Abstract
Purpose
There is controversial data regarding the effects of dietary antioxidative supplements on diabetic retinopathy (DR). We conducted a systematic review of both observational and randomized controlled clinical trials (RCTs) to clarify whether they are effective or not.
Methods
All observational and RCTs conducted by antioxidative supplements on DR published up to 1 January 2018 in PubMed, Web of Sciences, Scopus and Cochrane Library databases were included. Exclusion criteria were animal studies, and studies conducted in Type 1 diabetes mellitus (T1DM), children or pregnant women. Main outcome measures were reporting the incidence or progression of DR in T2DM by assessment of visual fields, and measurements of oxidative and antioxidative biomarkers. The quality of reporting of included articles and risk of bias were assessed.
Results
Finally, we reached 14 observational studies and 7 RCTs that conducted on 256,259 subjects. Due to severe methodological heterogeneity, only qualitative synthesis was carried. All studies were reported a significantly lower level of antioxidants and higher level of oxidative stress biomarkers in DR compared with others. There was an inverse significant correlation between vitamin C and malondialdehyde (MDA) (r = −0.81) or DNA damage (r = −0.41). These figures were statistically significant between vitamin E and MDA (r = 0.77) or superoxide dismutase (r = 0.44). Coefficient of correlation between MDA and zinc (−0.82), coenzyme Q10 (0.56), and magnesium (−0.73) was significant. Multi-oxidants trials were shown non-significant beneficial effects on DR.
Conclusions
Although our study supports the positive effects of antioxidative supplements on DR, more high quality studies are needed to confirm.
Electronic supplementary material
The online version of this article (10.1007/s40200-019-00434-x) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes mellitus, Diabetic retinopathy, Oxidative stress, Antioxidants
Introduction
Diabetic retinopathy (DR) that is definded as retinal micro-vascular lesion in subjects with diabetes classified as non-proliferative (NPDR) and proliferative diabetic retinopathy (PDR). NPDR can increase the risk for development of PDR and blindness. The prevalence of diabetic retinopathy (DR) as a common microvascular complication of diabetes is increasing worldwide. DR as the leading cause of vision loss in working-age adults affects 35% of patients with diabetes [1]. Based on report of International Diabetes Federation (IDF) there are approximately 425 million patients with diabetes in 2017 that expects to rise to 629 million by 2045. Studies in various countries have revealed an increasing trend in economic burden of DR [1, 2]. However, blindness due to DR can be prevented by appropriate screening and treatment. IDF is reported 76% reduction in the onset of DR through intensive control of hyperglycemia [1].
Within several pathways that have linked hyperglycemia to DR, oxidative stress has a crucial role in the pathogenesis of DR [3]. Oxidative stress is defined as a condition with an imbalance between oxidants and antioxidant defense system of the body [4]. This condition can be modified by enzymatic and non-enzymatic antioxidants [5–7]. Hyperglycemia-induced metabolic abnormalities including activation of polyol pathway, protein kinase C (PKC), advanced glycation end products (AGEs), and hexosamine pathway besides inflammatory mediators could increase production of reactive oxygen species (ROS) and oxidative stress that resulted in dysfunction of mitochondria, apoptosis and diabetic retinopathy [8]. Although circulating high glucose is the main investigator of DR development, other factors such as duration of diabetes, dyslipidemia and hypertension are also influenced on progression of DR [8].
Due to a potential association between diabetes’ complications and oxidative stress, it is logical to expect beneficial effects for antioxidants in improvement diabetes and its complications. The retina is disposed to damage by oxidative stress due to its polyunsaturated fatty acids-rich tissue, highest oxygen uptake than any tissue and high vascularization [9]. Although majority of the antioxidants can be consumed easily from foods, their effects on DR have been seen by influencing on one or more complex mechanisms [7]. Evidences have shown that DR can be controlled and prevented by several antioxidant supplements including vitamins C, E, D, beta-carotene, co-enzyme Q10, magnesium (Mg) and zinc (Zn) via the reduction of protein glycosylation, inhibition of LDL-oxidized formation; malondialdehyde (MDA), anti-inflammatory effects, or protection of insulin in release, production, and secretion [10–13]. Beneficial effects of antioxidants have shown in animal models of DR, but clinical trials have provided controversial results [14]. In this systematic review, we aimed to critically assess the effect of supplementations with antioxidants vitamins A, C, D, E, benfotiamine (B1), beta-carotene, alpha lipoic acid (ALA), co-enzyme Q10, selenium (Se), Mg, and Zn on DR in patients with type 2 diabetes mellitus (T2DM) based on current relevant data from both observational and randomized controlled trials (RCTs) conducted studies.
Methods
Data searches
All relevant available observational studies including cohort, case-control, and cross sectional studies as well as RCTs which reported the effects of antioxidative supplements, vitamins A, C, D, E, B1, ALA, beta-carotene, co-enzyme Q10, Se, Mg and Zn on diabetic retinopathy and published up to 1 January 2018 were included. To obtain all related studies, PubMed, Cochrane Library, Web of Science, and Scopus web databases were searched using search strategy referred in Supplementary 1.
In order to obtain required information, we sent maximum 3 e-mails to the corresponding authors of publications with insufficient data. According to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flowchart [15] the title and abstract of publications were examined after exclusion of duplicated articles. Finally, reference list of potentially eligible studies were assessed by hand searching for full-text retrieval. All steps were performed independently by two investigators. Any disagreement was resolved by the third reviewer.
Study selection
All observational studies or RCTs that reported the incidence or progression of DR in subjects with T2DM were included. All diagnostic methods such as visual fields’ assessment, by ophthalmoscopy, fluor-angiography, electro-retinography, visual acuity, beside measurements of oxidative biomarkers; MDA, and antioxidative biomarkers such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and total antioxidant status (TAS) were acceptable.
