Abstract
Background and objective
Stroke is a devastating condition with long-term comorbidities including metabolic abnormalities. Alpha lipoic acid (ALA), with its antioxidant properties, might improve metabolic status of patients, though current evidence is still inclusive. This systematic review of randomized controlled trials (RCTs) was conducted to summarize the existing evidence regarding the effects of ALA supplementation on fasting glucose and lipid profiles among patients with stroke.
Methods
We searched Cochrane Library, EMBASE, MEDLINE, and Web of Science from 1990 until April 5th, 2018. The relevant randomized-controlled articles, based on defined key words, were included in the analyses. Two independent researchers investigated study eligibility, extracted data, and assessed the risk of bias for included studies. Heterogeneity among included studies was tested using Q-test and I2 statistics. Random-effects models were applied to pool the data and standardized mean differences (WMD) were considered as summary effect size.
Results
A total of five studies (140 patients in each intervention group) were included in our meta-analysis. The findings showed that ALA supplementation significantly decreased fasting glucose levels (WMD −36.93 mg/dL; 95% CI, −65.58, −8.28; P = 0.01; I2 = 85.0%) in patients with stroke. We found no significant effect of ALA supplementation on triglycerides (WMD −7.45 mg/dL; 95% CI, −51.35, 36.45; P = 0.739; I2 = 83.9%), total cholesterol (WMD −23.23 mg/dL; 95% CI, −48.07, 1.62; P = 0.067; I2 = 80.5%), LDL-cholesterol (WMD −10.46 mg/dL; 95% CI, −21.01, 0.09; P = 0.052; I2 = 47.4%) and HDL-cholesterol levels (WMD −3.02 mg/dL; 95% CI, −20.18, 14.14; P = 0.730; I2 = 85.8%).
Conclusions
This meta-analysis suggested the beneficial impacts of ALA supplementation in improving fasting glucose of patients diagnosed with stroke.
Keywords: Alpha-lipoic acid, Lipid profiles, Stroke, Meta-analysis
Introduction
Stroke is a chronic devastating condition and one of the major causes of mortality worldwide [1]. The 2012 Behavioral Risk Factor Surveillance System (CDC) data showed that the prevalence of stroke among adults was 2.9% [2]. One of the common types of stroke, cerebral ischemia/reperfusion (I/R) injury involves different pathophysiological mechanisms including excitatory neurotransmitters release, increased intracellular Ca2+ levels, elevated inflammation and oxidative stress, and apoptosis [3–5]. Moreover, oxidative stress, which plays an important role in the pathogenesis of cerebral I/R injury [6], is produced by an imbalance between the production of reactive oxygen species (ROS) in the body and their effective removal by endogenous scavenger enzymes and protective antioxidants [7]. In addition, other risk factors of stroke are diabetes, hypertension, and hyperlipidemia which all have inflammation and oxidative stress as their mainstream causes [8–11].
Alpha-lipoic acid (ALA) is an antioxidant with many biological functions including reducing inflammation, scavenging ROS, chelating the transitional metal ions, and modulating the signal transduction of nuclear factor [12]. Several human trials have evaluated the possible lowering effects of ALA on fasting glucose and lipid profiles with inconclusive results. In a study by Zhao et al. [13], ALA supplementation for 3 weeks significantly reduced fasting glucose, triglycerides, and total- and LDL-cholesterol levels in patients had diagnosed with acute cerebral infarction. In another study, 12-week supplementation with 600 mg ALA had beneficial lowering effects on lipid profiles in patients who had experienced a stroke [14]. Despite reported anti-diabetic and anti-lipidemic effects of ALA supplementation in some randomized clinical trials (RCTs) [15–17], differences in study design, characteristics of study population, dosage of ALA used and duration of the interventions led to reported discrepant findings [18, 19]. In order to address the discrepancies among existing evidence determining whether ALA supplementation has a causal effect on fasting glucose and lipid profiles among patients with stroke, we aimed to systematically review these evidence and summarize the available findings in a meta-analysis.
Materials and methods
Methods
Search strategy
This study was designed and conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. We searched systematically different electronic databases including Cochrane Library, EMBASE, MEDLINE, and Web of Science databases from 1990 until April 2018. The authors used the following MeSH and text keywords to identify relevant RCTs have investigated the effect of ALA supplementation on fasting glucose and lipid: patients [“stoke” OR “cerebrovascular accident (CVA)” OR “cerebrovascular”], intervention [“alpha-lipoic acid (ALA)” OR “Acid, alpha-Lipoic” OR “ Lipoic Acid” OR “α-lipoic acid” AND “supplementation” OR “intake”], and outcomes [“fasting plasma glucose (FPG)” OR “glucose” “triglycerides” OR “total cholesterol” OR “low-density lipoprotein (LDL-cholesterol)” OR “LDL-C” OR “high density lipoprotein-cholesterol (HDL-cholesterol)” OR “HDL-C”]. Additionally, we searched the references of previously published systematic reviews and meta-analyses in this area and we communicated with related experts and research centers to detect any potential citation that was not captured based on the online searches. The authors included trials published in English.
