Abstract
Background
Right atrial pressure (RAP), a composite metric of right ventricular diastolic function, volume status, and right heart compliance, is a predictor of mortality in patients with heart failure due to acquired heart disease. Because patients with tetralogy of Fallot (TOF) might have abnormal right atrial and ventricular mechanics caused by myocardial injury and remodeling, we hypothesized that RAP would be associated with disease severity and cardiovascular adverse events in this population.
Methods and Results
We performed a cohort study of adults with TOF who underwent right heart catheterization at the Mayo Clinic Rochester between 1990 and 2017. The objective was to determine the association between RAP and multiple domains of disease severity in TOF (percentage of predicted peak oxygen consumption, atrial or ventricular arrhythmia, and heart failure hospitalization), as well as cardiovascular adverse events, defined as sustained ventricular tachycardia, resuscitated or aborted sudden death, heart transplantation, or death. Among 225 patients (113 male; mean age: 39±14 years), mean RAP was 10.7±5.2 mm Hg and median was 10 mm Hg (interquartile range: 7–13 mm Hg). Increasing RAP was associated with atrial or ventricular arrhythmias (odds ratio: 5.01; 95% CI, 1.22–23.49; P<0.001), heart failure hospitalization (odds ratio: 1.47; 95% CI, 1.10–2.39; P=0.033) per 5 mm Hg, and worsening exercise capacity (peak oxygen consumption; R 2=0.74, r=−0.86, P<0.001). RAP was a predictor of cardiovascular adverse events (hazard ratio: 1.28; 95% CI, 1.10–1.47; P=0.028) per 5 mm Hg.
Conclusions
In symptomatic patients with TOF, increasing RAP correlates with multiple domains of disease severity (risk stratification) and predicts future cardiovascular events (prognostication). These data have potential clinical implications in the target population of symptomatic TOF patients.
Keywords: diastolic dysfunction, exercise capacity, mortality, right atrial pressure, tetralogy of Fallot
Subject Categories: Congenital Heart Disease
Clinical Perspective
What Is New?
Right atrial pressure is the main determinant of left heart filling pressures, and it correlates with disease severity and mortality in patients with tetralogy of Fallot.
In tetralogy of Fallot patients with elevated pulmonary artery wedge pressure, right atrial pressure (and not pulmonary artery wedge pressure) was an independent predictor of cardiovascular adverse events.
What Are the Clinical Implications?
Elevated right atrial pressure is a hemodynamic characteristic of patients at high risk for adverse outcomes and thus can potentially be used to guide therapy in this population.
Introduction
Congenital heart disease is an important but understudied cause of heart failure and arrhythmia, and tetralogy of Fallot (TOF) is one of the most common congenital heart disease diagnoses in adults.1 Right ventricular (RV) systolic dysfunction frequently develops in adults with repaired TOF2, 3 and is one of the criteria used to guide the decision for pulmonary valve replacement.4, 5 RV systolic dysfunction in TOF is believed to be caused by cyanosis and RV pressure overload before TOF repair6 and RV volume and/or pressure overload caused by residual or recurrent hemodynamic lesions after TOF repair.7 RV diastolic dysfunction typically precedes systolic dysfunction,8, 9 suggesting that this might be an earlier indicator for ventricular dysfunction, but the assessment of RV diastolic function is challenging, and there are no validated noninvasive indexes of RV diastolic function in the TOF population.
Although there are robust invasive and noninvasive hemodynamic studies of left ventricular (LV) diastolic function,8, 9 the prevalence and clinical implications of RV diastolic dysfunction based on invasive hemodynamic data have not been studied in adults with TOF. Right atrial pressure (RAP) is a composite metric of right heart function that is related to RV diastolic function, volume status, and right atrial (RA) compliance. Increased RAP is a predictor of mortality in patients with heart failure due to acquired heart disease.10, 11 It seems likely that TOF patients might have abnormal RA and RV mechanics caused by myocardial injury and remodeling because of prior surgical and ongoing hemodynamic insult, thus causing abnormal RAP. We hypothesized that RAP, as assessed by the gold standard of invasive hemodynamic assessment, would be associated with disease severity and cardiovascular adverse events in adults with TOF.
Methods
Patient Selection
The data that support the findings of this study are available from the corresponding author on reasonable request. This is a retrospective cohort study, and the target population is symptomatic patients with repaired TOF. The Mayo Clinic institutional review board approved this study and waived informed consent for patients who provided research authorization. The MACHD (Mayo Adult Congenital Heart Disease) Registry was interrogated to identify all adults (aged ≥18 years) with repaired TOF who underwent right heart catheterization at the Mayo Clinic Rochester (Minnesota) from January 1, 1990, through December 31, 2017. Patients with tricuspid valve prostheses were excluded.
