Abstract
Background
Transcatheter aortic valve replacement (TAVR) has solidified the importance of a heart team and revolutionized patient selection for surgical aortic valve replacement (SAVR). It is unknown if hospital ability to offer TAVR impacts SAVR outcomes. We investigated outcomes after SAVR between TAVR and non‐TAVR centers.
Methods and Results
Hospitalizations of patients aged ≥50 years, undergoing elective SAVR between January 2012 and September 2015, in the National Readmission Database (NRD) were included. Multivariable logistic, linear, and generalized logistic regression models were used to adjust for patient and hospital characteristics and estimate association between undergoing SAVR at a TAVR center, compared with a non‐TAVR center. The association between TAVR volumes and these outcomes were also assessed. SAVR hospitalizations (n = 32 198) were identified; 22 066 (69%) at TAVR and 10 132 (31%) at non‐TAVR centers. SAVRs at TAVR centers had lower odds of inpatient mortality (odds ratio 0.67, 95% CI 0.55–0.82) and discharge to skilled nursing facility (odds ratio 0.92, 95% CI 0.85–0.99), compared with non‐TAVR centers. There was no difference in LOS (change in estimate −0.09, 95% CI −0.26 to 0.08) or 30‐day re‐admission (odds ratio 0.95, 95% CI 0.88–1.03). SAVRs performed at the highest TAVR volume centers had the lowest inpatient mortality, compared with non‐TAVR centers (odds ratio 0.43 95% CI 0.29–0.63).
Conclusions
Patients undergoing SAVR at TAVR centers are more likely to survive and have better discharge disposition than patients undergoing SAVR at non‐TAVR centers. Whether this represents benefits of a heart‐team approach to care or differences in patient selection for SAVR when TAVR is unavailable requires further study.
Keywords: aortic valve replacement, aortic valve stenosis, transcatheter aortic valve implantation
Subject Categories: Quality and Outcomes, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery, Valvular Heart Disease
Clinical Perspective
What Is New?
This study demonstrates improved inpatient outcomes after surgical aortic valve replacement (SAVR) at centers that offer both SAVR and transcatheter aortic valve replacement (TAVR) as compared with those that offer SAVR alone.
This study also shows that the highest volume TAVR centers have improved inpatient outcomes after SAVR when compared with smaller volume TAVR centers.
What Are the Clinical Implications?
As TAVR expands its reach to include patients across the entire surgical risk spectrum, it becomes imperative to understand the impact of TAVR on SAVR outcomes.
Introduction
Calcific aortic stenosis (AS) is a progressive disease which results in significant morbidity and mortality without valve replacement.1, 2 With advances in medical technology, the management of this disease process has benefitted from both surgical and interventional approaches. While surgical aortic valve replacement (SAVR) has been the standard of care for decades, transcatheter aortic valve replacement (TAVR) has now emerged as a suitable treatment strategy across the entire surgical risk spectrum.3, 4, 5, 6, 7, 8
In 2018, the American Association for Thoracic Surgery (AATS), the American College of Cardiology (ACC), the Society for Cardiovascular Angiography and Interventions (SCAI), and the Society of Thoracic Surgeons (STS) released an expert consensus on operator and institutional requirements for TAVR encompassing physician experience/expertise and the presence of a multidisciplinary approach.9 Additionally, in 2019, the Centers for Medicare and Medicaid Services released updated Medicare coverage criteria for individual hospitals seeking to perform TAVR based on AVR and catheterization volumes.10 As a result, many centers in the United States that offer SAVR are still unable to offer TAVR to their patients because of these national coverage criteria. As the Food Drug Administration recently approved the use of TAVR for low‐risk populations after results of the PARTNER 3 (The Placement of Aortic Transcatheter Valves Trial 3) and Medtronic Corevalve Evolut R System TAVR trials, it becomes more important than ever to continue to assess the utility of SAVR in contemporary populations, as well as the interplay between these 2 modalities of treatment.7, 8 More specifically, exploring the potential benefit to patients of hospitals offering both surgical and interventional modalities of treatment is crucial to optimizing patient care. Few studies, if any, have assessed whether outcomes after SAVR differ between TAVR and non‐TAVR centers, or whether the TAVR volume at TAVR centers impacts patient outcomes.
