Abstract
Background
Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation is uncertain. Fractional flow reserve (FFR) has never been clinically validated in aortic stenosis. The study aim was to analyze the clinical outcome of FFR‐guided revascularization in patients undergoing transcatheter aortic valve implantation.
Methods and Results
Patients with severe aortic stenosis and coronary artery disease at coronary angiography were included in this retrospective analysis and divided in 2 groups: angiography guided (122/216; 56.5%) versus FFR‐guided revascularization (94/216; 43.5%). Patients were clinically followed up and evaluated for the occurrence of major adverse cardiac and cerebrovascular events at 2‐year follow‐up. Most lesions in the FFR group resulted negative according to the conventional 0.80 cutoff value (111/142; 78.2%) and were deferred. The FFR‐guided group showed a better major adverse cardiac and cerebrovascular event–free survival compared with the angio‐guided group (92.6% versus 82.0%; hazard ratio, 0.4; 95% CI, 0.2–1.0; P=0.035). Patients with deferred lesions based on FFR presented better outcome compared with patients who underwent angio‐guided percutaneous coronary intervention (91.4% versus 68.1%; hazard ratio, 0.3; 95% CI, 0.1–0.6; P=0.001).
Conclusions
FFR guidance was associated with favorable outcome in this observational study in patients undergoing transcatheter aortic valve implantation. Randomized trials are needed to investigate the long‐term effects of FFR‐guided revascularization against angiographic guidance alone in patients with aortic stenosis.
Keywords: aortic valve stenosis, coronary artery disease, fractional flow reserve, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Coronary Artery Disease, Percutaneous Coronary Intervention, Revascularization, Valvular Heart Disease
Clinical Perspective
What Is New?
Fractional flow reserve–guided revascularization was associated with favorable outcome in a cohort of patients with severe aortic stenosis and coronary artery disease undergoing transcatheter aortic valve implantation.
What Are the Clinical Implications?
Coronary physiology may lead to a significant simplification of CAD management in transcatheter aortic valve implantation candidates, downgrading the number of lesions that require treatment.
These encouraging preliminary data warrant further validation of physiology‐guided myocardial revascularization in patients undergoing transcatheter aortic valve implantation in a prospective randomized fashion.
Obstructive coronary artery disease (CAD) is present in >60% of patients with severe aortic stenosis (AS) evaluated for transcatheter aortic valve implantation (TAVI).1, 2 However, the clinical significance and the best management of a bystander coronary lesion in this specific setting is unclear. The presence of CAD was reported to be associated with worse survival in patients undergoing TAVI.3, 4 However, the actual prognostic relevance of bystander CAD has been questioned by other investigators,5 and conversely, higher procedural complications rates have been reported when percutaneous coronary intervention (PCI) is performed during TAVI, with possible adverse implications in this setting of fragile patients.
Functional assessment of coronary obstructions by means of fractional flow reserve (FFR) demonstrated superiority over angiography alone in stable patients with CAD6, 7 and is highly recommended by the guidelines on myocardial revascularization.8 However, patients with significant valve disease have been excluded from all validation9, 10 and randomized studies11, 12 involving invasive physiological indices. Furthermore, some concerns have been raised on the actual safety of deferring coronary lesions with negative FFR. Therefore, at present, no validated invasive method to assess ischemia is available in patients with AS,13, 14 and significant discrepancy has been observed between angiographic and functional evaluation of coronary lesions in this clinical setting.13 We previously demonstrated the feasibility of measuring FFR systematically in a prospective cohort of patients with severe AS and concomitant CAD undergoing TAVI,15, 16 as well as the strong correlation between the standard ischemic FFR cutoffs and the presence of myocardial ischemia as assessed by stress myocardial scintigraphy using adenosine as stressor.17
The aim of this study was to evaluate the clinical outcome of patients undergoing TAVI who underwent FFR‐guided or angiography‐guided revascularization in a single‐center consecutive series. In particular, we sought to assess the safety of deferring intervention on coronary lesions on the basis of negative FFR in patients with AS.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients with severe AS and bystander CAD (diameter stenosis [DS] >30% at quantitative coronary analysis (QCA) in at least 1 of the main coronary branches) undergoing TAVI were included in this retrospective analysis of the Verona TAVI Registry. Physiology‐guided and angiography‐guided myocardial revascularization strategies were compared in 2 separate cohorts of patients undergoing TAVI to minimize the confounders of patient selection for each group.