Study End Points and Definitions
The primary study objective was to determine the association between RAP and disease severity indexes, assessed across 3 clinical domains: (1) aerobic capacity (percentage of predicted peak oxygen consumption [vo 2]), (2) arrhythmias, and (3) heart failure hospitalization. Atrial/ventricular arrhythmia was defined as documented atrial fibrillation, atrial flutter/tachycardia, or nonsustained ventricular tachycardia.12 Heart failure hospitalization was defined as admission for volume overload (pulmonary congestion and/or peripheral edema) requiring intravenous diuretics.13 The secondary study objective was to determine the association between RAP and cardiovascular adverse events, defined as sustained ventricular tachycardia, resuscitated or aborted sudden cardiac death, heart transplantation, or all‐cause mortality.
Exploratory analysis was performed to determine the correlation between RAP and echocardiographic indexes of RV diastolic function endorsed by the American Society of Echocardiography.14 These indexes are (1) tricuspid inflow early diastolic velocity/tricuspid inflow late diastolic velocity (E/A), (2) tricuspid inflow early diastolic velocity/tricuspid annular tissue Doppler early systolic velocity (E/e′), (3) tricuspid inflow deceleration time (DT), and (4) inferior vena cava (IVC) size. We included only patients in sinus rhythm and had less‐than‐moderate tricuspid regurgitation for this exploratory analysis.
Cardiac Catheterization
All studies were performed with patients on chronic medications in the fasted state and mild sedation using 7‐Fr fluid‐filled catheters. Catheter position was confirmed by appearance on fluoroscopy, characteristic pressure waveforms, and oximetry. Systemic arterial pressures and saturations were assessed at the time of left heart catheterization or via femoral or radial arterial cannulation in patients who did not undergo concurrent left heart catheterization. Pressure measurements were recorded at end expiration and represent an average of 3 beats for patients in sinus rhythm and 5 beats for patients in atrial fibrillation.15 Cardiac output was determined by the Fick technique using assumed O2 consumption and directly measured O2 contents in pulmonary and systemic circulations.16 Total pulmonary resistance was calculated as mean pulmonary artery (PA)/pulmonary blood flow index, PA compliance index by the ratio of RV stroke volume index/PA pulse pressure, and pulmonary elastance by the ratio of PA systolic pressure/stroke volume index.17, 18 LV transmural pressure, which reflects the net distending pressure that favors LV filling, was calculated as PA wedge press minus RAP, given that RAP is an accurate estimate of pericardial pressure.17, 19 These variables were indexed to body surface area. Hemodynamic pressure tracings were recorded, digitized (240 Hz), and stored for offline analysis. Offline review of hemodynamic tracings, angiographic images, and cardiac catheterization reports were performed in all patients.
Echocardiography
Two‐dimensional, M‐mode, and Doppler echocardiography were performed according to standard American Society of Echocardiography guidelines,14, 20 and only echocardiograms performed within 7 days from the time of cardiac catheterization were analyzed for this study. Offline measurements of tricuspid inflow and tissue Doppler indexes (apical view) and IVC size (subcostal short axis) were performed in all patients by an experienced sonographer. Dilated IVC was defined as IVC >21 mm, and reduced collapsibility during inspiration was defined as <50% decrease in IVC diameter during inspiration.14
Outcomes Assessment
Percentage of predicted peak vo 2 was assessed using upright treadmill cardiopulmonary exercise testing, with maximum effort defined as respiratory quotient >1.1.21 All exercise tests were required to be performed within 6 months from the time of cardiac catheterization. Atrial/ventricular arrhythmia and heart failure hospitalization status were ascertained in 100% of patients as of December 31, 2017, using the date of the last clinic visit. All‐cause mortality was ascertained using the Mayo Clinic registration database and Accurint, an institutionally approved location service, in 100% of patients as of December 31, 2017.
Statistical Analysis
Data are presented as mean±SD, median (interquartile range), or number (percentage). Between‐group comparisons were performed using the Fisher exact test, t test, or Wilcoxon rank sum test, as appropriate. Linear regression analysis was used to assess the relationship between RAP and RV afterload indexes (PA elastance, capacitance, and total pulmonary resistance), LV transmural pressure, and peak vo 2. We also used logistic regression and receiver operating characteristic curves to determine the optimal cutoff point of RAP that predicted a cardiovascular adverse event with the best sensitivity and specificity. For the exploratory analysis, logistic regression was used to assess the performance of the echocardiographic indexes of RV diastolic function to detect elevated RAP, defined as RAP >10 mm Hg. Cox regression was used to assess the correlation between RAP and cardiovascular adverse events, defined as sustained ventricular tachycardia, resuscitated or aborted sudden cardiac death, heart transplantation, or all‐cause mortality. Schoenfeld residual was used to assess Cox proportional hazards assumptions.
