Abstract
Background
Few patients survive after out‐of‐hospital cardiac arrest and any measure that improve circulation during cardiopulmonary resuscitation is beneficial. Animal studies support that resuscitative endovascular balloon occlusion of the aorta (REBOA) during cardiopulmonary resuscitation might benefit patients suffering from out‐of‐hospital cardiac arrest, but human data are scarce.
Methods and Results
We performed an observational study at the helicopter emergency medical service in Trondheim (Norway) to assess the feasibility and safety of establishing REBOA in patients with out‐of‐hospital cardiac arrest. All patients received advanced cardiac life support during the procedure. End‐tidal CO 2 was measured before and after REBOA placement as a proxy measure of central circulation. A safety‐monitoring program assessed if the procedure interfered with the quality of advanced cardiac life support. REBOA was initiated in 10 patients. The mean age was 63 years (range 50–74 years) and 7 patients were men. The REBOA procedure was successful in all cases, with 80% success rate on first cannulation attempt. Mean procedural time was 11.7 minutes (SD 3.2, range 8–16). Mean end‐tidal CO 2 increased by 1.75 kPa after 60 seconds compared with baseline (P<0.001). Six patients achieved return of spontaneous circulation (60%), 3 patients were admitted to hospital, and 1 patient survived past 30 days. The safety‐monitoring program identified no negative influence on the advanced cardiac life support quality.
Conclusions
To our knowledge, this is the first study to demonstrate that REBOA is feasible during non‐traumatic out‐of‐hospital cardiac arrest. The REBOA procedure did not interfere with the quality of the advanced cardiac life support. The significant increase in end‐tidal CO 2 after occlusion suggests improved organ circulation during cardiopulmonary resuscitation.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT03534011.
Keywords: aorta, cardiac arrest, occlusion, resuscitation
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest, Treatment
Clinical Perspective
What Is New?
To our knowledge, this study is the first to show that resuscitative endovascular balloon occlusion of the aorta is feasible during resuscitation of patients with non‐traumatic out‐of‐hospital cardiac arrest.
The resuscitative endovascular balloon occlusion of the aorta procedure does not negatively influence resuscitation quality.
The aortic occlusion increases central circulation.
What Are the Clinical Implications?
Patients with cardiac arrest can benefit from resuscitative endovascular balloon occlusion of the aorta during resuscitation.
This procedure can be performed in the pre‐hospital setting by a team of 2 people and could therefore also be performed in‐hospital if trained personnel are available.
Introduction
In Norway, the 30‐day survival rate after out‐of‐hospital cardiac arrest (OHCA) is currently 14%.1 Improved outcome after OHCA is achieved by many efforts, including education of the public for bystander cardiopulmonary resuscitation (CPR), the use of automatic external defibrillators and improved organization of emergency healthcare services. Basic and advanced CPR algorithms are relatively unchanged in recent years but have an increased emphasis on minimizing low‐flow periods during CPR2 to augment blood circulation to vital organs.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is used to treat non‐compressible hemorrhages.3, 4 Recently REBOA has been proposed as an adjunct treatment to patients with non‐traumatic cardiac arrest.3, 5 Preclinical studies have demonstrated that REBOA during CPR increase coronary artery blood flow and coronary perfusion pressure and rates of return of spontaneous circulation (ROSC).6, 7, 8, 9, 10, 11, 12, 13 It is shown that an increase in coronary perfusion pressure increases the probability of ROSC in humans.14 REBOA during CPR also increases carotid artery blood flow,9, 15 cerebral arterial blood flow,7, 8, 15, 16, 17 and cerebral perfusion pressure.7, 8, 15, 18
These findings suggest that the use of REBOA could be beneficial during OHCA and clinical investigations is warranted. Except for case stories, there is no clinical information on the use of REBOA in non‐traumatic OHCA.19, 20, 21 However, a prerequisite before initiating clinical controlled trials is to test the feasibility of REBOA, a highly invasive procedure, during CPR in a pre‐hospital setting. Thus, the primary aim of this study was to assess the feasibility of pre‐hospital REBOA in patients with cardiac arrest. A secondary aim was to observe changes in end‐tidal CO 2 (EtCO2) as proxy measure of the effect on circulation from use of REBOA in OHCA.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Settings
This is an observational study performed by the physician‐manned helicopter emergency medical service (HEMS) in Trondheim, Norway. The HEMS has a catchment population of about 700 000. Patients with OHCA that achieve ROSC are transported to St. Olav University Hospital, a tertiary 983‐bed hospital. The service has both a helicopter and a rapid response car at disposal, both manned by a board‐certified anesthesiologist and a paramedic. Eight physicians and 5 paramedics participated, and all completed a structured training program before entering the study. The training program included theoretical education, training on a special designed simulation mannequin, training during elective angiography procedures, and high‐fidelity simulation. Performance was evaluated with a global rating scale and all participants had to perform above a predefined score to complete the training program. Details of the training program have been reported previously.22
Patients were included between July 2018 and May 2019. All patients were resuscitated on scene according to the current guidelines for advanced cardiac life support (ACLS) provided by the Norwegian Resuscitation Council.23 All patients were endotracheally intubated and a mechanically chest compression machine (LUCAS CPR, Physio Control‐Inc, Lund, Sweden) was used in 30:2 mode.