Methods
Study Design and Population
The data that support the findings of this study are available from the corresponding author on reasonable request. This study was reviewed and approved by the University of North Carolina Institutional Review Board and the requirement for informed consent was waived. Hospitalizations for SAVR were identified using the National Readmission Database (NRD). The NRD database is a constituent of the Healthcare Cost and Utilization Project (HCUP) family of healthcare databases developed through a Federal‐State‐Industry partnership and sponsored by the Agency for Healthcare Research and Quality. The NRD includes >15 million discharges from 22 states and accounts for 51% of the total US resident population and 49% of all US hospitalizations. It is an all‐payer healthcare database in the United States that is nationally representative and contains verified patient linkage numbers which allows patients to be tracked across hospitals within a state, each year, allowing for all in‐state hospital readmissions to be captured.11 The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnostic and procedural codes were used to identify eligible patients.
Adults aged ≥50 years, diagnosed with AS (ICD‐9‐CM 424.1), and who underwent elective SAVR (35.21 and 35.22) between January 1, 2012 and September 30, 2015 (after which ICD‐10 codes were implemented) were eligible for inclusion. Patients also diagnosed with congenital aortic disorders (746.3), rheumatic aortic stenosis (395.0–395.9), or hypertrophic obstructive cardiomyopathy (425.11) or who underwent additional vascular procedures (00.61–00.69 and 36.00–36.99) such as coronary artery bypass grafting, or were not residents of the state they underwent surgery in were excluded. Only a patient's first SAVR within each year was included. Patients were categorized as either undergoing SAVR at a hospital that performed at least one TAVR (35.05 and 35.06) or at a non‐TAVR center that year. Yearly TAVR volume at TAVR centers was also calculated by counting the total number of TAVR procedures; in 2015, the total number of TAVR procedures from January to September were divided by 0.75 to estimate yearly volume.
The primary outcomes of interest were discharge disposition, average length of stay (LOS), and 30‐day readmission. Disposition was categorized as: (1) routine/home health care; (2) transfer to a short‐term hospital; (3) transfer to skilled nursing facility, intermediate care facility, or other care facility; and (4) died.
Statistical Analysis
Differences in the distributions of demographics and hospital characteristics of SAVR patients treated at TAVR and non‐TAVR centers were compared using Chi square and Student t tests as appropriate. Discharge disposition, LOS, and 30‐day readmission were assessed using the same methods. Patients who died during their index hospitalization were excluded from all readmission analyses. Quarterly trends in the proportion of SAVR procedures at TAVR and non‐TAVR centers were estimated using Poisson regression.
Multivariable generalized logistic, linear, and logistic regression were used to estimate the odds of discharge disposition, change in average LOS, and odds of 30‐day readmission after SAVR, respectively. Multivariable models were adjusted for year of surgery, sex, age, Charlson comorbidity index (CCI), primary insurance type, median household income for the patient's ZIP code, hospital type, and hospital size. CCI was calculated using the Deyo et al coding scheme.12 Age and CCI were modeled as restricted quadratic splines to allow for the most flexibility and fewest assumptions when modeling a continuous variable.
An additional analysis assessing the potential impact of TAVR volume was also performed. TAVR centers were stratified into low (<25 procedures per year), medium (25–100 procedures per year), and high (>100 procedures per year) TAVR centers. Patient outcomes were again compared with non‐TAVR centers using multivariable generalized logistic, linear, and logistic regression models described above. All analyses were performed using SAS 9.4 (SAS Inc., Cary, NC).
Results
There were 32 198 patients identified and included in this study; 22 066 (69%) underwent SAVR at a TAVR center and 10 132 (31%) underwent SAVR at a non‐TAVR center. Between 2012 and 2015, the proportion of SAVRs performed at TAVR centers increased from 65% to 72%, P<0.0001 (Figure 1). Patients treated at TAVR centers were similar in age, sex, CCI, and primary insurance type when compared with those treated at non‐TAVR centers (Table 1). Patients treated at TAVR centers were more likely to live in a zip code in the highest estimated income quartile (32% versus 23%, P<0.0001), more likely to be treated at an urban teaching hospital (85% versus 50%, P<0.0001), and more likely to be treated at a large hospital (78% versus 62%, P<0.0001).
Figure 1.