The angio‐guided group was composed of patients treated with TAVI between March 2010 and December 2014. The FFR‐guided group included patients undergoing TAVI between January 2015 and December 2018. In fact, from January 2015 onwards, a prospective study on functional CAD assessment in patients undergoing TAVI was initiated. Details on the study protocol were reported elsewhere.16
Patient Population
The TAVI procedure was performed in patients with severe symptomatic AS,18 with high surgical risk as predicted by Society of Thoracic Surgeons (STS) score19 ≥8%, or Logistic EuroSCORE20 ≥20%. Patients at lower surgical risk (EuroSCORE <20%) were treated with TAVI when presenting comorbidities not well captured by the risk scores, such as porcelain aorta, chest radiotherapy, severe obstructive pulmonary disease, organ transplantation, previous cardiac surgery, or advanced frailty as detected by a phenotype frailty index >3.21
To reduce confounders and to take into account the evolution of TAVI practice over time, patients with very high‐risk profile (STS score >15% or a Logistic EuroSCORE >50%), as well as patients with acute coronary syndromes were excluded.
Fifty‐four of the patients included in the FFR‐guided group belong to a series previously reported.16 The study was approved by the ethical review board of the University of Verona, and all patients provided their written informed consent.
TAVI Procedure
TAVI procedures were performed either by the percutaneous transfemoral or by surgical subclavian, or transapical approach. The Edwards SAPIEN‐XT or S3 (Edwards Lifesciences, Irvine, CA) or the Medtronic CoreValve, Evolut‐R, or Pro (Medtronic Inc., Minneapolis, MN) was used, according to the anatomic characteristics of the valve morphology as analyzed from the computed tomography scan.
Coronary Angiography and QCA
CAD was diagnosed by angiography, obtained either before or during the TAVI procedure.
The angiographic assessment was performed by QCA using the CASS‐II QCA package (Pie Medical Imaging, Maastricht, the Netherlands). The CAD severity was assessed by QCA and calculation of the SYNTAX score, in a previously validated core laboratory (NBR, Verona, Italy).22
According to QCA, coronaries were defined “unobstructed” if the %DS was ≤30%. Coronary obstructions with a %DS >30% and ≤70% were classified as intermediate lesions, and those with %DS >70% were considered severe.
Pressure Wire Measurements
Briefly, a pressure monitoring guidewire (Prestige Plus or Verrata Pressure Wire, Volcano Therapeutics, Rancho Cordova, CA) was advanced distally to the coronary artery stenosis after meticulous normalization. Hyperemia was obtained after administration of an intracoronary bolus of 150 to 250 μg of adenosine as previously reported.6, 23, 24, 25 An FFR value ≤0.80 was considered abnormal.8
In the majority of cases, FFR was measured before and after TAVI, as mandated by the study protocol,17 and in case of discrepancy between the 2 FFR measurements, a clinical decision was made according to the post‐TAVI FFR (Figure 1).
Figure 1.

A) Flowchart of study patients’ selection; B) PCI timing according to the study arms. DS indicates diameter stenosis; FFR, fractional flow reserve; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
PCIs and Antithrombotic Therapy
Before TAVI, all patients were pretreated with a loading dose of clopidogrel 300 mg and conventional doses of aspirin (100–160 mg). After valve and coronary stent implantation, patients received standard medications, including aspirin 100 to 160 mg/day and clopidogrel 75 mg/day for at least 6 months. Patients who did not undergo PCI had dual antiplatelet therapy for a minimum of 3 months. Patients treated with PCI having an indication to oral anticoagulation had triple antithrombotic therapy for 1 month and oral anticoagulation plus aspirin or clopidogrel thereafter.
Clinical Follow‐Up and Adverse Clinical Events Definition
The occurrence of any procedural‐related clinical complication was prospectively evaluated. After discharge, follow‐up was prospectively conducted during outpatients’ clinic visits at 1, 6, 12, and 24 months. In addition, adverse events were collected at longer‐term follow‐up with annual telephonic interviews and clinical controls (36, 48, 60, and 72 months) and confirmed with medical records consultation. The adjudication process was conducted retrospectively analyzing the events reported in the Verona TAVI Registry database. Two independent cardiologists examined every reported event. In case of ambiguity, a third cardiologist reviewed the case, and disagreement was resolved by consensus. All adverse clinical event definitions were defined according to Valve Academic Research Consortium‐2 recommendations.26
Cardiac death was defined as attributable to myocardial infarction, cardiac tamponade, worsening heart failure, sudden or unwitnessed death, and death of unknown cause.
Periprocedural (type 4a) myocardial infarction was defined as the occurrence of new ischemic symptoms or signs in addition to elevation of cardiac biomarkers (peak value exceeding 15x as the upper reference limit for troponin or 5x for creatine kinase‐MB) ≤72 hours after the index procedure.
Spontaneous myocardial infarction (type 1) was defined as detection of rise and/or fall of cardiac biomarkers together with the evidence of myocardial ischemia (symptoms, ECG changes, imaging evidences) or as sudden, unexpected cardiac death accompanied by ECG ischemia signs.
Coronary revascularization was defined as revascularization of the vessel subsequent to the index procedure by either PCI or bypass grafting.