Exclusion criteria were as following; (i) animal studies, (ii) studies on healthy population, type 1 DM, children and pregnant women. Review articles, letters to the editor, conference abstract, interview and thesis were also excluded. English language was considered to restriction of search.
Data extraction and quality assessment
The following data were extracted; first author, year of publication, study design, age and sex of participants, sample size, number of participants and patients with T2DM in observational studies, frequency and dosage of dietary supplement, duration of intervention, follow up, serum level of dietary supplement, outcome measures, and significant results.
The authors independently assessed the quality of all included observational and RCTs by STROBE (strengthening the reporting of observational studies in epidemiology) and Jadad scoring system [16, 17], respectively. Any disagreement was resolved by discussion and if not to reach consensus, third investigator solved the problem.
Five selected items from the recommended checklist of STROBE was used for quality assessment. The items included a) 1 point if confirmed diagnosis of diabetes based on accepted criteria such as American Diabetes Association (ADA), or medical records; (b) 1 point if provided inclusion and exclusion criteria; (c) 1 point if presented key elements of study design; d) 1 point if assessed taking of dietary supplements by a valid questionnaire, tool, or laboratory measurements; and (e) 1 point if described characteristics of participants. The range of score in the Jadad scale was between 0 and 5. The quality score lower than 3 points in both of observational studies and RCTs were classified as low quality or high risk of bias studies. Considerable heterogeneity among studies did not allow us to perform meta-analysis.
Results
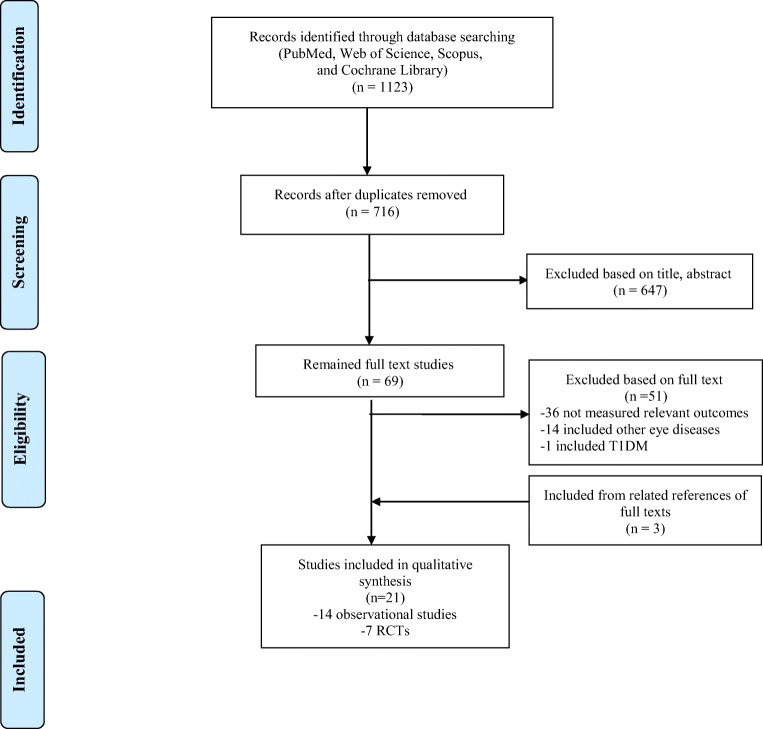
According to the PRISMA flowchart (Fig. 1), 21 articles are included in the present systematic review [10–12, 18–35]. Characteristics of the included observational studies and RCTs are shown in Table 1, and Table 2, respectively.
Fig. 1.
Flow diagram of the study selection process
Table 1.
Data extracted from eligible observational studies in qualitative systematic review
| Study | Design | Subjects | Sex/age years | Outcome measures | Significant Results/Odds Ratios | Quality score |
|---|---|---|---|---|---|---|
| Sinclair et al. 1992 [18] | Cross-sectional | Total 90 (40 healthy, 25 T2DM with DR, 25 T2DM without DR) | Both/> 60 | Fundoscopy, measurements of TBA reactivity, GSH, AA, DHA, TGs, TChol, glucose, fructosamine |
-↓AA in DR vs. Others (P < 0.01) -↑DHA/AA in DR vs. Without DR (P < 0.001), or controls (P < 0.0001) -Negative correlation between AA and fructosamine in DR (r = −0.46, P < 0.05) |
2 |
| Gurler et al. 2000 [19] | Case-control |
Total 85 (26 healthy, 34 T2DM, 25 T2DM + DR) |
Both/42–69 | MDA-like metabolite, erythrocyte GPx, SOD, VC, HbA1C |
-↓VC in T2DM + DR -↑MDA-like metabolite in T2DM + DR -↑HbA1C in T2DM with/without DR |
3 |
| Gupta et al. 2005 [20] | Case-control |
Total 132 (50 healthy, 40 T2DM without DR, 42 T2DM+ DR) |
Both/38–54 | MDA, SOD, GPx, VC, BG, HbA1C |
-↓Serum level VC in T2DM with/without DR vs. healthy -↑Serum level MDA, SOD, GPx in T2DM with/without DR vs. Healthy -↓SOD, VC with DR progression -Positive correlation between MDA, HbA1C and duration of DR |
4 |
| Yildirm et al. 2007 [21] | Case-control |
Total 75 (25 non-T2DM, 25 T2DM+ DR proliferative, 25 T2DM + DR non-proliferative) |
Both/50–77 | HbA1C, serum Cu, Zn, NO, GSH, AOPP level, SOD |
-↑AOPP in T2DM Proliferative -↑NO, Cu in T2DM vs. Non-T2DM -↓GSH in T2DM vs. Non-T2DM -↑HbA1C in T2DM vs. Non-T2DM |
2 |
| Kumari et al. 2008 [10] | Case-control |
Total 118 (32 healthy, 36 T2DM, 50 T2DM + DR) |
Both/48–68 | MDA, VE, VC, lipid profile, FBS, 2hppBS |
-↑MDA in T2DM with/without DR -↓VE, VC in T2DM especially T2DM+ DR -Positive correlation betwen MDA/VE (r = 0.77) -Negative correlation between MDA/VC (r = −0.81) -Positive correlation between MDA/FBS (r = 0.81) -Positive correlation between MDA/lipid profile except HDL-C |