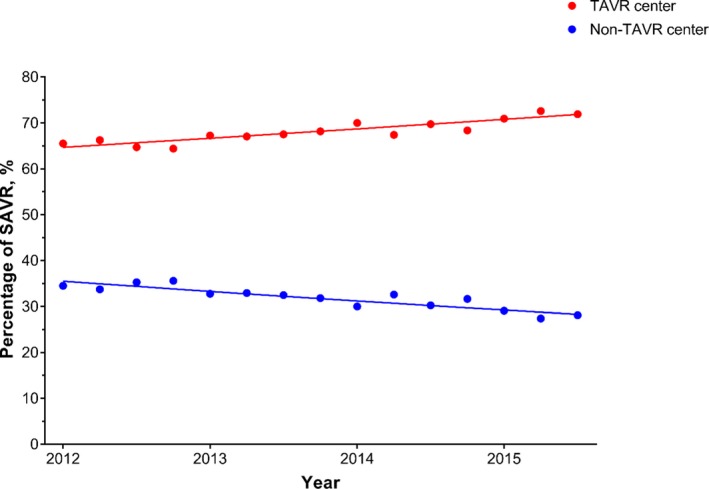
Trends in the proportion of SAVR procedures being done at TAVR and non‐TAVR centers. Percentage of SAVR performed at TAVR (red) and non‐TAVR (blue) centers during the study period (January 2012–September 2015). Over this time, the proportion of SAVRs performed at TAVR centers increased from 65% to 72%, P<0.0001. SAVR indicates surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Table 1.
Baseline Characteristics of Patients Undergoing Surgical Aortic Valve Replacement Between 2012 and 2015, Stratified by TAVR‐Center Status
| TAVR Center 22 066 (69%) | Non‐TAVR Center 10 132 (31%) | |
|---|---|---|
| Age, y, mean (SD) | 70 (9.6) | 70 (9.5) |
| Men, n (%) | 12 786 (58) | 5734 (57) |
| CCI, mean (SD) | 1.4 (1.4) | 1.3 (1.4) |
| CCI components, n (%) | ||
| Prior MI | 637 (3) | 246 (2) |
| Congestive heart failure | 6182 (28) | 2568 (25) |
| Peripheral vascular disease | 4236 (19) | 1491 (15) |
| Cerebrovascular disease | 333 (2) | 144 (1) |
| Dementia | 23 (<1) | 16 (<1) |
| COPD | 4402 (20) | 2063 (20) |
| Rheumatologic disease | 641 (3) | 301 (3) |
| Peptic ulcer disease | 26 (<1) | <11 |
| Diabetes mellitusa | 5954 (27) | 2958 (29) |
| Renal disease | 3077 (14) | 1137 (13) |
| Hemiplegia or paraplegia | 159 (1) | 50 (<1) |
| Cancerb | 548 (2) | 236 (2) |
| Liver diseasec | 202 (1) | 85 (1) |
| HIV/AIDS | 25 (<1) | <11 |
| Primary insurance, n (%) | ||
| Medicaid/Medicare | 15 477 (70) | 7196 (71) |
| Private | 6038 (27) | 2664 (26) |
| Other/self‐pay | 512 (2) | 243 (2) |
| Median household income,d n (%) | ||
| Low | 3718 (17) | 2179 (22) |
| Medium | 4974 (23) | 2770 (28) |
| High | 6023 (28) | 2683 (27) |
| Highest | 7026 (32) | 2337 (23) |
| Hospital type, n (%) | ||
| Urban, non‐teaching | 3288 (15) | 4722 (47) |
| Urban, teaching | 18 689 (85) | 5080 (50) |
| Rural | 89 (<1) | 330 (3) |
| Hospital bed size,e n (%) | ||
| Small | 1088 (5) | 1012 (10) |
| Medium | 3775 (17) | 2812 (28) |
| Large | 17 203 (78) | 6308 (62) |
CCI indicates Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; TAVR, transcatheter aortic valve replacement.
Includes patients diagnosed with complicated and/or uncomplicated disease.
Includes patients diagnosed with a malignancy (including leukemia and lymphoma) and/or metastatic solid tumor.
Includes patients diagnosed with mild, moderate, or severe liver disease.
Estimated median household income for the patient's zip code, stratified into quartiles.
Hospital size is based on the number of hospital beds; cut points were chosen within each region and hospital type strata so that approximately one third of hospitals would appear in each category.
Overall, 25 457 (79%) patients were discharged home after surgery (TAVR center: 17 619, 80%; non‐TAVR center: 7838, 77%), 196 (<1%) were transferred to another hospital (TAVR center: 157, 1%; non‐TAVR center: 39, <1%), 7026 (22%) were transferred to a skilled nursing facility (TAVR center: 3973, 18%; non‐TAVR center: 3053, 20%), and 530 (2%) died (TAVR center: 317, 1%; non‐TAVR center: 213, 2%) (Table 2). Median LOS was 6 days (IQR 5–8) at both TAVR and non‐TAVR centers. Among patients discharged alive (n=31 668), 3978 (13%) were readmitted within 30 days (TAVR center: 2693, 12%; non‐TAVR center: 1285, 13%).