Patient Inclusion
Inclusion criteria were aged 18 to 75 years, non‐traumatic cardiac arrest, and CPR initiated <10 minutes after onset of arrest. Exclusion criteria were pregnancy, known terminal illness, traumatic cardiac arrest (including avalanche victims), suspected intracerebral hemorrhage, accidental hypothermia, drowning, and strangulation. Patients too large to fit in the chest compression machine were excluded. Patients achieving sustained ROSC before aortic occlusion were excluded.
REBOA Procedure
For eligible patients, a REBOA catheter (7 Fr, 20 mm, Reboa Balloon Kit, Reboa Medical AS, Norway) was inserted via the femoral artery, guided by ultrasound (iViz, FUJIFILM SonoSite, WA, USA) and advanced 50 cm for an aortic balloon inflation in the aortic zone 1. To ensure arterial placement, ultrasound images of the guidewire were obtained before inserting the balloon catheter. The ultrasound images were stored and later reviewed by the principal investigator. Before and after occlusion of aorta a pulse check on left radial artery during chest compression was performed. The procedure is described in detail in Table S1.22 If ROSC was achieved, the aortic balloon was deflated slowly. If the patient re‐arrested, the ACLS‐guideline was followed and the aortic balloon re‐inflated. Clinical decisions for other ACLS procedures, whether to stay on scene or continue CPR during transport to hospital or to terminate CPR efforts were made by the HEMS‐physician. In case of death on scene, the physician performed a post‐mortem ultrasound examination to verify the position of the REBOA catheter.
Data Collection
Demographic variables and OHCA characteristics were obtained following the Utstein template24 and by a semi‐structured interview with the performing physician. Time of arrest was obtained from the emergency medical communications centrals (EMCC) database. Dispatch was at the time the HEMS team was contacted by the emergency medical communications centrals. ROSC was defined as restoration of spontaneous circulation with a palpable pulse in absence of chest compression, with an organized spontaneous ECG rhythm24 of ≥5 minutes. Time of REBOA procedural start was defined when the physician decides to perform the REBOA procedure and physician/paramedic start to prepare for the procedure. Time of occlusion was set when the balloon was inflated. Time of deflation was set when the team recognized ROSC and deflated the balloon. During resuscitation, variables was gathered and documented in a specifically designed checklist chart (Table S2, 22) by an available member of the resuscitation team.
End‐tidal CO2 (EtCO2) was measured before aortic occlusion, directly after occlusion, 30, 60, and 90 seconds after occlusion and after ROSC. Other data variables were gathered by the principal investigator from ambulance journals, emergency medical communications centrals database, transcripts from the monitor used (Tempus Pro, RDT, United Kingdom or Corpuls3, GS, Germany), HEMS run charts and by a semi‐structured interview with the performing physician.
The diameter of the femoral artery and vein was measured based on ultrasound images, before introduction of wire and at the greatest identified width. Two authors (JRB and TL) performed the individual measurements.
Statistical Analysis
Data analyses were performed applying IBM SPSS Statistics 25, STATA version 15.1, Matlab version 2016b and R version 3.6.0. Continuous variables are reported as mean±SD. Categorical variables are described as count and/or proportion. The mean change in end‐tidal CO2 after aortic balloon occlusion was modeled applying a linear mixed effect model, with time treated as a categorical variable.25 A P value of <0.05 was regarded as statistically significant.
Safety Monitoring Program
A 3‐step safety assurance system was used to ensure the safety of the procedure. First, direct feedback on safety concern from the pre‐hospital physician was given to the project group after each patient included. Second, a post‐intervention study of the ultrasound images was performed by an experienced interventional radiologist, the pre‐hospital physician and the project group. Third, a case review was performed using all available information by an external expert panel consisting of an interventional radiologist, an intensivist and a pre‐hospital physician. The safety monitoring group specifically focused on correct catheter placement and the quality of advanced resuscitation. The transcripts from the monitor used was examined specifically to disclose any increase in hands‐off time, change in chest compression rate or depth.
Ethics
The study was approved by the Regional Committees for Medical and Health Research Ethics (reference 2018/51/REK Midt). After arrival to the hospital the patient's next‐of‐kin were given information on the study and gave an oral and written consent. All patients who regained capacity to consent were asked to give a deferred informed consent. The study is registered in ClinicalTrials.gov (Identifier: NCT03534011).
Results
Demographic and Cardiac Arrest Characteristics
During the study period, the HEMS crew was dispatched 98 times due to OHCA. Twenty‐six patients were older than 75 years and 20 patients had obtained ROSC before the arrival of the HEMS crew. Thirty‐seven patients were not eligible for REBOA due to other exclusion criteria (Figure 1). In 5 per‐protocol eligible cases, REBOA was not initiated. Ten patients were finally included in this study. Baseline characteristics for these patients are described in Table 1.