5 |
| Samuel et al. 2010 [22] | Case-control |
Total 90 (30 healthy, 30 T2DM, 30 T2DM+ DR) |
Both/35–65 | MDA, erythrocyte SOD, GSH, VC, FBS |
-↑MDA in T2DM+ DR -↓SOD, GSH, VC in all T2DM especially T2DM + DR -Negative correlation between FBS and antioxidant enzymes in T2DM + DR |
2 |
| Aldebasi et al. 2011 [23] | Case-control |
Total 160 (60 T2DM, 100 T2DM+ DR) |
Both/ 61 | FBS, HbA1C, MDA, 8-OhdG,Cu-Zn SOD |
-↑MDA, 8-OhdG in T2DM+ DR vs. Others -↓Cu-Zn SOD in T2DM+ DR vs. Others -Negative correlation between MDA or HbA1C and Cu-Zn SOD (r = −0.24, r = −0.23) |
4 |
| Lam et al. 2011 [24] | Case-control |
Total 420 T2DM (46 without DR, 161 background DR, 207 non-PDR, 6 PDR) |
Both/ <75 | FBS, lipid profile, HbA1C, DNA Damage, serum VC, SOD, TAS, GPx, VE lipid standardized, allantoin, urine level F2-isoprostanes |
-Positive correlation between DNA damage and HbA1C (r = 0.32), and FBS (r = 0.52) -Negative correlation between DNA damage and VC (r = −0.41) -Negative correlation between DNA damage and TAS (r = −0.21) -Positive correlation between VE lipid standardized and allantoin (r = 0.33) |
4 |
| Ates et al., 2013 [11] | Case-control |
Total 100 (50 healthy, 50 T2DM + DR proliferative) |
Both/50–70 | Ubiquinone-10, total coenzyme Q10, ubiquinol-10/ubiquinone-10, MDA |
-Positive correlation between MDA and coenzyme Q10 (r = 0.557) -↑MDA in T2DM+ DR -↑Ubiquinone-10 in T2DM+ DR -↓Ubiquinol-10/ubiquinone-10 in T2DM+ DR |
4 |
| Roopa et al. 2013 [25] | Case-control |
Total 150 (50 healthy, 50 T2DM, 50 T2DM + DR) |
Both/ NA | MDA, VC, VE, hs-CRP |
-↑MDA and hs-CRP in T2DM with/without DR especially in DR -↓VC, VE in T2DM with/without DR especially in DR |
3 |
| Said et al. 2013 [26] | Case-control |
Total 120 (30 healthy, 30 T2DM, 30 T2DM+ PDR, 30 T2DM+ non-PDR) |
Both/40–70 | FBS, lipid profile, SOD, GPx, VE |
-↑Lipid profile (except HDL-C) in T2DM+ DR -↓SOD, GPx, VE in T2DM + DR especially proliferative -Positive correlation between GPx and VE (r = 0.34) -Positive correlation between SOD and VE (r = 0.44) |
3 |
| Kumari et al. 2014 [12] | Case-control |
Total 112 (40 healthy, 30 T2DM, 42 T2DM + DR) |
Both/50–70 | FBS, Mg, Zn, VC, VE, MDA |
-↓Mg, Zn in T2DM especially with DR -↑MDA in T2DM with/without DR -↓VC,VE in T2DM with/without DR -Negative correlation between MDA and Mg (r = −0.73), and Zn (r = −0.82) |
3 |
| Kundu et al. 2014 [27] | Case-control |
Total 150 (50 healthy, 50 T2DM, 50 T2DM + DR) |
Both/40–70 | FBS, HbA1C, MDA, erythrocyte GSH, VC |
-↑FBS, HbA1C, MDA, SBP in T2DM with/without DR -↓VC, erythrocyte GSH in T2DM with/without DR -Negative correlation between FBS and VC (r = −0.58) -Positive correlation between FBS and MDA (r = 0.47) |
4 |
| Longo-Mbenza et al. 2014 [28] | Case-control |
Total 192 (45 healthy, 84 T2DM, 66 T2DM+ DR) |
Both/37–70 | 8-isoprostane, 8-oHdG, serum GGT, antibody against OxLDL, TBARS, GSH, SOD, GPx, FBS, HOMA, TAS, Zn, Se, Mg, VC, VD, VE, ApoB, eye examination, visual acuity, Lipid profile |
-↑Lipid profile in T2DM vs. healthy -Highest oxidant markers and lowest antioxidants in T2DM+ DR vs. others -↑HOMA in T2DM vs. healthy |
5 |
T2DM Type 2 diabetes mellitus, DR Diabetic retinopathy, MDA Malondialdehyde, SOD Superoxide dismutase, GPx Glutathione peroxidase, VC, Vitamin C; BG Blood glucose, HbA1C Glycosylated hemoglobin, vs. versus, GSH Reduced glutathione, FBS Fasting blood sugar, VE Vitamin E, 2hppBS 2 h post prandial blood sugar, HDL-C High density lipoprotein cholesterol, 8-oHdG 8-hydroxydexoxyguanosine, GGT Gamma-glutamyltransferase, OxLDL Oxidized low density lipoprotein, TAS Total antioxidant status, Mg Magnesium, VD Vitamin D, ApoB Apolipoprotein B, TBARS Thiobarbituric acid reacting substances, HOMA Hemostasis model-based insulin resistance, NO Nitric oxide, AOPP Advanced oxidation protein products, Zn Zinc, Cu Copper, Se Selenium, SBP Systolic blood pressure, NA Non access, Hs-CRP High sensitivity c-reactive protein, AA Ascorbic acid, DHA Dehydroascorbic acid, TChol Total cholesterol, PDR Proliferative diabetic retinopathy
Table 2.