Table 2.
Crude and Adjusted Outcomes After SAVR Between Being Treated at a TAVR Center, Compared With a Non‐TAVR Center, on Discharge Disposition, 30‐Day Readmission, and LOS
| TAVR Center | Non‐TAVR Center | Crude | Adjusteda | |||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Discharge dispositionb | ||||||
| Routine | 17 619 (80) | 7838 (77) | Ref | ··· | Ref | ··· |
| Transfer, short term hospital | 157 (1) | 39 (<1) | 1.79 (1.26–2.55) | 0.001 | 2.09 (1.43–3.07) | 0.0002 |
| Transfer, skilled nursing facility | 3973 (18) | 3053 (20) | 0.87 (0.82–0.92) | <0.0001 | 0.92 (0.85–0.99) | 0.02 |
| Died | 317 (1) | 213 (2) | 0.66 (0.56–0.79) | <0.0001 | 0.67 (0.55–0.82) | <0.0001 |
| 30‐d readmissionc | 2693 (12) | 1285 (13) | 0.95 (0.88–1.02) | 0.15 | 0.95 (0.88–1.03) | 0.26 |
| Median (IQR) | Median (IQR) | CIE (95% CI) | P Value | CIE (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Length of stay, d | 6 (5–8) | 6 (5–8) | 0.04 (−0.12 to 0.20) | 0.63 | −0.09 (−0.26 to 0.08) | 0.29 |
CIE indicates change in estimate; IQR, interquartile range; LOS, length of stay; OR, odds ratio; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Adjusted for year of surgery, age, sex, primary insurance, median household income in the patient's zip code, Charlson comorbidity index, hospital teaching status, and hospital size; age, and Charlson comorbidity index were modeled as a restricted quadratic splines.
Compared with routine discharge.
Only assessed among patients discharged alive after SAVR.
After adjusting for demographics, comorbidities, and hospital characteristics, patients undergoing SAVR at TAVR centers, compared with non‐TAVR centers, were less likely to die (OR 0.67, 95% CI 0.55–0.82) and were less likely to be transferred to a skilled nursing facility (OR 0.92, 95% CI 0.85–0.99) (Table 2). There was no significant difference in average LOS, or 30‐day readmission. After adjustment, women were slightly more likely than men to be readmitted within 30 days of discharge, among those discharged alive (OR 1.13, 95% CI 1.06–1.21) and had poorer discharge dispositions (Table 3).
Table 3.
Incidence of Patient Outcomes After SAVR, Stratified by Sex
| Women n (%) | Men n (%) | OR (95% CI)a | P Value | |
|---|---|---|---|---|
| Discharge dispositionb | ||||
| Routine | 9838 (72) | 15 619 (84) | Ref | … |
| Transfer, short term hospital | 97 (1) | 99 (1) | 1.56 (1.17–2.07) | 0.003 |
| Transfer, skilled nursing facility | 3440 (25) | 2575 (14) | 1.96 (1.84–2.08) | <0.0001 |
| Died | 303 (2) | 227 (1) | 2.01 (1.68–2.40) | <0.0001 |
| 30‐d readmissionc | 1805 (14) | 2173 (12) | 1.13 (1.06–1.21) | 0.0004 |
| Median (IQR) | Median (IQR) | CIE (95% CI)a | P Value | |
|---|---|---|---|---|
| Length of stay, d | 6 (5–9) | 6 (5–8) | 0.51 (0.37–0.66) | <0.0001 |
CIE indicates change in estimate; IQR, interquartile range; OR, odds ratio; SAVR, surgical aortic valve replacement.
Adjusted for TAVR center status, year of surgery, age, primary insurance, median household income in the patient's zip code, Charlson comorbidity index, hospital teaching status, and hospital size; age, and Charlson comorbidity index were modeled as a restricted quadratic splines.
Compared with routine discharge.
Only assessed among patients discharged alive after SAVR.
In TAVR centers, the incidence of transfer to skilled nursing facility decreased over time (19% in 2012 to 17% in 2015, P=0.005) while the incidence of routine discharges and inpatient mortality remained consistent (78% in 2012 to 81% in 2015, P=0.07 and 2% in 2012 to 1% in 2015, P=0.07, respectively). The incidence of routine/home health discharges (78% in 2012 to 77% in 2015, P=0.71), transfers to skilled nursing facilities (20% in 2012 to 20% in 2015, P=0.45), and inpatient mortality (2% in 2012 to 2% in 2015, P=0.69) have remained consistent in patients treated at non‐TAVR centers. Average LOS decreased at TAVR centers (average LOS 8.5 days in 2012 to 7.4 days in 2015, P<0.0001) but remained consistent at non‐TAVR centers (average LOS 7.8 days in 2012 to 7.7 days in 2015, P=0.51).