Figure 1.
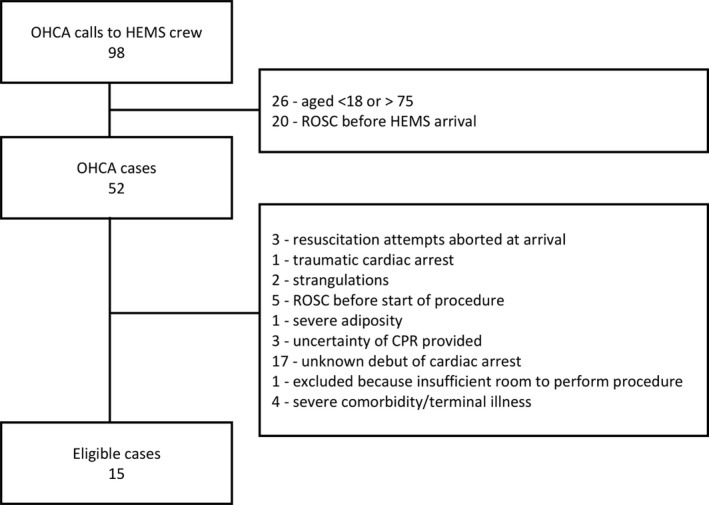
Flowchart of out‐of‐hospital cardiac arrest patients eligible for pre‐hospital resuscitative endovascular balloon occlusion of the aorta procedure. CPR indicates cardiopulmonary resuscitation; HEMS, helicopter emergency medical service; OHCA, out‐of‐hospital cardiac arrest; ROSC, return of spontaneous circulation.
Table 1.
Baseline Characteristics of Patients
| Baseline Characteristics | |
|---|---|
| Men, n (%) | 7 (70) |
| Age, mean (range), y | 62.7 (50–74) |
| Women | 59.0 (51–70) |
| Men | 64.3 (50–74) |
| Location, n (%) | |
| Public outdoors | 2 (20) |
| Public indoors | 1 (10) |
| Home indoors | 7 (70) |
| Time of day, n (%) | |
| Daytime (08–23) | 9 (90) |
| First monitored rhythm, n (%) | |
| Asystole | 6 (60) |
| PEA | 1 (10) |
| VF/VT | 3 (30) |
OHCA indicates out‐of‐hospital cardiac arrest; PEA, pulseless electrical activity; VF, ventricular fibrillation; VT, ventricular tachycardia.
The REBOA Procedure
The REBOA procedure was successful in all cases. The procedure was performed both indoors (n=8) and outside (n=2). One patient was included during nighttime. In 7 patients, a brief pause in chest compressions (10–20 seconds) was necessary to enable cannulation. In 2 patients, 2 cannulation attempts were needed, resulting in 80% success rate on the first attempt and 100% success rate on the second attempt. No major bleeding at puncture site, resistance to introduction of the equipment or equipment malfunction were experienced. In 2 patients, a left radial pulse present before occlusion disappeared after occlusion. The catheter was withdrawn 5 cm and the radial pulse reoccurred. In 2 patients, no radial pulse was found before occlusion, but a strong pulse was felt immediately after occlusion. Two patients achieved ROSC during the REBOA procedure, but before aortic occlusion. The guidewire and introducer were placed, and the procedure was temporarily paused. Both patients re‐arrested after a few minutes and the procedure was continued. Procedural data are presented in Table 2.
Table 2.
Relevant Procedural Data
| Mean | SD | Range | No. | |
|---|---|---|---|---|
| Dispatch to occlusion, min | 45.6 | 6.3 | 34 to 57 | 10 |
| Dispatch to ROSC, min | 53.3 | 8.2 | 37 to 58 | 6 |
| Procedure time, min | 11.7 | 3.2 | 8 to 16 | 10 |
| Occlusion time, min | 9.5 | 6.1 | 3 to 19 | 6 |
| Artery, diameter, mm | 5.9 | 1.2 | 3.6 to 7.4 | 10 |
| Vein, diameter, min | 9.3 | 2.8 | 5.0 to 12.9 | 10 |
Resuscitative endovascular balloon occlusion procedural times (minutes) and vessel size during resuscitation (mm). Occlusion times are only indicated for patients with return of spontaneous circulation. ROSC indicates return of spontaneous circulation.
Six out of 10 patients achieved ROSC, 3 were admitted to hospital and 1 survived >30 days. Four patients who achieved ROSC re‐arrested. Three re‐arrested after 5 to 6 minutes and 1 re‐arrested after 20 minutes. The 3 patients who experienced an early re‐arrest did not survive to hospital admission.
