Abstract
Background
Malignant profiles were identified by imaging profiles and unfavorable outcomes that have poor response to reperfusion therapy. Many trials have used this profile in their inclusion criteria including large‐vessel occlusion acute ischemic stroke trials. We aimed to redefine the cutoff values for malignant profile in acute ischemic stroke patients with large‐vessel occlusion regardless of reperfusion therapy.
Methods and Results
Consecutive acute ischemic stroke patients with anterior large‐vessel occlusion were prospectively extracted from the National Cerebral and Cardiovascular Center Stroke Registry between March 2014 and December 2017. Diffusion‐Weighted Imaging‐Alberta Stroke Program Early Computed Tomography Score (DWI‐ASPECTS) and diffusion‐weighted imaging lesion ischemic core volume (VolDWI) were measured in acute ischemic stroke patients with large‐vessel occlusion with or without treatment. Unfavorable outcome was defined as a modified Rankin Scale score 5 to 6 at 3 months, and optimal DWI‐ASPECTS and VolDWI for unfavorable outcome were assessed. In total, 198 patients (111 men, 77±13 years old) were enrolled. Median DWI‐ASPECTS was 7 (5‐9), and median VolDWI was 55 (6‐134) mL. Among the patients, 72 (36%) patients underwent reperfusion therapy, and 83 (42%) had unfavorable outcomes. The threshold values for a malignant profile on receiver operating characteristic curve analysis for DWI‐ASPECTS and VolDWI were 4 (area under the curve 0.78, P<0.01; sensitivity 0.71, specificity 0.75) and 71 mL (area under the curve 0.80, P<0.01; sensitivity 0.76, specificity 0.77), respectively.
Conclusions
The cutoff values for our redefined malignant profile were DWI‐ASPECTS 4 and VolDWI 71 mL with no selection bias for reperfusion therapy in the real‐world clinical practice.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov Unique identifier: NCT02251665
Keywords: large‐vessel occlusion, malignant profile, stroke
Subject Categories: Ischemic Stroke, Magnetic Resonance Imaging (MRI), Quality and Outcomes
Clinical Perspective
What Is New?
We found the cutoff values for predicting unfavorable outcome were Diffusion‐Weighted Imaging‐Alberta Stroke Program Early Computed Tomography Score (DWI‐ASPECTS) 4 and DWI lesion ischemic core volume 71 mL with no selection bias for reperfusion therapy in the real‐world clinical practice.
What Are the Clinical Implications?
The optimal DWI‐ASPECTS and treatable upper limit of core volumes are suggested from patient‐selected randomized controlled trials in most idealistic conditions.
Our results align with the previous highly selective randomized controlled trials, although favorable outcomes were still observed in endovascular treatment patients above this threshold, and DWI‐ASPECTS may not be an adequate substitute for DWI lesion ischemic core volume in cases with DWI‐ASPECTS ≤4.
In our study not all patients had attempted reperfusion therapy, and we found a variety of outcomes in large‐core patients; therefore, the optimal ischemic core volume threshold for unfavorable outcome may be higher than 71 mL in endovascular treatment patients.
Introduction
Recently, the efficacy and safety of endovascular treatment (EVT) in acute ischemic stroke (AIS) patients due to anterior large‐vessel occlusion (LVO) were established for acute‐onset including wake‐up stroke.1, 2 Especially in anterior LVO AIS, the volume of the magnetic resonance (MR) diffusion‐weighted imaging (DWI) lesion predicts poor outcomes after intravenous thrombolysis (IVT)3 and symptomatic intracerebral hemorrhage after EVT,4 and ischemic core volumes between 70 and 100 mL have been suggested to be reliable cutoffs to identify so‐called “malignant profiles.”5, 6
The optimal indication for EVT is currently determined by tissue‐based evaluation, which is imaging dependent and not time dependent. Thus, acute‐phase imaging of AIS has become increasingly important. Volumetrically assessing the ischemic core with an automated image postprocessing system within 5 minutes is extremely useful for deciding whether to perform EVT for LVO AIS patients.
In selected patients with AIS within 6 to 24 hours of last known normal who have LVO in the anterior circulation, obtaining MR imaging (MRI) DWI is recommended to aid in patient selection for EVT in the AHA/ASA (American Heart Association/American Stroke Association) Guideline,7 and DWI lesion ischemic core volume (VolDWI) is known to predict a poor outcome.8 However, because automated volume calculation software is not necessarily available in all facilities, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early Computed Tomography Score (DWI‐ASPECTS) has been reported as an alternative index. In addition, recent EVT randomized controlled trials have used imaging profile for inclusion criteria to exclude patients with a large ischemic core.
The aims of the present study were to clarify the correlation between DWI‐ASPECTS and VolDWI measured by automated software in anterior LVO AIS and to redefine the malignant profile for DWI‐ASPECTS and VolDWI regardless of reperfusion therapy in real‐world clinical practice.