Between 2012 and 2015, the median number of SAVRs per year at both TAVR (19 surgeries per year, IQR 10–32, range 1–154) and non‐TAVR centers has remained relatively consistent (7 surgeries per year, IQR 3–12, range 1–89). However, since the introduction of TAVR in the United States, the overall proportion of SAVRs being performed at TAVR centers has steadily increased (Figure 1).
In 2012, 5 hospitals classified as high volume TAVR centers (>100 TAVR procedures per year). By 2015, this number had increased to 41 hospitals that were high volume TAVR centers. The number of medium volume TAVR centers (25–100 procedures per year) increased from 33 hospitals in 2012 to 140 in 2015 and the number of small volume TAVR centers (<25 procedures per year) decreased from 151 hospitals in 2012 to 79 hospitals in 2015.
While high volume TAVR centers only constituted 8% of total SAVR volume in 2012, it increased to 20% of all SAVR volume in 2015 (Figure 2). Hospitals classified as low and medium volume TAVR centers in 2015 performed less SAVRs in 2015 than hospitals classified as low and medium centers in 2012 (low: 14 per year in 2012 [IQR 7–24] to 10 per year in 2015 [IQR 5–17]; medium: 39 per year in 2012 [IQR 32–49] to 20 per year in 2015 [IQR 14–31]). However, high volume TAVR centers in 2015 performed slightly more SAVR procedures than centers classified as high volume in 2012 (35 per year in 2012 [IQR 34–60] to 39 per year in 2015 [IQR 26–56]).
Figure 2.
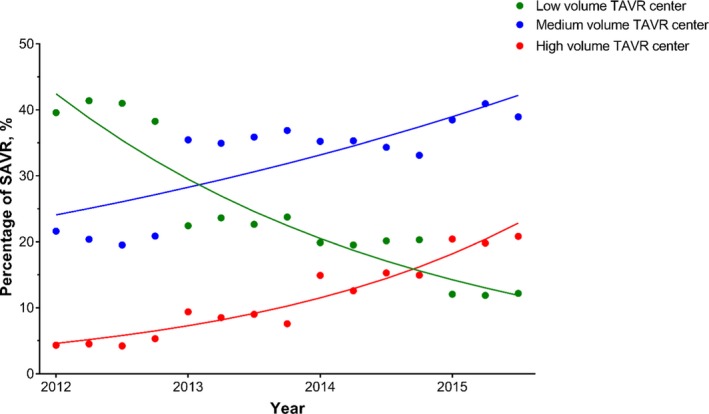
Trends in the proportion of SAVR procedures being done at TAVR centers, stratified by TAVR volume. Percentage of SAVR performed at low volume (green), medium volume (blue), and high volume (red) TAVR centers from January 2012 to September 2015. Centers classified as high and medium volume had an increase in the proportion of SAVR done over this time period while centers classified as low volume had a decrease. SAVR indicates surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Patient outcomes, stratified by TAVR volume, are depicted in Table 4. After adjustment, the highest TAVR volume centers had the lowest incidence of inpatient mortality after SAVR (Figure 3, Table 5). TAVR volume did not appear to affect differences in skilled nursing discharges between TAVR and non‐TAVR centers, P=0.10.
Table 4.
Incidence of Patient Outcomes After SAVR, Stratified by TAVR Center Designation and Volume
| Non‐TAVR Center 10 132 (31%) | Low Volume TAVR Center 7400 (23%) | Medium Volume TAVR Center 10 677 (33%) | High Volume TAVR Center 3989 (12%) | |
|---|---|---|---|---|
| Discharge disposition, n (%) | ||||
| Routine | 7838 (77) | 5830 (79) | 8555 (80) | 3234 (81) |
| Transfer, short term hospital | 39 (<1) | 48 (1) | 80 (1) | 29 (1) |
| Transfer, skilled nursing facility | 2042 (20) | 1409 (19) | 1876 (18) | 688 (17) |
| Died | 213 (2) | 113 (2) | 166 (2) | 38 (1) |
| 30‐d readmissiona, n (%) | 1285 (13) | 918 (13) | 1288 (12) | 487 (12) |
| LOS, d, median (IQR) | 6 (5–8) | 6 (5–8) | 6 (5–8) | 6 (5–8) |
IQR indicates interquartile range; LOS, length of stay; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Only assessed among patients discharged alive after SAVR.
Figure 3.
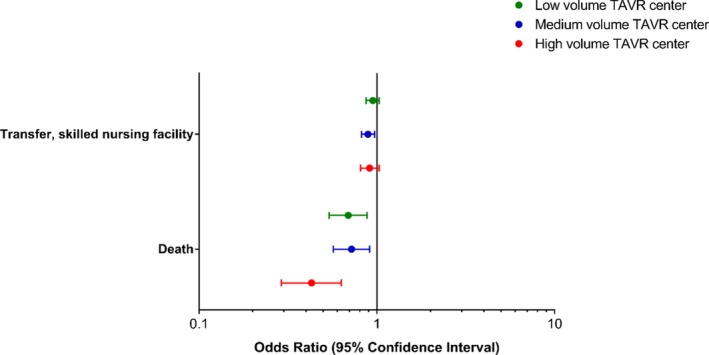
Adjusted odds of discharge disposition among patients treated at a TAVR center, compared with non‐TAVR center, stratified by TAVR volume. Odds ratios of transfer to skilled nursing facility and death at low volume, medium volume, and high volume TAVR centers. SAVR patients treated at TAVR centers of all volumes had a significantly lower odds of death at discharge as compared with patients at non‐TAVR centers, even after adjusting for hospital and patient characteristics. SAVRs performed at the highest TAVR volume centers also had the lowest inpatient mortality. TAVR centers also had lower rates of discharge to skilled nursing as compared with non‐TAVR centers; however, this was only statistically significant for medium volume centers. SAVR indicates surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Table 5.
Adjusted Association Between TAVR Center Volume, Compared With a Non‐TAVR Center, on Discharge Disposition, Length of Stay, and 30‐Day Readmission After SAVR
| Low volume OR (95% CI)a | Medium Volume OR (95% CI)a | High Volume OR (95% CI) a | P Valueb | |
|---|---|---|---|---|
| Discharge dispositionc | ||||
| Routine | Ref | Ref | Ref | ··· |
| Transfer, short term hospital | 1.85 (1.19–2.88) | 2.23 (1.46–3.40) | 2.60 (1.51–4.47) | 0.002 |
| Transfer, skilled nursing facility | 0.95 (0.87–1.03) | 0.89 (0.82–0.97) | 0.91 (0.81–1.03) | 0.10 |
| Died | 0.69 (0.54–0.88) | 0.72 (0.57–0.91) | 0.43 (0.29–0.63) | <0.0001 |
| 30‐d readmissiond | 0.94 (0.86–1.04) | 0.96 (0.87–1.05) | 0.98 (0.87–1.12) | 0.64 |
| CIE (95% CI)a | CIE (95% CI)a | CIE (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Length of stay, d | −0.02 (−0.22 to 0.19) | −0.19 (−0.38 to 0.00) | 0.01 (−0.25 to 0.28) | 0.14 |
CIE indicates change in estimate; OR, odds ratio; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Wald test assessing whether effect of undergoing surgery at TAVR center on outcomes differs across TAVR volume, degrees of freedom=3.
Adjusted for year of surgery, age, sex, primary insurance, median household income in the patient's zip code, Charlson comorbidity index, hospital teaching status, and hospital size; age, and Charlson comorbidity index were modeled as a restricted quadratic splines.
Compared with routine discharge.
Only assessed among patients discharged alive after SAVR.
Discussion
In this study comparing outcomes after SAVR in TAVR versus non‐TAVR centers, we found that patients who underwent SAVR at TAVR centers were similar in age, sex, and primary insurance coverage when compared with those who underwent SAVR at non‐TAVR centers. TAVR centers were more likely to be large, urban teaching hospitals and treat patients from higher median income areas. We also saw that the proportion of SAVRs performed at TAVR centers increased significantly over time. When comparing the 2 groups over an almost 4‐year period, our study found no difference in LOS or 30‐day readmission, even after adjusting for patient and hospital characteristics. However, those undergoing SAVR at TAVR centers had lower inpatient mortality and were less likely to be discharged to skilled nursing. Women were slightly more likely to be readmitted within 30 days of discharge and had poorer discharge dispositions. Between 2012 and 2015, the incidence of discharge to skilled nursing and average LOS declined among patients undergoing SAVR at TAVR centers, but there was no change over time at non‐TAVR centers.
As TAVR expands its reach to patients of lower surgical risk, it becomes imperative to investigate the impact of TAVR on SAVR populations. In our analysis, we found that patients undergoing SAVR at TAVR centers had lower inpatient mortality; moreover, as TAVR volume increased, there was an association with lower risk of inpatient mortality after SAVR. This is likely attributable to multiple factors. First, the addition of TAVR to a care facility in conjunction to SAVR allows for a multidisciplinary heart team approach for the treatment of severe AS, whereby cardiac surgeons, interventionalists, and other important role players may work in collaboration for more effective risk stratification, surgical planning, and treatment.13, 14 This strategy is also ideal for patients with multiple cardiac comorbidities such as severe AS in the presence of multi‐vessel coronary artery disease. The heart team approach to care has been incorporated into the guidelines of multiple professional organizations and is mandated by both the US Food and Drug Administration and the Centers for Medicare and Medicaid Services.8 It is possible that non‐TAVR centers are performing SAVR in higher surgical risk patients, since TAVR is not an available option at those centers, and thus leading to poorer outcomes.
Second, TAVR centers were also more likely to be high volume SAVR centers—which is associated with higher operator experience and known to have a favorable association with surgical outcomes.15, 16, 17 TAVR volume has also been associated with improved patient outcomes. In a recent study, Vemulapalli et al found an inverse volume‐outcomes relationship between TAVR volume and 30‐day mortality after transfemoral TAVR.18 In a study limited to Medicare patients, Kundi et al found a similar association between TAVR volume by quartile and both 30‐day and 1‐year mortality after SAVR.19 The current study differed in that we grouped TAVR centers into one cohort rather than quartiles, used an all‐payer database, and looked at additional markers of patient outcomes.20 Our study was similar in that we saw an inverse association between TAVR volume and inpatient mortality. However, results by Kundi et al showed SAVR volumes increased in non‐TAVR centers and low/medium volume TAVR centers and decreased in high volume TAVR centers. Our study, in contrast, showed a decrease in the proportion of SAVR at non‐TAVR centers and low volume TAVR centers and an increase in medium/high volume centers. As the current study population consisted of only 70% Medicare/Medicaid patients as compared with 100% in the Kundi et al study, these differences may be attributable to Medicare patients and those without private insurance being more likely to be referred to centers without a TAVR program and to smaller volume TAVR centers over time. A separate study by Kundi et al found that hospitals with higher SAVR mortality rates before offering TAVR went on to have worse 30‐day and 1‐year TAVR mortality once a TAVR program was initiated.21 Our results provide a corollary to this study in that we found that higher TAVR volumes, which is a known surrogate for improved TAVR outcomes, translated into better SAVR results. Whether this is because of patient selection for SAVR versus TAVR, operator experience, or resource availability is yet to be determined.
We also found that average LOS and skilled nursing facility discharges after SAVR decreased between 2012 and 2015 only at TAVR centers, but no change was seen at non‐TAVR centers. The relationship between improved outcomes and discharge disposition after SAVR has been previously reported. In a study by Henry et al, patients discharged to a skilled nursing facility were 2.5 times more likely to die at 1‐ and 2‐years post discharge, compared with those discharged home after valve surgery.22 The improvement in these quality indices are especially important in this current era of constrained healthcare resources. It is possible that the benefits of improved perioperative care and multidisciplinary heart team in TAVR centers extends to the surgical cohort as well, leading to a reduction in LOS and better discharge disposition for surgical patients at TAVR centers.
This study should be considered in light of a few limitations. First, we were unable to account for some clinical covariates (eg, Society of Thoracic Surgeons Predicted Risk of Mortality score) which could result in some unmeasured confounding and explain some of the differences we saw, particularly if they differed between TAVR and non‐TAVR centers. Second, we were unable to assess long‐term outcomes, as NRD only allows for limited follow‐up on patients. Third, comorbidities were identified using diagnosis codes, which likely underestimated the overall prevalence. Fourth, we are unable to determine if this study includes repeated observations of patients who underwent >1 SAVR during the study period. Fifth, we were unable to examine race or ethnicity which is often an important factor in assessing patient outcomes, as it is not included in NRD. Lastly, there was potential for coding errors and differences in coding practices across the hospitals included in the database. However, we suspect that these differences are random and would not be expected to differ between TAVR and non‐TAVR centers.