Changes in EtCO2 from time of occlusion (baseline) and at 30, 60 and 90 seconds are demonstrated in Figure 2. The results from the linear mixed effect model is demonstrated in Table 3. In 1 patient, the EtCO2‐value at time of occlusion was missing and the last observed value was carried forward. The estimated mean EtCO2 at aortic occlusion was 2.84 kPa and after 30 seconds the EtCO2 had increased by mean of 0.8 kPa (P=0.026). The EtCO2 increased by a mean of 1.75 kPa from baseline to an estimated mean of 4.6 kPa after 60 seconds (P<0.001).
Figure 2.
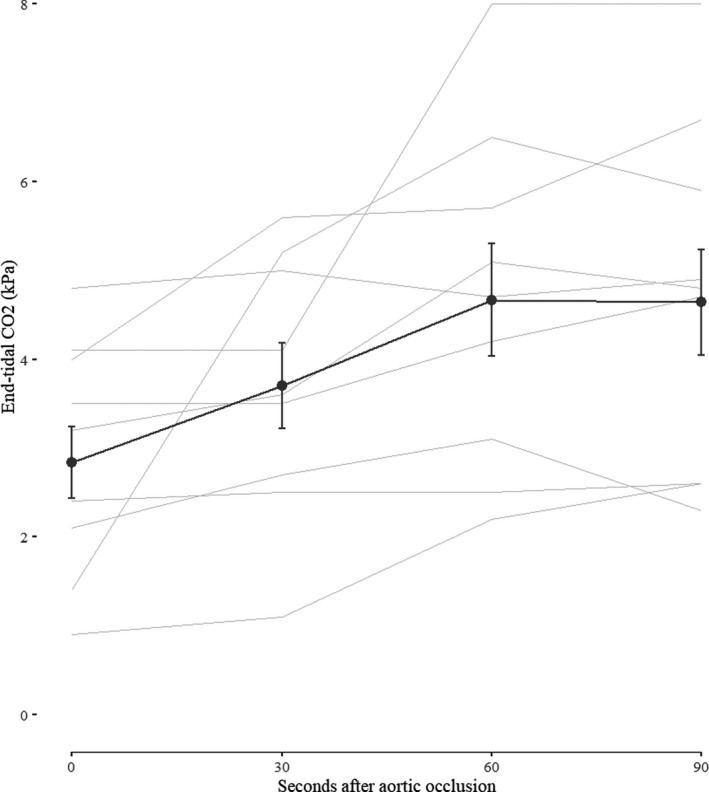
Observed changes in end‐tidal CO2 during the first 90 seconds after balloon occlusion of the aorta (mean±SE). Observed changes in end‐tidal CO2 for individual patients are plotted in the background (light grey), with the exception of 1 patient with missing observations at 30 and 60 seconds.
Table 3.
Changes in End‐Tidal CO2, Results From the Linear Mixed Effect Model
| Coef. | Estimate | 95% CI | P Value | |
|---|---|---|---|---|
| Parameters—fixed effects | ||||
| Intercept | β0 | 2.84 | (2.08–3.60) | |
| Change at 30 s | β1 | 0.79 | (0.09–1.49) | 0.026 |
| Change at 60 s | β2 | 1.75 | (0.90–2.60) | <0.001 |
| Change at 90 s | β3 | 1.80 | (0.77–2.83) | 0.001 |
| Parameters—random effects | ||||
| Variance random intercepts | b0,1 | 1.03 | (0.29–3.63) | |
| Variance random slopes | b1,j | 0.00022 | (0.00005–0.0009) | |
| Variance residual | εi,j | 0.50 | (0.25–0.99) | |
| Covariance (b0,1, b1,j) | 0.0046 | (−0.0097–0.0189) | ||
Model: yi,j=β0+β1×time 30 seconds+β2×time 60 seconds+β3×time 90 seconds+b0,1+b1,j×time+εi,j.
Linear mixed effect model with changes in end‐tidal CO2 (kPa) as dependent variable. The intercept corresponds to the estimated mean value at time of occlusion and the estimated mean changes at 30, 60, and 90 seconds are the added changes to baseline. Estimated with restricted maximum likelihood (unstructured covariance).
One patient was established on extra‐corporeal membrane oxygenation immediately after arrival to the hospital, before a coronary angiography was performed. The patient developed bowel ischemia on day 2 and a hemicolectomy was performed. The patient recovered successfully and was discharged after 8 days with a good cerebral outcome.
Safety Monitoring
The external safety monitoring group found no cases where the REBOA procedure influenced the ACLS quality. No intervention‐associated adverse events were found. It was noted that the REBOA procedure did not add unnecessary time on scene nor did it delay transport to hospital.
Size of Femoral Vessels
Ultrasound images before guidewire was placed were evaluated except for 1 patient where only images after guidewire placement was available. The mean width of the femoral artery was 5.9 mm (SD 1.2, 3.6–7.4) and the mean width of the femoral vein was 9.3 mm (SD 2.8, 5.0–12.9) (Table 2). Figure 3 shows 2 different ultrasound images from the femoral cannulation site.
Figure 3.
