Abstract
Background
The prevalence of adult congenital heart disease (ACHD) is increasing in the United States because of improved survival into adulthood. The unique physiology of ACHD commonly leads to multiorgan dysfunction, prompting interest in outcomes after multiorgan (heart+X) transplantation.
Methods and Results
We queried the SRTR (Scientific Registry of Transplant Recipients) database to examine 5‐year outcomes in ACHD patients (aged ≥18 years) who underwent dual organ (heart+kidney/liver/lung) transplantation between 2000 and 2016. Cox proportional hazards models were constructed to look at survival of dual organ transplant recipients versus heart‐only recipients in the ACHD population and heart+lung recipients versus heart‐only recipients in the ACHD populations and versus non‐ACHD recipients of heart+lung transplant. We then constructed a multivariable model to investigate independent risk factors for 5‐year mortality after multiorgan transplant. Overall, 5‐year mortality was greater for multiorgan (heart+kidney/liver/lung) transplant compared with heart‐only transplant. On further analysis, only heart+lung transplant was associated with increased mortality. Outcomes after heart+lung transplant were no different between the ACHD and non‐ACHD population. Risk factors for increased risk of 5‐year mortality in ACHD patients after multiorgan transplant included heart+lung transplant, previous cardiac surgery, and severe functional limitation.
Conclusions
The mortality risk associated with multiorgan heart transplant in ACHD patients is attributable primarily to heart+lung transplants. Multiorgan transplant in ACHD does not convey increased risk compared with the non‐ACHD population. Need for multiorgan transplant should not be an impediment to listing ACHD patients needing a heart transplant.
Keywords: adult congenital heart disease, heart failure, mortality, multiorgan transplant, transplantation
Subject Categories: Transplantation, Congenital Heart Disease, Mortality/Survival, Heart Failure
Clinical Perspective
What Is New?
This is the first investigation of outcomes after multiorgan transplant in adult congenital heart disease to include analysis of all organs commonly transplanted at the same time as the heart.
What Are the Clinical Implications?
Given the fact that adult congenital heart disease patients frequently experience noncardiac end‐organ dysfunction as a result of their heart disease, which may impact candidacy for heart transplant listing, it is important to know outcomes after multiorgan transplant to inform transplant decision making.
Prevalence of adult congenital heart disease (ACHD) has been increasing in the United States, and it is currently estimated that adults represent two‐thirds of the total congenital heart disease population. The majority of this growth is believed to be attributable to improved surgical techniques, which have allowed patients born with congenital heart disease to survive into adulthood.1, 2, 3 Recent reports estimate the US population of ACHD at 1.4 million in 2010, although this may underestimate the true population given variations in diagnosis coding in electronic health record systems.1, 2 The most common cause of mortality in ACHD is heart failure,2, 3 the incidence of which increases as ACHD patients age.4, 5 These trends have resulted in a dramatic 80% increase in heart failure–related admissions in the ACHD population over the last 2 decades.1
The only long‐term solution for end‐stage heart failure is cardiac transplantation. Although currently only ≈3% of adults that receive heart transplants in the United States have ACHD, this proportion is likely to grow.6 Because of the unique physiology imposed by unusual anatomy and palliative repairs, ACHD patients have a greater probability of multiorgan dysfunction requiring listing for multiorgan transplant, in particular heart/lung and heart/liver.1 Outcomes after multiorgan transplant in ACHD, however, remain poorly studied. In the present analysis, we sought to define outcomes after multiorgan transplant in ACHD to better inform heart transplant teams considering listing these high‐risk patients.
Methods
We will make the data and methods used in the analysis available to any researcher for purposes of reproducing the results or replicating the procedures presented here.
We utilized the heart, lung, kidney, and liver transplantation data tables of the SRTR (Scientific Registry of Transplant Recipients) database to perform this research. The SRTR data system includes data on all donor, wait‐listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities for the Organ Procurement and Transplantation Network and SRTR contractors. We obtained the SRTR database and approval from the University of Texas Southwestern Medical Center Institutional Review Board to conduct this research. Informed consent was waived because the database used is a government regulatory obligation. We defined dual organ recipients as those who received a lung, liver, or kidney transplant within ±7 days of a heart transplant. We used the heart transplantation outcome tables to evaluate the 5‐year survival status. We restricted analyses to adult recipients (aged ≥18 years) who underwent transplantation between 2000 and 2016.