Materials and Methods
Patients, Clinical Assessments, and Outcome Measures
Consecutive AIS patients were prospectively extracted from the National Cerebral and Cardiovascular Center Stroke Registry (ClinicalTrials.gov: NCT02251665),9 where MRI was implemented as first‐line pretreatment imaging. Patients who fulfilled the following eligibility criteria between March 2014 and December 2017 were included: (1) imaging within 24 hours of AIS onset; (2) patients with clinical outcome obtained at 3 months after onset; (3) baseline MR angiography to confirm an internal carotid artery (ICA) or middle cerebral artery (MCA) M1 proximal (horizontal segment of MCA within <5 mm from the terminal bifurcation of the ICA) occlusion; and (4) AIS confirmed by baseline MRI (3 T; DWI: 3 directions; b=0‐1000 s/mm2: 6‐mm contiguous slices). Because ASPECTS best indicates MCA M1 proximal occluded territories in scoring, patients with anterior cerebral artery (ACA), MCA M1 distal, M2 or M3, posterior circulation LVO were excluded from this analysis to fully match the area within DWI‐ASPECTS.
Clinical characteristics including baseline demographics (sex, age, premorbid modified Rankin Scale score,10 cerebrovascular risk factors), baseline National Institutes of Health Stroke Scale score,11 stroke subtype, imaging findings (arterial occlusion site on baseline MR angiography, baseline DWI‐ASPECTS,12 DWI and perfusion MRI, and VolDWI), treatment details (reperfusion therapy; use of IVT, EVT, and combination therapy of IVT and EVT), time metrics (times from stroke onset to qualifying imaging and times from stroke onset to reperfusion), and outcomes (modified Rankin Scale score 3 months after onset, symptomatic intracranial hemorrhage) were retrospectively collected and examined. Stroke subtype was determined according to the Trial of Org 10172 in Acute Stroke Treatment criteria13 by board‐certified stroke neurologists (K.F., Kanta T). Tandem occlusion was defined as both ICA and MCA occlusion. The time from stroke onset to qualifying imaging was defined as the time from symptom onset to the start of obtaining scout view imaging of baseline MRI. Onset to reperfusion time was defined as the time from symptom onset to successful reperfusion confirmed or procedure terminated with no reperfusion in the patients who underwent EVT. The time of onset was defined as either the time point when the symptom appeared or when “last‐known‐well” if the symptom onset was unknown.
Patients received IVT (0.6 mg/kg alteplase) according to the Japanese‐approved standard care protocol14 if eligible before EVT. All interventional procedures were performed by the Japanese Society for Neuroendovascular Therapy board‐certified neurointerventionalists.
A favorable outcome was defined as modified Rankin Scale score 0 to 2 at 3 months after onset, and an unfavorable outcome was defined as modified Rankin Scale score 5 to 6 at 3 months after onset. A malignant profile was defined as optimal DWI‐ASPECTS and VolDWI for unfavorable outcome. Successful reperfusion after EVT was defined as modified Thrombolysis in Cerebral Infarction scale grade 2b or 3.15 A symptomatic intracranial hemorrhage was defined as an increase of 4 points or more in the National Institutes of Health Stroke Scale score or death within 48 hours.16
Statistical Analyses
Two stroke neurologists (T.Y., K.F.) independently evaluated baseline DWI and DWI‐ASPECTS. When the readers’ assessments differed, final consensus was obtained with a third reader (Kanta T). Interobserver agreement for DWI‐ASPECTS was assessed using weighted‐κ and intraclass correlation coefficients. VolDWI was measured retrospectively using an automated processing system (RAPID version 4.7, iSchemaView, Menlo Park, CA) by apparent diffusion coefficient index <620×10−6 mm2/s, and unreliable blurry lesions which RAPID could not detect were manually measured (MIPAV, Ver 8.0.2, Bethesda, MA). The correlation between DWI‐ASPECTS and VolDWI was determined using the Spearman rank correlation coefficient. To identify the optimal VolDWI threshold, receiver operating characteristic curves were assessed. Significance was set at P<0.05 for all tests. The statistical analyses were performed by JMP 13.2.1 statistical software (SAS Institute Inc, Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board of NCVC (approved number M23‐073‐4). Written, informed consent for reperfusion therapy, as well as thrombectomy, was obtained from each patient or a relative if the patient had communication problems, and the opt‐out method for patient recruitment was used.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Total of 649 patients had AIS due to LVO, and of these, 198 patients met our study criteria. Patients were excluded based on missing cases for outcome at 3 months after onset (n=5), different occluded vessels (anterior cerebral artery, n=14; MCA M1 distal, n=152; M2 or M3, n=156; basilar artery, n=34; vertebral artery, n=20; posterior cerebral artery, n=50) or MRI contraindications (for example pacemaker implantation, n=20) (Figure 1). Of these, 198 patients (111 [56%] men; mean±standard deviation age, 77±13 years) were included. The baseline clinical characteristics of these patients are presented in Table 1. The median (interquartile range [IQR]) baseline National Institutes of Health Stroke Scale score was 21 (14‐27). Median (IQR) values for DWI‐ASPECTS and VolDWI were 5 (3‐8) and 55 (6‐134) mL, respectively. The median (IQR) time from stroke onset to qualifying imaging was 247 (64‐634) minutes. Reperfusion therapy was performed in 72 (36%), IVT in 39 (20%), EVT in 54 (27%), and combination therapy of IVT and EVT in 21 (11%) patients. A favorable outcome was observed in 48 (24%) patients, and unfavorable outcome was seen in 83 (42%), death at 3 months in 34 (17%), and symptomatic intracranial hemorrhage was seen in 12 (6%).
Figure 1.
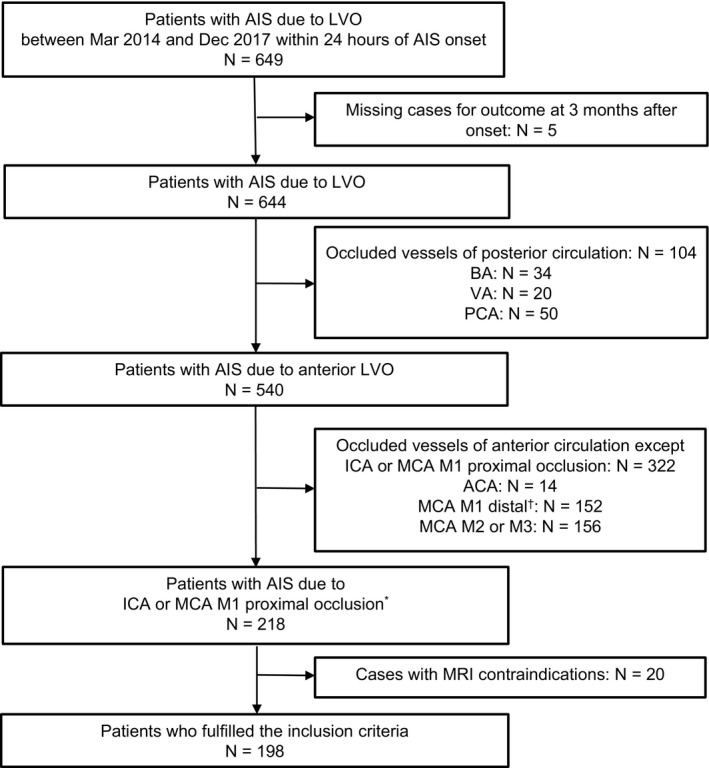
Flow diagram of patient selection. ACA indicates anterior cerebral artery; AIS, acute ischemic stroke; BA, basilar artery; ICA, internal carotid artery; LVO, large vessel occlusion; MCA, middle cerebral artery; MRI, magnetic resonance imaging; PCA, posterior cerebral artery; VA, vertebral artery. *MCA M1 proximal indicates horizontal segment of MCA within <5 mm from the terminal bifurcation of the ICA <5 mm. † MCA M1 distal indicates horizontal segment of MCA except M1 proximal.
Table 1.
Baseline Characteristics
| Total (n=198) | |
|---|---|
| Age, y | 79±13 |
| Male | 111 (56) |
| Premorbid modified Rankin scale score | 0 (0‐3) |
| Hypertension | 139 (70) |
| Diabetes mellitus | 39 (20) |
| Dyslipidemia | 84 (43) |
| Current smoking | 50 (25) |
| Atrial fibrillation | 129 (66) |
| Baseline NIH Stroke Scale score | 21 (14‐27) |
| Stroke subtype | |
| Large‐artery athrosclerosis | 29 (14) |
| Cardioembolism | 139 (70) |
| Others | 30 (16) |
| Imaging findings | |
| DWI‐ASPECTS | 5 (3‐8) |
| DWI‐ASPECTS ≤4 | 88 (44) |
| Diffusion and perfusion MRI | 7 (4) |
| DWI lesion ischemic core volume, mL | 55 (6‐134) |
| DWI lesion ischemic core volume >71 mL | 89 (45) |
| Arterial occlusion site on baseline MR angiography | |
| Internal carotid artery | 117 (59) |
| Middle cerebral artery M1 proximal | 73 (37) |
| Tandem | 8 (4) |
| Treatment details | |
| Reperfusion therapy | 72 (36) |
| Intravenous thrombolysis | 39 (20) |
| Endovascular treatment | 54 (27) |
| Combination therapy | 21 (11) |
| Time metrics | |
| Process measures, min | |
| Time from stroke onset to qualifying imaging | 247 (64‐634) |
| Outcomes | |
| Modified Rankin scale score 3 mo after onset | 4 (3‐5) |
| Favorable outcome | 48 (24) |
| Unfavorable outcome | 83 (42) |
| Symptomatic intracranial hemorrhage | 12 (6) |
Data are mean±SD for age; median (interquartile range) for premorbid modified Rankin scale score, baseline NIH Stroke Scale score, DWI‐ASPECTS, DWI ischemic core volume, time from stroke onset to qualifying imaging, and modified Rankin scale score at 3 mo after onset; and number of patients (%) for others. ASPECTS indicates Alberta Stroke Programme Early CT Score; CT, computed tomography; DWI‐ASPECTS, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early CT Score; MR, magnetic resonance; MRI, magnetic resonance imaging.
The interobserver intraclass correlation coefficient of DWI‐ASPECTS between readers was 0.766 (95% CI 0.693‐0.786), and the weighted‐κ statistic of DWI‐ASPECTS was 0.985 (95% CI, 0.981‐0.989), which showed excellent agreement. Figure 2 shows the box‐and‐whisker plot of baseline DWI‐ASPECTS and VolDWI. There was a strong negative correlation between DWI‐ASPECTS and VolDWI (ρ=−0.901; P<0.001). Although 3 stroke neurologists evaluated the DWI‐ASPECTS with an extremely high correlation, complete agreement was not obtained. The threshold values of DWI‐ASPECTS and VolDWI by receiver operating characteristic curve analysis for predicting unfavorable outcome were 4 (area under the curve [AUC] 0.78; sensitivity 0.71, specificity 0.75) and 71 mL (AUC 0.80; sensitivity 0.76, specificity 0.77), respectively (Figures 3 and 4). In total, there were 89 patients (45%) with VolDWI >71 mL and 88 patients (44%) with DWI‐ASPECTS ≤4.
Figure 2.
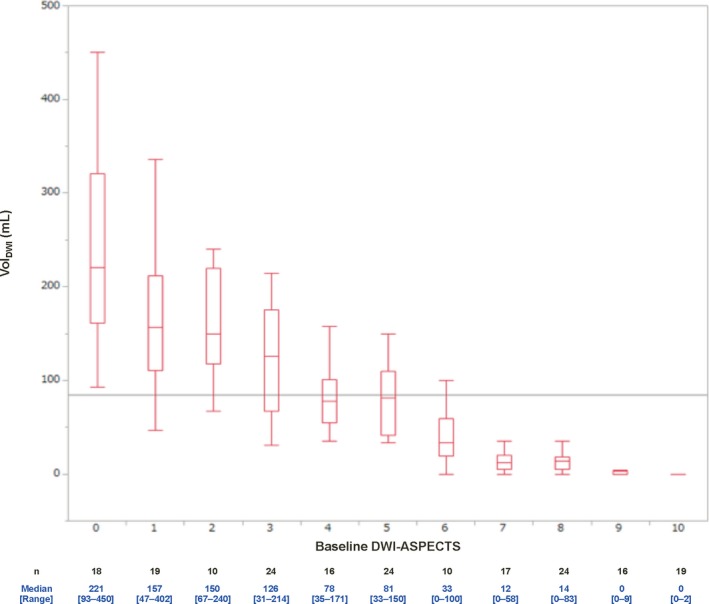
Box‐and‐whisker plot of baseline DWI‐ASPECTS and VolDWI. Boxes indicate interquartile range; whiskers, extreme values; horizontal lines in each box, median; and horizontal gray line, mean. CT indicates computed tomography; DWI, Diffusion‐Weighted Imaging; DWI‐ASPECTS, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early CT Score; VolDWI, DWI lesion ischemic core volume.
Figure 3.
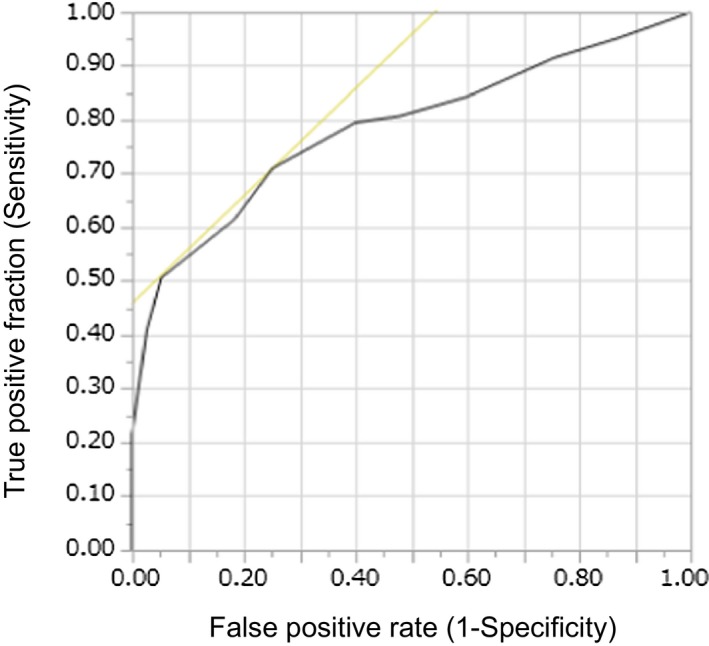
Receiver operating characteristic curves of DWI‐ASPECTS for predicting the malignant profile for the unfavorable outcomes. CT indicates computed tomography; DWI, diffusion‐weighted imaging; DWI‐ASPECTS, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early CT Score.
Figure 4.
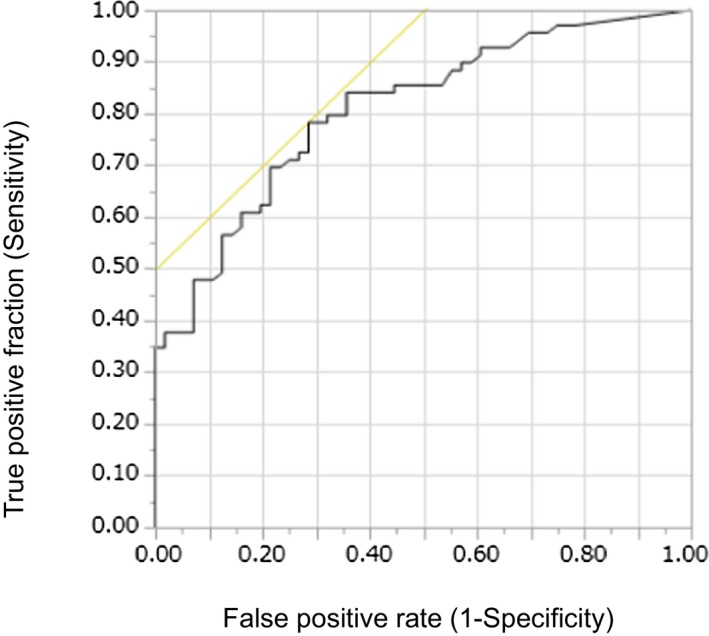
Receiver operating characteristic curves of VolDWI for predicting the malignant profile for the unfavorable outcomes. VolDWI indicates diffusion‐weighted imaging lesion ischemic core volume.
According to these results, we identified 2 groups with and without divergence between DWI‐ASPECTS and VolDWI. In the first group with DWI‐ASPECTS ≥7 (76 cases), the median VolDWI was 4 mL and the range was intermediate (0‐83 mL), which suggested that DWI‐ASPECTS can substitute for VolDWI in these cases. In the second group with DWI‐ASPECTS <7 (122 cases), the median VolDWI was 114 mL, but the range was wide (40‐450 mL) (Table 2). Examining the 39 AIS patients who underwent EVT in the second group, 56% (22/39) had favorable outcomes, and 13% (5/39) had unfavorable outcomes.
Table 2.
DWI‐ASPECTS Cutoff Points and Corresponding DWI Lesion Ischemic Core Volume
| DWI‐ASPECTS | DWI Lesion Ischemic Core Volume, mL | |||
|---|---|---|---|---|
| n | Median | IQR | Range | |
| <4 | 72 | 152 | 114 to 215 | 31 to 450 |
| ≥4 | 126 | 17 | 0 to 56 | 0 to 171 |
| <5 | 88 | 137 | 90 to 207 | 31 to 450 |
| ≥5 | 110 | 11 | 0 to 85 | 0 to 150 |
| <6 | 112 | 124 | 75 to 183 | 31 to 450 |
| ≥6 | 86 | 5 | 0 to 18 | 0 to 100 |
| <7 | 122 | 114 | 67 to 180 | 40 to 450 |
| ≥7 | 76 | 4 | 0 to 15 | 0 to 83 |
| <7 | 139 | 100 | 47 to 163 | 0 to 450 |
| ≥8 | 59 | 0 | 0 to 9 | 0 to 83 |
CT indicates computed tomography; DWI, diffusion‐weighted imaging; DWI‐ASPECTS, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early CT Score; IQR, interquartile range.
In the group with DWI‐ASPECTS ≤4 (88 cases), 3.4% (3/88) had favorable outcomes, and 67% (59/88) had unfavorable outcomes overall. Examining the 10 patients who underwent EVT in this group, we found 20% (2/10) had favorable outcomes, and 30% (3/10) had unfavorable outcomes. In the group with DWI‐ASPECTS ≤4 and VolDWI ≥71 mL (73 cases), only 1% (1/73) had favorable outcomes, and 78% (57/73) had unfavorable outcomes overall. Examining the 5 patients who underwent EVT in this group, we found 20% (1/5) had favorable outcomes, and 60% (3/5) had unfavorable outcomes. One patient who had a favorable outcome was 57 years old, and the lesion intensity was faint in the cortex with DWI‐ASPECTS 4 and VolDWI 102 mL.
The baseline clinical characteristics of patients who underwent EVT are presented in Table 3. There were 54 EVT eligible patients, and of these, the median (IQR) of DWI‐ASPECTS was 7 (5‐9), the median value of VolDWI (IQR) 17 (0‐58) mL, and the median time from stroke onset to qualifying imaging (IQR) 153 (71‐407) minutes. The rate of successful reperfusion after EVT was 93%. Threshold values by receiver operating characteristic curve analysis for DWI‐ASPECTS and VolDWI for unfavorable outcome in these patients were 5 (AUC 0.51; sensitivity 0.33, specificity 0.85) and 58 mL (AUC 0.65; sensitivity 0.56, specificity 0.80), respectively.
Table 3.
Baseline Characteristics of Patients Who Underwent Endovascular Treatment
| Total (n=54) | |
|---|---|
| Age, y | 73±13 |
| Male | 24 (44) |
| Premorbid modified Rankin scale score | 0 (0‐1) |
| Hypertension | 35 (66) |
| Diabetes mellitus | 11 (21) |
| Dyslipidemia | 20 (38) |
| Current smoking | 17 (32) |
| Atrial fibrillation | 35 (67) |
| Baseline NIH Stroke Scale score | 20 (13‐24) |
| Stroke subtype | |
| Large‐artery athrosclerosis | 5 (9) |
| Cardioembolism | 41 (76) |
| Others | 8 (15) |
| Imaging findings | |
| DWI‐ASPECTS | 7 (5‐9) |
| Diffusion and perfusion MRI | 4 (7) |
| DWI lesion ischemic core volume, mL | 17 (0‐58) |
| Arterial occlusion site on baseline MR angiography | |
| Internal carotid artery | 44 (81) |
| Middle cerebral artery M1 proximal | 4 (7) |
| Tandem | 6 (11) |
| Treatment details | |
| Intravenous thrombolysis | 21 (39) |
| Time metrics | |
| Process measures, min | |
| Time from stroke onset to qualifying imaging | 153 (71‐407) |
| Time from qualifying imaging to femoral puncture | 51 (37‐66) |
| Time from femoral puncture to reperfusion | 59 (37‐86) |
| Outcomes | |
| Modified Rankin scale score 3 mo after onset | 3 (1‐4) |
| Favorable outcome | 22 (45) |
| Unfavorable outcome | 45 (83) |
| Successful reperfusion | 50 (94) |
| Symptomatic intracranial haemorrhage | 2 (4) |
Data are mean±SD for age; median (interquartile range) for premorbid modified Rankin scale score, baseline NIH Stroke Scale score, DWI‐ASPECTS, DWI ischemic core volume, time from stroke onset to qualifying imaging, time from qualifying imaging to femoral puncture, time from femoral puncture to reperfusion, and modified Rankin scale score at 3 mo after onset; and number of patients (%) for others. ASPECTS indicates Alberta Stroke Programme Early CT Score; CT, computed tomography; DWI‐ASPECTS, Diffusion‐Weighted Imaging‐Alberta Stroke Program Early CT Score; MR, magnetic resonance; MRI, magnetic resonance imaging.
Table 2 shows each DWI‐ASPECTS cutoff point and corresponding volumes. The highest VolDWI in the 76 patients with DWI‐ASPECTS ≥7 was 83 mL, whereas the lowest VolDWI in the 72 patients with DWI‐ASPECTS <7 was 40 mL. And, the highest VolDWI in the 110 patients with DWI‐ASPECTS ≥5 was 150 mL, whereas the lowest VolDWI in the 88 patients with DWI‐ASPECTS <5 was 31 mL.
In 93 AIS patients with DWI‐ASPECTS >4 and VolDWI <71 mL, 31% (29/93) had favorable outcomes, and 19% (18/93) had unfavorable outcomes overall.
Discussion
This study reports the correlation between DWI‐ASPECTS and VolDWI measured by automated software for LVO AIS with no treatment bias. Baseline DWI‐ASPECTS and VolDWI measured by automated software showed a strong correlation in our study. The cutoff value for predicting an unfavorable outcome was 4 for DWI‐ASPECTS and 71 mL for VolDWI.
Interestingly, there were cases with low DWI‐ASPECTS scores despite the small VolDWI. In DWI‐ASPECTS ≥7 cases, DWI‐ASPECTS was useful to substitute for VolDWI, but when it turned to <7 (especially in DWI‐ASPECTS ≤4 cases), there was a significant divergence between DWI‐ASPECTS and VolDWI. In our results, 1 patient had DWI ASPECTS ≥7, and the VolDWI was ≥71 mL. The reason why divergence occurred in this case may be characterized by the category and assessment of DWI‐ASPECTS. DWI‐ASPECTS was derived from computed tomography (CT)‐ASPECTS as a tool to semiquantify the early ischemic changes. It was not originally devised as a substitute for VolDWI, and there are several problems with this quantification method as an evaluation of ischemic core volume. General limitations of DWI‐ASPECTS include overestimations in the penetrating branch territory of the MCA. Several of the ASPECT regions (caudate/internal capsule/lentiform/insular ribbon) involve small‐volume brain structures; therefore, a small‐volume lesion involving these regions can lead to a low score. In contrast, the fact that anterior cerebral artery and posterior cerebral artery territory are not included in ASPECTS may be 1 reason why large‐volume lesions have high ASPECT scores. Additionally, because DWI‐ASPECTS is a semiquantitative visually assessed measurement, differences in display output and brightness may also cause differences in the result. Especially in the present study, the following 2 limitations in scoring DWI‐ASPECTS were (1) punctate DWI high‐intensity signals and diffuse DWI high‐intensity signals are evaluated equally, and (2) DWI‐ASPECTS depends on the reader's impression. The current case was an acute onset ICA occlusion the core of which was too blurry in the ACA/parietal lobe area so that automated software could not detect and had DWI‐ASPECTS 8 with VolDWI as 83 mL.
The group with divergence between DWI‐ASPECTS and VolDWI included 88 patients with DWI‐ASPECTS scores ≤4. Of these, 17% (15/88) had VolDWI <71 mL, and unfavorable outcomes were seen in only 13% (2/15); 33% (5/15) were treated with EVT and had no unfavorable outcomes. From these results, it appears that DWI‐APECTS may not be an adequate substitute for VolDWI in cases with DWI‐ASPECTS ≤4. If the patients with DWI‐ASPECTS ≤4 were excluded from reperfusion therapy, nearly 20% of patients were erroneously excluded.
Similar situations were reported in previous studies, in which the cutoff value of DWI‐ASPECTS and VolDWI did not match. According to the study done by Terasawa et al, the cutoff value of DWI‐ASPECTS >7 detects DWI lesion volume ≤25 mL in anterior circulation AIS treated with IVT for manually outlined volumetric calculation.17 When these values, DWI‐ASPECTS >7 and VolDWI ≤25 mL, were applied to the condition reported in this study, a high correlation was observed in our data set (sensitivity 0.93, specificity 0.98). de Margerie‐Melon et al reported that DWI‐ASPECTS <4 or ≥7 may be used as reliable surrogates of VolDWI >100 or <70 mL, respectively, in AIS due to MCA occlusion in patients treated by IVT and/or EVT using a workstation (Advantage Workstation, GE Healthcare, Chalfont, UK) with an application for functional mapping calculation (READY View, GE Healthcare, Chalfont, UK),18 but they also concluded that DWI‐ASPECTS has limitations when lesion location is not considered. Our data set confirmed selection bias as reported in similar trials. Another problem seems to lie in the fact that most studies targeted their population as IVT‐ and/or EVT‐eligible AIS patients and excluded the medical treatment group. When the threshold values derived from favorable outcomes in the previous volumetric study17 (optimal VolDWI 25 mL) were applied, the sensitivity was 0.77, and the specificity was 0.73 (AUC 0.81). This result also confirms that our data‐set population was not different from those of previous volumetric studies.
Some of our results were also supported by recent studies. Compared with the HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) collaboration, 46% of all subjects had favorable outcomes, and 22% had unfavorable outcomes,19 and these clinical outcomes were almost equivalent to those in our study. In addition, according to our data, the cutoff value for the malignant profile was VolDWI 71 mL in 89 patients. Of these, 11% (10/89) underwent EVT, and 20% (2/10) of these achieved favorable outcomes; while 30% (3/10) had unfavorable outcomes. Similarly, in the HERMES collaboration's meta‐analysis, favorable outcomes occurred in 19% (9/48), and unfavorable outcomes occurred in 44% (21/48), of AIS patients who underwent EVT with ischemic core volume ≥70 mL. Compared with the HERMES collaboration meta‐analysis, our study included a higher proportion of AIS patients with ischemic core volume ≥70 mL (43% versus 12%) but a lower proportion treated with EVT.20 Although these results support our findings, there is a strong difference of backgrounds between HERMES collaboration's meta‐analysis and ours. Sixty‐six percent of the cores estimated in HERMES collaboration's meta‐analysis20 were done by CT perfusion regardless of its ischemic core size, whereas MR DWI was 100% used to measure the core in our study. MR DWI is known to have better detection than CT perfusion from its sensitivity and specificity, and even though CT perfusion cerebral blood flow has reasonable correlation with DWI,21 it still has some under/overcalls in certain cutoffs between cerebral blood flow <38% and cerebral blood flow <30%.22
Our study reflected real‐world clinical practice without selection bias and suggested that VolDWI ≥71 mL can still be a valuable indicator of the malignant profile regardless of EVT, but we also found a variety of outcomes in large ischemic core patients, and this deviation needs to be clarified. One reason is the difficulty of prediction in transition of brain edema after the ischemia. Minimum cytotoxic edema occurs after ischemia and is characterized by failure of ATP‐dependent ion transportation, cellular depolarization, and increased cell swelling. Despite the consequences of the recanalization, brain edema is complex to control, and its progress is difficult to predict. From our data set we came across cases that had large cores but did not have unfavorable outcomes. This might be explained by the inadequate lowering of apparent diffusion coefficient values and early renormalization, which produced no cell damage in the brain tissue even with slight edema. To elucidate these issues several randomized ongoing trials might clarify these varieties in large‐core patients such as the CHARM (Severe Cerebral Edema Following Large Hemispheric Infarction) trial (ClinicalTrials.gov: NCT02864953) for brain edema and SELECT (Optimize Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke) study (ClinicalTrials.gov: NCT02446587) for large cores.
Our study has some limitations. It was a single‐center, retrospective, nonrandomized, observational study. Nevertheless, it is a valuable study to explore alternative methods of automated software for AIS to evaluate ischemic core volume; our study may also be valuable as evidence for using EVT in facilities where automated software is unaffordable. In our present result, not all patients had attempted reperfusion therapy, and therefore, the optimal ischemic core volume threshold for unfavorable outcome may be higher than 71 mL in EVT patients. We excluded MCA M1 distal occlusion and other vessel occlusion cases that might be eligible for EVT in order to match the DWI‐ASPECTS evaluable territory. The limitation of RAPID software is that it cannot assess the very subtle DWI high intensity which the apparent diffusion coefficient index has not decreased enough, and manually outlined volumetric estimation may be beneficial in these cases. Further studies are required to include any kind of vessel‐occluded LVO to assess the ASPECTS and volumes.
Conclusions
In AIS due to ICA or MCA M1 proximal occlusion, a significant correlation was found between DWI‐ASPECTS and VolDWI. The cut‐off values for poor clinical outcomes were DWI‐ASPECTS 4 and VolDWI 71 mL. These results align with the previous highly selective randomized controlled trials, although favorable outcomes were still observed in EVT patients above this threshold. DWI‐ASPECTS may not be an adequate substitute for VolDWI in cases with DWI‐ASPECTS ≤4.
Sources of Funding
This study was supported by the Japan Agency for Medical Research and Development under Grant Number JP18ek0210109 (M.K.), Japan Society for the Promotion of Science Scientific Research Grant Number JP 17K00426 (M. Inoue), and The Bayer Scholarship for Cardiovascular Research 2018 (M. Inoue).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012558 DOI: 10.1161/JAHA.119.012558.)
References
- 1. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega‐Gutierrez S, McTaggart RA, Torbey MT, Kim‐Tenser M, Leslie‐Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; for the DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila GA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxte BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; for the DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 3. Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, Yamagami H, Okada Y, Hasegawa Y, Kario K, Okuda S, Nishiyama K, Naganuma M, Minematsu K, Toyoda K. Pretreatment ASPECTS on DWI predicts 3‐month outcome following rt‐PA: SAMURAI rt‐PA Registry. Neurology. 2010;75:555–561. [DOI] [PubMed] [Google Scholar]
- 4. Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, Thomalla G, Fiehler J, Berkefeld J, Neumann‐Haefelin T; for the MR Stroke Study Group Investigators . Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI‐ASPECTS. Stroke. 2009;40:2743–2748. [DOI] [PubMed] [Google Scholar]
- 5. Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, González RG. MRI‐based selection for intra‐arterial stroke therapy: value of pretreatment diffusion‐weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA, Chang CW, Warach S, Fazekas F, Inoue M, Tipirneni A, Hamilton SA, Zaharchuk G, Marks MP, Bammer R, Albers GW; for the DEFUSE 2 study investigators . MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 8. Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP; for the DEFUSE Investigators . Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 9. Toyoda K, Koga M, Yamagami H, Yokota C, Sato S, Inoue M, Tanaka T, Endo K, Fujinami J, Ihara M, Nagatsuka K, Minematsu K. Seasonal variations in neurological severity and outcomes of ischemic stroke: 5‐year single‐center observational study. Circ J. 2018;82:1443–1450. [DOI] [PubMed] [Google Scholar]
- 10. van Swieten JC, Koudstaal PJ, Visser MC, Schouten H, Van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 11. Meyer BC, Hemmen TM, Jackson CM, Lyden PD. Modified National Institutes of Health Stroke Scale for use in stroke clinical trials: prospective reliability and validity. Stroke. 2002;33:1261–1266. [DOI] [PubMed] [Google Scholar]
- 12. Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JH, Hudon ME, Tomanek A, Frayne R, Buchan AM; for the ASPECTS Study Group . Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion‐weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76:1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams H, Bendixen B, Kapelle L, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, Nakagawara J, Sakai N, Shiokawa Y, Tanahashi N, Yasaka M, Katayama Y, Miyamoto S, Ogawa A, Sasaki M, Suga S, Yamaguchi T; Japan Stroke Society . Guidelines for the intravenous application of recombinant tissue‐type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. 2013;22:571–600. [DOI] [PubMed] [Google Scholar]
- 15. Zaidat OO, Yoo AJ, Khatri P, Tomsik TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BFM, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers GW, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS; for the Cerebral Angiographic Revascularization Grading Collaborators, STIR Revascularization working group, and STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D; for the ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2007;358:1327–1335. [DOI] [PubMed] [Google Scholar]
- 17. Terasawa Y, Kimura K, Iguchi Y, Kobayashi K, Aoki J, Shibazaki K, Kaji R. Could clinical diffusion‐mismatch determined using DWI ASPECTS predict neurological improvement after thrombolysis before 3 h after acute stroke? J Neurol Neurosurg Psychiatry. 2010;81:864–868. [DOI] [PubMed] [Google Scholar]
- 18. de Margerie‐Mellon C, Turc G, Tisserand M, Naggara O, Calvet O, Legrand L, Meder JF, Mas JL, Baron JC, Oppenheim C. Can DWI‐ASPECTS substitute for lesion volume in acute stroke? Stroke. 2013;44:3565–3567. [DOI] [PubMed] [Google Scholar]
- 19. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miquel MA, Donnan GA, Roos YBWEM, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BCV, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; for the HERMES Collaborators . Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 20. Campbell BCV, Majoie CBLM, Albers GW, Menon BK, Yassi N, Sharma G, van Zwam WH, van Oostenbrugge RJ, Demchuk AM, Guillemin F, White P, Dávalos A, van der Lugt A, Butcher KS, Cherifi A, Marquering HA, Cloud G, Fernández JMM, Madigan J, Oppenheim C, Donnan GA, Roos YBWEM, Shankar J, Lingsma H, Bonafé A, Raoult H, Hernández‐Pérez M, Bharatha A, Jahan R, Jansen O, Richard S, Levy EI, Berkhemer OA, Soudant M, Aja L, Davis SM, Krings T, Tisserand M, Román LS, Tomasello A, Beumer D, Brown S, Liebeskind DS, Bracard S, Muir KW, Dippel DWJ, Goyal M, Saver JL, Jovin TG, Hill MD, Mitchell PJ; for the HERMES Collaborators . Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta‐analysis of individual patient‐level data. Lancet Neurol. 2019;18:46–55. [DOI] [PubMed] [Google Scholar]
- 21. Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. [DOI] [PubMed] [Google Scholar]
- 22. Cereda CW, Christensen S, Campbell BCV, Mishra NiK, Mlynash M, Levi C, Straka M, Wintermark M, Bammer R, Albers GW, Parsons MW, Lansberg MG. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab. 2016;36:1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