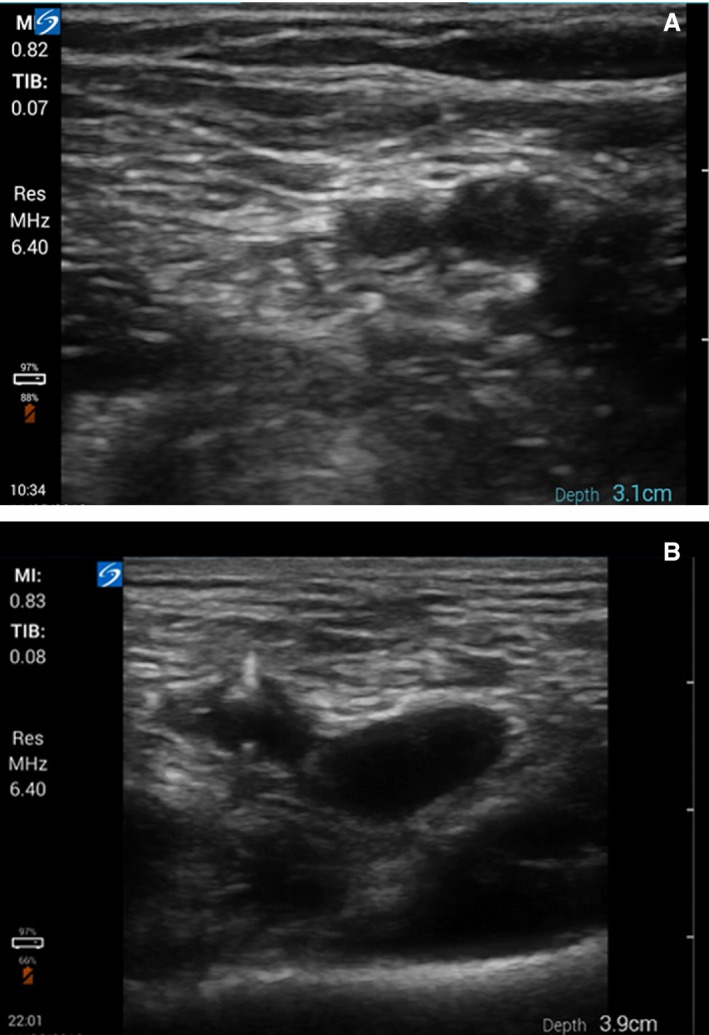
Ultrasound images from femoral cannulation site. A, A small artery (left) close to a larger vein (right). B, A greatly dilated vein (right) and a small artery (left), the guidewire is visible entering the artery.
Discussion
This is the first study to report that REBOA is feasible during OHCA in a pre‐hospital emergency service. The potential augmentation of circulation by the REBOA procedure is supported by a significant increase of EtCO2 after balloon inflation and the high proportion of immediate ROSC.
A pre‐hospital REBOA procedure is different from an in‐hospital procedure. Space may be constricted, light condition is often limited, weather conditions may interfere, the number of personnel is limited, there is no specialized intervention radiologist and there is no fluoroscopy to confirm placement of the catheter. Therefore, REBOA had to be tested in real life out‐of‐hospital settings to establish feasibility. Our observations agree with reports from trauma care26, 27 in that the REBOA procedure is feasible also in various out‐of‐hospital settings.
To implement REBOA in the pre‐hospital setting, we considered several issues related to the performance of the procedure. First, we had to either measure or calculate the length from the common femoral arterial puncture site to the aortic zone 1.28, 29, 30 Such calculations are not likely to be performed in an out‐of‐hospital scenario. We used a set length of guidewire and balloon catheter placement and found this to be adequate in the pre‐hospital setting. Second, ultrasound was used to avoid venous placement of the guidewire. Venous balloon inflation would decrease preload and must be avoided. The cannulation success rate found in this study implies that a structured training program and mandatory use of ultrasound22 can provide a high cannulation success rate in patients receiving CPR in the pre‐hospital environment. This observation agrees with reports from extra‐corporeal membrane oxygenation cannulation during cardiac arrest in the emergency department.31 Third, we used a non‐compliant, 20‐mm balloon, with an occlusion length of 30 mm. The descending aorta in the age span of eligible patients is ≈25 mm of width.32, 33 Using a 20‐mm balloon provides a nearly total obstruction of vascular volume without the risk of aortic rupture due to an oversized balloon, as earlier reported.34 Fourth, a simple pulse check in the left radial artery was performed in this study. A present radial pulse after occlusion of the aorta indicate that the balloon does not occlude left carotid circulation.