Stroke was defined as duration of a focal or global neurological deficit >24 hours or death attributable to neurological deficit as diagnosed by a heart team neurology specialist. Both ischemic and hemorrhagic stroke were included and diagnosed with computed tomography imaging. Disabling stroke was meant as a Modified Rankin Scale score of ≥2 at 90 days and an increase in at least 1 Modified Rankin Scale category from an individual's prestroke baseline.
Major adverse cardiac and cerebrovascular events (MACCE) were defined as the composite occurrence of cardiac death, periprocedural and spontaneous myocardial infarction (type 4a and 1), any coronary revascularization, or disabling stroke.
Life‐threatening bleeding, major vascular complications, acute kidney injury, and TAVI device failure were defined according to Valve Academic Research Consortium‐2 recommendations26 as the former events.
Study End Points
The primary end point was the MACCE‐free survival at follow up.
The secondary end points were (1) difference in MACCE‐free survival between patients with lesion treatment deferred on the basis of negative FFR (>0.80) and those who underwent angio‐guided PCI and (2) cumulative rate of MACCE stratified according to FFR values in patients with deferred lesion treatment.
Statistical Analysis
Categorical data are presented as numbers and percentages. Continuous data are presented as means and SDs for normally distributed variables and as median and interquartile range otherwise.
Differences between continuous variables were assessed using the t test for normally distributed variables and the Mann–Whitney U test otherwise. Categorical data were analyzed using Pearson's chi‐squared test or Fisher's exact test.
MACCE‐free survival was analyzed using Kaplan–Meier plots, and differences between groups were compared with the use of log‐rank test. Cox proportional hazards models were fitted to estimate hazard ratios with 95% CI for treatment comparison. A multivariate Cox analysis of the primary end point was performed to adjust for confounders.
A two‐sided P value of ≤0.05 was considered significant. All statistical analyses were performed with the use of SPSS 20.0 software (SPSS Inc., Chicago, IL).
Results
Study Population
Between March 2010 and December 2018, 243 patients with severe AS and bystander CAD underwent TAVI at Verona University Hospital out of a series of 526 patients undergoing TAVI (Table S1). Twenty‐three patients with CAD were excluded from the analysis because of the extremely high risk at baseline (STS >15% or Logistic‐EuroSCORE >50%). Clinical characteristics of this subgroup of patients are available in Table S2.
Additionally, 4 patients with abnormal FFR (≤0.80) but untreated coronary lesions were excluded from the analysis. In these cases, PCI was not performed because of high frailty and unfavorable coronary anatomy (Table S3).
After the application of exclusion criteria, 216 patients, 122 (56.5%) angio‐guided and 94 (43.5%) FFR‐guided, were included in the analysis (Figure 1).
STS score (5.1 [interquartile range, 3.1]% versus 4.5 [interquartile range, 3.2]%; P=0.17) and Logistic EuroSCORE (22.4±12.8% versus 19.1±10.9%; P=0.11) were not significantly different between the 2 groups (Table 1).
Table 1.
Baseline Characteristics
| Variables | Angiography‐Guided (122) | FFR‐Guided (94) | P Value |
|---|---|---|---|
| Age, y | 84 [8.3] | 84 [6.4] | 0.97 |
| Logistic EuroSCORE, % | 22.4±12.8 | 19.1±10.9 | 0.11 |
| EuroSCORE II, % | 6 [5.1] | 5.5 [4.1] | 0.07 |
| STS score, % | 5.13 [3.1] | 4.5 [3.2] | 0.17 |
| Male, n (%) | 61 (50.0) | 42 (44.7) | 0.48 |
| BMI, kg/m2 | 25.8±4.7 | 25.6±4.0 | 0.98 |
| COPD, n (%) | 27 (22.1) | 14 (14.9) | 0.17 |
| Diabetes mellitus, n (%) | 41 (33.6) | 27 (28.7) | 0.47 |
| Hypertension, n (%) | 107 (87.7) | 91 (96.8) | 0.15 |
| Previous AMI, n (%) | 33 (27.1) | 9 (9.6) | 0.001 |
| Atrial fibrillation, n (%) | 40 (32.8) | 29 (30.9) | 0.90 |
| Previous stroke, n (%) | 8 (6.6) | 5 (5.3) | 0.77 |
| Previous CABG, n (%) | 19 (15.5) | 11 (11.7) | 0.12 |
Categorical data are presented as numbers and percentages; continuous data are presented as means±standard deviations for normally distributed variables and as median [interquartile range] otherwise. AMI indicates acute myocardical infarction; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease.
TAVI Procedure
Procedural time (112.4±50.1 minutes versus 124.4±46.9 minutes; P=0.16); and fluoroscopy time (20.9±11.8 minutes versus 25.3±14.4 minutes; P=0.08) tended to be longer in the FFR‐guided group.
The vast majority of the TAVI procedures were performed via transfemoral access (86.3%), whereas transapical and transsubclavian approaches were used in 13.2% and 0.5% of the cases, respectively. Balloon‐expandable aortic valves were used in 165 of 216 (76.4%) patients.
