Abstract
Background
Data on racial disparities in major adverse cardiovascular events (MACE) and major hemorrhage (HEM) after percutaneous coronary intervention are limited. Factors contributing to these disparities are unknown.
Methods and Results
PRiME‐GGAT (Pharmacogenomic Resource to Improve Medication Effectiveness–Genotype‐Guided Antiplatelet Therapy) is a prospective cohort. Patients aged ≥18 years undergoing percutaneous coronary intervention were enrolled and followed for up to 1 year. Racial disparities in risk of MACE and HEM were assessed using an incident rate ratio. Sequential cumulative adjustment analyses were performed to identify factors contributing to these disparities. Data from 919 patients were included in the analysis. Compared with white patients, black patients (n=203; 22.1% of the cohort) were younger and were more likely to be female, to be a smoker, and to have higher body mass index, lower socioeconomic status, higher prevalence of diabetes mellitus and moderate to severe chronic kidney disease, and presentation with acute coronary syndrome and to undergo urgent percutaneous coronary intervention. The incident rates of MACE (34.1% versus 18.2% per 100 person‐years, P<0.001) and HEM (17.7% versus 10.3% per 100 person‐years, P=0.02) were higher in black patients. The incident rate ratio was 1.9 (95% CI, 1.3–2.6; P<0.001) for MACE and 1.7 (95% CI, 1.1–2. 7; P=0.02) for HEM. After adjustment for nonclinical and clinical factors, black race was not significantly associated with outcomes. Rather, differences in socioeconomic status, comorbidities, and coronary heart disease severity were attributed to racial disparities in outcomes.
Conclusions
Despite receiving similar treatment, racial disparities in MACE and HEM still exist. Opportunities exist to narrow these disparities by mitigating the identified contributors.
Keywords: coronary heart disease, health disparities, outcome, percutaneous coronary intervention, race
Subject Categories: Quality and Outcomes, Race and Ethnicity
Clinical Perspective
What Is New?
Despite receiving similar guideline‐recommended treatment, among patients undergoing percutaneous coronary intervention, black patients experienced higher incidence of major adverse cardiovascular events and major hemorrhage than white patients.
These racial disparities in outcomes were attenuated after adjustment for nonclinical (socioeconomic status) and clinical (comorbidities and coronary heart disease severity) factors.
What Are the Clinical Implications?
Black race is not an independent risk factor for adverse cardiovascular outcomes in coronary heart disease patients undergoing percutaneous coronary intervention.
Multifaceted implementation strategies are needed to attenuate racial disparities in outcome.
Introduction
Coronary heart disease (CHD) is a leading cause of morbidity, mortality, and medical costs in the United States.1 Although the prevalence of CHD is similar for black and white people,2 cardiovascular events, rehospitalization, and mortality are disproportionately higher for black patients.3, 4, 5, 6, 7, 8
Percutaneous coronary intervention (PCI) is an important therapeutic approach for CHD patients, especially those with an acute coronary syndrome (ACS).9, 10, 11 However, multiple reports have shown that black patients have a higher risk of cardiovascular events after PCI than white patients, with studies attributing these differences to higher prevalence of cardiovascular comorbidities,12 lower socioeconomic status (SES),13 and different treatment received during hospitalization of black patients.14
Given changing demographics, increasing minority groups including blacks, and the disproportionate CHD burden among black people, understanding differences in outcomes and elucidating reasons for the differences will facilitate the development of race‐specific interventions and eventually reduce race‐related disparities.
In this study, in patients undergoing PCI, we assess whether the risk of major adverse cardiovascular events (MACE) and major hemorrhage (HEM) differs by race, identify factors related to MACE and HEM, and determine whether these factors contribute to the racial disparities in outcomes.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Study Design and Participant Enrollment
PRiME‐GGAT (Pharmacogenomic Resource to Improve Medication Effectiveness–Genotype‐Guided Antiplatelet Therapy) is a prospective cohort study conducted under the approval of the institutional review board of the University of Alabama at Birmingham (UAB). Patients who were aged ≥18 years undergoing PCI at the UAB Hospital were enrolled after written informed consent was obtained.
Data Collection
Baseline characteristics
At baseline, patients were asked to identify their race and ethnicity. During the index PCI, a structured case report form was used to record patient demographics (age and sex), lifestyle (eg, smoking), and SES (annual income, education attainment, and health insurance), cardiovascular risk factors, and comorbidities. Serum creatinine at admission was used to calculate estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula.15
Patient characteristics at time of PCI
Vital signs (eg, blood pressure and heart rate) at time of PCI, PCI status (urgent or elective), indication for PCI, access site, coronary artery lesion location, periprocedural antiplatelet use, contrast volume, number of coronary arteries with ≥70% stenosis, and number and type of stents implanted were documented.
Follow‐Up and Studied Outcomes
Patients were followed for up to 1 year. Medical records were reviewed, and changes in medication use, laboratory parameters, any hospitalization, MACE, and HEM during follow‐up were documented. This approach ensured follow‐up for all patients, including patient encounters outside the UAB Health System. Specifically, we requested physical and/or electronic medical records from patients’ primary care physicians and cardiologists and/or hospital records in case of hospitalization. The laboratory parameters and medication changes for every encounter were collected. MACE outcomes were defined as a composite of all‐cause mortality, nonfatal myocardial infarction (MI), nonfatal ischemic stroke, transient ischemic attack, and stent thrombosis. Specifically, MI diagnosis was based on increase of cardiac troponin with at least 1 value above the 99th percentile upper reference limit plus ischemic symptoms and/or new or presumed new ST‐segment T‐wave changes or new left bundle‐branch block16; if a case with stent thrombosis was subsequently complicated by MI, we defined the event as stent thrombosis. HEM outcomes were defined as a composite of intracranial hemorrhage and/or gastrointestinal and other hemorrhage (eg, HEM related to access site, genital urinary tract HEM) that causes substantial hemodynamic compromise requiring treatment based on the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) criteria.17 MACE and HEM outcomes were adjudicated by independent cardiologists.