Data extracted from eligible RCTs in qualitative systematic review
| Study | Design | Subjects/ sample size | Age (yr) | Sex | Supplement | Duration (month) | Dosage | Indicator | Main results | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Bo-Jie Hu et al. 2011 [29] | Quasi-Experimental |
Total 90 (30 healthy, 30 non-PDR with treatment, 30 non-PDR non-treatment) |
59 | M/F | lutein + zeaxanthin | 3 | 6 mg + 0.5 mg | BCVA, CS, OCT |
After medication: -Improvement of VA -↑Sig CS -↓Sig Foveal thickness |
3 |
| Garcia-Medina et al. 2011 [30] | RCT |
Total 105 (62 T2DM with non-PDR with treatment, 43 T2DM with non-PDR without treatment) |
30–65 | M/F | Mixture: | 60 | 2tablets/day |
MDA, TAS, aBCVA, DR score |
-↓Sig MDA in both groups -↓Sig TAS in placebo and ↑NS in treatment group -NS change in aBCVA -↑Sig DR score in placebo and ↑NS in treatment group |
3 |
| VC, | 60 mg | |||||||||
| Alpha-Tocopherol | 10 mg | |||||||||
| Niacin | 10 mg | |||||||||
| b-Carotene | 3 mg | |||||||||
| Lutein, | 3 mg | |||||||||
| Zn | 13.5 mg | |||||||||
| Cu | 1 mg | |||||||||
| Se | 10 mcg | |||||||||
| Manganese | 1 mg | |||||||||
| Proteins | 130 mg | |||||||||
| CH | 60 mg | |||||||||
| Fat | 230 mg | |||||||||
| Nebbioso et al. 2012 [31] | RCT |
Total 32 (16 pre-DR in treatment, 16 pre-DR in placebo) |
57 | M/F |
Mixure: ALA, genistein, VC,VE, B complex |
1 | 400 + 80 + 30 + 5 + 15 mg |
FORT, d-ROM BAP |
-↑Sig. BAP by treatment -↑Sig. OP by treatment |
3 |
| Domanico et al. 2015 [32] | RCT |
Total 68 (34 Non-PDR with/without supplements) |
40–79 | M/F |
A mixture: Pycnogenol, Coenzyme Q10, VE |
6 |
1 tablet/ day (50+ 20+ 30 mg) |
CMT FORT |
-↑NS BCVA in both groups -↓Sig. FORT at 3 mo, ↓NS at 6 mo treatment -↓Sig. CMT at 3 mo, ↓NS at 6 mo treatment -NS correlation between FORT and CMT |
2 |
| Roig Revert et al. 2015 [33] |
RCT (Community-Based Study Design) |
Total 208 (68T2DM with DR, 62 T2DM without DR, 78 healthy) | 25–80 | M/F |
Mixture: DHA, vitamins E, C, B1, B2, B3, B6, B9, B12, lutein, zeaxanthin, glutathione, hydroxytyrosol, Se, Mn, Zn, Cu |
18 | Not clear |
MDA, TAS, HbA1C, BS, lipid profile, BCVA, retinal thickness, IOP |
At baseline: -↑Sig. Oxidative stress,inflammatory, vascular risk markers in T2DM + DR vs. others -↓Sig.TAS in T2DM + DR vs. others After treatment: -↓Sig MDA in T2DM+ DR -↑Sig TAS in T2DM + DR -↑Sig HbA1C in T2DM + DR -NS changes in lipid profile in T2DM ± DR -↓NS BCVA -↓NS IOP in T2DM + DR -↑Sig IOP in T2DM-DR -↑Sig RNFL in T2DM ± DR -↑NS MT in T2DM+ DR -↓NS MT in T2DM- DR |
2 |
| Rodríguez-Carrizalez et al. 2016 [34] | RCT |
Total 60 (20 Non-PDR in each group) |
60 | M/F |
Group 1: Coenzyme Q10 |
6 | 400 mg |
HbA1c, FBS, Lipid profile, LPO, Nitrites/ Nitrates, TAC, CAT, GPX, CVA, LEP |
-↓NS HbA1C, FBS, Tchol, LDL, TG by treatment -↑NS HDL by treatment -↓Sig. LPO, Nitrites/ Nitrates, CAT, GPx by treatment -↑Sig. TAC by treatment -NS change in CVA in all groups -NS decraese in LEP in all groups |
2 |
|
Group 2: Mixture of lutein, astaxanthin, zeaxanthin,VC,VE, Zn, Cu |
10 + 4 + 1 + 180 + 30 + 20 + 1 mg | |||||||||
| Group 3: placebo | ||||||||||
| Chatziralli et al. 2017 [35] | Quasi-experimental |
Total 282 (282 Insulin-dependent T2DM + DR in different stages) |
50–70 | M/F | VE | 3 | 300 mg | MDA, DR degree, HbA1C |
-↓Sig. MDA in all DR stages -↑Sig response to VE in PDR by treatment naïve vs. prior laser photocoagulation |
0 |
CMT Central macular thickness, FORT Free oxygen radical test, MDA Malondialdehyde, TAS Total antioxidant status, aBCVA Average best corrected visual acuity (average of both eyes of each patient on a decimal scale), LPO Lipo-peroxidation products, NS Non significant, d-ROM reactive oxygen metabolites, BAP Biological antioxidant potential, OP oscillatory potential, CS Contrast sensitivity, OCT optical coherence tomography, PDR proliferative diabetic retinopathy, MT Macular thickness, IOP Intraocular pressure, RNFL Retinal nerve fiber layer thickness, CAT Catalase, TAC total antioxidant capacity, LEP Left eye pressure, CVA Corrected visual acuity, CH Carbohydrate
Studies’ characteristics
All included studies were conducted in adult patients; 256,727 subjects. Among them, 14 studies [10–12, 18–28] were observational studies involving 163,913 participants with diabetes (163,445 with T2DM) and the remaining 7 articles were RCTs [29–35] with 92,814 total subjects with diabetes and 92,784 patients with T2DM. All of the studies were performed on adults aged 20–80 years in both genders. Most of the observational studies and RCTs were conducted to evaluate the effect of vitamins C or E on DR [10, 12, 18–20, 22, 24–28, 30–35]. It was not found any observational studies or RCTs to evaluate the effect of vitamin A on DR. Details of the included studies are described as below.