All regression models were adjusted for age at the time of catheterization, age at the time of TOF repair, TOF–pulmonary atresia diagnosis, severity of RV systolic dysfunction, tricuspid regurgitation, and pulmonary regurgitation, using manual backward stepwise model selection based on likelihood ratio P value. These variables were chosen a priori because of known association with clinical outcomes in patients with TOF.2, 3, 22 In addition, we adjusted for the effect of indication for cardiac catheterization, era effect, and pulmonary valve replacement during follow‐up in the Cox regression model assessing the relationship between RAP and cardiovascular adverse events. The indication for cardiac catheterization was modeled as a categorical variable (preoperative evaluation versus others), and the procedure era was also modeled as categorical variable (before January 2004 versus others). Kaplan–Meier analysis was used to assess the relationship between RAP and cardiovascular adverse event, and between‐group comparison was performed with a log‐rank test. The time of cardiac catheterization was used as the baseline for time‐to‐event analyses. P<0.050 was considered statistically significant. All statistical analyses were performed with JMP software (v14.0; SAS Institute).
Results
Clinical and Hemodynamic Data
A total of 231 ambulatory patients with TOF underwent right heart catheterization within the study period. Six were excluded because they had tricuspid valve prostheses. The indications for cardiac catheterization were preoperative evaluation (n=134, 60%), congestive heart failure (n=46, 21%), arrhythmia (n=75, 33%), and aborted sudden cardiac death (n=4, 2%). Age at the time of cardiac catheterization was 39±14 years, and 87 patients (39%) had TOF–pulmonary atresia (Table 1).
Table 1.
Baseline Clinical Characteristics
| N=225 | RAP ≤10 mm Hg (n=134) | RAP >10 mm Hg (n=94) | P Value | |
|---|---|---|---|---|
| Age at beginning of study, y | 34±16 | 33±12 | 35±13 | 0.136 |
| Male | 113 (50) | 63 (47) | 50 (53) | 0.105 |
| Age at TOF repair, y | 6 (3–14) | 5 (3–10) | 7 (4–17) | 0.092 |
| Prior palliative shunt | 141 (62) | 78 (58) | 63 (67) | 0.412 |
| TOF–pulmonary atresia | 87 (39) | 48 (36) | 39 (41) | 0.341 |
| Transannular patch repair | 122 (54) | 74 (55) | 48 (51) | 0.534 |
| Prior pulmonary valve replacement | 161 (72) | 92 (69) | 69 (73) | 0.348 |
| Prior heart failure hospitalization | 26 (12) | 8 (6) | 18 (19) | 0.003 |
| Loop diuretics | 47 (21) | 18 (13) | 29 (31) | 0.005 |
| RAAS antagonist | 56 (25) | 22 (16) | 34 (36) | <0.001 |
| β‐Blocker | 35 (16) | 12 (9) | 23 (24) | 0.004 |
| Comorbidities | ||||
| Atrial fibrillation | 63 (28) | 21 (16) | 42 (45) | <0.001 |
| Atrial flutter/tachycardia | 56 (25) | 26 (19) | 30 (32) | 0.026 |
| Chronic kidney disease | 17 (8) | 8 () | 9 (105) | 0.323 |
| Hypertension | 71 (32) | 37 (28) | 34 (36) | 0.481 |
| Hyperlipidemia | 95 (42) | 49 (37) | 46 (49) | 0.287 |
| Coronary artery disease | 25 (11) | 12 (9) | 13 (14) | 0.375 |
| Current or prior smoker | 45 (20) | 22 (16) | 23 (25) | 0.346 |
| Diabetes mellitus | 33 (15) | 8 (6) | 25 (27) | <0.001 |
| Obesity | 52 (23) | 28 (21) | 24 (26) | 0.219 |
Data are presented as mean±SD, median (interquartile range), or number (%). Chronic kidney disease is defined as stage ≥3 (creatinine clearance <60 mL/min). RAAS indicates renin–angiotensin–aldosterone system; RAP, right atrial pressure; TOF, tetralogy of Fallot.
Right Heart Filling Pressures
Mean RAP was 10.7±5.2 mm Hg, and median RAP was 10 mm Hg (interquartile range: 7–13 mm Hg; Table 2). There was good correlation between RAP and RV afterload, as measured by PA compliance, PA elastance, and total pulmonary resistance (Figure 1), but no correlation between RAP and RV outflow tract (RVOT) obstruction (r=0.003, P=0.218). Similarly, RAP correlated with LV transmural pressure and cardiac output (Figure 1). Because Fick‐derived stroke volume measures the net stroke volume (forward minus regurgitant flow) rather than the total stroke volume, sensitivity analyses were performed in the subset of patients without significant pulmonary regurgitation (excluding moderate or greater pulmonary regurgitation), and these analyses demonstrated similar correlations.
Table 2.