The increase in EtCO2 and high rate of ROSC after aortic occlusion suggest a centralization of the cardiac output. A brief pause to allow for cannulation was needed in 7 patients. Normal resuscitation effort was then continued while the rest of the equipment was inserted. Theoretically, the rise in EtCO2 could reflect reestablishment of effective CPR. However, the pause in compressions was ≈3 to 4 minutes before aortic occlusion and it is therefore likely that effective CPR was reestablished before aortic occlusion. Mean time from dispatch to balloon occlusion was 45.6 minutes, which imply that this centralization effect is seen even after a long low‐flow period. It is possible that this effect is even more pronounced if REBOA is applied earlier during ACLS efforts. The prolonged timespan after cardiac arrest is a potential cause for less effect from REBOA on outcome. In other settings it may be possible to perform REBOA earlier after cardiac arrest. One patient received CPR for a total of 58 minutes and had PEA at the time of balloon occlusion. Sustained ROSC was achieved, and the patient has survived with only minor sequela. This imply that REBOA could be an important therapeutic measure even in patients with prolonged ACLS.
Four patients who achieved ROSC re‐arrested. This suggest that deflation of the balloon is a critical moment and may increase the risk of re‐arrest. The increase in afterload provided by the balloon might increase coronary perfusion and oxygenate the myocardium, which can lead to an improvement in cardiac output. However, an elevated afterload created by the balloon will rapidly exhaust a stunned heart. Thus, the optimal time to perform balloon deflation is unknown. Studies from extra‐corporeal membrane oxygenation patients show that to reduce the afterload by unloading of the left ventricle can be beneficial in cardiogenic shock,35 but this is not feasible in this pre‐hospital setting. In this study, we choose to deflate the balloon slowly at the time of ROSC to avoid a sudden drop in afterload. We propose that a titrated deflation of the balloon using invasive arterial pressure measurements as target or the use of partial REBOA after ROSC might be beneficial in future studies.
In this study, several measures were done to ensure that the REBOA procedure did not preclude adequate ACLS. First, we used a chest compression machine to standardize the treatment. This was used in 30:2 mode, to enable less trained healthcare providers (ambulance personnel) to perform safe ventilations and limit the risk of barotrauma. Continuous or interrupted chest compressions are found to be of equal quality in ACLS.36 Second, the REBOA procedure was only initiated if paramedics from a ground ambulance were present, securing that standard ACLS was performed during the REBOA procedure. Third, in condition such as cold weather, insecure conditions on road and similar, the physician in charge could refrain from or abort the REBOA procedure.
The procedure was not performed in 5 eligible cases. In retrospect, it is difficult to determine the reason for this. It may imply that even with a strict protocol, there are numerous factors in the pre‐hospital setting that may interfere with the decision to do advanced interventions. This includes weather, temperature, safe surroundings, and compliance to protocol by physician and/or paramedic if rest and sleep have been sparse. These are expected and challenging factors in all pre‐hospital work.
Our findings on the size of the femoral vessels during CPR are consistent with other reports.37 The arteries are small and contracted, while the veins are dilated and almost double the size of the arteries. This illustrates the challenge of femoral arterial access during CPR and the importance of ultrasound‐guided cannulation.
We recognize that this study has several limitations. First, it is a single‐center study, with a small number of physicians and paramedics involved. Of these, only 5 physicians performed the procedure and all had recently participated in a structured REBOA training program.22 All physicians involved in this study are anesthesiologists familiar with the Seldinger technique, working in a HEMS system with a homogeneous set of skills and the results cannot be generalized to physicians with another training. However, the study is relevant to several services in Europe, where mainly anesthesiologists participate in the physician‐manned HEMS. Second, the study did not include autopsies in the non‐survivors, which could have screened for lesions attributed to the REBOA catheter. Third, this study only demonstrates the feasibility of REBOA in OHCA. Whether REBOA improves patient outcome must be demonstrated in clinical trials. In addition, the frequency of adverse effects from REBOA cannot be assessed in a small‐scale study. Finally, the specifics of our OHCA REBOA procedure was as decided by the study group. Further studies are needed to develop the performance of OHCA REBOA.
Conclusions
To our knowledge, this is the first study to assess the pre‐hospital use of REBOA on non‐traumatic cardiac arrest patients. This study shows that REBOA in non‐traumatic OHCA is feasible and that the REBOA procedure does not interfere with the quality of the ACLS. The use of REBOA as an adjunct treatment in OHCA significantly increased the EtCO2 after occlusion, which indicates an increase in centralized circulation during CPR. Whether or not REBOA during OHCA may improve patient outcome must be assessed in clinical trials.
Sources of Funding
This study was funded by the Norwegian Air Ambulance Foundation and the Department of Emergency Medicine and Pre‐Hospital Services, St. Olav University Hospital, Trondheim, Norway. The funders had no part in the design or execution of this study, nor the collection or management of the data, or in the preparation, review, and approval of the manuscript.
Disclosures
One of the authors (Dr Søvik) has stock ownership and a board position in Reboa Medical. Dr Brede, Dr Nordseth, and Dr Krüger are partly funded by the Norwegian Air Ambulance Foundation. The remaining authors have no disclosures to report.
Supporting information
Table S1. Procedure for Insertion of REBOA for OHCA
Table S2. Checklist REBOA
Acknowledgments
The authors wish to thank the participants in this study and the members of the safety monitoring group; Dr Daniel Bergum, Dr Martin Herje, Dr Odd‐Eirik Elden, and Dr Anne Fresvig.