Study selection
Two independent authors (RT, MA) screened selected trials in a two-step process before including them in the meta-analysis. In the first step, researchers reviewed the title and/or abstract to remove the duplicate studies and to determine whether selected trials are potentially eligible for this meta-analysis. In the second step, the full-texts of related RCTs were retrieved to evaluate the details based on the inclusion and exclusion criteria. In the case of a discrepancy among the authors, a third author (ZA) was discussed or it was resolved by consensus.
RCTs were selected for further analysis when they met the inclusion criteria including: being an original human RCT (either a parallel or crossover), the target subjects were patients with stroke, the intervention group received ALA supplements, being a placebo-controlled trial, the mean changes and standard deviations (SD) showing the effect of ALA on fasting glucose and lipid profiles including triglycerides, total-, LDL- and HDL-cholesterol could be extracted from selected RCT for both intervention and placebo groups. Non-RCTs, clinical trials without control groups, trial protocols without results, and the RCTs did not fulfill the least quality assessment value were excluded from this meta-analysis.
Data extraction and quality assessment
Quality assessment and data extraction were done using Cochrane Collaboration risk of bias tool and standard excel sheets, 2008 by two independent authors (RT and MA). Applying Cochrane tool, the quality of the selected RCTs was evaluated considering the following features: “randomization generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, and other probable sources of bias”. The data collected from each study included first authors’ name, year of publication, participants’ age, the location of study, the method of study, total sample size, number of subjects in intervention and placebo groups, dose of intervention, duration of intervention, type of intervention and placebo, the mean and SD for fasting glucose, triglycerides, total-, LDL-, and HDL-cholesterol concentrations. Any discrepancy was resolved through discussion with a third author (ZA or A.BH).
Statistical methods
All statistical analyses were done using STATA version 12.0 (Stata Corp, College Station, TX) and RevMan V.5.3 software (Cochrane Collaboration, Oxford, UK). Cochran’s Q test (with a P value of <0.1) and I-square test (I2 > 50% considered as homogenous) were used to demonstrate the significance of heterogeneity among included studies. Due to heterogeneity among included studies, random-effect models were applied in this meta-analysis. The weighted mean differences (WMD) and 95% confidence intervals for indicating effect sizes in both intervention and control groups. Sensitivity analyses were applied to evaluate the effect of each individual study on the pooled WMD using leave-one-out method. Subgroup analyses were performed to detect the source of heterogeneity. The subgroup analyses were conducted based on the following suspected potential variables: duration of the study (>4 weeks vs. ≤4 weeks) and total sample size (≥30 subjects vs. <30 subjects). Begg’s rank correlation and Egger’s test were conducted to verify any possible publication bias for the outcomes measured. P value less than 0.05 were considered as statistically significance level.
Results
Characteristics of included studies
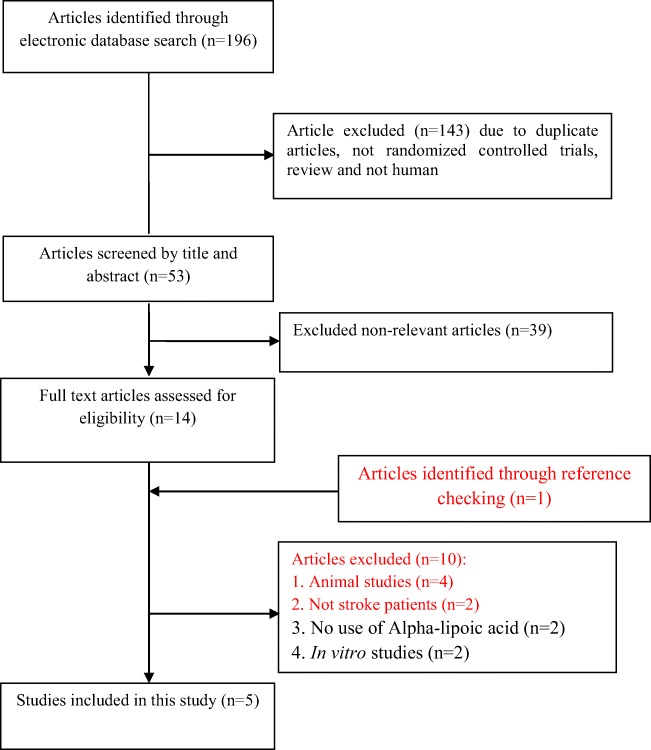
Our initial search identified 196 potential papers, after screening for our inclusion criteria and performing quality assessment, finally five studies were incorporated in this meta-analysis. Figure 1 illustrates the step by step process of studies selection. Two studies were double-blinded and the other three were randomized placebo-controlled trials. The effects of ALA supplementation on fasting glucose and HDL-cholesterol were individually investigated in three RCTs and lipid profiles (triglycerides, total- and LDL- cholesterol) were individually investigated in four RCTs. The sample size in each group (intervention/ placebo) varied from 14 to 46 individuals. Duration of the study ranged from 2 to 12 weeks. The detailed characteristics of included RCTs have been presented in Table 1.