Statistical Analysis
ANOVA was used to assess differences for continuous variables, and the χ2 test was used for categorical variables by race.
Incidence rates of MACE and HEM were calculated by dividing the number of events by person‐years of follow‐up accrued, and then we calculated incidence rate ratios (IRRs) for MACE and HEM for black and white patients. The influence of race on MACE and HEM was assessed using time‐to‐event analysis. Hazard ratios and 95% CIs for the adjusted Cox proportional hazards models were determined using the counting process format, permitting evaluation of time‐dependent risk intervals instead of a single event time.18, 19 This method allowed individuals to contribute >1 event for the analysis and supported the inclusion of time‐varying covariates (eg, comedications). Variance estimates and CIs were corrected for dependence of observation within patients with >1 event.
For all unadjusted and adjusted analyses, white patients served as the reference group. We evaluated the contribution of each of the following domains to observed racial differences in outcomes by sequential cumulative adjustment, as described previously.20 Specifically, in the first step (model 1), we adjusted for demographics (age and sex) and body mass index (calculated by weight in kilograms divided by height in square meters). In the second step (model 2), we adjusted for demographics, body mass index, and SES (annual income, educational attainment, and health insurance). In the third step (model 3), we adjusted for the prior 2 domains plus comorbidities (smoking, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, chronic kidney disease stage ≥3b, heart failure, prior history of PCI, coronary artery bypass grafting, and hemorrhage). In the fourth step (model 4), we adjusted for the prior 3 domains plus CHD severity (number of coronary arteries with ≥70% stenosis and presentation with ACS). In the fifth step (model 5), we adjusted for the prior 4 domains plus interventions received during index PCI (number of stents implanted and femoral artery access). In the final step (model 6), we adjusted for the prior 5 domains plus medication use at follow‐up (dual antiplatelet therapy [DAPT], statins, β‐blockers and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, oral anticoagulants, and proton pump inhibitors). The order of the assessed domains reflects the temporal relationships of these factors, adjusting first for patient demographics, SES, and clinical characteristics before admission, followed by treatment received during index hospitalization, and finally medication treatment during follow‐up. All analyses were performed using SAS v9.4 (SAS Institute), and a 2‐sided P<0.05 was considered statistically significant.
Results
Participant Enrollment
A total of 1024 participants were enrolled. Patients with self‐reported race other than black or white (n=15) and incomplete follow‐up (n=90) were excluded. Specifically, among the 90 patients with incomplete follow‐up, 24 (10.6%) were black and 66 (8.4%) were white (P=0.32). Consequently, data from 919 participants (mean age: 62.0±11.9 years) were included in these analyses.
Comparison of Baseline Characteristics by Race
Black participants (n=203) accounted for 22.1% of the cohort. As presented in Table 1, compared with white participants, black participants were younger and more likely to be female, to have higher body mass index and lower annual income and education attainment, and to be uninsured or underinsured. Black participants were also more likely to smoke (28.6% versus 21.6%, P=0.01) and to have diabetes mellitus (48.8% versus 40.4%, P=0.03) and chronic kidney disease stage ≥3b (16.7% versus 12.3%, P<0.001). White participants were more likely to have dyslipidemia (77.8% versus 69.0%, P=0.01), atrial fibrillation (13.4% versus 5.4%, P=0.002), and previous history of CHD (64.3% versus 55.2%, P=0.02) and to have undergone revascularization (PCI: 39.9% versus 31.0%, P=0.02; coronary artery bypass grafting: 28.9% versus 17.2%, P=0.001). White participants were more likely to be on aspirin therapy (72.3% versus 60.3%, P=0.002) and clopidogrel (32.3% versus 24.3%, P=0.04) at admission. The use of prasugrel or ticagrelor, statins, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, oral anticoagulants, and proton pump inhibitors at admission was similar across race groups.
Table 1.
Baseline Characteristics Among Black and White Patients Undergoing PCI
| Characteristic | All (n=919) | Black (n=203) | White (n=716) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean±SD | 62.0±11.9 | 58.8±11.9 | 63.0±11.8 | <0.001 |
| Female, n (%) | 260 (28.3) | 85 (41.9) | 175 (24.4) | <0.001 |
| BMI, kg/m2, mean±SD | 30.0±6.4 | 31.0±7.6 | 29.7±6.0 | 0.02 |
| SES | ||||
| Annual income <$50 000 | 517 (67.9) | 144 (88.9) | 373 (62.2) | <0.001 |