Association between dietary antioxidative supplements and DR in observational studies
Vitamin C
All 10 observational studies reported lower vitamin C (VC) levels in subjects with DR compared to those without DR [10, 12, 18–20, 22, 24, 25, 27, 28]. Gupta et al. [20] found a significant acceleration in oxidative stress biomarkers and a reduction in VC level with DR progression. Kumari et al., and Lam et al., reported an inverse significant correlation between malondialdehyde (MDA) and DNA damage; as oxidative stress biomarkers and VC that equaled −0.81, and − 0.41, respectively [10, 24].
Vitamin E
All 6 observational studies that assessed vitamin E (VE) level showed a significant lower serum level in subjects with DR than others [10, 12, 24–26, 28]. Kumari et al., and Lam et al. [10, 24] found positive significant associations between MDA and allantoin as oxidative stress biomarkers and VE; r = 0.77, and 0.33, respectively. Said et al. [26] reported a significant lower level of SOD and GPx in DR with greater reduction in proliferative DR. In addition, they found significant positive correlations between SOD/GPx with VE; r = 0.44, and 0.34, respectively.
Other antioxidative dietary supplements
In one observational study that assessed the correlation between vitamin D (VD) and DR [28] lower level of vitamin in patients with DR than other participants was reported. Three studies showed lower levels of Zn in patients with DR compared to those without DR [12, 23, 28] and one study showed no significant differences in the serum levels of Zn between patients with proliferative DR and non-proliferative DR [21]. Kumari et al. [12] revealed a significant inverse correlation between MDA and Zn (r = −0.82). Just in one study was reported the lower level of coenzyme Q10 in DR than others [11]. They also found a significant positive correlation between MDA and coenzyme Q10 (r = 0.56). Researchers of two studies [12, 28] found lowest level of Mg in DR versus others. In addition, it was reported an inverse significant association between MDA and Mg (r = −0.73) [12]. In one study was observed lower serum level of Se in T2DM especially in patients without DR than control group [28].
Association between dietary antioxidative supplements and DR in RCTs
All of RCTs were used mixture of antioxidants except Chatziralli et al. [35] that supplemented VE for patients with DR. In most RCTs, VE was recommended in combination with VC [30, 31, 33, 34]. Intake of vitamins C and E concomitant other antioxidative supplements for 18 months follow up was shown non-significant effect on best-corrected visual acuity (BCVA), intraocular pressure (IOP), and retinal nerve fiber layer thickness (RNFL) [33]. Sole intake of 300 mg VE for 3 months could significantly decrease serum level of MDA that was associated with significant good response to VE in those had PDR without prior laser therapy [35]. In one study, an improvement of visual acuity, and foveal thickness with mixture of lutein and zeaxanthin was observed [29]. Dosage of lutein in this trial was 6 mg with a follow-up duration of 3 months. Intake of 3 mg lutein mixed with other antioxidants including VC, alpha-tocopherol, niacin, beta-carotene, Zn, and Se for 60 months [30] was not showed any significant changes in BCVA. Percentage of onset/ progression of DR in T2DM with DR were retarded from 61% to 39% and from 92% to 8%, respectively after 18 months follow up with/ without supplementation. These figures were worsened in those without DR from 9% to 91% and from 35% to 65%, respectively after 18 months follow up with/ without supplementation. Coenzyme Q10 was consumed with/without other antioxidative supplements in two studies [32, 34]. Treatment with mixture of 20 mg coenzyme Q10 and other antioxidants could be resulted in non-significant increase in BCVA and significant decrease in free oxygen radical test (FORT) and central macular thickness (CMT) after 6 months follow up [32]. Sole intake of 400 mg coenzyme Q10 could non-significantly improve level of HbA1C, lipid profile, visual acuity, and eye pressure, while could significantly improve level of oxidative and antioxidative biomarkers [34]. Mixture of ALA with other antioxidants including genistein and vitamins was shown a significant beneficial effect on antioxidant potential (BAP) test and electroretinogram oscillatory potential (OP) values [31].