(J Am Heart Assoc. 2019;8:e014394 DOI: 10.1161/JAHA.119.014394.)
The abstract of this work was presented at the Resuscitation Science Symposium, November 16–17, 2019, in Philadelphia, PA.
References
- 1. Tjelmeland IBM, Nilsen JE, Kramer‐Johansen J, Andersson LJ, Bratland S, Hafstad AK, Haug B, Langoergen J, Larsen AI, Lindner T, Soereide E, Skogvoll E. Norsk hjertestansregister Årsrapport for 2017 med plan for forbedringstiltak. 71. Available at: https://www.kvalitetsregistre.no/sites/default/files/8_arsrapport_2017_norsk_hjertestansregister.pdf. Accessed November 26, 2018.
- 2. Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low‐flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osborn LA, Brenner ML, Prater SJ, Moore LJ. Resuscitative endovascular balloon occlusion of the aorta: current evidence. Open Access Emerg Med. 2019;11:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes CW. Use of an intra‐aortic balloon catheter tamponade for controlling intra‐abdominal hemorrhage in man. Surgery. 1954;36:65–68. [PubMed] [Google Scholar]
- 5. Daley J, Morrison JJ, Sather J, Hile L. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non‐traumatic cardiac arrest. Am J Emerg Med. 2017;35:731–736. [DOI] [PubMed] [Google Scholar]
- 6. Sesma J, Sara MJ, Espila JL, Arteche A, Saez MJ, Labandeira J. Effect of intra‐aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20:453–462. [DOI] [PubMed] [Google Scholar]
- 7. Nozari A, Rubertsson S, Wiklund L. Improved cerebral blood supply and oxygenation by aortic balloon occlusion combined with intra‐aortic vasopressin administration during experimental cardiopulmonary resuscitation. Acta Anaesthesiol Scand. 2000;44:1209–1219. [DOI] [PubMed] [Google Scholar]
- 8. Nozari A, Rubertsson S, Wiklund L. Intra‐aortic administration of epinephrine above an aortic balloon occlusion during experimental CPR does not further improve cerebral blood flow and oxygenation. Resuscitation. 2000;44:119–127. [DOI] [PubMed] [Google Scholar]
- 9. Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40:171–180. [DOI] [PubMed] [Google Scholar]
- 10. Rubertsson S, Bircher NG, Alexander H. Effects of intra‐aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med. 1997;25:1003–1009. [DOI] [PubMed] [Google Scholar]
- 11. Barton C, Manning JE, Batson N. Effect of selective aortic arch perfusion on median frequency and peak amplitude of ventricular fibrillation in a canine model. Ann Emerg Med. 1996;27:610–616. [DOI] [PubMed] [Google Scholar]
- 12. Manning JE, Murphy CA, Hertz CM, Perretta SG, Mueller RA, Norfleet EA. Selective aortic arch perfusion during cardiac arrest: a new resuscitation technique. Ann Emerg Med. 1992;21:1058–1065. [DOI] [PubMed] [Google Scholar]
- 13. Paradis NA, Rose MI, Gawryl MS. Selective aortic perfusion and oxygenation: an effective adjunct to external chest compression‐based cardiopulmonary resuscitation. J Am Coll Cardiol. 1994;23:497–504. [DOI] [PubMed] [Google Scholar]
- 14. Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 15. Nozari A, Rubertsson S, Gedeborg R, Nordgren A, Wiklund L. Maximisation of cerebral blood flow during experimental cardiopulmonary resuscitation does not ameliorate post‐resuscitation hypoperfusion. Resuscitation. 1999;40:27–35. [DOI] [PubMed] [Google Scholar]
- 16. Spence PA, Lust RM, Chitwood WR, Iida H, Sun YS, Austin EH. Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res. 1990;49:217–221. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki A, Taki K, Kamiya K, Miyake T. Cerebral blood flow during open‐chest cardiac massage with occlusion of the descending aorta in dogs. Resuscitation. 1985;13:69–75. [DOI] [PubMed] [Google Scholar]
- 18. Gedeborg R, Silander HC, Rubertsson S, Wiklund L. Cerebral ischaemia in experimental cardiopulmonary resuscitation—comparison of epinephrine and aortic occlusion. Resuscitation. 2001;50:319–329. [DOI] [PubMed] [Google Scholar]
- 19. Aslanger E, Golcuk E, Oflaz H, Yilmaz A, Mercanoglu F, Bugra Z, Umman B, Nisanci Y. Intraaortic balloon occlusion during refractory cardiac arrest. A case report. Resuscitation. 2009;80:281–283. [DOI] [PubMed] [Google Scholar]
- 20. Deakin CD, Barron DJ. Haemodynamic effects of descending aortic occlusion during cardiopulmonary resuscitation. Resuscitation. 1996;33:49–52. [DOI] [PubMed] [Google Scholar]
- 21. McGreevy D, Dogan E, Toivola A, Bilos L, Pirouzran A, Nilsson K, Hörer T. Endovascular resuscitation with aortic balloon occlusion in non‐trauma cases: first use of ER‐REBOA in Europe. J Endovasc Resusc Trauma Manag. 2017;1:42. [Google Scholar]
- 22. Brede JR, Lafrenz T, Krüger AJ, Soevik E, Steffensen T, Kriesi C, Steinert M, Klepstad P. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in non‐traumatic out‐of‐hospital cardiac arrest: evaluation of an educational programme. BMJ Open. 2019;9:e027980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norwegian Resuscitation Council . Norsk Resuscitasjonsråd. Available at: http://nrr.org/no/. Accessed September 9, 2017.
