HF power of HRV which were lost when adjusted for co-variables related to cardiovascular risk. In contrary to this, the association of IL-6 with HRV parameters in the present study remained stable even after adjusting for various co-variables (Table 4). These differences may be attributed to the fact that Herder et al. [7] assessed this association on a sample of recently diagnosed T2DM patients (< 1 year) in contrary to our sample where duration of DM was 8.5 ± 6.32 years. It has been revealed that duration of diabetes determines the development of diabetic complications and that systemic inflammation usually sets in the later course of the disease [32]. These discrepancies also indicate that cardiac autonomic dysfunction during the initial years of diabetes (< 1 year) may not be determined by systemic inflammation whereas it may be accounted as a potential determinant of autonomic imbalance in the later stages of the disease. However, Lieb et al. [33] found a significant correlation between IL-6 and cardiac autonomic function in patients with recent-onset and established diabetes both. Another recent study [28] also conforms to the findings of Herder et al. [7] and reported that association between IL-6 and sympatho-vagal balance was partially mediated by cardiovascular risk factors. These variations in the findings of the present and previous research [7, 34] suggest that the relationship between IL-6 and cardiac autonomic function remains complex and needs to be further explored by more robust designs accounting for each and every aspect of the disease. In line with the findings of the present study, robust relationship has been observed between IL-6 and measures of autonomic function in patients with long-standing type 1 diabetes mellitus (TIDM) [35] which again suggests the importance of disease duration with respect to association of IL-6 and autonomic nervous system (ANS).
IL-18 belongs to the IL-1 family of cytokines with mainly pro-inflammatory properties [36]. The present study illustrated a significant inverse association between IL-18 and cardiac vagal control (RMSSD, pNN50, HF power) even after adjusting for potential confounders (Table 4). These findings are consistent with the findings of a previous investigation [7] where a significant inverse association was observed between IL-18 and HF power and RMSSD in a sample of recent-onset T2DM patients. Findings of ours and their study [7] suggest that association between IL-18 and measures of vagal cardiac control remains significant in both recent-onset and long-term diabetes and clearly registers the possible role of IL-18 in the pathogenesis of diabetic CAN. Although the underlining biological plausibility behind these findings are unknown, however, down regulation of IL-18 by activation of the parasympathetic nervous system has been reported [37]. Also, a strong link between IL-18 and stress [38] further underlines the speculation that IL-18 does play a role in causing adverse autonomic modulation in diseased conditions which themselves may be considered a stress stimulus to the human body. It has been reported that IL-18 is elevated in other cardiac conditions and it appears to likely participate in the pathophysiology of cardiovascular diseases [39]. It is believed that the activation of IL-18 by activation of the hypothalamus-pituitary axis (HPA) is mediated by the ANS which underlines the possible association between IL-18 and cardiac autonomic control [37].
In the present study, hsCRP was found to be inversely associated with cardiac autonomic function measures such as RMSSD, TP and LF power when adjusted for co-variables. These findings corroborate well with the previous findings [40] where high hsCRP levels have altered cardiac autonomic function in T2DM patients. In contrary, a recent study [7] showed that association between CRP levels and measures of sympathetic and parasympathetic tone were confounded by anthropometric, metabolic and lifestyle variables whereas in the present study, association between hsCRP and cardiac autonomic function measures (RMSSD, TP, and LF power) remained robust after adjustment of co-variables, however, its relationship with CARTs got influenced by co-variables in the regression analysis (Table 4). Similar to the present findings, CRP levels have shown inverse cross-sectional relationship with cardiac vagal activity (HF power) in young adults after adjusting demographic and clinical confounders [41]. Low levels of LF power have also found to be associated with higher levels of CRP in T1DM patients in adjusted analysis [42].
Findings of the present study showed that serum levels of inflammatory biomarkers were inversely associated with measures of cardiac vagal activity or/and global variability in HR. However, no significant relationship was observed between subclinical inflammation and cardiac sympathetic activity in the present sample which may suggest that the involvement of inflammation-mediated sympathetic nervous system activity is not present in the early stages of CAN (majority of patients in the present study were in early stage of CAN) and that it may be involved in the later course of diabetic CAN. Conversely, experimental findings suggest immunomodulatory functions of the autonomic nervous system (ANS) [43]. Vagal stimulation, in particular, may reduce inflammatory reactions by inhibiting tissue macrophage activation and this process is known as “cholinergic anti-inflammatory pathway” [43]. On the other hand, high sympathetic activity may favour inflammatory reactions [44]. Inflammation products have also potential to influence ANS activity by stimulating autonomic related centres in the hypothalamus and limbic system [45].