Discussion
This systematic review of fourteen observational studies and seven RCTs demonstrated in patients with T2DM and DR was shown lower level of antioxidants parameters and higher concentrations of oxidative stress biomarkers than those without DR. Based on RCTs, it seemed taking mixture of antioxidative supplements can be helpful for DR.
In our included observational studies, serum level of VC and VE in patients with T2DM and DR was lower than those with healthy individuals. Some evidences showed that VC and VE can protect retina against DR through (i) scavenging of free radicals, (ii) decreasing production of ROS, (iii) suppression lipid peroxidation (or MDA formation), and (iv) reducing leukocyte adhesion in retinal vessels [3, 6, 7, 36–38]. Since, reported beneficial effects of mixed VC with VE in observational studies or trials may be related to intake of fruits and vegetables as a main source of mixture of micronutrients and existence possibility a synergism interaction between antioxidative supplements, it is reasonable to respond better to the mixture mode of dietary antioxidants than sole intake [39]. Therefore, it is supposed suitable type and appropriate dosage of supplements beside suitable selection in combination with each other can be helpful. The beneficial effects of combination of VC with VE have confirmed in several complications of diabetes [6, 40]. The mixture of VC and VE in diet of animal model of DR could be resulted in the reduction of retinal neovascularization by increasing antioxidant defense system and decreasing ROS levels [40]. However, administration of VC and VE with other antioxidants could be led to better response than sole intake of VC or VE due to prevent activation of PKC, and decrease production of nitric oxide and formation of micro-vascular lesions in the retina [40]. Our findings in three RCTs have shown similar effects [30–32]. Mixture of VC and VE with niacin, beta-carotene, lutein, zeaxanthin, ALA, B complex, genistein and some trace elements have demonstrated some vision protections that was significant in retinal thickness and non-significant in visual acuity in our included RCTs. It should be noted that not only total daily dosage of VC (30–180 mg) and VE (5–30 mg), but also duration of studies were inappropriate in our multi-antioxidants’ mixture. Although in some studies, a single high dose of vitamins C and E was taken, high intake does not necessarily mean a full absorption [41]. It was established that development of many pathological changes in diabetes has occurred few years before their presentation in clinic. On the other word, it may need more than 5 years to reverse the pathological changes by antioxidative supplementation [42]. Therefore, it was not observed any significant changes in visual acuity after a few months.
Administration of ALA in diabetic rats has ameliorated activation of nuclear translocation factor κB (NF-κB), scavenging ROS, decreasing retinal level of vascular endothelial growth factor (VEGF), and protection of retina against ischemic injury [43, 44]. ALA can protect mitochondria against oxidative damage via prevention of mitochondrial dysfunction, apoptosis of capillary cell of retina, and development of DR in experimental study on rats [43]. In addition, ALA has beneficial effects on contrast sensitivity in type 1 and two diabetes mellitus [45]. In our study, administration of 400 mg ALA with other antioxidants including genistein, vitamins C, E and B complex was significantly increased Biological antioxidant potential (BAP) test and oscillatory potential (OP) values after treatment [31].
Lutein and zeaxanthin are both carotenoid with antioxidative properties. Lutein and zeaxanthin are most important carotenoids that exist in peripheral and fovea of retina, respectively. It was demonstrated a special effect for lutein and zeaxanthin on binding protein gene expression through enhancing synaptic connections of cells in nervous system. Thus, it resulted in increasing contrast sensitivity [46]. In addition, lutein and zeaxanthin can protect retina against neovascularization by decreasing permeability and leakage of vessels as well as prevention of combining free radicals with retinal collagen [47]. However, overdose of carotenoid supplementation could cause apoptotic cell death and should be used with caution [48]. In our study, combination of lutein with zeaxanthin and other antioxidants was improved visual acuity, foveal thickness, and contrast sensitivity in DR [29, 30, 32, 34]. Possible protecting mechanisms against oxidative stress by multi-antioxidants may related to synergistic interaction between vitamins C and E, carotenoids, and trace elements such as Se, Zn, copper (Cu), and manganese (Mn) in scavenging free radicals, and ameliorating the mitochondrial dysfunction [32, 44].
Coenzyme Q10 has a key role in oxidative phosphorylation of mitochondria by electron carrying [49]. Several studies were demonstrated its beneficial effects on endothelial function in patients with T2DM, cardiovascular diseases and animal model of diabetic nephropathy [50–52]. Coenzyme Q10 can reduce oxidative stress by recoupling endothelial nitric oxide synthase or through influence on respiratory chain of mitochondria. In two RCTs, 20 mg coenzyme Q10 combined with VE and pycnogenol or 400 mg sole intake was associated with a non-significant improvement in visual acuity and central macular thickness in patients with NPDR [33, 34].
There is lack of evidence on long-term safety of antioxidative nutrients on DR. Accordingly; it is better to increase intake of nutrients from natural food sources (8–10 servings of fruits and vegetables) according to American Diabetes Association recommendation [53].
Our study has some strengths and limitations. The most important strength of this study is that this study assessed critically all observational studies and RCTs on the effect of dietary antioxidative supplementation in patients with T2DM and it was the first systematic review in this topic. However, some potential limitations should be addressed. First, most of included studies had small sample size and participants consumed varied dosage of multi-antioxidants not sole intake that resulted in methodological heterogeneity. So, we could not perform meta-analysis. Second, the quality of included observational and RCTs in this study was varied. Out of twenty-one included studies, just eleven observational studies and three trials had high-quality.
Conclusion
This systematic review has attempted to offer antioxidative supplements as the target therapeutic agents for prevention or retarding the progression of DR. Antioxidants could effect on the development of DR by targeting multiple steps of oxidative stress and mitochondrial damage. Therefore, it is expected that supplementation with antioxidants may prevent DR in patients with T2DM. However, most evidences on beneficial effects of dietary antioxidative supplements have been encouraged by animal studies. Due to lack of well-designed and high quality longitudinal and RCTs, more studies are needed to confirm our findings.