FFR Assessment
Angiographic data are shown in Table 2.
Table 2.
CAD Details
| Variables | Angiography‐Guided (122) | FFR‐Guided (94) | P Value |
|---|---|---|---|
| SYNTAX score | 12.1±10.3 | 11.2±6.2 | 0.24 |
| QCA (%DS), all lesions, % | 62.4±22.6 | 56.4±12.8 | 0.023 |
| PCI/patients, n (%) | 43 (35.2) | 24 (25.5) | 0.19 |
| PCI after valve implantation, n (%) | 36 (29.5) | 21 (22.3) | 0.44 |
| 3 months DAPT, n (%) | 68 (55.7) | 67 (71.2) | 0.06 |
| 6 months DAPT, n (%) | 54 (44.3) | 27 (28.7) | |
| CAD degree/patient | |||
| Single VD, n (%) | 73 (59.8) | 52 (55.3) | 0.18 |
| 2‐VD, n (%) | 24 (19.7) | 24 (25.5) | |
| 3‐VD, n (%) | 21 (17.2) | 13 (13.8) | |
| Vessel disease/patient | |||
| LM, n (%) | 8 (6.6) | 3 (3) | 0.08 |
| LAD, n (%) | 69 (56.6) | 68 (72.3) | 0.04 |
| LCx, n (%) | 53 (43.4) | 41 (43.8) | 0.81 |
| RCA, n (%) | 47 (38.5) | 29 (31) | 0.53 |
| VG, n (%) | 7 (5.7) | 1 (1.1) | 0.08 |
| No. of total lesions | n=184 | n=142 | |
| Intermediate (%DS 30–70), n (%) | 145 (78.8) | 114 (80.2) | 0.93 |
| No. lesions treated, n (%) | 15 (8.2) | 8 (5.6) | |
| Severe (%DS ≥70), n (%) | 39 (21.2) | 28 (19.7) | 0.98 |
| No. of lesions treated, n (%) | 39 (100.0) | 23 (82.1) | |
| Total lesions treated, n (%) | 54 (29.3) | 31 (21.8) | 0.13 |
| Proximal segment, n (%) | 37 (68.5) | 19 (61.3) | 0.51 |
| LM or LAD, n (%) | 32 (59.2) | 18 (58.1) | 0.93 |
| No. of stents implanted, n (%) | 49 (26.6) | 28 (19.7) | 0.46 |
| QCA pre‐PCI | |||
| DS, % | 59.8±14.9 | 63.1±19.7 | 0.001 |
| Lesion length, mm | 14.8±8.2 | 10.5±4.9 | 0.18 |
| MLD, mm | 0.9±0.6 | 1.0±0.7 | 0.31 |
| D‐ref, mm | 2.7±0.7 | 2.8±0.6 | 0.71 |
Categorical data are presented as numbers and percentages; continuous data are presented as means±SDs for normally distributed variables and as median [interquartile range] otherwise. DS indicates diameter stenosis; DAPT, double antiplatelet therapy; D‐ref, reference diameter; LAD, left anterior descending; LCx, left circumflex; LM, left main; MLD, minimal luminal diameter; PCI, percutaneous coronary intervention; QCA, quantitative coronary analysis; RCA, right coronary artery; VD, vessel disease; VG, venous graft.
FFR was obtained in 142 coronary obstructions in 94 patients and was measured more often in the left anterior descending artery territory (58.1%). The mean FFR value at baseline was 0.87±0.12 and 0.87±0.08 following TAVI (P=0.74). The majority of the lesions (111/142, 78.2%) resulted negative (FFR >0.8) and were thus deferred.
Notably, the physiological assessment resulted in a significant downgrading of the number of lesions requiring treatment compared with the initial angiographic evaluation (1.5±0.7 versus 0.6±0.4; P<0.001). No major clinical event related to the pressure wire or the intracoronary adenosine administration occurred.
Percutaneous Coronary Intervention
Overall, PCI was performed in 67 of 216 patients, 43 of 67 in the angiographic‐group and 24 of 67 in the FFR group.
In the angiography guided group, a total of 54 of 184 lesions (29.3%) were treated with PCI. In 39 (72.2%) cases, the indication for PCI was the angiographic lesion severity only, whereas in 15 (27.8%) cases PCI was guided by the presence of some chest pain or inducible ischemia on noninvasive stress tests in angiographic borderline lesions (DS% 30–70).
In the FFR‐guided group, PCI was performed in 31 coronary lesions in 24 patients with abnormal FFR (Table 2).
The number of treated vessels tended to be lower in the FFR group (21.8% versus 29.3%; P=0.13).
PCI was performed more often in the left main or left anterior descending (58.9%) artery compared with other coronary vessels and during the same TAVI procedure rather than in a staged fashion (48 of 67 patients; 71.6%) (Figure 1).