Invasive and Noninvasive Hemodynamic Data
| Results (N=225) | |
|---|---|
| Echocardiography | |
| Moderate or greater tricuspid regurgitationa | 51 (23) |
| Moderate or greater pulmonary regurgitationa | 121 (56) |
| Moderate or greater RV enlargementa | 161 (73) |
| Moderate or greater RV systolic dysfunctiona | 75 (34) |
| RVSP, mm Hg | 64±23 |
| Tricuspid regurgitation velocity, m/s | 3.6±0.8 |
| Assumed RAP, mm Hg | 10±4 |
| Pulmonary valve peak velocity, m/s | 2.9±1.0 |
| RA volume index, mL/m2 | 59±22 |
| Moderate or greater RA enlargementa | 131 (59) |
| LA volume index, mL/m2 | 31±8 |
| Moderate or greater LA enlargementa | 36 (16) |
| Medial E/e′ | 11±4 |
| Lateral E/e′ | 7±3 |
| LV ejection fraction, % | 59±10 |
| Catheterization | |
| RAP, mm Hg | 10 (7–13) |
| RVEDP, mm Hg | 14 (11–17) |
| RVSP, mm Hg | 62 (50–86) |
| PA systolic pressure, mm Hg | 41 (31–52) |
| PA diastolic pressure, mm Hg | 11 (7–16) |
| Mean PA pressure, mm Hg | 23 (17–30) |
| PAWP, mm Hg | 14±5 |
| PA compliance index, mL×m−2/mm Hg | 1.46 (0.94–2.07) |
| PA elastance index, mm Hg/mL×m2 | 1.01 (0.72–1.58) |
| TPR index, mm Hg/L×min−1 | 10.2 (7.2–14.3) |
| PVR, index, WU×m2 | 3.6 (2.4–6.6) |
| LV transmural pressure, mm Hg | 3 (2–5) |
| Cardiac index, L/min×m2 | 2.3±0.7 |
| MAP, mm Hg | 86±15 |
| Mixed venous saturation, % | 69±8 |
| Aortic saturation, % | 96 (94–98) |
Data are presented as mean±SD, median (interquartile range), or number (%). E indicates mitral inflow early velocity; e′, tissue Doppler early velocity; LA, left atrium; LV, left ventricle; MAP, mean arterial pressure; PA, pulmonary artery; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RAP, right atrial pressure; RV, right ventricular; RVEDP, right ventricular end‐diastolic pressure; RVSP, right ventricular systolic pressure; TPR, total pulmonary resistance; WU, Wood units.
Qualitative echocardiographic assessment.
Figure 1.
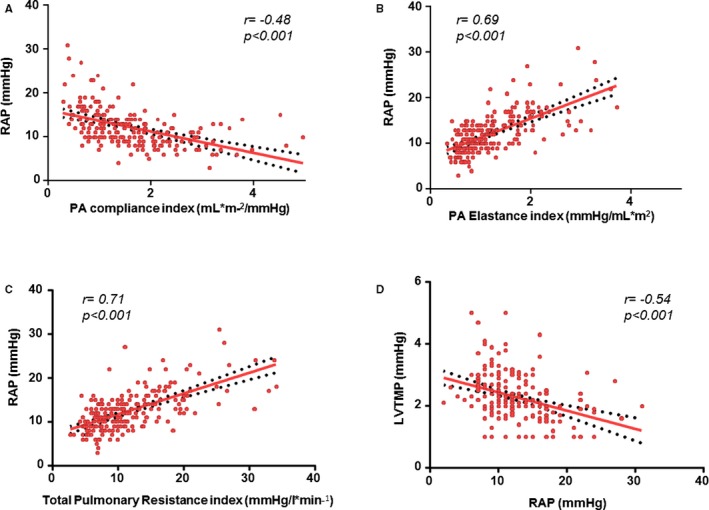
Linear regression of right atrial pressure (RAP) and pulmonary artery (PA) capacitance index (A), PA elastance index (B), total pulmonary resistance (C), and left ventricular transmural pressure (LVTMP) (D). Note that increase in PA elastance index and total pulmonary resistance denote increase in right ventricular (RV) afterload, whereas decrease in PA compliance denotes a decrease in RV afterload.
RAP and Disease Severity Indexes
Of 225 patients, 95 (42%) experienced atrial/ventricular arrhythmias (63 with atrial fibrillation, 56 with atrial flutter/tachycardia, and 50 with nonsustained ventricular tachycardia) during the follow‐up period. RAP was associated with atrial/ventricular arrhythmia occurrence (odds ratio: 5.01; 95% CI, 1.22–23.49; P<0.001 per 5‐mm Hg increase in RAP). An exploratory analysis was performed to assess the correlation between RAP and ventricular arrhythmias. Again, we noted an association between RAP and ventricular arrhythmia occurrence (odds ratio: 3.22; 95% CI, 1.09–16.12; P=0.024 per 5‐mm Hg increase in RAP). There were 26 patients (12%) with heart failure hospitalizations, and RAP was associated with heart failure hospitalization (odds ratio: 1.47; 95% CI, 1.10–2.39; P=0.033 per 5‐mm Hg increase in RAP). Of the 128 patients (57%) with exercise test data, the mean peak vo 2 and percentage of predicted peak vo 2 were 21±6 mL/kg per minute and 62±15%, respectively. There was an inverse correlation between RAP and percentage of predicted peak vo 2 (R 2=0.74, r=−0.86, P<0.001; Figure 2.
Figure 2.
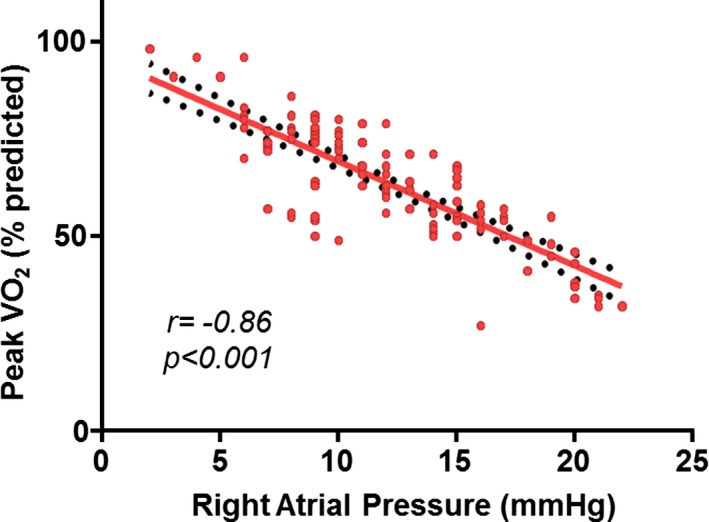
Linear regression of right atrial pressure and peak oxygen consumption (vo 2).
RAP and Cardiovascular Adverse Events
Mean follow‐up from the time of cardiac catheterization was 7.3±5.8 years, yielding total follow‐up of 1642 patient‐years. In total, 97 patients (43%) underwent pulmonary valve replacement during follow‐up. During this period, there were 28 cases of sustained ventricular tachycardia, 4 cases of aborted sudden cardiac death, 4 patients who underwent heart transplant, and 35 patients who died. The cause of death was end‐stage heart failure (n=20), arrhythmic death (n=6), postoperative death after cardiac surgery (n=3), bleeding‐ or stroke‐related death (n=1), malignancy (n=1), sepsis (n=1), and unknown (n=3). A cardiovascular adverse event end point occurred in 55 patients (24%), yielding an event rate of 3.2 per 100 patient‐years.
The 10‐year rate of freedom from cardiovascular adverse events was 74% for the entire cohort. RAP >10 mm Hg provided the best prediction of cardiovascular adverse events (area under the curve: 0.752; sensitivity: 74%; specificity: 76%). Patients with RAP >10 mm Hg had lower event‐free survival compared with those with RAP ≤10 mm Hg (54% versus 85%, P<0.001; Figure 3. RAP was a statistically significant predictor of cardiovascular adverse events (hazard ratio: 1.28; 95% CI, 1.10–1.47; P=0.028; Table 3.
Figure 3.
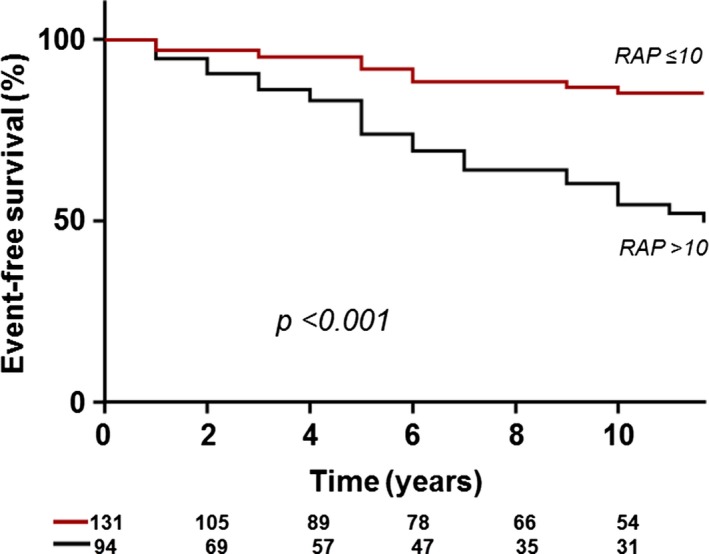
Kaplan–Meier analysis showing event‐free survival. RAP indicates right atrial pressure.
Table 3.
Multivariable Predictors of Cardiovascular Adverse Events
| Full Model | Final Model | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| RAP (per 5 mm Hg) | 1.34 (1.05–1.69) | 0.035 | 1.28 (1.10–1.47) | 0.028 |
| Age at cardiac catheterization (per 1 y) | 1.05 (1.01–1.07) | 0.016 | 1.04 (1.01–1.07) | 0.014 |
| Age at TOF repair (per 1 y) | 0.99 (0.97–1.01) | 0.534 | … | … |
| Indication for cardiac catheterization | 1.17 (0.86–2.14) | 0.133 | … | … |
| Procedure era before 2004 | 1.08 (0.73–4.66) | 0.234 | … | … |
| PVR during follow‐up | 1.13 (0.64–3.55) | 0.298 | … | … |
| TOF–pulmonary atresia diagnosis | 1.02 (0.56–1.79) | 0.881 | … | … |
| Moderate or greater RV systolic dysfunction | 1.83 (0.95–3.53) | 0.074 | 1.81 (1.00–3.04) | 0.046 |
| Moderate or greater tricuspid regurgitation | 1.73 (0.84–3.84) | 0.151 | … | … |
| Moderate or greater pulmonary regurgitation | 1.62 (0.48–2.52) | 0.286 | … | … |
HR indicates hazard ratio; PVR, pulmonary valve replacement; RAP, right atrial pressure; RV, right ventricle; TOF, tetralogy of Fallot.
Echocardiographic Predictors of RAP
Of the 231 patients in the study, 135 (58%) met the inclusion criteria for exploratory analysis, and the echocardiographic indexes of diastolic function are shown in Table S1. Of all diastolic function indexes assessed, IVC size and collapsibility provided the best detection of elevated RAP (RAP >10 mm Hg) based on area under the curve (Table S2). Dilated IVC had the best sensitivity to detect elevated RAP (sensitivity: 93%; specificity: 58%; area under the curve: 0.766), whereas dilated IVC with reduced inspiratory collapse had the best specificity to detect elevated RAP (sensitivity: 77%; specificity: 98%; area under the curve: 0.742).
Of the 129 patients with IVC size and collapsibility data, 44 (33%) had normal IVC size and collapsibility, 55 (41%) had dilated IVC or reduced collapsibility, and 36 (27%) had dilated IVC and reduced collapsibility, and median RAP was significantly different among the 3 groups: 6 mm Hg (interquartile range: 4–7 mm Hg), 9 mm Hg (interquartile range: 6–11 mm Hg), and 14 mm Hg (interquartile range: 12–16 mm Hg), respectively; P<0.001). Similarly, the 10‐year event‐free survival rate was significantly different among the 3 groups (88%, 64%, and 57%, respectively; P<0.001; Figure S1).
Discussion
In this study of symptomatic patients with TOF who underwent right heart catheterization, increasing RAP—an integrated measure of right heart compliance—was associated with worsening exercise capacity disease severity and cardiovascular adverse events. These data suggest that RAP can be used for risk stratification in symptomatic TOF patients and can have potential clinical implications regarding type and timing of intervention. Furthermore, assessment of IVC size and collapsibility can be used for noninvasive estimation of RAP and correlated with event‐free survival.
The prevalence of RV diastolic dysfunction in the TOF population is unknown but is likely common considering the cumulative effect of myocardial injury caused by cyanosis before repair, hypoxic injury at the time of surgery, and residual or recurrent hemodynamic lesion after repair.7 In a multicenter study of 556 adult TOF patients, RV diastolic dysfunction—defined based on tricuspid inflow and tricuspid annulus tissue Doppler indexes—was present in 52% of patients.23 In a study of 38 TOF patients, Gatzoulis et al24 defined restrictive RV physiology (advanced RV diastolic dysfunction) by the presence of diastolic forward flow in the PA during atrial systole. Based on this definition, the authors identified restrictive RV physiology in 53% of the participants. Subsequent studies using the same definition have reported restrictive RV physiology in 20% to 40% of TOF patients.23, 24, 25, 26, 27, 28 In contrast to prior studies that defined RV diastolic function on the basis of noninvasive indexes, the current study assessed right heart filling pressures in symptomatic TOF patients based on invasive hemodynamic data.
RAP and Prognosis
RAP correlated with exercise capacity, arrhythmias, and heart failure hospitalization, which are validated markers of cardiovascular disease severity.21, 29, 30 RAP was also an predictor of all‐cause mortality. Chronic pulmonary regurgitation is the most common hemodynamic lesion in the adult TOF population.30 Consequently, current risk stratification indexes are centered around RV volumetric assessment.4, 5 Some studies have demonstrated an association between RV afterload and mortality,30 and thus the guidelines recommend RV systolic pressure as one of the criteria for RVOT intervention.4, 5 In contrast, the relationship among RV diastolic function, symptoms, and clinical outcomes has not been extensively investigated. The available literature on RV diastology has mostly centered on noninvasive markers of restrictive RV physiology,23, 24, 25, 26, 27, 28 but the correlation of these noninvasive indexes with invasive hemodynamic data has not been investigated in a robust way. The current study shows that right heart filling pressures, as defined by the gold standard of invasive hemodynamic assessment, correlated with current disease severity indexes and predicted future outcomes. This novel finding has potential clinical implications, such as improvement of the current risk stratification metrics, and provides a potential therapeutic target to improve outcomes.
Potential Mechanism for the Detrimental Effects of High RAPs
Unlike the intuitive chain of causality between RV systolic dysfunction and adverse outcomes, the relationship between RV diastolic dysfunction and adverse outcomes is less clear‐cut. We postulate that high RAP in TOF reflects greater pericardial restraint from right heart overload, which is then coupled with reduction in LV transmural pressure. LV transmural pressure, which can be estimated as the difference between intracavitary LV pressure and pericardial pressure, more accurately reflects the distending pressure that determines true LV preload or end‐diastolic volume.17, 19 RAP is a close estimate for intrapericardial pressure,19 so as RAP increases at a given LV filling pressure, there is progressive “underfilling” of the LV, which may impair stroke volume and cardiac output reserve according to the Frank‐Starling principle. This may explain the robust relationship we observed between RAP and peak vo 2; the latter is defined as the product of cardiac output and arteriovenous O2 content difference, according to the Fick principle. We speculate that high filling pressures and cardiac output limitation in TOF patients with high RAP explain the reduced exercise capacity, arrhythmia, heart failure hospitalization, and cardiovascular adverse events observed in this study.
Clinical Implications and Future Directions
The current guideline recommendations for RVOT intervention are based on symptoms, RV volumes, RV ejection fraction, and the severity of RV outflow lesion.4, 5 The current study showed significantly worse outcomes in patients with high RAP (RV diastolic dysfunction and/or RA dysfunction) despite having similar severity of RVOT lesion (pulmonary regurgitation and stenosis). These data suggest that RAP might be considered when deciding on timing of RVOT intervention in patients with borderline indications for intervention, especially when symptoms (exercise intolerance, arrhythmia, and heart failure) seem disproportionate to the severity of RVOT lesion. Furthermore, RAP may also be considered during sudden death risk stratification because of its correlation with occurrence of ventricular arrhythmia occurrence.
It is important to highlight the lack of correlation between RAP and outflow tract obstruction, suggesting that the association between RAP and RV afterload is more likely due to pulmonary vascular dysfunction (both PA and capillaries) and left atrial hypertension. Therefore, in addition to identifying and treating branch PA stenosis, the current data raise the possibility that medical therapies targeting the pulmonary vasculature tested in acquired heart diseases may also hold promise in TOF.31, 32, 33, 34
Limitations
The current study was based on a selected cohort of patients who underwent cardiac catheterization, and this approach may limit the generalizability of the results. Although this study will not be appropriate for describing the prevalence of diastolic dysfunction, the clinical implications of RV diastolic dysfunction reported in this study are still pertinent when dealing with symptomatic patients. A disproportionately large number of patients had TOF–pulmonary atresia—more than would be expected in a contemporary TOF cohort. We adjusted for TOF–pulmonary atresia diagnosis in our statistical models, but this difference in population demographics may affect the generalizability of our results. Finally, our risk model was based on qualitative echocardiographic indexes of right heart function (RV dysfunction, tricuspid and pulmonary regurgitation) rather than quantitative cardiac magnetic resonance imaging data.
Conclusions
In adults with repaired TOF, increasing RAP correlates with worse exercise capacity and greater disease severity including arrhythmic burden and risk of heart failure and also predicts future cardiovascular adverse events. These data provide new insight in the pathophysiology of disease progression for symptomatic patients with TOF and can be used to improve risk stratification in this population.
Sources of Funding
Dr Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448‐01. Dr Borlaug is supported by National Heart, Lung, and Blood Institute RO1 HL128526 and U10 HL110262. Dr Reddy is supported by NHLBI grant T32 HL007111. Dr Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Disclosures
None.
Supporting information
Table S1. Echocardiographic Indexes of Right Ventricular Diastolic Function
Table S2. Echocardiographic Predictors of Right Atrial Pressure >10 mm Hg
Figure S1. Kaplan–Meier curves comparing event‐free survival based on inferior vena cava (IVC) size and collapsibility (IVC hemodynamics).
(J Am Heart Assoc. 2019;8:e014148 DOI: 10.1161/JAHA.119.014148.)
References
- 1. Budts W, Roos‐Hesselink J, Radle‐Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo‐Leiro MG, Walker F, Frogoudaki AA. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown‐Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2016;37:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El‐Harasis MA, Connolly HM, Miranda WR, Qureshi MY, Sharma N, Al‐Otaibi M, DeSimone CV, Egbe A. Progressive right ventricular enlargement due to pulmonary regurgitation: clinical characteristics of a “low‐risk” group. Am Heart J. 2018;201:136–140. [DOI] [PubMed] [Google Scholar]
- 3. Egbe AC, Miranda WR, Said SM, Pislaru SV, Pellikka PA, Borlaug BA, Kothapalli S, Connolly HM. Risk stratification and clinical outcomes after surgical pulmonary valve replacement. Am Heart J. 2018;206:105–112. [DOI] [PubMed] [Google Scholar]
- 4. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;139:e637–e697. [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 6. Munkhammar P, Cullen S, Jogi P, de Leval M, Elliott M, Norgard G. Early age at repair prevents restrictive right ventricular (RV) physiology after surgery for tetralogy of Fallot (TOF): diastolic RV function after TOF repair in infancy. J Am Coll Cardiol. 1998;32:1083–1087. [DOI] [PubMed] [Google Scholar]
- 7. Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, Smith GC, Tat T, Pennell DJ, Gatzoulis MA. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right‐to‐left ventricular interaction. J Am Coll Cardiol. 2002;40:2044–2052. [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD; Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 9. Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart. 2005;91:681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Austin C, Alassas K, Burger C, Safford R, Pagan R, Duello K, Kumar P, Zeiger T, Shapiro B. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest. 2015;147:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unverferth DV, Magorien RD, Moeschberger ML, Baker PB, Fetters JK, Leier CV. Factors influencing the one‐year mortality of dilated cardiomyopathy. Am J Cardiol. 1984;54:147–152. [DOI] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 13. Egbe AC, Connolly HM, Khan AR, Niaz T, Said SS, Dearani JA, Warnes CA, Deshmukh AJ, Kapa S, McLeod CJ. Outcomes in adult Fontan patients with atrial tachyarrhythmias. Am Heart J. 2017;186:12–20. [DOI] [PubMed] [Google Scholar]
- 14. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 15. Miranda WR, Borlaug BA, Hagler DJ, Connolly HM, Egbe AC. Haemodynamic profiles in adult Fontan patients: associated haemodynamics and prognosis. Eur J Heart Fail. 2019;21:803–809. [DOI] [PubMed] [Google Scholar]
- 16. LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. [DOI] [PubMed] [Google Scholar]
- 17. Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyberg JV, Taichman GC, Smith ER, Douglas NWS, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure—an intraoperative study. Circulation. 1986;73:428–432. [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 21. Egbe AC, Driscoll DJ, Khan AR, Said SS, Akintoye E, Berganza FM, Connolly HM. Cardiopulmonary exercise test in adults with prior Fontan operation: the prognostic value of serial testing. Int J Cardiol. 2017;235:6–10. [DOI] [PubMed] [Google Scholar]
- 22. Geva T, Mulder B, Gauvreau K, Babu‐Narayan SV, Wald R, Hickey K, Powell AJ, Gatzoulis MA, Valente AM. Preoperative predictors of death and sustained ventricular tachycardia after pulmonary valve replacement in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Circulation. 2018;138:2106–2115. [DOI] [PubMed] [Google Scholar]
- 23. Aboulhosn JA, Lluri G, Gurvitz MZ, Khairy P, Mongeon FP, Kay J, Valente AM, Earing MG, Opotowsky AR, Lui G, Gersony DR, Cook S, Child J, Ting J, Webb G, Landzberg M, Broberg CS; Alliance for Adult Research in Congenital Cardiology (AARCC) . Left and right ventricular diastolic function in adults with surgically repaired tetralogy of Fallot: a multi‐institutional study. Can J Cardiol. 2013;29:866–872. [DOI] [PubMed] [Google Scholar]
- 24. Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781. [DOI] [PubMed] [Google Scholar]
- 25. Kutty S, Valente AM, White MT, Hickey K, Danford DA, Powell AJ, Geva T. Usefulness of pulmonary arterial end‐diastolic forward flow late after tetralogy of Fallot repair to predict a “restrictive” right ventricle. Am J Cardiol. 2018;121:1380–1386. [DOI] [PubMed] [Google Scholar]
- 26. Cullen S, Shore D, Redington A. Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery. Circulation. 1995;91:1782–1789. [DOI] [PubMed] [Google Scholar]
- 27. Lu JC, Cotts TB, Agarwal PP, Attili AK, Dorfman AL. Relation of right ventricular dilation, age of repair, and restrictive right ventricular physiology with patient‐reported quality of life in adolescents and adults with repaired tetralogy of Fallot. Am J Cardiol. 2010;106:1798–1802. [DOI] [PubMed] [Google Scholar]
- 28. Samyn MM, Kwon EN, Gorentz JS, Yan K, Danduran MJ, Cava JR, Simpson PM, Frommelt PC, Tweddell JS. Restrictive versus nonrestrictive physiology following repair of tetralogy of Fallot: is there a difference? J Am Soc Echocardiogr. 2013;26:746–755. [DOI] [PubMed] [Google Scholar]
- 29. Egbe AC, Connolly HM, Miranda WR, Ammash NM, Hagler DJ, Veldtman GR, Borlaug BA. Hemodynamics of Fontan failure: the role of pulmonary vascular disease. Circ Heart Fail. 2017;10:e004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valente AM, Gauvreau K, Assenza GE, Babu‐Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, Landzberg MJ, Mulder B, Powell AJ, Wald R, Geva T. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 32. Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K, Sigman J, Arneson C, Galie N. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM‐C study): a randomized controlled trial. Chest. 2012;142:1383–1390. [DOI] [PubMed] [Google Scholar]
- 34. Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The beta‐adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction. Circ Res. 2019;124:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Echocardiographic Indexes of Right Ventricular Diastolic Function
Table S2. Echocardiographic Predictors of Right Atrial Pressure >10 mm Hg
Figure S1. Kaplan–Meier curves comparing event‐free survival based on inferior vena cava (IVC) size and collapsibility (IVC hemodynamics).