NO is a soluble gas, continuously synthesized from the amino acid L-arginine in endothelial cells by the constitutive calcium calmodulin-dependent enzyme NOS [46]. NO plays a crucial role in maintaining the integrity of vascular endothelium by inhibiting platelet aggregation, leucocyte endothelial adhesion and vascular smooth muscle proliferation [47]. NOS enzymes which produce NO have 3 isoforms: neuronal NOS, eNOS and inducible NOS [48]. Out of these, eNOS is predominantly expressed in endothelial cells and adequate levels of endothelial NO are important to preserve normal vascular physiology. Diminished NO bioavailability is associated with endothelial dysfunction and thus increases the susceptibility to atherosclerotic disease [49]. In the present study, both serum NO and eNOS were found to be positively associated with measures of cardiac vagal control which suggest their possible role in cardiac vagal modulation in T2DM patients. These findings are in contrary to the findings of Tiftikcioglu et al. [50] where no significant association was observed between biomarkers of endothelial function and parameters of cardiac autonomic function in T2DM. However, it should be noted that the biomarkers (von Willebrand factor and E-selectin) evaluated by them were different from the present study. Nevertheless, NO had illustrated strong associations with cardiac autonomic control and its role in modulating cardiac vagal control in healthy humans is established [51]. Previous literature [52] has suggested mutual complex interactions between endothelial dysfunction and ANS imbalance which are considered to be a potential basis for the development of various cardiovascular diseases. The structural proximity of the endothelium and the ANS may partially explain the behaviour of their inter-relationship. Adventitial layer of the vascular smooth muscle receive ANS input and can affect the process of endoluminal atherosclerosis via several mechanisms [53]. The pathological process of diabetes seems to have an adverse effect on both vascular function and the ANS. Autonomic dysfunction was found to be associated with increased pulse wave velocity, vascular smooth muscle thickness and impaired arterial compliance in a study on T2DM patients [54]. Thus, the pathophysiological process of autonomic neuropathy is closely related to endothelial dysfunction in T2DM. Modulation of cholinergic neurogenic vasodilation was found to be mediated by NO in a previous experimental research [55] where acetylcholine was found to diffuse across the vascular endothelium to release NO. Therefore, it may be inferred that increased vagal tone can cause excessive vasodilation through NO production.
Unlikely the NO and eNOS, no independent association between ET-1 and indices of cardiac autonomic control was observed in the present study which suggests that it might not be involved in the pathophysiology of diabetic CAN. In the present study, the only correlation between ET-1 and ∆HR was confounded by WC in the regression analysis which means that clinical risk factors partially determine the association between ET-1 and cardiac autonomic dysfunction. A previous study [56] has illustrated similar findings and suggested that although there exists a strong correlation between ET levels and echocardiographic parameters of cardiac function, no significant relationship essentially exists between ET-1 and measures of CAN. Thus, it may be believed that ET may adversely affect cardiac function but not cardiac autonomic function, however, physiological plausibility for the same is not known and should be explored by future research.
The major limitation of this study is its cross-sectional design which does not allow examining the cause and effect relationship between systemic inflammation, endothelial dysfunction, and CAN. Therefore, prospective design should be employed by future research to holistically examine the relationship between these systems. The present T2DM sample was not free from co-morbidities which might have confounded the findings, however, still two most prevalent co-morbidities i.e. hypertension and dyslipidemia were adjusted in the regression analysis for sorting the same. Moreover, patients were on drugs which could modulate autonomic function and could affect the relationship between biomarkers and cardiac autonomic control. Nevertheless, two most important drugs which could potentially confound the results (metformin and statins) were adjusted in the regression analysis.