No difference in overall MACCE‐free survival was observed between patients who underwent PCI before TAVI or during the TAVI procedure immediately after the valve implantation (82.0% versus 90.2%; hazard ratio, 0.6; 95% CI, 0.5–1.2; P=0.35). Furthermore, no ischemic intraprocedural complications were observed during the valve implantation among patients with severe CAD who underwent post‐TAVI PCI.
Clinical Outcomes
At a mean follow‐up time of 24.2±17.4 months, patients in the FFR‐guided group demonstrated a better MACCE‐free survival compared with the angiography‐guided group (92.6% versus 82.0%; hazard ratio, 0.4; 95% CI, 0.2–1.0, P=0.035; Figure 2).
Figure 2.
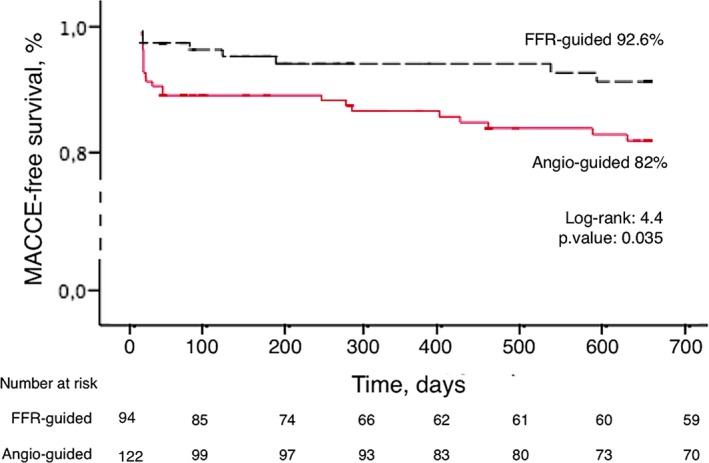
Kaplan–Meier survival analysis of patients for the FFR (fractional flow reserve)‐guided group vs the angio‐guided group. MACCE indicates major adverse cardiac and cerebrovascular events.
Kaplan–Meier curves showed an early postprocedure separation, driven by higher rates of type 4a myocardial infarction and cardiac death in the angio‐guided group. The 30‐day and long‐term clinical outcomes are reported in Tables 3 and 4.
Table 3.
In‐Hospital and 30‐Day Clinical Outcomes
| Variables | Angiography‐Guided (122) | FFR‐Guided (94) | P Value |
|---|---|---|---|
| Death, n (%) | 6 (4.9%) | 1 (1%) | 0.40 |
| Cardiac death, n (%) | 4 (3.3%) | 0 | 0.25 |
| Type 4a MI, n (%) | 7 (5.7%) | 3 (3.2%) | 0.12 |
| Type 1 MI, n (%) | 1 (0.8%) | 0 | 0.34 |
| Urgent PCI, n (%) | 0 | 0 | ··· |
| New elective PCI, n (%) | 0 | 0 | ··· |
| Stroke, n (%) | 2 (1.6%) | 0 | 0.12 |
| AKI stage 2 to 3, n (%) | 4 (3.3%) | 3 (3.2%) | 0.87 |
| LT bleeding, n (%) | 4 (3.3%) | 3 (3.2%) | 0.87 |
| MVC, n (%) | 12 (9.8%) | 4 (4.3%) | 0.18 |
Categorical data are presented as numbers and percentages. AKI indicates acute kidney injury; LT, life‐threatening; MI, myocardial infarction; MVC, major vascular complications; PCI, percutaneous coronary intervention.
Table 4.
Long‐Term Clinical Outcomes: All Events and Hierarchical (MACCE) Variables
| 2‐Year Follow‐Up | Angiography‐Guided (122) | FFR‐Guided (94) | P Value | 95% CI | HR |
|---|---|---|---|---|---|
| Death, n (%) | 25 (20.5%) | 16 (17%) | 0.30 | 0.4 to 1.4 | 0.7 |
| Cardiac death, n (%) | 6 (4.9%) | 3 (3.2%) | 0.35 | 0.2 to 1.9 | 0.6 |
| AMI, n (%) | 9 (7.4%) | 4 (4.3%) | 0.71 | 0.3 to 2.5 | 0.8 |
| New elective PCI, n (%) | 1 (0.8%) | 1 (1.1%) | 0.34 | 0.2 to 1.7 | 1.8 |
| Stroke, n (%) | 6 (4.9%) | 1 (1.1%) | 0.17 | 0.0 to 1.9 | 0.2 |
| MACCE, n (%) | 22 (18%) | 7 (7.4%) | 0.04 | 0.2 to 1.0 | 0.4 |
Categorical data are presented as numbers and percentages. AMI indicates acute myocardial infarction (both periprocedural and spontaneous); MACCE, major adverse cardiac and cerebrovascular events; PCI, percutaneous coronary intervention.