Electronic supplementary material
(DOCX 17 kb)
Acknowledgements
This paper is the outcome of an in-house study without any financial support.
Abbreviations
- NPDR
Non-proliferative diabetic retinopathy
- PDR
Proliferative diabetic retinopathy
- DR
Diabetic retinopathy
- IDF
International Diabetes Federation
- PKC
Protein kinase C
- AGEs
Advanced glycation end products
- ROS
Reactive oxygen species
- Mg
Magnesium
- Zn
Zinc
- MDA
Malondialdehyde
- B1
Benfotiamine
- ALA
Alpha lipoic acid
- Se
Selenium
- T2DM
Type 2 diabetes mellitus
- RCTs
Randomized controlled trials
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- GPx
Glutathione peroxidase
- SOD
Superoxide dismutase
- TAS
Total antioxidant status
- STROBE
Strengthening the reporting of observational studies in epidemiology
- ADA
American Diabetes Association
- VC
Vitamin C
- VE
Vitamin E
- VD
Vitamin D
- BCVA
Best-corrected visual acuity
- IOP
Intraocular pressure
- RNFL
Retinal nerve fiber layer thickness
- FORT
Free oxygen radical test
- CMT
Central macular thickness
- NF-κB
Nuclear translocation factor- κB
- VEGF
Vascular endothelial growth factor
- BAP
Biological antioxidant potential
- OP
Oscillatory potential
- Cu
Copper
- Mn
Manganese
Author’s contribution
Both OTM and NN helped to collect data, data interpretation, and drafting article. OTM and EAL equally designed, conceived of the study, data analysis and interpretation. SN and RBJ helped to written drafting article, and critical revision of the article. BL conceived of the study, helped to data interpretation, and drafting article. All authors read and approved the final draft of the article.
Funding
Any funding.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
The original article has been corrected.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ozra Tabatabaei-Malazy and Edris Ardeshirlarijani equally contributed as first author.
Change history
11/5/2019
Please note the following corrections to the original article. The 2nd author affiliation should read Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Contributor Information
Ozra Tabatabaei-Malazy, Email: tabatabaeiml@sina.tums.ac.ir.
Edris Ardeshirlarijani, Email: ediardeshir@gmail.com.
Nazli Namazi, Email: nazli.namazi@yahoo.com.
Shekoufeh Nikfar, Email: shekoufeh.nikfar@gmail.com.
Reza Baradar Jalili, Email: reza.jalili@ubc.ca.
Bagher Larijani, Email: emrc@tums.ac.ir.
References
- 1.IDF diabetes atlas, 8th edition (2017). Available at: www.IDF.org/e-library/epidemiology-research/diabetes-atlas.html. Date access: 29 Aug.
- 2.Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic retinopathy. Prim Care. 2015;42(3):451–464. doi: 10.1016/j.pop.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. CardiovascDiabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Investig. 2015;45(11):1161–1174. doi: 10.1111/eci.12534. [DOI] [PubMed] [Google Scholar]
- 7.Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17(4):554–582. doi: 10.18433/J3ZG6R. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. BiochemicaBiophysicaActa. 1852;2015:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complicat. 2012;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kumari S, Panda S, Mangaraj M, Mandal MK, Mahapatra PC. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Cli Biochem. 2008;23:158–162. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ates O, Bilen H, Keles S, Alp HH, Keles MS, Yildirim K, et al. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int J Ophthalmol. 2013;6:675–679. doi: 10.3980/j.issn.2222-3959.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari S, Pradhan T, Panda TK. Trace minerals and oxidative stress in diabetic retinopathy. BJMS. 2014;13:175–179. [Google Scholar]
- 13.Jee D. Han Kd, Kim EC. Inverse association between high blood 25-hydroxyvitamin D levels and diabetic retinopathy in a representative Korean population. PLoS One. 2014;9:e115199. doi: 10.1371/journal.pone.0115199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviewsand Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halpern SH, Douglas MJ. Jadad scale for reportingrandomized controlled trials. Evidence-based Obstetric Anesthesia. 2005:237–8. 10.1002/9780470988343.app1.