Fig. 1.
Literature search and review flowchart for selection of studies
Table 1.
Characteristics of included studies
| Authors (Ref) | Publication year | Sample size (control/intervention) | Side effect/drop out (control/intervention) | Country/population | Intervention (name and daily dose) | Duration | Usage of intervention | Age (control, intervention) | Presented data |
|---|---|---|---|---|---|---|---|---|---|
| Mohammadi et al. [14] | 2017 | 34/33 | NR/(6/7) | Iran/patients who experienced a stroke | 600 mg ALA supplementation | 12 week | Capsule | 64.23 ± 8.01, 62.33 ± 6.19 | TC, TG, LDL-C, HDL-C |
| Zhao et al. [13] | 2014 | 44/46 | NR/− | China/acute cerebral infarction | 600 mg ALA supplementation | 3 week | Ampule | 71.6 | Glucose, TC, TG, LDL-C |
| Oprea et al. [35] | 2013 | 14/14 | NR/− | Romania/post-acute stroke patients | 600 mg ALA supplementation | 2 week | Pills | 67.07 ± 2.9, 64 ± 2.9 | Glucose, TC, TG, LDL-C, HDL-C |
| Manolescu et al. [20] | 2013 | 14/14 | NR/− | Romania/post-acute stroke patients | 600 mg ALA supplementation | 2 week | Pills | 67.1 ± 2.9, 64 ± 2.9 | TC, TG, LDL-C, HDL-C |
| Mohammadi et al. [36] | 2018 | 34/33 | NR/(6/7) | Iran/patients who experienced stroke | 600 mg ALA supplementation | 12 week | Capsule | 64.23 ± 8.01, 62.33 ± 6.19 | Glucose |
ALA alpha-lipoic acid; LDL-C low density lipoprotein-cholesterol; HDL-C high density lipoprotein-cholesterol; TC total cholesterol; TG triglycerides; NR not reported
The quality of included clinical trials was judged by two authors, using Cochrane Collaboration risk of bias tool which is shown in Fig. 2. The findings of risk of bias assessment indicated that 2 studies were at unclear risk of bias, and 3 studies were at high risk bias based on the judgments of author.
Fig. 2.

The methodological quality of included studies (risk of bias)
Effects of ALA supplementation on fasting glucose and lipid profiles
The pooled effect sizes for different outcomes, using random-effect model, indicated that ALA supplementation significantly decreased fasting glucose concentrations (WMD −36.93 mg/dL; 95% CI, −65.58, −8.28; P = 0.01; I2 = 85.0%) (Fig. 3 & Table 2).
Fig. 3.
a-e. Meta-analysis glycemic control standardized mean differences estimates for (a) fasting glucose, (b) for triglycerides, (c) for total cholesterol, (d) for LDL-cholesterol, and (e) for HDL-cholesterol in alpha-lipoic acid supplements and placebo groups (CI = 95%)
Table 2.
Estimation of the standardized difference means of related indictors with CI 95% between the intervention and placebo groups
| Variable | Number of study | Standardized Mean difference (mg/dL) | CI 95% | P value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | Q |
P value Heterogeneity |
||||||
| Glucose | Intervention group (after vs. before) | 3 | −67.96 | −155.17, 19.25 | 0.127 | 98.7 | 150.34 | <0.001 |
| Placebo group (after vs. before) | 3 | −28.88 | −76.27, 18.52 | 0.232 | 96.2 | 53.26 | <0.001 | |
| Change intervention group vs. placebo group | 3 | −36.93 | −65.58, −8.28 | 0.012 | 85.0 | 13.37 | <0.01 | |
| Triglycerides | Intervention group (after Vs. before) | 4 | −36.86 | −117.78, 44.06 | 0.372 | 95.1 | 60.98 | <0.001 |
| Placebo group (after Vs. before) | 4 | −21.68 | −53.71, 10.35 | 0.185 | 78.5 | 13.98 | 0.003 | |
| Change intervention group Vs. placebo group | 4 | −7.45 | −51.35, 36.45 | 0.739 | 83.9 | 18.62 | <0.001 | |
| Total cholesterol | Intervention group (after Vs. before) | 4 | −40.12 | −128.84, 48.59 | 0.375 | 98.5 | 195.70 | <0.001 |
| Placebo group (after Vs. before) | 4 | −19.37 | −80.91, 42.18 | 0.537 | 97.2 | 108.87 | <0.001 | |
| Change intervention group Vs. placebo group | 4 | −23.23 | −48.07, 1.62 | 0.067 | 80.5 | 15.41 | <0.01 | |
| LDL-cholesterol | Intervention group (after Vs. before) | 4 | −24.69 | −68.41, 19.04 | 0.269 | 92.6 | 40.57 | <0.001 |
| Placebo group (after Vs. before) | 4 | −16.37 | −43.10, 10.36 | 0.230 | 85.2 | 20.29 | <0.001 | |
| Change intervention group Vs. placebo group | 4 | −10.46 | −21.01, 0.09 | 0.052 | 47.4 | 5.70 | 0.127 | |
| HDL-cholesterol | Intervention group (after Vs. before) | 3 | 2.09 | −8.62, 12.80 | 0.702 | 84.4 | 12.82 | <0.01 |
| Placebo group (after Vs. before) | 3 | 1.50 | −2.28, 5.28 | 0.437 | 0.0 | 1.24 | 0.537 | |
| Change intervention group Vs. placebo group | 3 | −3.02 | −20.18, 14.14 | 0.730 | 85.8 | 14.13 | <0.01 | |
CI, confidence interval
Serum triglyceride WMD −7.45 mg/dL; 95% CI, −51.35, 36.45; P = 0.739; I2 = 83.9%), total cholesterol (WMD −23.23 mg/dL; 95% CI, −48.07, 1.62; P = 0.067; I2 = 80.5%), LDL-cholesterol (WMD −10.46 mg/dL; 95% CI, −21.01, 0.09; P = 0.052; I2 = 47.4%), and HDL-cholesterol levels (WMD −3.02 mg/dL; 95% CI, −20.18, 14.14; P = 0.730; I2 = 85.8%) were not significantly affected by ALA supplementation (Fig. 3). Detailed meta-analysis results for the effects of ALA supplementation on fasting glucose and lipid profiles at baseline and the end of follow-up in both intervention and control groups have been summarized in Table 2.
Sensitivity analyses did not reveal any significant difference in the pooled WMD for the effect of ALA on triglycerides and total cholesterol, indicating the removal of each study was not a significant influence on the findings of meta-analysis and the results were robust. However, we found that the pooled WMD for fasting glucose differed significantly between the pre-sensitivity WMD (−36.93 mg/dL; 95% CI, −65.58, −8.28) and post-sensitivity WMD (−38.26 mg/dL; 95% CI, −84.05, 7.50) when Oprea et al. [35] study was removed. For LDL-cholesterol, we also observed significant difference between the pre-sensitivity pooled WMD (−10.46 mg/dL; 95% CI, −21.01, 0.09) and post-sensitivity pooled WMD (−13.65 mg/dL; 95% CI, −25.09, −2.21) after removing Manolescu et al. [20] study. Also, we found for HDL-cholesterol a significant difference between the pre-sensitivity pooled WMD (−3.02 mg/dL; 95% CI, −20.18, 14.14) and post-sensitivity pooled WMD (−11.39 mg/dL; 95% CI, −21.77, −1.02) after removing Mohammadi et al. [14] study (Table 3).
Table 3.
The assess of contribution one by one trials in association between alpha-lipoic acid supplementation and fasting glucose and lipid profiles using sensitivity analysis
| Variable | Pre-sensitivity analysis | Upper & lower of effect size | Post-sensitivity analysis | ||||
|---|---|---|---|---|---|---|---|
| No. of studies included | Pooled WMD (random effect) | 95% CI | Pooled WMD (random effect) | 95% CI | Excluded studies | ||
| Glucose | 3 | −36.93 | −65.58, −8.28 | Upper | −38.26 | −84.05, 7.50 | Oprea |
| Lower | −49.66 | −75.95, −23.38 | Mohammadi 2018 | ||||
| Triglycerides | 4 | −7.45 | −51.35, 36.45 | Upper | 12.86 | −22.55, 48.28 | Zhao |
| Lower | −22.29 | −74.49, 29.91 | Manolescu | ||||
| Total cholesterol | 4 | −23.23 | −48.07, 1.62 | Upper | −13.51 | −27.64, 0.61 | Zhao |
| Lower | −27.99 | −56.13, 0.14 | Oprea | ||||
| LDL-cholesterol | 4 | −10.46 | −21.01, 0.09 | Upper | −5.49 | −21.44, 10.44 | Mohammadi 2017 |
| Lower | −13.65 | −25.09, −2.21 | Manolescu | ||||
| HDL-cholesterol | 3 | −3.02 | −20.18, 14.14 | Upper | 0.65 | −20.35, 21.66 | Manolescu |
| Lower | −11.39 | −21.77, −1.02 | Mohammadi 2017 | ||||
The finding of subgroup analysis based on sample size indicated that ALA supplementation led to a significant greater promotion in triglycerides levels in the strata <30 subjects (WMD 31.08 mg/dL; 95% CI, 6.32, 55.85, I2: 0.0) compared with the strata ≥30 subjects (WMD 0.57; 95% CI, −1.19, 1.32, I2: 80.6).
Subgroup analysis showed a significant greater reduction in total cholesterol concentrations using the clinical trials with ≥30 subjects (WMD −35.42 mg/dL; 95% CI, −68.62, −2.23, I2: 88.5) compared with <30 subjects (WMD −6.57 mg/dL; 95% CI, −29.25, 16.10, I2: 0.0). Similarly, ALA supplementation showed greater reduction in LDL-cholesterol levels in the subgroup with ≥30 subjects (WMD −17.69 mg/dL; 95% CI, −30.09, −5.29, I2: 0.0) compared with the trials <30 subjects (WMD 8.45 mg/dL; 95% CI, −11.62, 28.52, I2: 0.0). The detailed results of subgroup analyses have been presented in Table 4.
Table 4.
The effects of alpha-lipoic acid supplementation and glucose control and lipid profiles with CI 95% between based on subgroup analysis
| Variable | Number of WMD included | Subgroups | Pooled effect estimate | 95% CI | I2 (%) | Overall I2 (%) | |
|---|---|---|---|---|---|---|---|
| Glucose | Duration of intervention (weeks) | 1 | >4 weeks | −16.20 | −26.19, −6.21 | – | 85.0 |
| 2 | ≤4 weeks | −49.67 | −75.95, −23.39 | 57.5 | |||
| Sample size (subjects) | 2 | ≥30 subjects | −38.27 | −84.06, 7.52 | 91.9 | ||
| 1 | <30 subjects | −36.18 | −60.58, −11.78 | – | |||
| Triglycerides | Duration of intervention (weeks) | 1 | >4 weeks | −18.32 | −44.12, 7.48 | – | 83.9 |
| 3 | ≤4 weeks | −4.78 | −69.74, 60.19 | 87.6 | |||
| Sample size (subjects) | 2 | ≥30 subjects | −47.48 | −111.58, 16.61 | 80.6 | ||
| 2 | <30 subjects | 31.08 | 6.32, 55.85 | 0.0 | |||
| Total cholesterol | Duration of intervention (weeks) | 1 | >4 weeks | −17.92 | −35.98, 0.14 | – | 80.5 |
| 3 | ≤4 weeks | −24.13 | −59.14, 10.88 | 82.3 | |||
| Sample size (subjects) | 2 | ≥30 subjects | −35.42 | −68.62, −2.23 | 88.5 | ||
| 2 | <30 subjects | −6.57 | −29.25, 16.10 | 0.0 | |||
| LDL-cholesterol | Duration of intervention (weeks) | 1 | >4 weeks | −14.33 | −28.40, −0.26 | – | 47.4 |
| 3 | ≤4 weeks | −5.50 | −21.44, 10.45 | 60.3 | |||
| Sample size (subjects) | 2 | ≥30 subjects | −17.69 | −30.09, −5.29 | 0.0 | ||
| 2 | <30 subjects | 8.45 | −11.62, 28.52 | 0.0 | |||
| HDL-cholesterol | Duration of intervention (weeks) | 1 | >4 weeks | 10.33 | 5.78, 14.88 | – | 85.8 |
| 2 | ≤4 weeks | −11.40 | −21.77, −1.02 | 0.0 | |||
| Sample size (subjects) | 1 | ≥30 subjects | 10.33 | 5.78, 14.88 | – | ||
| 2 | <30 subjects | −11.40 | −21.77, −1.02 | 0.0 | |||
CI confidence interval
Publication bias
Begg’s and Egger’s tests showed no significant publication bias assessing the effects of ALA on fasting glucose (Begg’s: Z = -0.52, P = 0.60 and Egger’s: B = -4.59, P = 0.28), triglycerides (Begg’s: Z = -0.68, P = 0.49 and Egger’s: B = -2.68, P = 0.71), total cholesterol (Begg’s: Z = 0.00, P = 1.00 and Egger’s: B = 4.71, P = 0.15), and LDL-cholesterol (Begg’s: Z = 0.68, P = 0.49 and Egger’s: B = 1.61, P = 0.56).
But, there was evidence of the possible publication bias on the effects of ALA supplements on HDL-cholesterol (Begg’s: Z = -0.52, P = 0.60 and Egger’s: B = -4.20, P = 0.02). Therefore, we used non parametric method (Duval and Tweedie) to include the results of censored studies. The results showed that pooled effect sizes changed significantly for HDL-cholesterol between pre and post including the results of censored trials.
Discussion
The findings of current meta-analysis depict that ALA supplementation significantly decreased serum glucose and total cholesterol levels and might improve metabolic profile of patients recovered from stroke. To our best knowledge, this is the first meta-analysis of RCTs evaluating the effect of ALA supplementation on fasting glucose and lipid profiles in patients with stroke.
Effects of ALA supplementation on fasting glucose
Therapeutic lifestyle modification as an acceptable strategy to control chronic disease risk factors including dyslipidemia and hyperglycemia has not been a totally successful approach due to poor adherence and persistence [21, 22]. Current meta-analysis showed significant lowering effect of ALA supplementation on serum glucose levels in patients with stroke. Data on the effects of ALA supplementation on glycemic control are limited; few studies have evaluated the beneficial effects of antioxidants supplementation on glycemic control in patients diagnosed with metabolic disorders. In a meta-analysis conducted by Tabrizi et al. [23], selenium supplementation led to a significant reduction in insulin concentrations and improved insulin sensitivity in patients with metabolic syndrome and related disorders, though no significant effect on fasting glucose and HOMA-IR was reported. Moreover, in a systematic review conducted by Cruz et al. [24], zinc supplementation significantly improved insulin sensitivity in obese subjects. In another clinical trial, taking ascorbic acid by diabetic patients significantly decreased fasting glucose levels [25]. Although, coenzyme Q10 supplementation to patients with diabetes did not affect glycemic control [26]. The beneficial effects of ALA supplementation on lowering fasting glucose may be related to its role in modulating adenosine monophosphate-activated protein kinase (AMPK) [27]. ALA intake has been shown to decrease fasting glucose levels by activating AMPK in skeletal muscle [28] and beta-cells [29].
Effects of ALA supplementation on lipid profiles
The results of current meta-analysis revealed that ALA supplementation might be effective in lowering total cholesterol levels in patients with stroke. Zhao et al. [13] demonstrated the beneficial effects of ALA supplementation for 3 weeks, in significantly decreasing serum triglycerides, total- and LDL-cholesterol levels among patients diagnosed with acute cerebral infarction. Moreover, a 12-week supplementation with 600 mg ALA by patients experienced a stroke showed lowering impact on lipid profiles [14]. There are still studies with discrepant results. Li et al. [18] did not find any significant reduction in lipid profile of overweight individuals following ALA supplementation (1200 mg/day) for 8 weeks. Moreover, ALA supplementation at a dosage of 600 mg/day for 8 weeks to hemodialysis patients did not improve their lipid profiles [30]. Different geographical latitudes where study conducted might further complicate the effect of ALA supplementation on lipid profiles. In addition, study design, sample size, baseline circulating levels of ALA, different dosages of ALA used along with characteristics of study participants, including comorbidities might explain the discrepancies among existing studies. If exists, the beneficial effects of ALA intake on lipid profiles may be related to elevating AMPK activity in peripheral tissues including skeletal muscle which directly inhibits fatty acid synthesis, while concomitantly increases β-oxidation of fatty acids as well [31, 32]. Furthermore, gene expression of the two rate-limiting enzymes in fatty acid synthesis, acetyl-CoA carboxylase and fatty acid synthase decreases in response to ALA administration [31, 33]. Moreover, a remarkable reduction in plasma proportion convertase subtilisin/kexin type 9 concentrations and an elevation in hepatic LDL receptor protein, following ALA supplementation, may decrease total cholesterol levels [34].
There are several strengths for this study. All included studies were placebo-controlled randomized trials with acceptable methodological quality and the least probable chance of bias. Further, we relied on independent judgment in which different reviewers independently performed the systematic review process. The current study had a few limitations. We were unable to evaluate the dose-response association between supplementation and metabolic profiles. One of the major limitations of the study was the inclusion of studies with relatively small sample size that could influence type-2 statistical error.
Conclusions
Overall, this meta-analysis demonstrated the beneficial effects of ALA supplementation for improving metabolic abnormalities in patients have recovered from stroke. In order to improve stroke, additional prospective studies regarding the effect of ALA supplementation on fasting glucose and lipid profiles with a proper dosage range are necessary.
Acknowledgements
The present study was supported by a grant (no. 97-01-106-17829) from the Vice-chancellor for Research, SUMS, Shiraz, and Iran.
Abbreviations
- ALA
Alpha-lipoic acid
- LDL-C
Low density lipoprotein-cholesterol
- HDL-C
High density lipoprotein-cholesterol
- TC
Total cholesterol
- TG
Triglycerides
Author contributions
ZA, MA and RT contributed in conception, design, statistical analysis and drafting of the manuscript. A-BH, NM, KB-L, RT, S-TH, FK and FR contributed in conception, data collection and manuscript drafting. The final version was confirmed by all authors for submission.
Funding
The research grant provided by Research Deputy of Shiraz University of Medical Sciences (SUMS).
Data availability
The primary data for this study is available from the authors on direct request.
Compliance with ethical standards
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reza Tabrizi, Email: kmsrc89@gmail.com.
Afshin Borhani-Haghighi, Email: Afshin.Borhani-Haghighi1@gmail.com.
Naghmeh Mirhosseini, Email: namirhossini@gmail.com.
Kamran B. Lankarani, Email: lankaran@sums.ac.ir
Ahmad Naghibzadeh-Tahami, Email: anaghibzadeh61@gmail.com.
Maryam Akbari, Email: m.akbari45@yahoo.com.
Seyed Taghi Heydari, Email: heidaryt@sums.ac.ir.
Mojgan Sangari, Email: mjnsanjari@gmail.com.
Fariba Kolahdooz, Email: fariba.kolahdooz@ualberta.ca.
Fariba Raygan, Email: raygan.fariba2@gmail.com.
Zatollah Asemi, Email: asemi_r@yahoo.com.
References
- 1.Vasiliadis AV, Zikic M. Current status of stroke epidemiology in Greece: a panorama. Neurol Neurochir Pol. 2014;48:449–457. doi: 10.1016/j.pjnns.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Jauch Edward C., Saver Jeffrey L., Adams Harold P., Bruno Askiel, Connors J.J. (Buddy), Demaerschalk Bart M., Khatri Pooja, McMullan Paul W., Qureshi Adnan I., Rosenfield Kenneth, Scott Phillip A., Summers Debbie R., Wang David Z., Wintermark Max, Yonas Howard. Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 3.Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhao NN, Zeng KX, Wang YL, Sheng PJ, Tang CZ, Xiao P, et al. Research on the nutrition and cognition of high-risk stroke groups in community and the relevant factors. Eur Rev Med Pharmacol Sci. 2017;21:5408–5414. doi: 10.26355/eurrev_201712_13928. [DOI] [PubMed] [Google Scholar]
- 5.Santoro L, De Matteis G, Fuorlo M, Giupponi B, Martone AM, Landi F, et al. Atherosclerosis and cardiovascular involvement in celiac disease: the role of autoimmunity and inflammation. Eur Rev Med Pharmacol Sci. 2017;21:5437–5444. doi: 10.26355/eurrev_201712_13932. [DOI] [PubMed] [Google Scholar]
- 6.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Hanafy KA, Selim MH. Antioxidant strategies in neurocritical care. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:44–55. doi: 10.1007/s13311-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.STR.22.3.312. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon S, Rodgers A. Blood pressure, antihypertensive treatment and stroke risk. J Hypertens Suppl. 1994;12:S5–14. [PubMed] [Google Scholar]
- 10.Zhong LL, Ding LS, He W, Tian XY, Cao H, Song YQ, et al. Systolic hypertension related single nucleotide polymorphism is associated with susceptibility of ischemic stroke. Eur Rev Med Pharmacol Sci. 2017;21:2901–2906. [PubMed] [Google Scholar]
- 11.Liu J, Yang H, Yu B. The correlation between blood calcium level, hematoma volume, stroke severity and prognosis in patients with acute cerebral hemorrhage. Eur Rev Med Pharmacol Sci. 2016;20:4119–4123. [PubMed] [Google Scholar]
- 12.Seifar F, Khalili M, Khaledyan H, Amiri Moghadam S, Izadi A, Azimi A, et al. Alpha-lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: a review. Nutr Neurosci. 2017:1–11. [DOI] [PubMed]
- 13.Zhao L, Hu FX. Alpha-lipoic acid treatment of aged type 2 diabetes mellitus complicated with acute cerebral infarction. Eur Rev Med Pharmacol Sci. 2014;18:3715–3719. [PubMed] [Google Scholar]
- 14.Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid comsumption improve lipid profile in patients with stroke? A randomized, double blind, placebo-controlled clinical trial. Iranian Red Crescent Med J. 2017;19.