| High school or less | 505 (56.2) | 128 (65.6) | 377 (53.6) | 0.003 |
| Insurance | ||||
| Medicaid/no insurance | 130 (14.7) | 42 (21.8) | 88 (12.7) | 0.002 |
| Private/Medicare | 757 (85.3) | 151 (78.2) | 606 (87.3) | |
| Comorbidities | ||||
| Current smoker | 213 (23.2) | 58 (28.6) | 155 (21.6) | 0.01 |
| Hypertension | 785 (85.4) | 180 (88.7) | 605 (84.5) | 0.14 |
| Dyslipidemia | 697 (75.8) | 140 (69.0) | 557 (77.8) | 0.01 |
| Diabetes mellitus | 388 (42.2) | 99 (48.8) | 289 (40.4) | 0.03 |
| Atrial fibrillation | 107 (11.6) | 11 (5.4) | 96 (13.4) | 0.002 |
| CHD | 572 (62.2) | 112 (55.2) | 460 (64.3) | 0.02 |
| Heart failure | 245 (26.7) | 50 (24.6) | 195 (27.2) | 0.46 |
| eGFR distribution | ||||
| ≥60 | 648 (70.5) | 154 (75.9) | 494 (69.0) | <0.001 |
| 45 to <60 | 149 (16.2) | 15 (7.4) | 134 (18.7) | |
| 30 to <45 | 73 (7.9) | 12 (5.9) | 61 (8.5) | |
| <30 | 49 (5.3) | 22 (10.8) | 27 (3.8) | |
| Prior event history | ||||
| MI | 290 (31.6) | 68 (33.5) | 222 (31.0) | 0.50 |
| Stroke/TIA | 125 (13.6) | 33 (16.3) | 92 (12.9) | 0.21 |
| Hemorrhage | 125 (13.6) | 30 (14.8) | 95 (13.3) | 0.58 |
| PCI | 349 (38.0) | 63 (31.0) | 286 (39.9) | 0.02 |
| CABG | 242 (26.3) | 35 (17.2) | 207 (28.9) | 0.001 |
| Medications on admission | ||||
| Antiplatelet | ||||
| Aspirin | 591 (69.6) | 114 (60.3) | 477 (72.3) | 0.002 |
| Clopidogrel | 259 (30.5) | 46 (24.3) | 213 (32.3) | 0.04 |
| Prasugrel or ticagrelor | 47 (5.5) | 8 (4.2) | 39 (5.9) | 0.37 |
| Statins | 570 (62.0) | 115 (56.7) | 455 (63.6) | 0.07 |
| β‐Blocker | 515 (56.0) | 106 (52.2) | 409 (57.1) | 0.21 |
| ACEI/ARB | 461 (50.2) | 96 (47.3) | 365 (51.0) | 0.35 |
| Proton pump inhibitor | 341 (37.1) | 71 (35.0) | 270 (37.7) | 0.48 |
| Oral anticoagulants | 77 (8.4) | 12 (5.9) | 65 (9.1) | 0.15 |
Data are shown as n (%) except as noted. Data missing for annual income (n=157; black, n=41 [20.2%], and white, n=116 [16.2%]; P=0.19); education (n=21; black, n=8 [3.9%], and white, n=13 [1.8%]; P=0.10); insurance (n=32; black, n=10 [4.9%], and white, n=22 [3.1%]; P=0.22). ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate (in mL/min/1.73 m2); MI, myocardial infarction; PCI, percutaneous coronary intervention; SES, socioeconomic status; TIA, transient ischemic attack.
Comparison of PCI Periprocedural Characteristics by Race
As shown in Table 2, compared with white participants, black participants were more likely to present with ACS (86.2% versus 76.8%, P<0.001), to undergo urgent PCI (71.4% versus 52.3%, P<0.001), and to receive prasugrel or ticagrelor loading during index PCI (69.5% versus 56.3%, P=0.001). There were no significant differences in access site, numbers of coronary arteries with ≥70% stenosis and stents implanted, proportion of drug‐eluting stents implanted, and use of glycoprotein IIb/IIIa receptor inhibitors across race groups.
Table 2.
Periprocedural Characteristics Among Black and White Patients Undergoing PCI
| PCI Periprocedural Characteristic | All (n=919) | Black (n=203) | White (n=716) | P Value |
|---|---|---|---|---|
| Vital sign | ||||
| SBP, mm Hg, mean±SD | 142±23 | 145±23 | 141±24 | <0.001 |
| DBP, mm Hg, mean±SD | 84±14 | 88±14 | 83±14 | <0.001 |
| HR, beats/min, mean±SD | 74±18 | 75±16 | 74±19 | 0.32 |
| Procedure characteristics | ||||
| PCI precipitating condition | ||||
| STEMI | 190 (20.7) | 48 (23.7) | 142 (19.8) | <0.001 |
| Non‐STEMI | 276 (30.0) | 86 (42.3) | 190 (26.5) | |
| Unstable angina | 259 (28.2) | 41 (20.2) | 218 (30.5) | |
| Stable angina | 194 (21.1) | 28 (13.8) | 166 (23.2) | |
| PCI status | ||||
| Urgent | 519 (56.5) | 145 (71.4) | 374 (52.3) | <0.001 |
| Elective | 400 (43.5) | 58 (28.6) | 342 (47.8) | |
| Femoral artery access | 773 (84.1) | 163 (80.3) | 610 (85.2) | 0.06 |
| Coronary artery lesion location | ||||
| Left main | 50 (4.1) | 5 (2.0) | 45 (4.6) | <0.001 |
| Left anterior descending | 444 (36.3) | 78 (30.8) | 366 (37.7) | |
| Left circumflex | 291 (23.8) | 84 (33.2) | 207 (21.3) | |
| Right coronary artery | 372 (30.4) | 72 (28.5) | 300 (30.9) | |
| Graft | 53 (4.3) | 7 (2.8) | 46 (4.7) | |
| No. of coronary arteries ≥70% stenosis, mean±SD | 1.8 (1.2) | 1.9 (1.3) | 1.8 (1.1) | 0.11 |
| No. of stents, mean±SD | 1.2 (0.6) | 1.3 (0.6) | 1.2 (0.6) | 0.38 |
| Drug‐eluting stent | 826 (91.5) | 171 (88.1) | 655 (92.4) | 0.10 |
| Contrast, mL, mean±SD | 146 (66) | 146 (71) | 146 (65) | 0.89 |
| PCI periprocedural antiplatelet medication | ||||
| Glycoprotein IIb/IIIa receptor inhibitors | 161 (17.5) | 37 (18.2) | 124 (17.3) | 0.76 |
| Clopidogrel loading | 372 (40.5) | 89 (43.8) | 283 (39.5) | 0.27 |
| Prasugrel or ticagrelor loading | 544 (59.2) | 141 (69.5) | 403 (56.3) | 0.001 |
Data are shown as n (%) except as noted. DBP indicates diastolic blood pressure; HR, heart rate; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST‐segment–elevation myocardial infarction.
Comparison of Medication Use at Discharge and Follow‐Up by Race
At discharge, the institution of guideline‐recommended DAPT, statins, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers was similar in both race groups (Table 3). By the end of follow‐up, however, the use of β‐blockers was significantly lower among black than white participants (49.3% versus 59.6%, P=0.009). In addition, white participants were more likely to receive oral anticoagulants at discharge (12.3% versus 6.4%, P=0.02) and at follow‐up (11.4% versus 6.4%, P=0.03).
Table 3.
Medications Use at Discharge and Follow‐Up Among Black and White Patients
| All (n=919) | Black (n=203) | White (n=716) | P Value | |
|---|---|---|---|---|
| Medications at discharge | ||||
| DAPT | 915 (99.6) | 200 (98.5) | 715 (99.9) | 0.97 |
| Aspirin+clopidogrel | 612 (66.6) | 134 (66.0) | 478 (66.8) | |
| Aspirin+prasugrel or ticagrelor | 303 (33.0) | 66 (32.5) | 237 (33.1) | |
| Statins | 839 (91.6) | 188 (92.6) | 651 (91.3) | 0.55 |
| β‐Blocker | 779 (85.0) | 172 (84.7) | 607 (85.1) | 0.89 |
| ACEI/ARB | 624 (67.9) | 139 (68.5) | 485 (67.7) | 0.85 |
| Proton pump inhibitor | 350 (38.1) | 76 (37.4) | 274 (38.3) | 0.96 |
| Oral anticoagulants | 101 (11.0) | 13 (6.4) | 88 (12.3) | 0.02 |
| Medications at time of event or last follow‐up | ||||
| DAPT | 821 (89.7) | 175 (86.3) | 646 (90.8) | 0.89 |
| Aspirin+clopidogrel | 604 (66.0) | 128 (63.1) | 476 (66.9) | |
| Aspirin+prasugrel or ticagrelor | 217 (23.7) | 47 (23.2) | 170 (23.9) | |
| Statins | 833 (91.0) | 185 (91.1) | 648 (91.0) | 0.96 |
| β‐Blocker | 524 (57.3) | 100 (49.3) | 424 (59.6) | 0.009 |
| ACEI/ARB | 610 (66.7) | 134 (66.0) | 476 (66.9) | 0.82 |
| Proton pump inhibitor | 368 (40.2) | 75 (36.9) | 293 (41.1) | 0.31 |
| Oral anticoagulants | 95 (10.3) | 13 (6.4) | 82 (11.4) | 0.03 |
Data are shown as n (%). ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; DAPT, dual antiplatelet therapy.
Within each race group, compared with medication prescribed at discharge, medication adherence at the end of follow‐up was lower. The use of DAPT declined among black participants (98.5% versus 86.3%, P=0.04) and white participants (99.9% versus 90.8%, P<0.001), as did the use of β‐blockers (black: 84.7% versus 49.3%, P<0.001; white: 85.1% versus 59.6%, P<0.001). The decrease in adherence to DAPT and β‐blocker therapy did not differ by race (P=0.78 for DAPT; P=0.19 for β‐blocker). The use of statins, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and proton pump inhibitors remained similar across time points in both race groups.
Comparison of Incidence Rates of MACE and HEM by Race
The average follow‐up time was 0.8±0.3 year. As presented in Table 4, compared with white participants, the incidence rate of MACE for black participants was significantly higher (34.1% versus 18.2% per 100 person‐years; IRR: 1.9; 95% CI, 1.3–2.6), which was driven by differences in nonfatal MI (20.7% versus 9.4% per 100 person‐years; IRR: 2.2; 95% CI, 1.4–3.3). Black participants had numerically higher incidence rates of all‐cause mortality (7.9% versus 5.1% per 100 person‐years; IRR: 1. 6; 95% CI, 0.8–3.0) and nonfatal ischemic stroke (4.3% versus 1.7% per 100 person‐years; IRR: 2.5; 95% CI, 0.9–6.7) but without statistically significant difference.
Table 4.
Incidence Rates of MACE and HEM at 1 Year After PCI (Per 100 Person‐Years)
| All Patients (n=919) | Black (n=203) | White (n=716) | IRR (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Follow‐up, y | 756.9 | 164.3 | 592.6 | |||||
| Average follow‐up, y | 0.8±0.3 | 0.8±0.3 | 0.8±0.3 | 0.47 | ||||
| Event (n) | Rate (%) | Event (n) | Rate (%) | Event (n) | Rate (%) | |||
| MACE | 164 | 21.7 | 56 | 34.1 | 108 | 18.2 | 1.9 (1.3–2.6) | <0.001 |
| All‐cause mortality | 43 | 5.7 | 13 | 7.9 | 30 | 5.1 | 1.6 (0.8–3.0) | 0.19 |
| Nonfatal MI | 90 | 11.9 | 34 | 20.7 | 56 | 9.4 | 2.2 (1.4–3.3) | <0.001 |
| Nonfatal ischemic stroke | 17 | 2.2 | 7 | 4.3 | 10 | 1.7 | 2.5 (0.9–6.7) | 0.07 |
| TIA | 8 | 1.1 | 1 | 0.6 | 7 | 1.2 | 0.5 (0.02–3.3) | 0.60 |
| Stent thrombosis | 6 | 0.8 | 1 | 0.6 | 5 | 0.8 | 0.7 (0.03–5.2) | 0.84 |
| HEM | 90 | 11.9 | 29 | 17.7 | 61 | 10.3 | 1.7 (1.1–2.7) | 0.02 |
| Gastrointestinal hemorrhage | 42 | 5.5 | 18 | 11.0 | 24 | 4.1 | 2.7 (1.5–5.0) | 0.002 |
| Intracranial hemorrhage | 5 | 0.7 | 0 | 0 | 5 | 0.8 | 0 (0–3.0) | 0.29 |
| Other hemorrhage | 43 | 5.7 | 11 | 6.7 | 32 | 5.4 | 1.2 (0.6–2.4) | 0.53 |
For MACE: 11 patients had 2 nonfatal MI events; 2 patients had 2 nonfatal ischemic stroke events; 1 patient had 2 TIA events. For HEM: 16 patients had 2 events and 1 patient had 4 events. HEM indicates major hemorrhage; IRR, incident rate ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Compared with white participants, black participants also had a significantly higher incidence rate of HEM (17.7% versus 10.3% per 100 person‐years; IRR: 1.7; 95% CI, 1.1–2.7), driven by differences in gastrointestinal hemorrhage (11.0% versus 4.1% per 100 person‐years; IRR: 2.7; 95% CI, 1.5–5.0). Black participants also had a higher incidence rate of other hemorrhage but without a statistically significant difference (6.7% versus 5.4% per 100 person‐years; IRR: 1.2; 95% CI, 0.6–2.4). Among other hemorrhage, 12 events (in 3 black patients and 9 white patients) were related to the access site, with no significant differences by race (black versus white: 1.8% versus 1.5% per 100 person‐years; IRR: 1.2; 95% CI, 0.3–4.3).