Safety of FFR‐Guided Deferral
In 70 of 94 patients (74.5%), coronary revascularization was deferred because of negative FFR values. This subgroup presented better outcomes at 24 months compared with patients who underwent angiography‐guided PCI (91.4% versus 68.1%; hazard ratio, 0.3; 95% CI, 0.1–0.6; P=0.001; Figure 3A). No significant difference was observed in the MACCE‐free survival rate of deferred patients stratified according to the FFR values (Figure 3B).
Figure 3.

A, Kaplan–Meier survival analysis of patients deferred to medical therapy on the basis of FFR (fractional flow reserve) values >0.8 and patient treated with PCI (percutaneous coronary intervention) on the basis of angiographic guidance; (B) Kaplan–Meier survival analysis of deferred patients stratified according to the FFR values. MACCE indicates major adverse cardiac and cerebrovascular events.
Additional subanalyses of patients stratified according to different FFR thresholds (<0.75; 0.75–0.85; >0.85) and to %DS (30%–50% versus 51%–70% versus 71%–100%) confirmed the favorable outcome of the FFR‐guided management strategy. Furthermore, patients with FFR >0.80 had a similar long‐term outcome compared with those without coronary obstructive disease (Figures S1 through S3).
Discussion
The main findings of this retrospective observational study are the following:
Patients who underwent TAVI with FFR‐guided revascularization presented a better MACCE‐free survival at 24 months compared with those who underwent angiography‐guided revascularization (92.6% versus 82.0%; P=0.035). This finding is in line with the milestone studies performed in patients with CAD without AS but needs to be confirmed in dedicated randomized studies.
FFR assessment yielded a substantial reclassification of CAD in patients undergoing TAVI, with a significant downgrading of the vessels requiring treatment.
Bystander intermediate coronary lesions resulted FFR negative in 78.2% of cases and were safely deferred without ischemic complications during the TAVI procedure and long term.
Currently, considering that noninvasive functional evaluation frequently is not feasible in these patients, who additionally experience angina only rarely, clinical decision making in case of bystander CAD is mostly guided by the angiographic severity.27 Guidelines, in fact, largely recommend PCI without scientific evidence (IIa‐C).8
Angiographic CAD assessment presents important limitations in patients with AS, and previous studies demonstrated the modest correlation between angiography and functional indexes in AS, especially for coronary lesions located on the left anterior descending artery, upholding the general assumption that the larger the myocardial mass, the higher the likelihood of an anatomic‐hemodynamic mismatch in the assessment of a given stenosis.28
At the same time, the reliability of intracoronary physiological indices has been questioned in severe AS, given the faulty intracoronary resting conditions and the impaired capacity to achieve maximal hyperemia. The blunted vasodilator ability in AS may be caused by a combination of microvascular dysfunction27, 29 and myocardial hypertrophy, resulting in a suboptimal response to adenosine.1 Furthermore, the compensatory increase in resting coronary flow observed in AS may lead to the exhaustion of the vasodilatory reserve with a consequent possible underestimation of the true ischemic potential of a coronary stenosis.
For these reasons, the validation of FFR in the presence of AS is a priority, to exclude ischemic events over time.
Our previous research has demonstrated the feasibility and reliability of FFR obtained with intracoronary adenosine in patients with severe AS and preserved left ventricular function,30 and also that the same conventional FFR cutoffs used in stable CAD are able to detect inducible ischemia in AS with similar sensitivity and specificity compared with the adenosine‐stress myocardial scintigraphy.17
In our series, a trend toward a better event‐free survival among patients assessed by FFR was observed. This result, although not powered to demonstrate a clinical difference between FFR‐guided and angiography guided revascularization, replicates the findings of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) trial (composite event‐free survival at 2 years of 91.6% in the FFR‐guided PCI group)6 and endorses a physiology‐guided CAD management strategy in patients with AS undergoing TAVI.
Additionally, these findings support the concept of the FFR‐related simplification of CAD management in TAVI patients, with a significant downgrading of the number of vessels needing treatment and a lower rate of PCI in the FFR‐guided group.
Importantly, the favorable outcome of patients deferred on the basis of FFR >0.80 support the use of this clinical cutoff, recommended by clinical guidelines in patients with CAD, even in the presence of severe AS.31 The FFR‐guided decision‐making strategy, by reducing the number of lesions requiring treatment, may offer the advantage to limit the exposure of the TAVI population to the PCI‐related procedural risk, including the need for a longer and more aggressive antiplatelet regimen. This is especially relevant in elderly patients with multiple comorbidities.
Limitations
The main limitations of our study are the following:
This is a nonrandomized retrospective observational study. Therefore, on the basis of our results, no definitive conclusions can be drawn on the safety and clinical benefit of FFR‐guided myocardial revascularization in patients undergoing TAVI.