- 18.Sinclair AJ, Girling AJ, Gray L, Lunec J, Barnett AH. An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. Gerontology. 1992;38(5):268–274. doi: 10.1159/000213339. [DOI] [PubMed] [Google Scholar]
- 19.Gürler B, Vural H, Yilmaz N, Oguz H, Satici A, Aksoy N. The role of oxidative stress in diabetic retinopathy. Eye (Lond) 2000;14(Pt 5):730–735. doi: 10.1038/eye.2000.193. [DOI] [PubMed] [Google Scholar]
- 20.Gupta MM, Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J Physiol Pharmacol. 2005;49(2):187–192. [PubMed] [Google Scholar]
- 21.Yildirim Z, Uçgun NI, Kiliç N, Gürsel E, Sepici-Dinçel A. Antioxidant enzymes and diabetic retinopathy. Ann N Y Acad Sci. 2007;1100:199–206. doi: 10.1196/annals.1395.019. [DOI] [PubMed] [Google Scholar]
- 22.Samuel TV, Murthy DSJ, Dattatreya K, Babu PS, Johncy SS. Impaired antioxidant defence mechanism in diabetic retinopathy. J Clin Diagn Res. 2010;4:3430–3436. [Google Scholar]
- 23.Aldebasi Y, Mohieldin A, Almansour Y, Almoteri B. Imbalance of oxidant/antioxidant status and risk factors for Saudi type 2 diabetic patientswith retinopathy. Br J Med Med Res. 2011;1:371–384. doi: 10.9734/BJMMR/2011/479. [DOI] [Google Scholar]
- 24.Lam CS, Benzie IF, Choi SW, Chan LY, Yeung VT, Woo GC. Relationships among diabetic retinopathy, antioxidants, and glycemic control. Optom Vis Sci. 2011;88(2):251–256. doi: 10.1097/OPX.0b013e318208494a. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni RP, Kodliwadmath MV. Oxidative stress and high sensitivity c-reactive protein in diabetic retinopathy. Int J Pharm Bio Sci. 2013;4:1306–1310. [Google Scholar]
- 26.Said NS, Hadhoud KM, Nada WM, El Tarhouny SA. Superoxide dismutase, glutathione peroxidase and vitamin E in patients with diabetic retinopathy. Life Sci. 2013;1:1851–1856. [Google Scholar]
- 27.Kundu D, Mandal T, Nandi M, Osta M, Bandyopadhyay U, Ray D. Oxidative stress in diabetic patients with retinopathy. Ann Afr Med. 2014;13(1):41–46. doi: 10.4103/1596-3519.126951. [DOI] [PubMed] [Google Scholar]
- 28.Longo-Mbenza B, MvituMuaka M, Masamba W, MuizilaKini L, Longo Phemba I, Kibokela Ndembe D, et al. Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: a new phenotype in Central Africa? Int J Ophthalmol. 2014;7(2):293–301. doi: 10.3980/j.issn.2222-3959.2014.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4(3):303–306. doi: 10.3980/j.issn.2222-3959.2011.03.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21(5):637–643. doi: 10.5301/EJO.2010.6212. [DOI] [PubMed] [Google Scholar]
- 31.Nebbioso M, Federici M, Rusciano D, Evangelista M, Pescosolido N. Oxidative stress in preretinopathic diabetes subjects and antioxidants. Diabetes Technol Ther. 2012;14(3):257–263. doi: 10.1089/dia.2011.0172. [DOI] [PubMed] [Google Scholar]
- 32.Domanico D, Fragiotta S, Cutini A, Carnevale C, Zompatori L, Vingolo EM. Circulating levels of reactive oxygen species in patients with nonproliferativediabetic retinopathy and the influence of antioxidant supplementation: 6-monthfollow-up. Indian J Ophthalmol. 2015;63(1):9–14. doi: 10.4103/0301-4738.151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roig-Revert MJ, Lleó-Pérez A, Zanón-Moreno V, Vivar-Llopis B, Marín-Montiel J, Dolz-Marco R, et al. Valencia Study On Diabetic Retinopathy (VSDR). Enhanced oxidative stress and other potential biomarkers for retinopathy in type 2 diabetics: beneficial effects of the nutraceutic supplements. Biomed Res Int. 2015:408180. 10.1155/2015/408180. [DOI] [PMC free article] [PubMed]
- 34.Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Pacheco-Moisés FP, Román-Pintos LM, et al. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: a phase IIa, randomized, double-blind, and placebo-controlled study. Redox Rep. 2016;21(4):155–163. doi: 10.1179/1351000215Y.0000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatziralli IP, Theodossiadis G, Dimitriadis P, Charalambidis M, Agorastos A, Migkos Z, et al. The effect of vitamin E on oxidative stress indicated by serum malondialdehyde in insulin-dependent type 2 diabetes mellitus patients with retinopathy. Open Ophthalmol J. 2017;11:51–58. doi: 10.2174/1874364101711010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J Pharm Pharm Sci. 2012;15:447–466. doi: 10.18433/J32W29. [DOI] [PubMed] [Google Scholar]
- 37.de Lopes JCC, Atallah AN, Valente O, Fernandes MTV. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database Syst Rev. 2008;1. 10.1002/14651858.CD006695.pub2. [DOI] [PubMed]
- 38.Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, et al. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxidative Med Cell Longev. 2017;2017:9702820–9702815. doi: 10.1155/2017/9702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A novel management of diabetes by means of strong antioxidants’combination. Journal of Medical Hypotheses and Ideas. 2013;7:25–30. doi: 10.1016/j.jmhi.2012.12.002. [DOI] [Google Scholar]
- 40.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia, VII, effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 41.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg D. Clinical trials of antioxidants in atherosclerosis: are we doing the right thing? Lancet. 1995;346:36–38. doi: 10.1016/S0140-6736(95)92657-7. [DOI] [PubMed] [Google Scholar]
- 43.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 44.Barlett HF, Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthalmic Physiol Opt. 2008;28:503–523. doi: 10.1111/j.1475-1313.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 45.Gębka A, Serkies-Minuth E, Raczyńska D. Effect of the administration of alpha-lipoic acid on contrast sensitivity in patients with type 1 and type 2diabetes. Mediat Inflamm. 2014;2014:131538–131537. doi: 10.1155/2014/131538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahl W, Sies H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors. 2001;15:95–98. doi: 10.1002/biof.5520150209. [DOI] [PubMed] [Google Scholar]
- 47.Kvansakul J, Rodriguez-Carmona M, Edger DF, Barker FM, Kopoke W, Schalch W, et al. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006;26:362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 48.Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp Eye Res. 2008;86:70–80. doi: 10.1016/j.exer.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7(Suppl):S168–S174. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32:810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 52.Sourris KC, Harcourt BE, Tang PH, Morley AL, Huynh K, Penfold SA, et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic Biol Med. 2012;52:716–723. doi: 10.1016/j.freeradbiomed.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 53.American Diabetes Association Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S1–S193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb)