Abstract
Background
The choice of optimal drug‐eluting stent therapy for patients with diabetes mellitus (DM) undergoing percutaneous coronary intervention remains uncertain. We aimed to assess the long‐term clinical outcomes after percutaneous coronary intervention with biodegradable polymer sirolimus‐eluting stents (BP‐SES) versus durable polymer everolimus‐eluting stents (DP‐EES) in patients with DM.
Methods and Results
In a prespecified subgroup analysis of the BIOSCIENCE (Ultrathin Strut Biodegradable Polymer Sirolimus‐Eluting Stent Versus Durable Polymer Everolimus‐Eluting Stent for Percutaneous Coronary Revascularization) trial (NCT01443104), patients randomly assigned to ultrathin‐strut BP‐SES or thin‐strut DP‐EES were stratified according to diabetic status. The primary end point was target lesion failure, a composite of cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularization, at 5 years. Among 2119 patients, 486 (22.9%) presented with DM. Compared with individuals without DM, patients with DM were older and had a greater baseline cardiac risk profile. In patients with DM, target lesion failure at 5 years occurred in 74 patients (cumulative incidence, 31.0%) treated with BP‐SES and 57 patients (25.8%) treated with DP‐EES (risk ratio, 1.23; 95% CI, 0.87–1.73 [P=0.24]). In individuals without DM, target lesion failure at 5 years occurred in 124 patients (16.8%) treated with BP‐SES and 132 patients (16.8%) treated with DP‐EES (risk ratio, 0.98; 95% CI, 0.77–1.26 [P=0.90; P for interaction=0.31]). Cumulative 5‐year incidence rates of cardiac death, target vessel myocardial infarction, clinically indicated target lesion revascularization, and definite stent thrombosis were similar among patients with DM treated with BP‐SES or DP‐EES. There was no interaction between diabetic status and treatment effect of BP‐SES versus DP‐EES.
Conclusions
In a prespecified subgroup analysis of the BIOSCIENCE trial, we found no difference in clinical outcomes throughout 5 years between patients with DM treated with ultrathin‐strut BP‐SES or thin‐strut DP‐EES.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01443104.
Keywords: biodegradable polymer, diabetes mellitus, drug‐eluting stent, long‐term outcomes, ultrathin stent strut
Subject Categories: Percutaneous Coronary Intervention, Stent, Revascularization
Clinical Perspective
What Is New?
At 5 years, ultrathin‐strut biodegradable polymer sirolimus‐eluting stents provide similar long‐term efficacy and safety outcomes compared with thin‐strut durable polymer everolimus‐eluting stents in patients with diabetes mellitus undergoing percutaneous coronary revascularization.
Despite improvements in evidence‐based medical therapy and recent iterations in drug‐eluting stent technology including ultrathin‐strut stent platforms and biodegradable polymer coatings, patients with diabetes mellitus remain at increased long‐term risk for adverse clinical outcomes after percutaneous coronary intervention compared with individuals without diabetes mellitus.
What Are the Clinical Implications?
At long‐term follow‐up, ultrathin‐strut biodegradable polymer sirolimus‐eluting stents represent a safe and effective alternative to thin‐strut durable polymer everolimus‐eluting stents for the treatment of patients with diabetes mellitus undergoing percutaneous coronary revascularization, but further research is still warranted to determine the optimal drug‐eluting stent therapy in this high‐risk patient subgroup.
Introduction
Among patients undergoing percutaneous coronary intervention (PCI), patients with diabetes mellitus (DM) represent a high‐risk subset compared with individuals without DM and are at increased long‐term risk of death, myocardial infarction (MI), and repeat revascularization.1, 2, 3 Newer‐generation drug‐eluting stents (DES) are currently recommended for treatment of patients with DM undergoing PCI owing to a lower risk of repeat revascularization compared with bare‐metal stents and early‐generation thick‐strut permanent polymer‐based DES.4 Among contemporary available DES, thin‐strut durable polymer everolimus‐eluting stents (DP‐EES) were shown to provide the best safety and efficacy profile in patients with DM.5 However, long‐term persistence of durable polymer coatings has been associated with delayed vascular healing attributed to a chronic inflammatory process,6 translating into a persisting risk of late adverse clinical events.7
Biodegradable polymer DES (BP‐DES) were introduced with the purpose of controlling antiproliferative drug release while allowing subsequent polymer degradation, thus potentially eliminating triggers of a chronic inflammatory tissue response induced by durable polymer coatings and restoring the stent phenotype to an inert bare‐metal stent. Among patients with DM, thick‐strut BP‐DES have been shown to provide comparable long‐term clinical outcomes and significantly lower rates of definite or probable stent thrombosis compared with first‐generation thick‐strut durable polymer sirolimus‐eluting stents.8 Recently, ultrathin‐strut BP‐SES demonstrated similar safety and efficacy outcomes compared with thin‐strut DP‐EES at 1‐year follow‐up in patients with DM,9 but whether potential differences between BP‐SES and DP‐EES may emerge during extended follow‐up, when drug elution and polymer degradation are completed, remains unknown. We therefore performed a subgroup analysis of patients included in the BIOSCIENCE (Ultrathin Strut Biodegradable Polymer Sirolimus‐Eluting Stent Versus Durable Polymer Everolimus‐Eluting Stent for Percutaneous Coronary Revascularization) trial to assess the effect of DM on long‐term outcomes in patients treated with ultrathin‐strut BP‐SES versus thin‐strut DP‐EES.
Methods
Study Design and Study Population
We performed a prespecified subgroup analysis of patients with insulin‐dependent or noninsulin‐dependent DM enrolled in the BIOSCIENCE trial. Detailed descriptions of the study rationale, design, inclusion and exclusion criteria, methods, and data management have been previously reported.10 Briefly, BIOSCIENCE was an investigator‐initiated, prospective, multicenter, single‐blind, randomized, noninferiority trial including patients with stable coronary artery disease or acute coronary syndrome and minimal exclusion criteria. Patients with at least one >50% diameter coronary stenosis or in‐stent restenosis in a native coronary vessel or bypass graft suitable for PCI were randomly allocated in a 1:1 ratio to treatment with BP‐SES or DP‐EES. The final 5‐year results of the BIOSCIENCE trial were recently reported.11 The study protocol complied with the Declaration of Helsinki and was approved by the institutional ethics committees at each participating site. All enrolled patients provided written informed consent for participation. The trial is registered with ClinicalTrials.gov (NCT01443104). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Procedures
The BP‐SES (Orsiro, Biotronik AG) consists of an ultrathin‐strut (60 μm for stent diameters ≤3.0 mm and 80 μm for stent diameters >3.0 mm) cobalt‐chromium L‐605 metallic stent platform covered by both a permanent silicon‐carbide passive coating that reduces interaction between the metallic stent surface and the surrounding tissue, and a biodegradable poly‐L‐lactic acid polymer active coating that elutes sirolimus at a reduced dose of 1.4 μg/mm2 stent surface over a 12‐ to 14‐week period.12 The asymmetrical polymer matrix completely degrades after ≈2 years.12 The DP‐EES (Xience Prime/Xpedition, Abbott Vascular) consists of a thin‐strut (81 μm) L‐605 cobalt‐chromium stent platform coated with a durable poly‐n‐butyl‐methacrylate and vinylidine fluoride and hexafluoropropylene co‐polymer that releases everolimus. Eligible patients were randomized using a web‐based system and stratified according to the study center and to the presence or absence of ST‐segment elevation MI. PCI was performed according to current guidelines at the time of study enrollment. Unfractionated heparin (5 000 IU or 70–100 IU/kg of body weight) or bivalirudin, and glycoprotein IIb/IIIa inhibitors were administrated at the operator's discretion. Dual antiplatelet therapy, consisting of acetylsalicylic acid (>250 mg) combined with clopidogrel (loading dose, 600 mg; maintenance dose, 75 mg QD), prasugrel (loading dose, 60 mg; maintenance dose, 10 mg QD), or ticagrelor (loading dose, 180 mg; maintenance dose, 90 mg BID), was administered before or at the time of PCI and continued for a recommended period of 12 months.
Definition
All patients previously diagnosed and treated for insulin‐dependent and noninsulin‐dependent DM, as well as patients with DM under diet control, were considered for the present analysis. The presence or absence of DM at presentation was determined by the enrolling physician based on the information collected from the patient and medical records.
Study End Points
The primary end point was target lesion failure (TLF), defined as the composite of cardiac death, target vessel MI, or clinically indicated target lesion revascularization (TLR), within 5 years. All primary and secondary end point definitions are outlined in detail in Data S1. Data collection and monitoring have been previously described.10 Any adverse event was independently adjudicated by a clinical events committee blinded to treatment assignment.
Statistical Analyses
Patients enrolled in the BIOSCIENCE trial and randomly assigned to treatment with BP‐SES or DP‐EES were stratified according to diabetic status. All analyses were performed in the intention‐to‐treat population, consisting of all patients who underwent randomization, irrespective of the treatment received. Baseline characteristics of study patients were presented as frequencies and percentages for categorical variables and means±SDs for continuous variables. Treatment effects for baseline characteristics were calculated using unpaired t tests, chi‐square tests, or Fisher exact tests, except when specified, for per‐patient analyses, and general (continuous variables) or generalized linear mixed models (counts numbers) accounting for the nonindependence of lesions within the same patient for per‐lesion analyses. Kaplan‐Meier estimates were used to construct survival curves for time‐to‐event outcomes that were compared by means of the log‐rank test. All events were censored beyond 1825 days of follow‐up. The Mantel‐Cox method with 2‐sided P values from log‐rank test was used to calculate rate ratios (RRs) with 95% CIs. Approximate Mantel‐Haenszel chi‐square tests for effect modification were used to determine the interaction between diabetic status and randomized stent type. A P value of 0.05 was considered statistically significant. Stata software (version 14.2, StataCorp) was used for all statistical analyses.
Results
Between March 1, 2012, and May 31, 2013, 2119 patients with 3139 lesions were enrolled in the BIOSCIENCE trial. A total of 1063 patients (257 patients with DM and 806 patients without DM) were randomly assigned to treatment with BP‐SES and 1056 patients (229 patients with DM and 827 patients without DM) were randomly assigned to treatment with DP‐EES (Figure 1). Among 486 patients (22.9%) with DM, 257 patients with 396 lesions were randomized to treatment with BP‐SES and 229 patients with 331 lesions were allocated to treatment with DP‐EES. Among the remaining 1633 (77.1%) patients without DM, 806 patients with 1198 lesions were randomly assigned to treatment with BP‐SES and 827 patients with 1214 lesions were randomized to treatment with DP‐EES (Figure 1). At 5 years, follow‐up data were available for 1012 (95.2%) patients treated with BP‐SES (240 patients with DM and 772 patients without DM) and for 1014 (96.0%) patients treated with DP‐EES (221 patients with DM and 793 patients without DM) (Figure 1).
Figure 1.
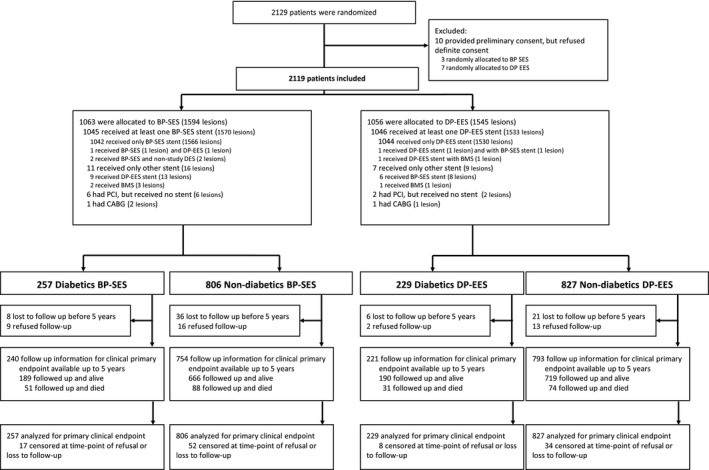
Patient flow according to CONSORT statement. BMS indicates bare‐metal stents; BP‐SES, biodegradable polymer sirolimus‐eluting stents; CABG, coronary artery bypass grafting; DP‐EES, durable polymer everolimus‐eluting stents; PCI, percutaneous coronary intervention.
Baseline clinical characteristics are summarized in Table 1 and were well balanced between treatment arms in both patients with DM and patients without DM. Compared with individuals without DM, patients with DM were older and had a greater baseline cardiac risk profile, including a higher prevalence of cardiovascular risk factors (hypertension, hypercholesterolemia), peripheral artery disease, chronic renal failure, and history of PCI, coronary artery bypass graft surgery, or cerebrovascular event. Most patients with DM (n=345, 71%) were taking oral hypoglycemic agents, whereas 161 (33.1%) patients presented with insulin‐dependent DM, including 90 (35.0%) patients allocated to BP‐SES and 71 (31%) patients allocated to DP‐EES. Nearly half of the patients with DM (n=217, 44.7%) presented with acute coronary syndrome (114 patients allocated to BP‐SES and 103 patients allocated to DP‐EES). At baseline, cardiac medications, including antiplatelet agents, statins, angiotensin‐converting enzyme inhibitors, and β‐blockers, were more commonly prescribed to patients with DM than patients without DM. Notably, 114 (23.6%) patients with DM and 282 (17.5%) individuals without DM were on dual antiplatelet therapy at 5‐year follow‐up. Baseline angiographic and procedural characteristics are summarized in Table 2 and were similar between both treatment arms with respect to target lesion characteristics, including target vessel location and number of lesions treated.
Table 1.
Baseline Clinical Characteristics
| Patients With DM | Patients Without DM | |||||
|---|---|---|---|---|---|---|
| All | BP‐SES | DP‐EES | All | BP‐SES | DP‐EES | |
| Patients, No. | N=486 | n=257 | n=229 | N=1633 | n=806 | n=827 |
| Age, mean±SD, y | 67.9±10.5 | 68.6±10.7 | 67.1±10.4 | 65.5±11.7 | 65.3±11.8 | 65.6±11.6 |
| Male sex, No. (%) | 370 (76.1) | 198 (77.0) | 172 (75.1) | 1262 (77.3) | 620 (76.9) | 642 (77.6) |
| Body mass index, mean±SD, kg/m2 | 25.5±4.8 | 25.8±4.6 | 29.2±5.0 | 27.1±4.3 | 27.1±4.3 | 27.1±4.3 |
| DM, No. (%) | ||||||
| Orally treated | 345 (71.0) | 179 (69.6) | 166 (72.5) | NA | NA | NA |
| Insulin‐treated | 161 (33.1) | 90 (35.0) | 71 (31.0) | NA | NA | NA |
| Hypertension, No. (%) | 409 (84.3) | 221 (86.0) | 188 (82.5) | 1025 (62.8) | 507 (63.0) | 518 (62.6) |
| Hypercholesterolemia, No. (%) | 361 (74.3) | 189 (73.5) | 172 (75.1) | 1067 (65.4) | 523 (65.0) | 544 (65.8) |
| Current smoker, No. (%) | 109 (22.4) | 56 (17.8) | 53 (23.1) | 500 (30.7) | 253 (31.4) | 247 (29.9) |
| Family history of CAD, No. (%) | 127 (26.2) | 62 (20.1) | 65 (28.5) | 460 (28.3) | 230 (28.7) | 230 (27.8) |
| Previous MI, No. (%) | 123 (25.3) | 64 (24.9) | 59 (25.8) | 304 (18.6) | 159 (19.7) | 145 (17.5) |
| Previous PCI, No. (%) | 187 (38.5) | 103 (40.1) | 84 (36.7) | 430 (26.3) | 222 (27.5) | 208 (25.2) |
| Previous CABG, No. (%) | 84 (17.3) | 55 (17.4) | 29 (12.7) | 127 (7.8) | 58 (7.2) | 69 (8.3) |
| Atrial fibrillation, No. (%) | 42 (8.6) | 23 (8.9) | 19 (8.3) | 121 (7.4) | 60 (7.4) | 61 (7.4) |
| Previous stroke or TIA, No. (%) | 30 (6.2) | 13 (5.1) | 17 (7.4) | 66 (4.0) | 26 (3.2) | 40 (4.8) |
| Peripheral vascular disease, No. (%) | 71 (14.6) | 42 (16.3) | 29 (12.7) | 105 (6.4) | 53 (6.6) | 52 (6.3) |
| Renal failure (GFR <60 mL/min), No. (%) | 105 (23.0)* | 58 (24.5)† | 47 (17.4)‡ | 177 (11.4)§ | 93 (12.1)∥ | 84 (10.8)¶ |
| Left ventricular ejection fraction, mean±SD, % | 54.4±13.7# | 53.6±13.6** | 55.1±13.9†† | 56.2±11.9‡‡ | 56.3±11.6§§ | 56.1±12.2∥∥ |
| Clinical presentation, No. (%) | ||||||
| Unstable angina | 34 (7.0) | 16 (6.2) | 18 (7.9) | 118 (7.2) | 62 (7.7) | 56 (6.8) |
| NSTEMI | 126 (25.9) | 68 (26.5) | 58 (25.3) | 445 (27.3) | 219 (27.2) | 226 (27.3) |
| STEMI | 57 (11.7) | 30 (11.7) | 27 (11.8) | 351 (21.5) | 182 (22.6) | 169 (20.4) |
| Stable angina | 192 (39.5) | 103 (40.1) | 89 (38.9) | 569 (34.9) | 268 (33.3) | 301 (36.4) |
| Silent ischemia | 77 (15.8) | 40 (15.6) | 37 (16.2) | 149 (9.1) | 74 (9.2) | 75 (9.1) |
| Baseline medications, No. (%) | ||||||
| Aspirin | 343 (70.9) | 179 (70.2) | 164 (71.6) | 895 (55.7)¶¶ | 432 (54.4)## | 463 (56.9)*** |
| Clopidogrel | 95 (19.6) | 43 (16.9) | 52 (22.7) | 193 (12.0)¶¶ | 86 (10.8)## | 107 (13.2)*** |
| Prasugrel | 20 (4.1) | 11 (4.3) | 9 (3.9) | 60 (3.7)¶¶ | 32 (4.0)## | 28 (3.4)*** |
| Ticagrelor | 22 (4.5) | 9 (3.5) | 13 (5.7) | 67 (4.2)¶¶ | 29 (3.7)## | 38 (4.7)*** |
| Any dual antiplatelet therapy | 114 (23.6) | 54 (21.2) | 60 (26.2) | 282 (17.5)¶¶ | 127 (16.0)## | 155 (19.1)*** |
| Vitamin K oral anticoagulant | 42 (8.7) | 23 (9.0) | 19 (8.3) | 94 (5.8)¶¶ | 50 (6.3)## | 44 (5.4)*** |
| Nonvitamin K oral anticoagulant | 2 (0.4) | 2 (0.8) | 0 (0) | 4 (0.2)¶¶ | 1 (0.1)## | 3 (0.4)*** |
| Any anticoagulant therapy | 44 (9.1) | 25 (9.8) | 19 (8.3) | 98 (6.1)¶¶ | 51 (6.4)## | 47 (5.8)*** |
| Statins | 336 (69.6) | 178 (69.8) | 158 (69.3) | 789 (49.1)¶¶ | 384 (48.4)## | 405 (49.8)*** |
| ACE inhibitors or ARBs | 182 (37.7) | 100 (39.2) | 82 (36.0) | 366 (22.8)¶¶ | 171 (21.6)## | 195 (24.0)*** |
| β‐Blockers | 274 (56.7) | 153 (60.0) | 121 (53.1) | 695 (43.3)¶¶ | 343 (43.3)## | 352 (43.3)*** |
P values from Fisher exact tests and unpaired t tests, respectively. ACE indicates angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BP‐SES, biodegradable polymer sirolimus‐eluting stents; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DM, diabetes mellitus; DP‐EES, durable polymer everolimus‐eluting stents; GFR, glomerular filtration rate; MI, myocardial infarction; NA, not applicable; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
*n=457; †n=237; ‡n=220; §n=1546; ∥n=771; ¶n=775; #n=377; **n=199; ††n=178; ‡‡n=1320; §§n=653; ∥∥n=667; ¶¶n=1607; ##n=794; ***n=813.
Table 2.
Angiographic and Procedural Characteristics
| Patients, No | Patients With DM | Patients Without DM | Interaction P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | BP‐SES | DP‐EES | P Value | All | BP‐SES | DP‐EES | P Value | ||
| N=486 | n=257 | n=229 | N=1633 | n=806 | n=827 | ||||
| Lesions, No. | 727 | 396 | 331 | 2412 | 1198 | 1214 | |||
| Target vessel location per lesion, No. (%) | 0.68 | 0.38 | 0.45 | ||||||
| Left main coronary artery | 19 (2.6) | 10 (2.5) | 9 (2.7) | 37 (1.5) | 19 (1.6) | 18 (1.5) | |||
| Left anterior descending artery | 274 (37.7) | 141 (35.6) | 133 (40.2) | 1054 (43.7) | 508 (42.4) | 546 (45.0) | |||
| Left circumflex artery | 162 (22.3) | 96 (24.2) | 66 (19.9) | 549 (22.8) | 274 (22.9) | 275 (22.7) | |||
| Right coronary artery | 236 (32.5) | 127 (32.1) | 109 (32.9) | 721 (29.9) | 378 (31.6) | 343 (28.3) | |||
| Bypass graft | |||||||||
| Saphenous vein graft | 31 (4.3) | 19 (4.8) | 12 (3.6) | 0.44 | 47 (1.9) | 19 (1.6) | 28 (2.3) | 0.20 | <0.001 |
| Arterial graft | 5 (0.7) | 3 (0.8) | 2 (0.6) | 0.80 | 4 (0.2) | 0 (0.0) | 4 (0.3) | 0.12 | 0.53 |
| No. of treated lesions per patient¶ | 1.50±0.74 | 1.54±0.78 | 1.45±0.69 | 0.39 | 1.48±0.77 | 1.49±0.79 | 1.47±0.74 | 0.76 | 0.53 |
| No. of treated lesions per patient, No. (%) | 0.68 | 0.68 | 0.46 | ||||||
| 1 | 305 (62.8) | 155 (60.3) | 150 (65.5) | 1066 (65.3) | 528 (65.5) | 538 (65.1) | |||
| 2 | 132 (27.2) | 73 (28.4) | 59 (25.8) | 401 (24.6) | 193 (23.9) | 208 (25.2) | |||
| 3 | 39 (8.0) | 22 (8.6) | 17 (7.4) | 131 (8.0) | 62 (7.7) | 69 (8.3) | |||
| ≥4 | 10 (2.1) | 7 (2.7) | 3 (1.3) | 35 (2.1) | 23 (2.9) | 12 (1.5) | |||
| Type of intervention per lesion, No. (%)** | 0.07 | 0.71 | 0.84 | ||||||
| PCI | 699 (96.1) | 376 (94.9) | 323 (97.6) | 2301 (95.4) | 114 (95.2) | 1160 (95.6) | |||
| Balloon angioplasty | 28 (3.9) | 20 (5.1) | 8 (2.4) | 101 (4.2) | 49 (4.1) | 52 (4.3) | |||
| CABG | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.1) | 2 (0.2) | 1 (0.1) | |||
| Failed PCI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (0.3) | 6 (0.5) | 1 (0.1) | |||
| Baseline TIMI flow per lesion, No. (%) | 0.08 | 0.32 | <0.001 | ||||||
| 0 or 1 | 107 (15.0) | 54 (13.8) | 53 (16.4) | 496 (20.9) | 258 (22.1) | 238 (19.8) | |||
| 2 | 94 (13.1) | 43 (11.0) | 51 (15.7) | 352 (14.9) | 177 (15.1) | 175 (14.6) | |||
| 3 | 514 (71.9) | 294 (75.2) | 220 (67.9) | 1520 (64.2) | 734 (62.8) | 786 (65.6) | |||
| TIMI flow postintervention per lesion, No. (%) | 0.56 | 0.58 | 0.60 | ||||||
| 0 or 1 | 3 (0.4) | 1 (0.3) | 2 (0.6) | 7 (0.3) | 3 (0.3) | 4 (0.3) | |||
| 2 | 4 (0.6) | 3 (0.8) | 1 (0.3) | 29 (1.2) | 17 (1.4) | 12 (1.0) | |||
| 3 | 715 (99.0) | 388 (99.0) | 327 (99.1) | 2357 (98.5) | 1163 (98.3) | 1194 (98.7) | |||
| Restenotic lesion per lesion, No. (%) | 55 (7.6) | 35 (8.8) | 20 (6.0) | 0.16 | 125 (5.2) | 71 (5.9) | 54 (4.5) | 0.10 | 0.06 |
| Total occlusion per lesion, No. (%) | 98 (13.5) | 60 (15.2) | 38 (11.5) | 0.15 | 433 (18.0) | 218 (18.2) | 215 (17.8) | 0.79 | 0.19 |
| Thrombus aspiration per lesion, No. (%) | 24 (3.3) | 14 (3.5) | 10 (3.0) | 0.70 | 247 (10.3) | 132 (11.0) | 115 (9.5) | 0.21 | <0.001 |
| Thrombus aspiration (only STEMI) per lesion, No. (%) | 18 (22.2)* | 11 (26.8)# | 7 (17.5)¶¶ | 0.32 | 190 (40.1)‖‖‖ | 105 (42.2)§§§§ | 85 (37.8)***** | 0.33 | 0.07 |
| No. of stents per lesion, mean (SD) | 1.30±0.61 | 1.32±0.64 | 1.28±0.57 | 0.71 | 1.33±0.63 | 1.30±0.60 | 1.36±0.66 | 0.28 | 0.86 |
| Total stent length per lesion, mm | 26.61±16.44 | 26.65±17.22 | 26.56±15.51 | 0.87 | 26.69±16.01 | 25.67±14.75 | 27.70±17.11 | <0.001 | 0.27 |
| Maximum stent diameter per lesion, mm | 3.02±0.50 | 3.03±0.48 | 3.01±0.53 | 0.52 | 3.05±0.49 | 3.05±0.49 | 3.04±0.48 | 0.34 | 0.32 |
| Left main coronary artery | 3.51±0.44† | 3.50±0.47** | 3.53±0.44## ** | 0.89 | 3.67±0.38¶¶¶ | 3.59±0.42† | 3.76±0.31††††† | 0.58 | 0.51 |
| Left anterior descending artery | 2.93±0.45‡ | 2.96±0.44†† | 2.89±0.46*** | 0.21 | 2.97±0.42### | 3.00±0.44‖‖‖‖ | 2.94±0.41‡‡‡‡‡ | 0.020 | 0.86 |
| Left circumflex artery | 2.80±0.41§ | 2.80±0.40‡‡ | 2.80±0.43††† | 0.98 | 2.85±0.44**** | 2.84±0.45¶¶¶¶ | 2.86±0.43§§§§§ | 0.67 | 0.85 |
| Right coronary artery | 3.21±0.50‖ | 3.22±0.46§§ | 3.21±0.55‡‡‡ | 0.75 | 3.25±0.50†††† | 3.24±0.50#### | 3.26±0.50‖‖‖‖‖ | 0.63 | 0.60 |
| Bypass graft | 3.13±0.57¶ | 3.10±0.53‖‖ | 3.18±0.65§§§ | 0.81 | 3.35±0.53‡‡‡‡ | 3.22±0.55† | 3.44±0.52¶¶¶¶¶ | 0.21 | 0.55 |
| Maximum pressure per lesion, atm. | 14.00±3.46 | 13.92±3.61 | 14.11±3.29 | 0.55 | 13.69±3.41 | 13.71±3.46 | 13.66±3.35 | 0.69 | 0.26 |
| Overlapping stents per lesion, No. (%) | 145 (20.7) | 81 (21.5) | 64 (19.8) | 0.57 | 521 (22.6) | 234 (20.5) | 287 (24.7) | 0.02 | 0.60 |
| Direct stenting per lesion, No. (%) | 184 (26.3) | 90 (23.9) | 94 (29.1) | 0.18 | 683 (29.7) | 338 (29.6) | 345 (29.7) | 0.72 | 0.05 |
| Bifurcation treatment per lesion, No. (%) | 105 (14.5) | 46 (11.6) | 59 (17.8) | 0.04 | 417 (17.4) | 216 (18.2) | 201 (16.6) | 0.31 | 0.004 |
| Type of stent per lesionⱡ, No. (%) | 0.58 | 0.41 | 0.97 | ||||||
| Study stent BP‐SES | 372 (53.2) | 369 (98.1) | 3 (0.9) | 1138 (49.5) | 1132 (99.2) | 6 (0.5) | |||
| Study stent DP‐EES | 327 (46.8) | 7 (1.9) | 320 (99.1) | 1160 (50.4) | 6 (0.5) | 1154 (99.5) | |||
| Other drug‐eluting stent | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 2 (0.2) | 0 (0.0) | |||
| Bare‐metal stent | 1 (0.1) | 0 (0.0) | 1 (0.3) | 4 (0.2) | 3 (0.3) | 1 (0.1) | |||
| IABP per patient, No. (%) | 2 (0.4) | 1 (0.4) | 1 (0.4) | 0.93 | 3 (0.2) | 2 (0.2) | 1 (0.1) | 0.56 | NA |
| Vasopressors per patient, No. (%) | 4 (0.8) | 3 (1.2) | 1 (0.4) | 0.39 | 8 (0.5) | 6 (0.7) | 2 (0.2) | 0.17 | 0.92 |
Data are expressed as number (percentage) or mean±SD. P values from ¶Poisson regression and ǂchi‐square test for per‐patient analyses, otherwise P values from mixed models for the per‐lesion analyses, accounting for lesions nested within patients. General linear mixed models for continuous variables and generalized linear mixed models for counts numbers. ⱡ Two patients randomized to biodegradable polymer sirolimus‐eluting stents (BP‐SES) with both BP‐SES and another drug‐eluting stent within the same lesion; 1 patient randomized to durable polymer everolimus‐eluting stents (DP‐EES) with DP‐EES and BP‐SES within the same lesion; 1 patient randomized to DP‐EES with DP‐EES and bare‐metal stents within the same lesion. P value for any nonrandomized stent implanted per patient. CABG indicates coronary artery bypass grafting; DM, diabetes mellitus; IABP, intra‐aortic balloon pump; NA, not applicable; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction. *N=81; †N=19; ‡N=262; §N=156; ‖N=228; ¶N=34; #N=396; **N=10; ††N=133; ‡‡N=90; §§N=123; ‖‖N=20; ¶¶N=40; ##N=9; ***N=129; †††N=66; ‡‡‡N=105; §§§N=14; ‖‖‖N=474; N=36; ###N=1002; ****N=521; ††††N=692; ‡‡‡‡N=50; §§§§N=249; ‖‖‖‖N=483; ¶¶¶¶N=259; ####N=361; *****N=225; †††††N=17; ‡‡‡‡‡N=519; §§§§§N=262; ‖‖‖‖‖N=331; ¶¶¶¶¶N=31.
At 5 years, TLF occurred in 131 patients (cumulative incidence, 28.5%) with DM and 256 patients (16.8%) without DM (RR, 1.87; 95% CI, 1.51–2.31 [P<0.001]) (Table 3). The 5‐year cumulative incidence rates of cardiac death (12.3% versus 6.8%; RR, 1.95 [95% CI, 1.41–2.71]), target vessel MI (11.2% versus 5.4%; RR, 2.10 [95% CI, 1.47–2.98]), and clinically indicated TLR (16.4% versus 8.6%; RR, 2.01 [95 CI, 1.51–2.69]) were significantly higher among patients with DM compared with individuals without DM (P<0.001 for all) (Table 3). The cumulative 5‐year rates of definite stent thrombosis were significantly higher among patients with DM compared with individuals without DM (2.8% versus 1.3%; RR, 2.10 [95% CI, 1.03–4.28]; P=0.04) (Table 3).
Table 3.
Clinical Outcomes at 5‐Year Follow‐up in Patients With DM vs Patients Without DM
| Overall | Patients With DM | Patients Without DM | Interaction P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients With DM | Patients Without DM | RR (95% CI) (Patients With vs Patients Without DM) | P Value | BP‐SES | DP‐EES | RR (95% CI) (BP‐SES vs DP‐EES) | P Value | BP‐SES | DP‐EES | RR (95% CI) (BP‐SES vs DP‐EES) | P Value | ||
| Patients, No. | n=486 | n=1633 | n=257 | n=229 | n=806 | n=827 | |||||||
| All‐cause death | 82 (17.5) | 162 (10.6) | 1.78 (1.37–2.32) | <0.001 | 51 (20.9) | 31 (13.8) | 1.55 (0.99–2.42) | 0.053 | 88 (12.0) | 74 (9.3) | 1.25 (0.92–1.71) | 0.151 | 0.447 |
| Cardiac death | 56 (12.3) | 101 (6.8) | 1.95 (1.41–2.71) | <0.001 | 35 (14.9) | 21 (9.5) | 1.56 (0.91–2.69) | 0.102 | 46 (6.6) | 55 (7.0) | 0.88 (0.60–1.31) | 0.531 | 0.091 |
| MI (any) | 72 (16.5) | 145 (9.9) | 1.77 (1.34–2.35) | <0.001 | 35 (15.3) | 37 (17.7) | 0.89 (0.56–1.41) | 0.612 | 64 (9.0) | 81 (10.9) | 0.82 (0.59–1.13) | 0.225 | 0.775 |
| Q wave | 18 (4.2) | 38 (2.9) | 1.69 (0.96–2.96) | 0.065 | 10 (4.4) | 8 (3.9) | 1.17 (0.46–2.98) | 0.737 | 22 (3.4) | 16 (2.4) | 1.45 (0.76–2.77) | 0.252 | 0.711 |
| Non–Q wave | 56 (12.9) | 113 (7.4) | 1.74 (1.27–2.40) | 0.001 | 26 (11.4) | 30 (14.3) | 0.81 (0.48–1.37) | 0.436 | 46 (6.1) | 67 (8.7) | 0.71 (0.49–1.03) | 0.071 | 0.680 |
| Target vessel MI | 49 (11.2) | 82 (5.4) | 2.10 (1.47–2.98) | <0.001 | 26 (11.4) | 23 (11.0) | 1.07 (0.61–1.89) | 0.806 | 36 (4.8) | 46 (6.0) | 0.81 (0.52–1.25) | 0.343 | 0.438 |
| Q wave | 13 (2.9) | 22 (1.4) | 2.07 (1.04–4.11) | 0.034 | 8 (3.4) | 5 (2.4) | 1.49 (0.49–4.58) | 0.479 | 13 (1.8) | 9 (1.1) | 1.51 (0.65–3.54) | 0.336 | 0.986 |
| Non–Q wave | 36 (8.3) | 62 (4.1) | 2.02 (1.34–3.04) | 0.001 | 18 (8.0) | 18 (8.6) | 0.95 (0.49–1.82) | 0.868 | 24 (3.1) | 38 (5.0) | 0.65 (0.39–1.09) | 0.099 | 0.380 |
| Cardiac death or MI | 116 (25.3) | 231 (15.3) | 1.81 (1.45–2.26) | <0.001 | 63 (26.3) | 53 (24.1) | 1.12 (0.77–1.61) | 0.558 | 105 (14.4) | 126 (16.3) | 0.86 (0.67–1.12) | 0.264 | 0.261 |
| Repeat revascularization (any) | 117 (26.8) | 266 (17.6) | 1.62 (1.30–2.01) | <0.001 | 59 (26.0) | 58 (27.6) | 0.98 (0.68–1.40) | 0.896 | 129 (17.3) | 137 (17.9) | 1.00 (0.78–1.27) | 0.975 | 0.927 |
| PCI | 112 (25.7) | 250 (16.5) | 1.64 (1.31–2.05) | <0.001 | 56 (24.7) | 56 (26.6) | 0.95 (0.66–1.38) | 0.805 | 121 (16.2) | 129 (16.8) | 0.99 (0.78–1.27) | 0.959 | 0.859 |
| CABG | 11 (2.7) | 28 (1.9) | 1.39 (0.69–2.79) | 0.351 | 5 (2.3) | 6 (3.1) | 0.78 (0.24–2.58) | 0.686 | 12 (1.7) | 16 (2.2) | 0.79 (0.37–1.67) | 0.536 | 0.988 |
| TLR (any) | 74 (17.0) | 142 (9.5) | 1.90 (1.43–2.52) | <0.001 | 41 (18.2) | 33 (15.8) | 1.20 (0.76–1.90) | 0.435 | 69 (9.4) | 73 (9.5) | 0.99 (0.72–1.38) | 0.976 | 0.515 |
| Clinically indicated TLR | 71 (16.4) | 129 (8.6) | 2.01 (1.51–2.69) | <0.001 | 38 (16.9) | 33 (15.8) | 1.10 (0.69–1.76) | 0.680 | 65 (8.9) | 64 (8.4) | 1.07 (0.76–1.52) | 0.684 | 0.928 |
| PCI | 65 (15.0) | 116 (7.7) | 2.04 (1.50–2.76) | <0.001 | 35 (15.6) | 30 (14.3) | 1.11 (0.68–1.81) | 0.669 | 59 (8.0) | 57 (7.5) | 1.09 (0.76–1.58) | 0.626 | 0.959 |
| CABG | 8 (1.9) | 18 (1.2) | 1.57 (0.68–3.62) | 0.281 | 4 (1.8) | 4 (2.1) | 0.94 (0.23–3.83) | 0.936 | 8 (1.1) | 10 (1.3) | 0.85 (0.33–2.14) | 0.722 | 0.898 |
| Target vessel revascularization (any) | 88 (20.2) | 174 (11.5) | 1.85 (1.43–2.38) | <0.001 | 42 (18.7) | 44 (21.0) | 0.90 (0.59–1.38) | 0.635 | 83 (11.3) | 79 (10.3) | 1.11 (0.82–1.52) | 0.491 | 0.430 |
| PCI (any) | 83 (19.0) | 159 (10.5) | 1.90 (1.46–2.47) | <0.001 | 39 (17.4) | 42 (20.0) | 0.87 (0.56–1.35) | 0.541 | 77 (10.4) | 73 (9.5) | 1.12 (0.81–1.54) | 0.494 | 0.369 |
| CABG (any) | 9 (2.2) | 21 (1.4) | 1.52 (0.70–3.31) | 0.292 | 4 (1.8) | 4 (2.1) | 0.94 (0.23–3.83) | 0.936 | 8 (1.1) | 10 (1.3) | 0.85 (0.33–2.14) | 0.722 | 0.898 |
| Clinically indicated target vessel revascularization | 86 (19.8) | 162 (10.8) | 1.94 (1.49–2.52) | <0.001 | 44 (19.5) | 44 (20.9) | 0.95 (0.63–1.45) | 0.821 | 86 (11.7) | 88 (11.4) | 1.03 (0.77–1.39) | 0.843 | 0.764 |
| PCI | 81 (18.6) | 150 (9.9) | 1.96 (1.50–2.57) | <0.001 | 41 (18.2) | 42 (19.9) | 0.92 (0.60–1.42) | 0.721 | 79 (10.6) | 80 (10.4) | 1.04 (0.76–1.42) | 0.793 | 0.657 |
| CABG | 8 (1.9) | 18 (1.2) | 1.57 (0.68–3.62) | 0.281 | 5 (2.3) | 4 (2.1) | 1.19 (0.31–4.48) | 0.801 | 9 (1.3) | 12 (1.6) | 0.79 (0.33–1.87) | 0.593 | 0.615 |
| Cerebrovascular event | 25 (5.7) | 50 (3.3) | 1.77 (1.09–2.86) | 0.019 | 11 (4.8) | 14 (6.7) | 0.73 (0.33–1.61) | 0.429 | 26 (3.6) | 24 (3.1) | 1.14 (0.65–1.98) | 0.649 | 0.364 |
| Transient ischemic attack | 0 (0.0) | 17 (1.1) | 0.10 (0.01–1.66) | 0.018 | 0 (0.0) | 0 (0.0) | NA | NA | 11 (1.5) | 6 (0.8) | 1.92 (0.71–5.19) | 0.188 | NA |
| Strokea | 25 (5.7) | 36 (2.4) | 2.47 (1.48–4.13) | <0.001 | 11 (4.8) | 14 (6.7) | 0.73 (0.33–1.61) | 0.429 | 16 (2.2) | 20 (2.6) | 0.84 (0.43–1.62) | 0.601 | 0.786 |
| Ischemic stroke | 24 (5.5) | 34 (2.3) | 2.51 (1.49–4.24) | <0.001 | 10 (4.4) | 14 (6.7) | 0.66 (0.29–1.49) | 0.311 | 15 (2.1) | 19 (2.5) | 0.83 (0.42–1.63) | 0.584 | 0.672 |
| Intracerebral hemorrhagic stroke | 1 (0.2) | 2 (0.1) | 1.73 (0.16–19.35) | 0.651 | 1 (0.4) | 0 (0.0) | 2.67 (0.11–65.22) | 1.000 | 1 (0.1) | 1 (0.1) | 1.05 (0.06–17.26) | 0.975 | 0.410 |
| TLFb | 131 (28.5) | 256 (16.8) | 1.87 (1.51–2.31) | <0.001 | 74 (31.0) | 57 (25.8) | 1.23 (0.87–1.73) | 0.244 | 124 (16.8) | 132 (16.8) | 0.98 (0.77–1.26) | 0.901 | 0.307 |
| Target vessel failurec | 143 (31.1) | 296 (19.4) | 1.76 (1.44–2.15) | <0.001 | 79 (33.1) | 64 (28.9) | 1.17 (0.84–1.63) | 0.348 | 141 (19.0) | 155 (19.7) | 0.95 (0.76–1.19) | 0.656 | 0.305 |
| Death, MI, or any repeat revascularizationd . | 195 (41.4) | 438 (28.2) | 1.66 (1.40–1.96) | <0.001 | 104 (42.4) | 91 (40.4) | 1.08 (0.81–1.43) | 0.597 | 221 (29.0) | 217 (27.5) | 1.07 (0.88–1.29) | 0.506 | 0.942 |
| Definite stent thrombosis | 12 (2.8) | 20 (1.3) | 2.10 (1.03–4.28) | 0.038 | 7 (3.0) | 5 (2.5) | 1.33 (0.42–4.24) | 0.626 | 9 (1.2) | 11 (1.4) | 0.85 (0.35–2.06) | 0.726 | 0.548 |
| Definite or probable stent thrombosis | 51 (11.7) | 87 (5.7) | 2.05 (1.45–2.90) | <0.001 | 26 (11.5) | 25 (11.9) | 0.98 (0.57–1.70) | 0.950 | 36 (4.7) | 51 (6.6) | 0.73 (0.48–1.12) | 0.147 | 0.402 |
Data are expressed as number of first events (number) and cumulative incidence (percentage). Rate ratios (RRs) (95% CI) estimated using the Mantel‐Cox method with 2‐sided P values from log‐rank test. All events were censored beyond 1825 days. Continuity correction with P value from Fisher exact test in case of zero events. Interaction P value testing for the modifying effect of diabetes mellitus (DM) (yes or no) on the RR of biodegradable polymer sirolimus‐eluting stents (BP‐SES) vs durable polymer everolimus‐eluting stents (DP‐EES), using an approximate Mantel‐Haenszel chi‐square test (df=1). CABG indicates coronary artery bypass grafting; NA, not applicable; PCI, percutaneous coronary intervention; TLF, target lesion failure.
Includes ischemic stroke, intracerebral hemorrhagic stroke, and unclear cause of cerebrovascular event.
Primary end point, defined as the composite of cardiac death, target vessel Q‐wave or non–Q wave myocardial infarction (MI), clinically indicated target lesion revascularization (TLR).
Defined as the composite of cardiac death, any Q‐wave or non–Q wave myocardial infarction, and any target vessel revascularization.
Patient‐oriented composite end point.
At 5 years of follow‐up, the primary composite end point of TLF occurred in 74 patients (31.0%) treated with BP‐SES and 57 patients (25.8%) treated with DP‐EES (RR 1.23; 95% CI 0.87–1.73 [P=0.24]) among patients with DM, and in 124 patients (16.8%) treated with BP‐SES and 132 patients (16.8%) treated with DP‐EES (RR, 0.98; 95% CI, 0.77–1.26 [P=0.90]) in individuals without DM (P for interaction=0.31) (Table 3, Figure 2). Cumulative incidence rates of cardiac death (14.9% versus 9.5%; RR, 1.56 [95% CI, 0.91–2.69]; P=0.10), target vessel MI (11.4% versus 11.0%; RR, 1.07 [95% CI, 0.61–1.89]; P=0.81), and clinically indicated TLR (16.9% versus 15.8%; RR, 1.10 [95% CI, 0.69–1.76]; P=0.68) were comparable between patients with DM treated with BP‐SES or DP‐EES (Table 3, Figure 2). The cumulative incidence of definite stent thrombosis at 5 years was 3.0% and 2.5% in patients with DM allocated to treatment with BP‐SES and DP‐EES, respectively (RR, 1.33; 95% CI, 0.42–4.24 [P=0.63]), and 1.2% and 1.4% in individuals without DM allocated to treatment with BP‐SES and DP‐EES, respectively (RR, 0.85; 95% CI, 0.35–2.06 [P=0.73; P for interaction=0.55]) (Table 3).
Figure 2.
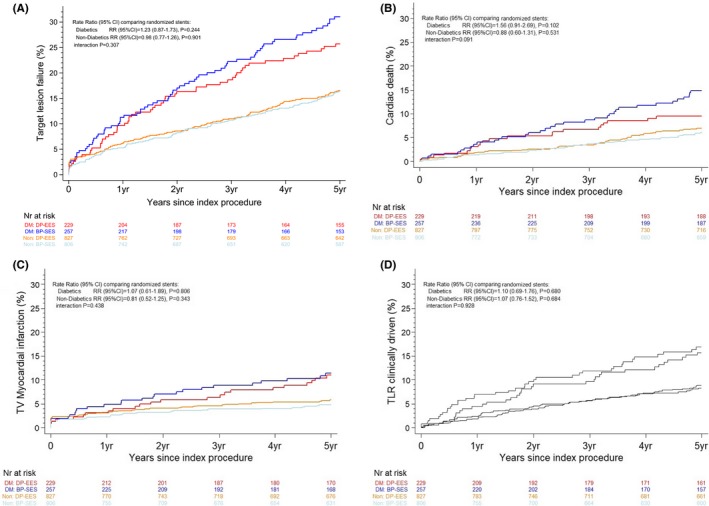
Time to event curves for the composite end point target lesion failure and individual components of the primary end point up to 5 years in patients with diabetes mellitus (DM) vs patients without DM. A, Target lesion failure; B, cardiac death; C, target vessel (TV) myocardial infarction; D, clinically driven target lesion revascularization (TLR). Blue lines indicate biodegradable polymer sirolimus‐eluting stent, patients with DM subgroup (BP‐SES, DM); red lines indicate durable polymer everolimus‐eluting stent, patients with DM subgroup (DP‐EES, DM); grey lines indicate biodegradable polymer sirolimus‐eluting stent, patients without DM subgroup (BP‐SES, Non); and orange lines indicate durable polymer everolimus‐eluting stent, patients without DM subgroup (DP‐EES, Non). RR indicates rate ratio.
Discussion
In a prespecified subgroup analysis of the large‐scale, multicenter, randomized BIOSCIENCE trial, BP‐SES provided similar long‐term clinical benefit compared with DP‐EES regarding the composite of TLF in unselected patients with DM undergoing PCI. To our knowledge, the present study is the first to assess the effect of BP‐DES compared with contemporary durable biocompatible polymer‐based DES among patients with DM throughout 5 years.
Contemporary second‐generation DES have reached a high level of safety and efficacy, and the importance of stent‐related relative to disease‐related events has decreased, thereby underscoring the paramount importance of secondary prevention of the underlying disease process. The present analysis confirms that, despite significant improvements in evidence‐based medical management and iterative developments in DES technology, patients with DM remain at increased risk for TLF, mainly driven by higher rates of TLR, following PCI with newer‐generation DES compared with individuals without DM, irrespective of the underlying coronary artery disease complexity.13 Diabetic status affects short‐ and long‐term clinical outcomes after PCI through multiple mechanisms, including disturbances that accelerate atherosclerosis progression, patient comorbidities, and anatomical factors that increase coronary artery disease complexity (eg, multivessel disease, diffuse lesions, high grades of calcification and tortuosity, higher incidence of left main disease, bifurcation lesions and chronic total occlusions, smaller reference vessel diameter, longer lesions, and greater plaque burden).14, 15 In addition, the proinflammatory milieu of DM enhances the vasculo‐proliferative response to stent‐mediated arterial injury.16 Thus, DM is associated with both a higher rate of recurrent ischemic events related to atherosclerotic disease progression and worse clinical outcomes related to stent failure following PCI.14 In the present analysis, though optimal secondary prevention medical therapy was more commonly prescribed in patients with DM than in individuals without DM, all‐comer patients with DM treated with newer‐generation biocompatible DES experienced higher rates of TLF, cardiac death, target vessel MI, clinically indicated TLR and definite stent thrombosis at 5 years of follow‐up compared with patients without DM. TLF accounted for approximately two thirds of the patient‐oriented clinical outcome, a composite of death, MI, or any repeat revascularization, among both patients with and those without DM, and the proportion of target lesion‐related events to patient‐related events were comparable in both the DM and non‐DM groups. Of note, even with the latest‐generation biocompatible DES, patients with DM remain at higher risk of long‐term definite or probable stent thrombosis compared with individuals without DM.
The choice of optimal DES therapy for patients with DM undergoing PCI remains an unresolved issue.17, 18, 19 Newer‐generation DES with thinner‐strut cobalt‐ or platinum‐chromium platforms, more biocompatible permanent polymer coatings and alternative antiproliferative drugs were shown to significantly reduce the risk of repeat revascularization without compromising safety outcomes, including stent thrombosis, compared with bare‐metal stents and early‐generation thick‐strut permanent polymer‐based DES in both patients with DM5 and patients without DM.20 Available evidence supports the use of thin‐strut DP‐EES in patients with DM undergoing PCI. In a large‐scale meta‐analysis,5 thin‐strut DP‐EES were shown to provide the most favorable efficacy and safety profile in patients with DM compared with early‐generation thick‐strut DP‐DES and thin‐strut durable polymer zotarolimus‐eluting stents with a 2‐, 3‐, 4‐, and 9‐fold risk reduction in target vessel revascularization, TLR, MI, and definite or probable stent thrombosis, respectively. Similarly, in a recent meta‐analysis of 18 trials including 17 000 patient‐years of follow‐up,21 thin‐strut DP‐EES were associated with significant reductions by 18% in major adverse cardiovascular events, 43% in MI, and 46% in stent thrombosis among patients with DM, whereas thin‐strut durable polymer zotarolimus‐eluting stents were associated with an 89% increased risk of TLR, compared with early‐generation thick‐strut DP‐DES. Furthermore, thin‐strut DP‐EES demonstrated a trend towards lower TLR and target vessel revascularization rates and a greater clinical benefit over early‐generation thick‐strut DP‐DES with regards to major adverse cardiovascular events and stent thrombosis among insulin‐dependent individuals with DM.21 However, these findings are only hypothesis‐generating because of the limited available outcome data on zotarolimus‐eluting stents in the diabetic population.
BP‐DES, with controlled drug release followed by subsequent degradation of the polymer coating, were developed to overcome the chronic inflammatory stimulus and hypersensitivity reactions elicited by durable polymer coatings that result in delayed vascular healing.6 Conceptually, the biodegradable polymer‐based DES technology represents an attractive treatment option for patients with DM and avoids persistent arterial injury through polymer biodegradation, thereby potentially reducing persisting late adverse clinical event rates associated with newer‐generation DP‐DES.7 Recently, BP‐SES demonstrated comparable clinical outcomes with regards to TLF and definite or probable stent thrombosis compared with thin‐strut DP‐EES at 1‐year follow‐up among patients with DM.9 However, longer‐term safety and efficacy data after percutaneous revascularization with available BP‐DES in patients with DM are limited and whether potential differences between BP‐DES and DP‐DES may emerge at extended follow‐up remains unknown. In a patient‐level pooled meta‐analysis,8 biodegradable polymer biolimus‐eluting stents with a thick‐strut stainless steel platform were associated with comparable clinical outcomes compared with first‐generation thick‐strut durable polymer sirolimus‐eluting stents at 4 years, but demonstrated a more favorable safety profile with significantly lower definite or probable stent thrombosis rates, driven by significantly lower rates of very late stent thrombosis among patients treated with BP‐DES. In a recent predefined subgroup analysis of the NOBORI 2 multicenter registry including 888 patients with DM treated with thick‐strut biodegradable polymer biolimus‐eluting stents,22 TLF rates were significantly higher in patients with DM than in individuals without DM, and in insulin‐dependent than in noninsulin‐dependent patients with DM, at 5‐year follow‐up. Notwithstanding, definite and probable stent thrombosis rates at 5 years were similar among patients with DM versus individuals without DM, or insulin‐dependent versus noninsulin‐dependent patients with DM with extremely low very late stent thrombosis events in both patients with DM and insulin‐dependent DM.22 The present analysis extends previous knowledge by providing long‐term randomized outcome data after PCI with newer‐generation ultrathin‐strut BP‐DES in patients with DM. At 5‐year follow‐up, we found that ultrathin‐strut BP‐SES were associated with similar clinical outcomes, including stent thrombosis, compared with thin‐strut DP‐EES among patients with DM, without significant interaction between diabetic status and treatment effect of BP‐SES and DP‐EES. These findings are consistent with the long‐term results reported in the overall study population11 and suggest that ultrathin‐strut BP‐DES may represent a valuable treatment option even in the high‐risk DM subgroup with similar long‐term efficacy and safety outcomes compared with thin‐strut durable polymer‐based DES.
In addition to the polymer coating characteristics, stent strut thickness is another major component of DES design that potentially affects clinical outcomes after PCI in patients with DM. A recent large‐scale study‐level meta‐analysis demonstrated lower rates of TLF with ultrathin‐strut DES compared with contemporary‐generation thin‐strut DES.23 Available evidence on the effect of ultrathin‐strut DES on clinical outcomes in patients with DM is limited. In a subgroup analysis of the SORT OUT VII (Scandinavian Organization for Randomized Trials With Clinical Outcome) trial,24 no significant differences with regards to rates of TLF, cardiac death, MI, and TLR were observed among patients with DM allocated to ultrathin‐strut BP‐SES or thick‐strut biodegradable polymer biolimus‐eluting stents at 2‐year follow‐up, despite numerically lower rates of definite or probable stent thrombosis among patients with DM treated with BP‐SES. However, these findings warrant confirmation from dedicated and adequately powered clinical trials comparing newer‐generation ultrathin‐strut and thin‐strut DES in the diabetic population.
Study Limitations
The present analysis must be interpreted in view of several limitations. First, as per the design of the main trial, randomization was not stratified according to diabetic status. Second, diabetic status was assessed based on the patient clinical history at admission, and patients with newly diagnosed DM during hospitalization or follow‐up were not included. Furthermore, levels of glycohemoglobin were not available for the entire diabetic population. Third, although prespecified, this subgroup analysis was not powered for the reported end points and the results can only be interpreted as hypothesis‐generating. Fourth, we did not stratify the present analysis according to insulin‐dependent or noninsulin‐dependent DM because of the limited total number of patients with DM included in the trial.
Conclusions
In the prespecified subgroup analysis of the BIOSCIENCE trial, long‐term clinical outcomes in patients with DM treated with BP‐SES or DP‐EES were comparable throughout 5 years. Despite iterative developments in DES technology, patients with DM remain at increased long‐term TLF risk compared with individuals without DM.
Sources of Funding
BIOSCIENCE was an investigator‐initiated trial supported by a dedicated research grant from Biotronik, Bülach, Switzerland. The study principal investigators (Windecker and Pilgrim) and statistician (Heg) are responsible for the design and conduct of the trial and all analyses. The funding source was not involved in the study design, data collection, or data management, and had no role in the study results analysis and interpretation, writing of the article, or decision to submit for publication.
Disclosures
Iglesias reports institutional research grants from Biotronik, Abbott Vascular, Philips Volcano and Astra Zeneca, and personal fees from Biotronik, Philips Volcano, Astra Zeneca, Terumo, Medtronic, and Cardinal Health, outside the submitted work. Roffi reports institutional research grants from Terumo, Boston Scientific, Medtronic, Abbott Vascular, and Biotronik, outside the submitted work. Jüni is a tier 1 Canada research chair in clinical epidemiology of chronic diseases. This research was completed, in part, with funding from the Canada Research Chairs Programme. Jüni also serves as an unpaid member of the steering group for trials funded by AstraZeneca, Biotronik, Biosensors, St Jude Medical, and The Medicines Company. Windecker received research grants to his institution from Abbott, Amgen, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Medicines Company, and St Jude. Pilgrim received research grants to his institution from Biotronik, Boston Scientific, and Edwards Lifesciences, and speaker fees from Biotronik and Boston Scientific. The remaining authors have no disclosures to report.
Supporting information
Data S1. Study end points definitions.
(J Am Heart Assoc. 2019;8:e013607 DOI: 10.1161/JAHA.119.013607.)
References
- 1. Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, Bakhai A, Cohen DJ, Kuntz RE, Ho KK. Beyond restenosis: five‐year clinical outcomes from second generation coronary stent trials. Circulation. 2004;110:1226–30. [DOI] [PubMed] [Google Scholar]
- 2. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents. JAMA. 2005;293:2126–30. [DOI] [PubMed] [Google Scholar]
- 3. Lee TT, Feinberg L, Baim DS, Holmes DR, Aroesty JM, Carrozza JP Jr, Cohen DJ, Ho KK, Cutlip DE. Effect of diabetes mellitus on five‐year clinical outcomes after single‐vessel coronary stenting (a pooled analysis of coronary stent clinical trials). Am J Cardiol. 2006;98:718–17. [DOI] [PubMed] [Google Scholar]
- 4. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 5. Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, Williams DO, Slater J, Cutlip DE, Feit F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow‐up from randomised trials. BMJ. 2012;345:e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 7. Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus‐ and everolimus‐eluting coronary stents: final 5‐year report of the RESOLUTE all‐comers trial. Circ Cardiovasc Interv. 2015;8:e002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Waha A, Stefanini GG, King LA, Byrne RA, Serruys PW, Kufner S, Meier B, Jüni P, Kastrati A, Windecker S. Long‐term outcomes of biodegradable polymer versus durable polymer drug‐eluting stents in patients with diabetes a pooled analysis of individual patient data from 3 randomized trials. Int J Cardiol. 2013;168:5162. [DOI] [PubMed] [Google Scholar]
- 9. Franzone A, Pilgrim T, Heg D, Roffi M, Tüller D, Vuilliomenet A, Muller O, Cook S, Weilenmann D, Kaiser C, Jamshidi P, Räber L, Stortecky S, Wenaweser P, Jüni P, Windecker S. Clinical outcomes according to diabetic status in patients treated with biodegradable polymer sirolimus‐eluting stents versus durable polymer everolimus‐eluting stents: prespecified subgroup analysis of the BIOSCIENCE trial. Circ Cardiovasc Interv. 2015;8:e002319. [DOI] [PubMed] [Google Scholar]
- 10. Pilgrim T, Roffi M, Tüller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P, Heg D, Jüni P, Windecker S. Randomized comparison of biodegradable polymer sirolimus‐eluting stents versus durable polymer everolimus‐eluting stents for percutaneous coronary revascularization: rationale and design of the BIOSCIENCE trial. Am Heart J. 2014;168:256–61. [DOI] [PubMed] [Google Scholar]
- 11. Pilgrim T, Piccolo R, Heg D, Roffi M, Tüller D, Muller O, Moarof I, Siontis GC, Cook S, Weilenmann D, Kaiser C, Cuculi F, Hunziker L, Eberli FR, Jüni P, Windecker S. Ultrathin‐strut, biodegradable‐polymer, sirolimus‐eluting stents versus thin‐strut, durable‐polymer, everolimus‐eluting stents for percutaneous coronary revascularisation: 5‐year outcomes of the BIOSCIENCE randomised trial. Lancet. 2018;392:737–746. [DOI] [PubMed] [Google Scholar]
- 12. Iglesias JF, Roffi M, Degrauwe S, Secco GG, Aminian A, Windecker S, Pilgrim T. Orsiro cobalt‐chromium sirolimus‐eluting stent: present and future perspectives. Expert Rev Med Devices. 2017;14:773–88. [DOI] [PubMed] [Google Scholar]
- 13. Koskinas KC, Siontis GC, Piccolo R, Franzone A, Haynes A, Rat‐Wirtzler J, Silber S, Serruys PW, Pilgrim T, Räber L, Heg D, Jüni P, Windecker S. Impact of diabetic status on outcomes after revascularization with drug‐eluting stents in relation to coronary artery disease complexity: patient‐level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9:e003255. [DOI] [PubMed] [Google Scholar]
- 14. Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J. 2011;32:2748–57. [DOI] [PubMed] [Google Scholar]
- 15. Bernelli C, Chan J, Chieffo A. Drug‐eluting stent outcomes in diabetes. Expert Rev Cardiovasc Ther. 2014;12:95–109. [DOI] [PubMed] [Google Scholar]
- 16. Biondi‐Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41:1071–1077. [DOI] [PubMed] [Google Scholar]
- 17. Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus‐eluting and Paclitaxel‐eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. [DOI] [PubMed] [Google Scholar]
- 18. Kastrati A, Massberg S, Ndrepepa G. Is diabetes the Achilles’ heel of limus‐eluting stents? Circulation. 2011;124:869–72. [DOI] [PubMed] [Google Scholar]
- 19. Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, Park DW, Kang SJ, Lee CW, Lee JH, Choi SW, Seong IW, Lee BK, Lee NH, Cho YH, Shin WY, Lee SJ, Lee SW, Hyon MS, Bang DW, Park WJ, Kim HS, Chae JK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Choi YJ, Cheong SS, Yang TH, Jang JS, Her SH, Park SJ; ESSENCE‐DIABETES Study Investigators . Randomized comparison of everolimus‐eluting stent versus sirolimus‐eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE‐DIABETES): results from the ESSENCE DIABETES trial. Circulation. 2011;124:886–92. [DOI] [PubMed] [Google Scholar]
- 20. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short‐ and long‐term outcomes with drug‐eluting and bare‐metal coronary stents: a mixed‐treatment comparison analysis of 117 762 patient‐years of follow‐up from randomized trials. Circulation. 2012;125:2873–2891. [DOI] [PubMed] [Google Scholar]
- 21. Bavishi C, Baber U, Panwar S, Pirrotta S, Dangas GD, Moreno P, Tamis‐Holland J, Kini AS, Sharma SK. Efficacy and safety of everolimus and zotarolimus‐eluting stents versus first‐generation drug‐eluting stents in patients with diabetes: a meta‐analysis of randomized trials. Int J Cardiol. 2017;230:310–318. [DOI] [PubMed] [Google Scholar]
- 22. Wiemer M, Stoikovic S, Samol A, Dimitriadis Z, Ruiz‐Nodar JM, Birkemeyer R, Monsegu J, Finet G, Hildick‐Smith D, Tresukosol D, Novo EG, Koolen JJ, Barbato E, Danzi GB; NOBORI 2 investigators . Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow‐up. Cardiovasc Diabetol. 2017. Feb 10;16(1):23 DOI: 10.1186/s12933-017-0500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer‐generation ultrathin strut drug‐eluting stents versus older second‐generation thicker strut drug‐eluting stents for coronary artery disease. Circulation. 2018;138:2216–2226. [DOI] [PubMed] [Google Scholar]
- 24. Ellert J, Christiansen EH, Maeng M, Raungaard B, Jensen SE, Kristensen SD, Veien KT, Junker AB, Jakobsen L, Aarøe J, Terkelsen CJ, Kahlert J, Villadsen AB, Bøtker HE, Jensen LO. Impact of diabetes on clinical outcomes after revascularization with sirolimus‐eluting and biolimus‐eluting stents with biodegradable polymer from the SORT OUT VII trial. Catheter Cardiovasc Interv. 2019;93:567–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Study end points definitions.