- 15.Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588. [PubMed] [Google Scholar]
- 16.Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity (Silver Spring) 2015;23:313–321. doi: 10.1002/oby.20966. [DOI] [PubMed] [Google Scholar]
- 17.Okanovic A, Prnjavorac B, Jusufovic E, Sejdinovic R. Alpha-lipoic acid reduces body weight and regulates triglycerides in obese patients with diabetes mellitus. Med Glas (Zenica) 2015;12:122–127. doi: 10.17392/798-15. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Yan W, Hu X, Huang Y, Wang F, Zhang W, et al. Effects of oral alpha-lipoic acid administration on body weight in overweight or obese subjects: a crossover randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2017;86:680–687. doi: 10.1111/cen.13303. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira AM, Rondo PH, Luzia LA, D'Abronzo FH, Illison VK. The effects of lipoic acid and alpha-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Res Clin Pract. 2011;92:253–260. doi: 10.1016/j.diabres.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Manolescu BN, Berteanu M, Cinteza D. Effect of the nutritional supplement ALAnerv(R) on the serum PON1 activity in post-acute stroke patients. Pharmacol Rep. 2013;65:743–750. doi: 10.1016/S1734-1140(13)71054-5. [DOI] [PubMed] [Google Scholar]
- 21.Chapman K. Can people make healthy changes to their diet and maintain them in the long term? A review of the evidence. Appetite. 2010;54:433–441. doi: 10.1016/j.appet.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Roumen C, Blaak EE, Corpeleijn E. Lifestyle intervention for prevention of diabetes: determinants of success for future implementation. Nutr Rev. 2009;67:132–146. doi: 10.1111/j.1753-4887.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 23.Tabrizi R, Akbari M, Moosazadeh M, Lankarani KB, Heydari ST, Kolahdooz F, et al. The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2017;49(11):826–830. doi: 10.1055/s-0043-119544. [DOI] [PubMed] [Google Scholar]
- 24.Andrade VH, Mata AM, Borges RS, Costa-Silva DR, Martins LM, Ferreira PM, et al. Current aspects of polycystic ovary syndrome: a literature review. Rev Assoc Med Bras (1992) 2016;62:867–871. doi: 10.1590/1806-9282.62.09.867. [DOI] [PubMed] [Google Scholar]
- 25.Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17:554–582. doi: 10.18433/J3ZG6R. [DOI] [PubMed] [Google Scholar]
- 26.Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40:413–418. doi: 10.1111/jcpt.12280. [DOI] [PubMed] [Google Scholar]
- 27.Karadag C, Yoldemir T, Yavuz DG. Effects of vitamin D supplementation on insulin sensitivity and androgen levels in vitamin-D-deficient polycystic ovary syndrome patients. J Obstet Gynaecol Res. 2017. [DOI] [PubMed]
- 28.Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARgamma expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017;36:54–59. doi: 10.1016/j.nut.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Mirmasoumi G, Fazilati M, Foroozanfard F, Vahedpoor Z, Mahmoodi S, Taghizadeh M, et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018;126(4):222–228. doi: 10.1055/s-0043-119751. [DOI] [PubMed] [Google Scholar]
- 30.Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012;22:244–250. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Qu F, Wang FF, Yin R, Ding GL, El-Prince M, Gao Q, et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med (Berl) 2012;90:911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 32.Irani Mohamad, Seifer David, Grazi Richard, Irani Sara, Rosenwaks Zev, Tal Reshef. Vitamin D Decreases Serum VEGF Correlating with Clinical Improvement in Vitamin D-Deficient Women with PCOS: A Randomized Placebo-Controlled Trial. Nutrients. 2017;9(4):334. doi: 10.3390/nu9040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khani B, Mardanian F, Fesharaki SJ. Omega-3 supplementation effects on polycystic ovary syndrome symptoms and metabolic syndrome. J Res Med Sci. 2017;22:64. doi: 10.4103/jrms.JRMS_644_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrier B, Wen S, Zigouras S, Browne RW, Li Z, Patel MS, et al. Alpha-lipoic acid reduces LDL-particle number and PCSK9 concentrations in high-fat fed obese Zucker rats. PLoS One. 2014;9:e90863. doi: 10.1371/journal.pone.0090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oprea E, Berteanu M, Cinteza D, Manolescu BN. The effect of the ALAnerv nutritional supplement on some oxidative stress markers in postacute stroke patients undergoing rehabilitation. Appl Physiol Nutr Metab. 2013;38:613–620. doi: 10.1139/apnm-2012-0436. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid supplementation modulate cardiovascular risk factors in patients with stroke? A randomized, double-blind clinical trial. Int J Prev Med. 2018;9:34. doi: 10.4103/ijpvm.IJPVM_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.