The design of the study, including 2 consecutive groups of patients with severe AS and CAD, aimed to reduce the possible confounders between 2 different CAD management strategies (angiography guided versus FFR‐guided). Nonetheless, a certain selection bias, mainly related to the different time frame of inclusion between the 2 groups, cannot be excluded in our analysis, that should be considered hypothesis generating. In fact, the clinical and procedural characteristics were not identical between the 2 groups because of the changes acquired with experience over time. Nevertheless, with these important limitations in mind, our analysis suggests the safety and potential benefits of the physiologically guided strategy.
The sample size is relatively small, and our preliminary experience must be confirmed by adequately powered prospective randomized trials. Nonetheless, to the best of our knowledge, this is the first report on the clinical outcome of a consecutive series of patients undergoing TAVI managed with FFR‐guided CAD assessment.
Events were not adjudicated by an independent adjudication committee. However, accurate follow‐up was performed prospectively within the Verona TAVI registry. Moreover, all the events were prospectively reviewed by independent investigators for this analysis.
Conclusions
Our analysis supports the use of FFR to assess CAD in patients with AS undergoing TAVI. FFR guidance yielded a 92.6% MACCE‐free survival at 24 months and an overall simplification of CAD management with a significant downgrading of the number of lesions requiring treatment. On the basis of these preliminary observations, a nationwide, randomized clinical trial (FAITAVI [Functional Assessment in TAVI], Clinicaltrial.gov: NCT03360591) has been initiated to compare FFR with angiography guided revascularization in patients undergoing TAVI.
Author Contributions
All authors participated in the research and preparation of the manuscript.
Disclosures
None.
Supporting information
Table S1. Population Details According to the 2 Study Periods
Table S2. Clinical Characteristics of Excluded High‐Risk Patients
Table S3. Baseline, Angiographic, and Follow‐Up Variables of 4 FFR‐Guided Patients Excluded
Figure S1. Fractional flow reserve (FFR)‐guided patients: no significant difference observed in the clinical outcome stratifying the coronary lesions according to the FFR values (FFR <0.75 vs 0.75–0.85 vs >0.85).
Figure S2. Patients with fractional flow reserve (FFR)‐guided deferred coronary lesions presented similar outcome compared with patients with “unobstructed” coronary arteries (HR, 3.1; 95% CI, 0.4–24.7; P=0.29) despite a significantly worse angiographic severity (%DS, 52.7±7.6 vs 16.1±6.2; P<0.001).
Figure S3. Major adverse cardiac and cerebrovascular event (MACCE)‐free survival according to 3 %DS‐subgroups (30–50% vs 51–70% vs 71–100%) in fractional flow reserve (FFR)‐guided (A) and angio‐guided patients (B).
(J Am Heart Assoc. 2019;8:e012618 DOI: 10.1161/JAHA.119.012618.)
References
- 1. Danson E, Hansen P, Sen S, Davies J, Meredith I, Bhindi R. Assessment, treatment, and prognostic implications of CAD in patients undergoing TAVI. Nat Rev Cardiol. 2016;13:276–285. [DOI] [PubMed] [Google Scholar]
- 2. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators . Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 3. Gautier M, Pepin M, Himbert D, Ducrocq G, Iung B, Dilly MP, Attias D, Nataf P, Vahanian A. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. EuroIntervention. 2011;7:549–555. [DOI] [PubMed] [Google Scholar]
- 4. Snow TM, Ludman P, Banya W, DeBelder M, MacCarthy PM, Davies SW, Di Mario C, Moat NE. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: the United Kingdom TAVI Registry. Int J Cardiol. 2015;199:253–260. [DOI] [PubMed] [Google Scholar]
- 5. Masson JB, Lee M, Boone RH, Al Ali A, Al Bugami S, Hamburger J, John Mancini GB, Ye J, Cheung A, Humphries KH, Wood D, Nietlispach F, Webb JG. Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:165–173. [DOI] [PubMed] [Google Scholar]
- 6. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 7. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius‐Winkler S, Mobius‐Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF; FAME 2 Trial Investigators . Fractional flow reserve‐guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 8. Authors/Task Force members , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 9. De Bruyne B, Paulus WJ, Pijls NH. Rationale and application of coronary transstenotic pressure gradient measurements. Cathet Cardiovasc Diagn. 1994;33:250–261. [DOI] [PubMed] [Google Scholar]
- 10. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 11. Davies JE, Sen S, Dehbi HM, Al‐Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Härle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva P, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria‐Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J. Use of the instantaneous wave‐free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 12. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P; FAME 2 Trial Investigators . Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 13. Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Barbierato M, Caprioglio F, Vassanelli C, Ribichini F. Coronary physiology in patients with severe aortic stenosis: comparison between fractional flow reserve and instantaneous wave‐free ratio. Int J Cardiol. 2017;243:40–46. [DOI] [PubMed] [Google Scholar]