Conclusion
To summarize, biomarkers of systemic inflammation and endothelial function are associated with measures of CAN in T2DM patients. Higher IL-6 levels were associated with reduced global HRV whereas higher hsCRP levels were associated with both impaired cardiac vagal function and global variability while IL-18 showed negative associations with cardiac vagal control. Higher NO and eNOS were associated with favourable cardiac vagal activity whereas ET-1 does not seem to determine cardiac autonomic dysfunction in T2DM. These findings suggest that there is some pathophysiological link between subclinical inflammation, endothelial dysfunction and cardiac autonomic dysfunction in T2DM. Hence, the biomarkers of subclinical inflammation and endothelial dysfunction should be routinely investigated in T2DM patients for timely prevention and management of CAN.
Electronic supplementary material
(DOCX 11 kb)
(DOCX 20 kb)
(DOCX 19 kb)
Acknowledgements
Authors would like to admit gratitude to University Grants Commission (UGC), India for providing fellowship to the author PB during this study. A sincere thank to Jamia Millia Islamia (A Central University) and UGC for providing adequate financial help during the study.
Abbreviations
- CAN
Cardiac autonomic neuropathy
- T2DM
Type 2 diabetes mellitus
- IL-6
Interleukin-6
- IL-18
Interleukin-18
- hsCRP
high sensitivity C-reactive protein
- NO
Nitric oxide
- eNOS
Endothelial nitric oxide synthase
- ET-1
Endothelin-1
- CVD
Cardiovascular disease
- HRV
Heart rate variability
- HR
Heart rate
- DBT
Deep breathing test
- VM
Valsalva Maneuvre
- HUT
Head-up tilt
- BP
Blood Pressure
- HGT
Hand grip test
- 30/15 ratio
ratio of largest and shortest R-R interval at 30th and 15th second of head-up tilt test
- ECG
Electrocardiography
- FFT
Fast Fourier transform
- mean NN
average of N-N intervals
- SDNN
Standard deviation of N-N intervals
- RMSSD
Root mean square of successive differences between adjacent R-R intervals
- pNN50
percentage of consecutive N-N intervals that vary by more than 50 ms
- TP
Total power
- LF
Low frequency power
- HF
High frequency power
- LF/HF ratio
Ratio of low and high frequency power
- CV
Coefficient of variability
- HbA1c
Glycosylated hemoglobin
- WC
Waist circumference
- CI
Confidence intervals
- VIF
Variance inflation factor
- T1DM
Type 1 diabetes mellitus
- CARTs
Cardiovascular autonomic reflex tests
- ANS
Autonomic nervous system
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pooja Bhati, Email: pooja.bhati092@gmail.com.
Rizwan Alam, Email: mdrizwan2001@gmail.com.
Jamal Ali Moiz, Email: jmoiz@jmi.ac.in.
M. Ejaz Hussain, Email: ehusain@jmi.ac.in.
References
- 1.Fisher VL, Tahrani AA. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes Metab Syndr Obes. 2017;10:419–434. doi: 10.2147/DMSO.S129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25, 979 participants from four UK prospective cohort studies. Diabetes Care. 2012;5:396–403. doi: 10.2337/dc11-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charvat J, Michalova K, Chlumsky J, et al. The association between left ventricle diastolic dysfunction and endothelial dysfunction and the results of stress myocardial SPECT in asymptomatic patients with type 2 diabetes. J Int Med Res. 2005;33:473–482. doi: 10.1177/147323000503300501. [DOI] [PubMed] [Google Scholar]
- 4.Herder C, Lankisch M, Ziegler D, Rathmann W, Koenig W, Illig T, Döring A, Thorand B, Holle R, Giani G, Martin S. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA survey F3 (Augsburg, Germany) Diabetes Care. 2009;32:680–682. doi: 10.2337/dc08-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Känel R, Nelesen RA, Mills PJ, Ziegler MG, Dimsdale JE. Relationship between heart rate variability, interleukin-6, and soluble tissue factor in healthy subjects. Brain Behav Immun. 2008;22:461–468. doi: 10.1016/j.bbi.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med. 2014;276:667–671. doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- 7.Herder C, Schamarek I, Nowotny B, Carstensen-Kirberg M, Straßburger K, Nowotny P, Kannenberg JM, Strom A, Püttgen S, Müssig K, Szendroedi J. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart. 2017;103:63–70. doi: 10.1136/heartjnl-2015-309181. [DOI] [PubMed] [Google Scholar]