Factors Contributing to Racial Disparities in MACE and HEM
Compared with white participants, black participants had a 1.9‐fold higher risk of MACE (hazard ratio: 1.86; 95% CI, 1.24–2.80). Inclusion of demographics and body mass index did not attenuate the race–MACE association (Figure A). Incorporation of SES and comorbidities attenuated the race–MACE association by 22% and 5%, respectively. After controlling for CHD severity, the race–MACE association was attenuated by 26%. Interventions received during index PCI and medication use at follow‐up had no further impact on race–MACE association. These findings indicate that SES and CHD severity were the 2 key factors contributing to racial disparities in MACE.
Figure 1.
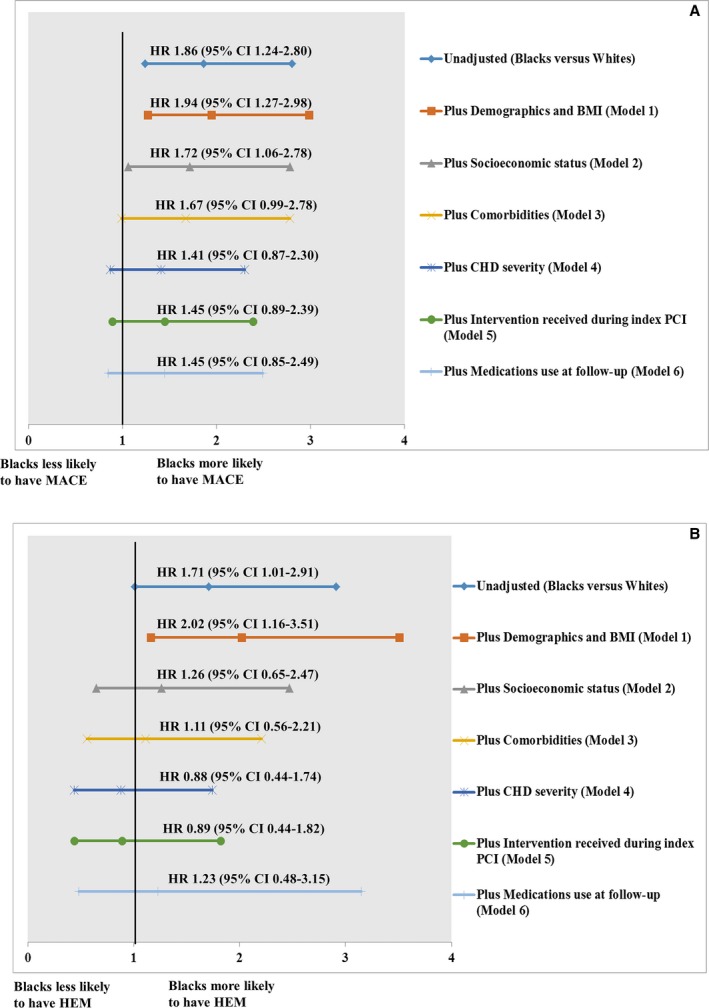
Factors influencing racial disparities in major adverse cardiovascular events (A) and in major hemorrhage (B). Hazard ratios (HRs) are provided with 95% CIs. The vertical line indicates the reference value of 1. In all models, values for black patients are compared with white patients. BMI indicates body mass index; CHD, coronary heart disease; HEM, major hemorrhage; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention.
Compared with white participants, black participants had a 1.7‐fold higher risk of HEM (hazard ratio: 1.71; 95% CI, 1.01–2.91; FigureB). Incorporation of SES attenuated the race–HEM association by 76%. After controlling for comorbidities and CHD severity, the race–MACE association was attenuated by 15% and 23%, respectively. These findings indicate that SES, comorbidities, and CHD severity were the key factors contributing to racial disparities in HEM.
Discussion
To our knowledge, this study is the first to compare post‐PCI risks of both MACE and HEM by race in a prospective inception cohort. We found that black participants had a higher incidence of MACE and HEM than white participants. However, these differences were attenuated after adjustment for nonclinical (SES) and clinical (comorbidities and CHD severity) factors. These findings suggest that racial disparities in outcomes are attributable to differences in nonclinical and clinical factors.
Factors Associated With Racial Disparities in MACE
Racial disparities in MACE after PCI have been reported, and the reasons for these disparities are diverse, including differences in cardiovascular risk factors and 30‐day revascularization rates12; clinical, angiographic, and SES factors13; and treatment received during hospitalization.14 Our current analysis also demonstrates a higher 1‐year risk of MACE among black participants. Our approach is unique because we incorporated the influence of factors across 6 domains: demographics, SES, comorbidities, CHD severity, treatment received during PCI, and medication use at follow‐up. Our results elucidate the role of SES and CHD severity in racial disparities in MACE after PCI.
Prior studies suggest that widowhood or insurance status13 and median household income21 are the key SES factors for racial disparities in MACE after PCI. These studies evaluated the influence of individual SES indicators on outcomes. As done previously,20 we combined annual income, educational attainment, and health insurance into 1 SES domain because it is unlikely that a single SES indicator is adequate for predicting cardiovascular risk.22 Concordant with previous results, our study shows the importance of SES in attenuating the unadjusted racial differences in outcomes. SES may influence outcomes through several mechanisms including greater disease burden and poor lifestyle or health behaviors.22, 23 Indeed, both our and prior studies show that lower SES is more prevalent among black cohorts and is associated with a greater prevalence of risk factors and comorbidities.24, 25 Understanding the complex interplay of SES, lifestyle, and comorbidities is vital to developing interventions to attenuate these disparities.22, 23
Unlike prior reports,3, 12, 13 our study did not show a significant influence of comorbidities on race–MACE association. We hypothesize that the inclusion of the CHD severity measure captures the joint pathological effects of multiple long‐standing comorbidities (eg, smoking and diabetes mellitus) on CHD progression. Investigators have incorporated CHD severity using varying definitions, including number of stenoses, length and location of lesions, presentation with ACS, and undergoing urgent PCI.3, 12, 13, 20, 26 We included number of coronary arteries with ≥70% stenosis and ACS presentation as indicators of CHD severity because they can directly reflect the burden of coronary lesions and associated ischemic risk.27, 28 Because this measure did not differ by race, our results support attribution of racial disparity in MACE to differences in ACS presentation. Prior studies also have shown that black patients are more likely to present with ACS and experience stent thrombosis and death after PCI.3, 26 The reasons for these differences are probably multifactorial and may be related to differences in SES (eg, insurance status).
We did not observe a significant impact of treatment received during PCI or medications used after discharge on race–MACE association. Unlike prior studies, which focused on medication use only during PCI hospitalization or at discharge,8, 29 we assessed the impact of adherence to guideline‐recommended medications in the PCI periprocedural period, at discharge, and during follow‐up regarding racial disparities in outcomes. These results suggest that racial disparities in outcomes due to differences in treatment received can be attenuated with implementation of similar treatment protocols.
Factors Associated With Racial Disparities in HEM
Our study shows that black patients have a higher risk of HEM for up to 1 year after PCI. Although increased bleeding risk in black patients receiving fibrinolysis is well documented,30, 31, 32, 33 the data on bleeding risk after PCI for black and white patients are limited. To our knowledge, only 1 study has specifically addressed racial disparities in bleeding and mortality after PCI. Mehta et al reported that black patients receiving primary PCI or fibrinolysis for ST‐segment–elevation MI had a higher in‐hospital major bleeding risk than white patients over the ≈3‐day hospital stay.34 These findings collectively indicate that black patients are vulnerable with either reperfusion strategy. Our study elucidates the influence of (and interplay among) SES, comorbidity, and CHD severity in explaining racial disparities in HEM. Lower SES was associated with higher bleeding risk in another PCI study35 and among atrial fibrillation patients on warfarin therapy.36 We recognize that higher prevalence of diabetes mellitus and moderate and severe chronic kidney disease among black patients may also explain the higher incidence of HEM observed in our study.37, 38 Periprocedural bleeding complications following PCI are common in patients presenting with ACS.39, 40 Administration of more potent P2Y12 inhibitors (prasugrel or ticagrelor loading during index PCI) may be associated with increased risk of bleeding.41, 42 In our study, black participants were more likely to present with ACS and to receive prasugrel or ticagrelor loading during index PCI. However, at discharge and during follow‐up, the use of prasugrel or ticagrelor was similar across races, as was the use of proton pump inhibitors. This result suggests that the higher risk of HEM in black patients is independent of DAPT. How other factors (eg, uncontrolled hypertension43 or CYP2C19 genotype44) contribute to racial differences in HEM was not assessed in this study, and we recognize this limitation.
The strengths of this study are the prospective design and the documentation of both clinical and nonclinical factors. The medical records of all participants presenting to other hospitals were obtained, reviewed, and adjudicated, enhancing capture of events and medication changes. We recognize that despite the data‐curation efforts, unmeasured factors could have led to residual confounding. These factors include severity of comorbid conditions (eg, uncontrolled hypertension), genetics,45 neighborhood effects,46 and inflammatory burden,47 among others. We have not measured mental illness (eg, depression), which can influence outcomes in patients with CHD.48, 49 Moreover, SES is a complex construct including assessment of income, education, employment, and social support (eg, marital status).50 We recognize these limitations.
Conclusions
Despite receiving similar guideline‐recommended treatment, black patients undergoing PCI experienced higher incidences of MACE and HEM than their white counterparts. The reasons for these differences are multifactorial. Our results highlight the contributions of SES and CHD severity to race–MACE association and of SES, comorbidities, and CHD severity to race–HEM association. Further studies are needed to understand the mechanisms and interplay of these factors and to develop interventions to reduce these disparities.
Sources of Funding
This work was supported in part by grants from the University of Alabama at Birmingham Health Service Foundation's General Endowment Fund and Hugh Kaul Personalized Medicine Institute and the National Heart, Lung, and Blood Institute (RO1HL092173; K24HL133373) and the National Institutes of Health Clinical and Translational Science Award program (UL1TR000165).
Disclosures
None.
Acknowledgments
We are grateful to all patients who participated in the study.
(J Am Heart Assoc. 2019;8:e012874 DOI: 10.1161/JAHA.119.012874.)
References
- 1. National Center for Health Statistics . National Health Interview Survey, 2015. Public‐use data file and documentation: NCHS tabulations. Available at: http://www.cdc.gov/nchs/nhis/nhis_2015_data_release.htm. Accessed February 28, 2017.
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Glorioso TJ, Armstrong EJ, Maddox TM, Plomondon ME, Grunwald GK, Bradley SM, Tsai TT, Waldo SW, Rao SV, Banerjee S, Nallamothu BK, Bhatt DL, Rene AG, Wilensky RL, Groeneveld PW, Giri J. Comparative outcomes after percutaneous coronary intervention among black and white patients treated at US Veterans Affairs hospitals. JAMA Cardiol. 2017;2:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hess CN, Kaltenbach LA, Doll JA, Cohen DJ, Peterson ED, Wang TY. Race and sex differences in post‐myocardial infarction angina frequency and risk of 1‐year unplanned rehospitalization. Circulation. 2017;135:532–543. [DOI] [PubMed] [Google Scholar]
- 5. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S, Fonarow GC, Mukamal KJ, Liang L, Schulte PJ, Smith EE, DeVore A, Hernandez AF, Peterson ED, Bhatt DL. Sex and race/ethnicity‐related disparities in care and outcomes after hospitalization for coronary artery disease among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:S36–S44. [DOI] [PubMed] [Google Scholar]
- 7. Pradhan J, Schreiber TL, Niraj A, Veeranna V, Ramesh K, Saigh L, Afonso L. Comparison of five‐year outcome in African Americans versus Caucasians following percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;72:36–44. [DOI] [PubMed] [Google Scholar]
- 8. Iantorno M, Torguson R, Kolm P, Gajanana D, Suddath WO, Rogers T, Bernardo NL, Ben‐Dor I, Gai J, Satler LF, Garcia‐Garcia HM, Weintraub WS, Waksman R. Relation of sex and race to outcomes in patients undergoing percutaneous intervention with drug‐eluting stents. Am J Cardiol. 2019;123:913–918. [DOI] [PubMed] [Google Scholar]
- 9. Fox KA, Poole‐Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ. Interventional versus conservative treatment for patients with unstable angina or non‐ST‐elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–751. [DOI] [PubMed] [Google Scholar]
- 10. Fox KA, Poole‐Wilson P, Clayton TC, Henderson RA, Shaw TR, Wheatley DJ, Knight R, Pocock SJ. 5‐year outcome of an interventional strategy in non‐ST‐elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366:914–920. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 12. Thomas KL, Honeycutt E, Shaw LK, Peterson ED. Racial differences in long‐term survival among patients with coronary artery disease. Am Heart J. 2010;160:744–751. [DOI] [PubMed] [Google Scholar]
- 13. Batchelor W, Kandzari DE, Davis S, Tami L, Wang JC, Othman I, Gigliotti OS, Haghighat A, Singh S, Lopez M, Giugliano G, Horwitz PA, Chandrasekhar J, Underwood P, Thompson CA, Mehran R. Outcomes in women and minorities compared with white men 1 year after everolimus‐eluting stent implantation: insights and results from the PLATINUM Diversity and PROMUS Element Plus Post‐Approval Study Pooled Analysis. JAMA Cardiol. 2017;2:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popescu I, Vaughan‐Sarrazin MS, Rosenthal GE. Differences in mortality and use of revascularization in black and white patients with acute MI admitted to hospitals with and without revascularization services. JAMA. 2007;297:2489–2495. [DOI] [PubMed] [Google Scholar]
- 15. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 17. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 18. Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, Acton RT, Allon M. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ake CF, Carpenter AL. Extending the use of PROC PHREG in survival analysis. Proceedings of the 11th Annual Western Users of SAS Software, Inc. Users Group Conference, Cary, NC: SAS Institute Inc.; 2003. [Google Scholar]
- 20. Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaglia MA Jr, Steinberg DH, Pinto Slottow TL, Roy PK, Bonello L, Delabriolle A, Lemesle G, Okabe T, Torguson R, Kaneshige K, Xue Z, Suddath WO, Kent KM, Satler LF, Pichard AD, Lindsay J, Waksman R. Racial disparities in outcomes following percutaneous coronary intervention with drug‐eluting stents. Am J Cardiol. 2009;103:653–658. [DOI] [PubMed] [Google Scholar]
- 22. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA, Sperling LS. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 24. Pool LR, Ning H, Lloyd‐Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6:e006027 DOI: 10.1161/JAHA.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris AA, Ko YA, Hutcheson SH, Quyyumi A. Race/ethnic and sex differences in the association of atherosclerotic cardiovascular disease risk and healthy lifestyle behaviors. J Am Heart Assoc. 2018;7:e008250 DOI: 10.1161/JAHA.117.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collins SD, Torguson R, Gaglia MA Jr, Lemesle G, Syed AI, Ben‐Dor I, Li Y, Maluenda G, Kaneshige K, Xue Z, Kent KM, Pichard AD, Suddath WO, Satler LF, Waksman R. Does black ethnicity influence the development of stent thrombosis in the drug‐eluting stent era? Circulation. 2010;122:1085–1090. [DOI] [PubMed] [Google Scholar]
- 27. Berger JS, Petersen JL, Brown DL. Vascular disease burden and in‐hospital outcomes among patients undergoing percutaneous coronary intervention in New York State. Circ Cardiovasc Interv. 2009;2:317–322. [DOI] [PubMed] [Google Scholar]
- 28. Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, Detre KM, Wilensky RL. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143–149. [DOI] [PubMed] [Google Scholar]