- 24. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D'Este K, Finn J, Halperin H, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports. Circulation. 2004;110:3385–3397. [DOI] [PubMed] [Google Scholar]
- 25. Fitzmaurice G, Laird N, Ware J. Chapter 8. Linear mixed effect models In: Applied Longitudinal Analysis. 2nd ed JohnWiley & Sons, Inc, Hoboken, New Jersey; 2011. [Google Scholar]
- 26. Butler FK, Holcomb JB, Shackelford S, Barbabella S, Bailey JA, Baker JB, Cap AP, Conklin CC, Cunningham CW, Davis M, DeLellis SM, Dorlac WC, DuBose JJ, Eastridge B, Fisher AD, Glasser JJ, Gurney J, Jenkins DA, Johannigman J, King DR, Kotwal RS, Littlejohn LF, Mabry RL, Martin MJ, Miles EA, Montgomery HR, Northern DM, O'Connor KC, Rasmussen TE, Riesberg JC, Spinella PC, Stockinger Z, Strandenes G, Via DK, Weber MA. Advanced resuscitative care in tactical combat casualty care: TCCC guidelines change 18‐01:14 October 2018. J Spec Oper Med. 2018;18:37–55. [DOI] [PubMed] [Google Scholar]
- 27. Sadek S, Lockey DJ, Lendrum RA, Perkins Z, Price J, Davies GE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre‐hospital setting: an additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation. 2016;107:135–138. [DOI] [PubMed] [Google Scholar]
- 28. Okada Y, Narumiya H, Ishi W, Iiduka R. Anatomical landmarks for safely implementing resuscitative balloon occlusion of the aorta (REBOA) in zone 1 without fluoroscopy. Scand J Trauma Resusc Emerg Med. 2017;25:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linnebur M, Inaba K, Haltmeier T, Rasmussen TE, Smith J, Mendelsberg R, Grabo D, Demetriades D. Emergent non‐image‐guided resuscitative endovascular balloon occlusion of the aorta (REBOA) catheter placement: a cadaver‐based study. J Trauma Acute Care Surg. 2016;81:453–457. [DOI] [PubMed] [Google Scholar]
- 30. MacTaggart JN, Poulson WE, Akhter M, Seas A, Thorson K, Phillips NY, Desyatova AS, Kamenskiy AV. Morphometric roadmaps to improve accurate device delivery for fluoroscopy‐free resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2016;80:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voicu S, Henry P, Malissin I, Jean‐Guillaume D, Koumoulidis A, Magkoutis N, Yannopoulos D, Logeart D, Manzo‐Silberman S, Peron N, Deye N, Megarbane B, Sideris G. Improving cannulation time for extracorporeal life support in refractory cardiac arrest of presumed cardiac cause—comparison of two percutaneous cannulation techniques in the catheterization laboratory in a center without on‐site cardiovascular surgery. Resuscitation. 2018;122:69–75. [DOI] [PubMed] [Google Scholar]
- 32. McComb BL, Munden RF, Duan F, Jain AA, Tuite C, Chiles C. Normative reference values of thoracic aortic diameter in American College of Radiology Imaging Network (ACRIN 6654) arm of the National Lung Screening Trial. Clin Imaging. 2016;40:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, Thanassoulis G, Isselbacher EM, Hoffmann U, O'Donnell CJ. Distribution, determinants and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol. 2013;111:1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Søvik E, Stokkeland P, Storm BS, Åsheim P, Bolås O. The use of aortic occlusion balloon catheter without fluoroscopy for life‐threatening post‐partum haemorrhage. Acta Anaesthesiol Scand. 2012;56:388–393. [DOI] [PubMed] [Google Scholar]
- 35. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Santo PD, Mathew R, So DY, Takeda K, Garan AR, Karmpaliotis D, Takayama H, Kirtane AJ, Hibbert B. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. [DOI] [PubMed] [Google Scholar]
- 36. Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillantcourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373:2203–2214. [DOI] [PubMed] [Google Scholar]
- 37. Hilty WM, Hudson PA, Levitt MA, Hall JB. Real‐time ultrasound‐guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29:331–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Procedure for Insertion of REBOA for OHCA
Table S2. Checklist REBOA