- 8.Cha Seon-Ah, Yun Jae-Seung, Lim Tae-Seok, Min Kyoungil, Song Ki-Ho, Yoo Ki-Dong, Park Yong-Moon, Ahn Yu-Bae, Ko Seung-Hyun. Diabetic Cardiovascular Autonomic Neuropathy Predicts Recurrent Cardiovascular Diseases in Patients with Type 2 Diabetes. PLOS ONE. 2016;11(10):e0164807. doi: 10.1371/journal.pone.0164807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 10.Tousoulis D, Kampoli AM. Tentolouris Nikolaos Papageorgiou C, Stefanadis C. the role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 11.Schalkwijk C. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 12.Kreusser MM, Haass M, Buss SJ, Hardt SE, Gerber SH, Kinscherf R, Katus HA, Backs J. Injection of nerve growth factor into stellate ganglia improves norepinephrine reuptake into failing hearts. Hypertension. 2006;47:209–215. doi: 10.1161/01.HYP.0000200157.25792.26. [DOI] [PubMed] [Google Scholar]
- 13.Archer CR, Robinson EL, Drawnel FM, Roderick HL. Endothelin-1 promotes hypertrophic remodelling of cardiac myocytes by activating sustained signalling and transcription downstream of endothelin type a receptors. Cell Signal. 2017;36:240–254. doi: 10.1016/j.cellsig.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Pinter A, Horvath T, Sarkozi A, Kollai M. Relationship between heart rate variability and endothelial function in healthy subjects. Auton Neurosci. 2012;169:107–112. doi: 10.1016/j.autneu.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Truccolo AB, Dipp T, Eibel B, Ribeiro RA, Casali KR, Irigoyen MC, Gus I, Pellanda LC, Plentz RD. Association between endothelial function and autonomic modulation in patients with chagas disease. Arq Bras Cardiol. 2013;100:135–140. doi: 10.5935/abc.20130026. [DOI] [PubMed] [Google Scholar]
- 17.Ulleryd MA, Prahl U, Börsbo J, Schmidt C, Nilsson S, Bergström G, Johansson ME. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One. 2017;12:e0174974. doi: 10.1371/journal.pone.0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 19.Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen R, Coumel P, Fallen E, Kennedy H, Kleiger RE, Lombardi F. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the north American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Davis JE, Mcdonald JM, Jarett L. A high-performance liquid chromatography method for hemoglobin A1c. Diabetes. 1978;27:102–107. doi: 10.2337/diab.27.2.102. [DOI] [PubMed] [Google Scholar]
- 21.Ambade VN, Sharma YV, Somani BL. Methods for estimation of blood glucose: a comparative evaluation. Med J Armed Forces India. 1998;54:131–133. doi: 10.1016/S0377-1237(17)30502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: risk factors, diagnosis and treatment. World J Diabetes. 2018;9:1–24. doi: 10.4239/wjd.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen ST, Witte DR, Fleischer J, Andersen H, Lauritzen T, Jørgensen ME, Jensen TS, Pop-Busui R, Charles M. Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes: ADDITION-Denmark. Diabetes Care. 2018;41:2586–2594. doi: 10.2337/dc18-1411. [DOI] [PubMed] [Google Scholar]
- 24.Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg. 2014;3:2–7. doi: 10.1016/j.amsu.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kepez A, Doğan Z, Mammadov C, et al. Evaluation of association between hyperlipidemia and heart rate variability in subjects without apparent cardiovascular disease. Kosuyolu Heart Journal. 2015;18:29–33. [Google Scholar]
- 26.Manzella D, Grella R, Esposito K, Giugliano D, Barbagallo M, Paolisso G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens. 2004;7:223–227. doi: 10.1016/j.amjhyper.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Millar PJ, Floras JS. Statins and the autonomic nervous system. Clin Sci. 2014;126:401–415. doi: 10.1042/CS20130332. [DOI] [PubMed] [Google Scholar]
- 28.Thompson CG, Kim RS, Aloe AM, Becker BJ. Extracting the variance inflation factor and other multicollinearity diagnostics from typical regression results. Basic Appl Soc Psychol. 2017;39:81–90. [Google Scholar]
- 29.Souza JR, Oliveira RT, Blotta MH, Coelho OR. Serum levels of interleukin-6 (Il-6), interleukin-18 (Il-18) and C-reactive protein (CRP) in patients with type-2 diabetes and acute coronary syndrome without ST-segment elevation. Arq Bras Cardiol. 2008;90:94–99. doi: 10.1590/s0066-782x2008000200004. [DOI] [PubMed] [Google Scholar]