- 14. Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Gottin L, Zanetti C, Faggian G, Ribichini F. Physiologic evaluation of coronary lesions using instantaneous wave‐free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2018;13:1512–1519. [DOI] [PubMed] [Google Scholar]
- 15. Zivelonghi C, Pesarini G, Scarsini R, Lunardi M, Piccoli A, Ferrero V, Gottin L, Vassanelli C, Ribichini F. Coronary catheterization and percutaneous interventions after transcatheter aortic valve implantation. Am J Cardiol. 2017;120:625–631. [DOI] [PubMed] [Google Scholar]
- 16. Pesarini G, Scarsini R, Zivelonghi C, Piccoli A, Gambaro A, Gottin L, Rossi A, Ferrero V, Vassanelli C, Ribichini F. Functional assessment of coronary artery disease in patients undergoing transcatheter aortic valve implantation: influence of pressure overload on the evaluation of lesions severity. Circ Cardiovasc Interv. 2016;9:e004088. [DOI] [PubMed] [Google Scholar]
- 17. Scarsini R, Cantone R, Venturi G, De Maria GL, Variola A, Braggio P, Lunardi M, Pesarini G, Ferdeghini M, Piccoli A, Feola M, Kharbanda RK, Banning AP, Ribichini F. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int J Cardiol. 2019;19:S0167‐S5273. [DOI] [PubMed] [Google Scholar]
- 18. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz ER, Rosenhek R, Sjögren J, Mas PT, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JS; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:S2739–S2791. [Google Scholar]
- 19. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2‐isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 20. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 21. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 22. Tomai F, Ribichini F, De Luca L, Petrolini A, Ghini AS, Weltert L, Spaccarotella C, Proietti I, Trani C, Nudi F, Pighi M, Vassanelli C. Randomized comparison of Xience V and multi‐link vision coronary stents in the same multivessel patient with chronic kidney disease (RENAL‐DES) study. Circulation. 2014;129:1104–1112. [DOI] [PubMed] [Google Scholar]
- 23. Wiegerinck EM, van de Hoef TP, Rolandi MC, Yong Z, van Kesteren F, Koch KT, Vis MM, de Mol BA, Piek JJ, Baan J. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e002443. [DOI] [PubMed] [Google Scholar]
- 24. De Bruyne B, Gould KL. Standardized hyperemic stress for fractional flow reserve. Circ Cardiovasc Interv. 2013;6:602–603. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, Bresnahan JF, Rihal CS, Lerman LO, Lerman A. Long‐term outcomes of fractional flow reserve‐guided vs. angiography‐guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB; Valve Academic Research Consortium‐2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 27. Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985). 2009;106:113–121. [DOI] [PubMed] [Google Scholar]
- 28. Di Gioia G, Scarsini R, Strisciuglio T, De Biase C, Zivelonghi C, Franco D, De Bruyne B, Ribichini F, Barbato E. Correlation between angiographic and physiologic evaluation of coronary artery narrowings in patients with aortic valve stenosis. Am J Cardiol. 2017;120:106–110. [DOI] [PubMed] [Google Scholar]
- 29. Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. [DOI] [PubMed] [Google Scholar]
- 30. Scarsini R, De Maria GL, Di Gioia G, Kotronias RA, Aurigemma C, Zimbardo G, Burzotta F, Leone AM, Pesarini G, Trani C, Crea F, Kharbanda RK, De Bruyne B, Barbato E, Banning A, Ribichini F. The influence of aortic valve obstruction on the hyperemic intracoronary physiology: difference between resting Pd/Pa and FFR in aortic stenosis. J Cardiovasc Transl Res. 2019. Available at: 10.1007/s12265-019-09890-5. Accessed September 5, 2019. [DOI] [PubMed] [Google Scholar]
- 31. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio‐Thoracic Surgery (EACTS). 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.30165437 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Population Details According to the 2 Study Periods
Table S2. Clinical Characteristics of Excluded High‐Risk Patients
Table S3. Baseline, Angiographic, and Follow‐Up Variables of 4 FFR‐Guided Patients Excluded
Figure S1. Fractional flow reserve (FFR)‐guided patients: no significant difference observed in the clinical outcome stratifying the coronary lesions according to the FFR values (FFR <0.75 vs 0.75–0.85 vs >0.85).
Figure S2. Patients with fractional flow reserve (FFR)‐guided deferred coronary lesions presented similar outcome compared with patients with “unobstructed” coronary arteries (HR, 3.1; 95% CI, 0.4–24.7; P=0.29) despite a significantly worse angiographic severity (%DS, 52.7±7.6 vs 16.1±6.2; P<0.001).
Figure S3. Major adverse cardiac and cerebrovascular event (MACCE)‐free survival according to 3 %DS‐subgroups (30–50% vs 51–70% vs 71–100%) in fractional flow reserve (FFR)‐guided (A) and angio‐guided patients (B).