Conclusions
Our study suggests that patients undergoing SAVR at TAVR centers have lower inpatient mortality and discharges to skilled nursing facilities. Moreover, when TAVR centers were stratified by case load, TAVR volume was inversely associated with post‐SAVR inpatient mortality. Between 2012 and 2015, improvements in quality indices for SAVR (LOS, skilled nursing facility discharges) only occurred at TAVR centers. Whether these patterns are because of improved resources and expertise at TAVR centers or are a result of patient selection for SAVR based on TAVR availability is still unknown. These data highlight the utility of centers which are able to offer comprehensive care for patients with aortic valve disease. Furthermore, given the continued decline in the percentage of patients receiving SAVR at non‐TAVR centers, the differences in outcomes may continue to diverge. Additional research is needed to better understand whether the improved SAVR outcomes seen at TAVR centers—particularly high volume TAVR centers—are related to more appropriate patient selection or higher quality of care.
Disclosures
Dr Strassle has received salary support from researchEZ LLC and Dr Arora's spouse has a proprietary role in researchEZ LLC. Dr Cavender has received consulting fees from Edwards Lifesciences. Dr Vavalle received consulting fees from Edwards Lifesciences. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e013794 DOI: 10.1161/JAHA.119.013794.)
References
- 1. Généreux P, Stone GW, O'Gara PT, Marquis‐Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67:2263–2288. [DOI] [PubMed] [Google Scholar]
- 2. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99:396–400. [DOI] [PubMed] [Google Scholar]
- 3. Schwarz F, Baumann P, Manthey J, Hoffman M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. [DOI] [PubMed] [Google Scholar]
- 4. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 5. Smith CR, Leon MB, Mack MJ, Miller C, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 6. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 7. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie C, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros W, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 8. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchetche D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 9. Bavaria JE, Tommaso CL, Brindis RG, Carroll JD, Deeb GM, Feldman TE, Gleason TG, Horlick EM, Kavinsky CJ, Kumbhani DJ, Miller DC, Seals AA, Shahian DM, Shemin RJ, Sundt TM 3rd, Thourani VH. 2018 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: operator and institutional requirements for transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73:385. [DOI] [PubMed] [Google Scholar]
- 10. DHHS Centers for Medicare & Medicaid Services . Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG‐ 00430R)[serial online]. 2019. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=293&type=Open&bc=ACAAAAAAQCAA&. Accessed August 1, 2019.
- 11. Agency for Healthcare Research and Quality . NRD Overview. Healthcare Cost and Utilization Project (HCUP)[serial online]. August 2018. Available at: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed September 1, 2018.
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 13. Holmes DR, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;5:903–907. [DOI] [PubMed] [Google Scholar]
- 14. Holmes DR, Mack MJ. Transcatheter valve therapy: a professional society overview from the American College of Cardiology Foundation and the Society of Thoracic surgEons. Ann Thorac Surg. 2011;92:380–389. [DOI] [PubMed] [Google Scholar]
- 15. Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 16. Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Umegaki T, Kunisawa S, Nakajima Y, Kamibayashi T, Fushimi K, Imanaka Y. Volume‐outcome relationship in surgical aortic valve replacement for patients with aortic valve stenosis: a retrospective analysis of administrative data. J Heart Cardiovasc Res. 2017;1:1–7. [Google Scholar]
- 18. Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, Kumbhani DJ, Ruiz CE, Thourani VH, Hanzel G, Gleason TG, Herrmann HC, Brindis RG, Bavaria JE. Procedural volume and outcomes for transcatheter aortic‐valve replacement. N Engl J Med. 2019;380:2541–2550. [DOI] [PubMed] [Google Scholar]
- 19. Kundi H, Strom JB, Valsdottir LR, Elmariah S, Popma JJ, Shen C, Yeh RW. Trends in isolated surgical aortic valve replacement according to hospital‐based transcatheter aortic valve replacement volumes. JACC Cardiovasc Interv. 2018;11:2148–2156. [DOI] [PubMed] [Google Scholar]
- 20. Strassle PD, Arora S, Vavalle JP. Trends in isolated surgical aortic valve replacement according to hospital‐based transcatheter aortic valve replacement volumes. JACC Cardiovasc Interv. 2018;11:2148–2156.30343022 [Google Scholar]
- 21. Kundi H, Popma JJ, Khabbaz KR, Chu LM, Strom JB, Valsdottir LR, Shen C, Yeh RW. Association of hospital surgical aortic valve replacement quality with 30‐day and 1‐year mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2019;4:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henry L, Halpin L, Hunt S, Holmes SD, Ad N. Patient disposition and long‐term outcomes after valve surgery in octogenarians. Ann Thorac Surg. 2012;94:744–750. [DOI] [PubMed] [Google Scholar]