- 30.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 31.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, Williams B. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 33.Lieb DC, Parson HK, Mamikunian G, Vinik AI. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp Diabetes Res. 2011;2012:878760–878768. doi: 10.1155/2012/878760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen CS, Vistisen D, Jørgensen ME, Witte DR, Brunner EJ, Tabák AG, Kivimäki M, Roden M, Malik M, Herder C. Adiponectin, biomarkers of inflammation and changes in cardiac autonomic function: Whitehall II study. Cardiovasc Diabetol. 2017;16:153. doi: 10.1186/s12933-017-0634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Clemente JM, Vilardell C, Broch M, Megia A, Caixas A, Gimenez-Palop O, Richart C, Simon I, Martinez-Riquelme A, Arroyo J, Mauricio D. Lower heart rate variability is associated with higher plasma concentrations of IL-6 in type 1 diabetes. Eur J Endocrinol. 2007;157:31–38. doi: 10.1530/EJE-07-0090. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw. 2000;11:483–486. [PubMed] [Google Scholar]
- 37.Sugama S, Conti B. Interleukin-18 and stress. Brain Res Rev. 2008;58:85–95. doi: 10.1016/j.brainresrev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.La Fratta I, Tatangelo R, Campagna G, Rizzuto A, Franceschelli S, Ferrone A, Patruno A, Speranza L, De Lutiis MA, Felaco M, Grilli A. The plasmatic and salivary levels of IL-1β, IL-18 and IL-6 are associated to emotional difference during stress in young male. Sci Rep. 2018;8:3031. doi: 10.1038/s41598-018-21474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Okamura H, Iwasaki T. Increased circulating interleukin-18 in patients with congestive heart failure. Heart. 2002;88:296–297. doi: 10.1136/heart.88.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aso Y, Wakabayashi S, Nakano T, Yamamoto R, Takebayashi K, Inukai T. High serum high-sensitivity C-reactive protein concentrations are associated with relative cardiac sympathetic overactivity during the early morning period in type 2 diabetic patients with metabolic syndrome. Metabolism. 2006;55:1014–1021. doi: 10.1016/j.metabol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanza GA, Pitocco D, Navarese EP, Sestito A, Sgueglia GA, Manto A, Infusino F, Musella T, Ghirlanda G, Crea F. Association between cardiac autonomic dysfunction and inflammation in type 1 diabetic patients: effect of beta-blockade. Eur Heart J. 2007;28:814–820. doi: 10.1093/eurheartj/ehm018. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 44.März P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose-John S. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 46.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 47.Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine-nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- 48.Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 49.Grange RW, Isotani EI, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- 50.Tiftikcioglu BI, Bilgin S, Duksal T, Kose S, Zorlu Y. Autonomic neuropathy and endothelial dysfunction in patients with impaired glucose tolerance or type 2 diabetes mellitus. Medicine. 2016;95:e3340. doi: 10.1097/MD.0000000000003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- 52.Amiya E, Watanabe M, Komuro I. The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 2014;7:109–119. doi: 10.3400/avd.ra.14-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnstock G. Local mechanisms of blood flow control by perivascular nerves and endothelium. Int J Hypertens. 1990;8:S95–106. [PubMed] [Google Scholar]
- 54.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–2362. doi: 10.1161/01.CIR.0000128695.49900.12. [DOI] [PubMed] [Google Scholar]
- 55.Loke KE, Sobey CG, Dusting GJ, Woodman OL. Cholinergic neurogenic vasodilatation is mediated by nitric oxide in the dog hindlimb. Cardiovasc Res. 1994;28:542–547. doi: 10.1093/cvr/28.4.542. [DOI] [PubMed] [Google Scholar]
- 56.Erbas T, Gedik O, Koray Z, Erbas B, Kabakci G, Aksöyek S. Plasma big-endothelin levels, cardiac autonomic neuropathy, and cardiac functions in patients with insulin-dependent diabetes mellitus. Clin Cardiol. 2000;23:259–263. doi: 10.1002/clc.4960230407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)
(DOCX 20 kb)
(DOCX 19 kb)