- 29. Edmund Anstey D, Li S, Thomas L, Wang TY, Wiviott SD. Race and sex differences in management and outcomes of patients after st‐elevation and non‐ST‐elevation myocardial infarct: results from the NCDR. Clin Cardiol. 2016;39:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sane DC, Califf RM, Topol EJ, Stump DC, Mark DB, Greenberg CS. Bleeding during thrombolytic therapy for acute myocardial infarction: mechanisms and management. Ann Intern Med. 1989;111:1010–1022. [DOI] [PubMed] [Google Scholar]
- 31. Berkowitz SD, Granger CB, Pieper KS, Lee KL, Gore JM, Simoons M, Armstrong PW, Topol EJ, Califf RM. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation. 1997;95:2508–2516. [DOI] [PubMed] [Google Scholar]
- 32. Mehta RH, Marks D, Califf RM, Sohn S, Pieper KS, Van de Werf F, Peterson ED, Ohman EM, White HD, Topol EJ, Granger CB. Differences in the clinical features and outcomes in African Americans and whites with myocardial infarction. Am J Med. 2006;119:70.e1–8. [DOI] [PubMed] [Google Scholar]
- 33. Mehta RH, Stebbins A, Lopes RD, Rao SV, Bates ER, Pieper KS, Armstrong PW, Van de Werf F, White HD, Califf RM, Alexander JH, Granger CB. Race, bleeding, and outcomes in STEMI patients treated with fibrinolytic therapy. Am J Med. 2011;124:48–57. [DOI] [PubMed] [Google Scholar]
- 34. Mehta RH, Parsons L, Rao SV, Peterson ED. Association of bleeding and in‐hospital mortality in black and white patients with ST‐segment‐elevation myocardial infarction receiving reperfusion. Circulation. 2012;125:1727–1734. [DOI] [PubMed] [Google Scholar]
- 35. Patel NJ, Pau D, Nalluri N, Bhatt P, Thakkar B, Kanotra R, Agnihotri K, Ainani N, Patel N, Patel N, Shah S, Kadavath S, Arora S, Sheikh A, Badheka AO, Lafferty J, Alfonso C, Cohen M. Temporal trends, predictors, and outcomes of in‐hospital gastrointestinal bleeding associated with percutaneous coronary intervention. Am J Cardiol. 2016;118:1150–1157. [DOI] [PubMed] [Google Scholar]
- 36. Cressman AM, Macdonald EM, Yao Z, Austin PC, Gomes T, Paterson JM, Kapral MK, Mamdani MM, Juurlink DN. Socioeconomic status and risk of hemorrhage during warfarin therapy for atrial fibrillation: a population‐based study. Am Heart J. 2015;170:133–140, 140.e1–3. [DOI] [PubMed] [Google Scholar]
- 37. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 39. Budaj A, Eikelboom JW, Mehta SR, Afzal R, Chrolavicius S, Bassand JP, Fox KA, Wallentin L, Peters RJ, Granger CB, Joyner CD, Yusuf S. Improving clinical outcomes by reducing bleeding in patients with non‐ST‐elevation acute coronary syndromes. Eur Heart J. 2009;30:655–661. [DOI] [PubMed] [Google Scholar]
- 40. Moscucci M, Fox KA, Cannon CP, Klein W, Lopez‐Sendon J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–1823. [DOI] [PubMed] [Google Scholar]
- 41. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 42. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 43. Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Otsuka T, Tomita H, Origasa H. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J‐RHYTHM Registry. J Am Heart Assoc. 2016;5:e004075 DOI: 10.1161/JAHA.116.004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grosdidier C, Quilici J, Loosveld M, Camoin L, Moro PJ, Saut N, Gaborit B, Pankert M, Cohen W, Lambert M, Beguin S, Morange PE, Bonnet JL, Alessi MC, Cuisset T. Effect of CYP2C19*2 and *17 genetic variants on platelet response to clopidogrel and prasugrel maintenance dose and relation to bleeding complications. Am J Cardiol. 2013;111:985–990. [DOI] [PubMed] [Google Scholar]
- 45. Cavallari LH, Lee CR, Beitelshees AL, Cooper‐DeHoff RM, Duarte JD, Voora D, Kimmel SE, McDonough CW, Gong Y, Dave CV, Pratt VM, Alestock TD, Anderson RD, Alsip J, Ardati AK, Brott BC, Brown L, Chumnumwat S, Clare‐Salzler MJ, Coons JC, Denny JC, Dillon C, Elsey AR, Hamadeh IS, Harada S, Hillegass WB, Hines L, Horenstein RB, Howell LA, Jeng LJB, Kelemen MD, Lee YM, Magvanjav O, Montasser M, Nelson DR, Nutescu EA, Nwaba DC, Pakyz RE, Palmer K, Peterson JF, Pollin TI, Quinn AH, Robinson SW, Schub J, Skaar TC, Smith DM, Sriramoju VB, Starostik P, Stys TP, Stevenson JM, Varunok N, Vesely MR, Wake DT, Weck KE, Weitzel KW, Wilke RA, Willig J, Zhao RY, Kreutz RP, Stouffer GA, Empey PE, Limdi NA, Shuldiner AR, Winterstein AG, Johnson JA. Multisite investigation of outcomes with implementation of CYP2C19 genotype‐guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Udell JA, Desai NR, Li S, Thomas L, de Lemos JA, Wright‐Slaughter P, Zhang W, Roe MT, Bhatt DL. Neighborhood socioeconomic disadvantage and care after myocardial infarction in the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes. 2018;11:e004054. [DOI] [PubMed] [Google Scholar]
- 47. Ranjit N, Diez‐Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi‐ethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. [DOI] [PubMed] [Google Scholar]
- 48. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1‐year mortality after acute myocardial infarction: insights from the TRIUMPH Registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation. 2017;135:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure‐Smith N, Freedland KE, Jaffe AS, Leifheit‐Limson EC, Sheps DS, Vaccarino V, Wulsin L. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369. [DOI] [PubMed] [Google Scholar]
- 50. Schultz WM, Hayek SS, Samman Tahhan A, Ko YA, Sandesara P, Awad M, Mohammed KH, Patel K, Yuan M, Zheng S, Topel ML, Hartsfield J, Bhimani R, Varghese T, Kim JH, Shaw L, Wilson P, Vaccarino V, Quyyumi AA. Marital status and outcomes in patients with cardiovascular disease. J Am Heart Assoc. 2017;6:e005890 DOI: 10.1161/JAHA.117.005890. [DOI] [PMC free article] [PubMed] [Google Scholar]