Statistical Analysis
Continuous variables were skewed and are presented as medians [quartile 1, quartile 3]. Categorical variables are presented as frequency (percentage). To assess differences in patient characteristics between recipients of a multiorgan transplant versus heart‐only, we performed Wilcoxon rank‐sum and Pearson chi‐squared tests. Survival time was calculated per standard time‐to‐event analyses (ie, subjects were followed until a confirmed date of death) or censored either at the last known date of life or the end of the study outcome (1 or 5 years) accordingly. We constructed Cox proportional hazards models and Kaplan–Meier plots to compare 5‐year survival curves of: (1) dual organ recipients versus heart‐only recipients in the ACHD population, (2) heart+lung recipients versus heart‐only recipients in the ACHD population, and (3) ACHD versus non‐ACHD recipients of heart+lung. We additionally constructed a multivariable Cox model to assess the effect of heart+lung transplant versus heart‐only transplant on the risk of 1‐ and 5‐year mortality, while accounting for additional clinical and demographic confounders. We pursued these analyses for only the heart+lung cohort because prohibitively small event counts in the heart+liver and heart+kidney cohorts precluded this type of analysis. To do so, we identified the significant risk factors in bivariate analyses and considered them for the multivariable model by step‐wise selection. Missing categorical variables were coded as “unknown.” To compare rates of mortality between groups in which the sample size/event count precluded survival analysis, we utilized Fisher's exact test hypothesis tests assuming 2‐sided alternative and a type 1 error rate of 5%. Analyses were performed in SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Results
Heart‐Only Versus Dual Organ Transplant in ACHD
There were 834 heart transplant recipients with ACHD who qualified for analysis, 134 (16%) of whom were multiorgan transplant recipients. Patient characteristics are depicted in Table 1. At 5 years after transplant, 35 (26.1%) of multiorgan recipients and 115 (16.4%) of heart‐only recipients had died. Overall, having a multiorgan transplant was associated with a greater 5‐year mortality compared with having a heart‐only transplant (hazard ratio=1.60; 95% CI, 1.10–2.34; P=0.015).
Table 1.
Characteristics of Transplant Recipientsa
| Heart Only (n=700) | Heart+Lung (n=84) | P Value | Heart+Liver (n=36) | P Value | Heart+Kidney (n=14) | P Value | |
|---|---|---|---|---|---|---|---|
| Age, y | 34.0 [24.0, 45.0] | 33.5 [27.0, 39.5] | 0.974 | 32 [27, 40] | 0.825 | 43 [36, 49] | 0.0108 |
| Sex (male) | 428 (61.14%) | 36 (42.86%) | 0.0013 | 20 (55.56%) | 0.5029 | 9 (64.29%) | 0.8111 |
| Body mass index | 23.0 [20.0, 28.0] | 22.0 [22.0, 24.0] | 0.0011 | 23 [20, 26] | 0.9283 | 24.5 [22, 28] | 0.4914 |
| White | 624 (89.14%) | 73 (86.9%) | 0.5372 | 32 (88.89%) | 1.0000 | 10 (71.43%) | 0.0610 |
| Education | 0.3459 | 0.3917 | 0.3351 | ||||
| High school or less | 52 (66.00%) | 462 (61.90%) | 13 (36.11%) | 3 (21.43%) | |||
| College degree | 24 (21.86%) | 153 (28.57%) | 21 (58.33%) | 8 (57.14%) | |||
| Unknown | 8 (12.14%) | 85 (9.52%) | 2 (5.56%) | 3 (21.43%) | |||
| Working for income | 0.2171 | 0.0061 | 0.1207 | ||||
| Yes | 86 (12.29%) | 5 (5.95%) | 3 (8.33%) | 0 (0%) | |||
| No | 400 (57.14%) | 53 (63.1%) | 30 (83.33%) | 12 (85.71%) | |||
| Unknown | 214 (30.57%) | 26 (30.95%) | 3 (8.33%) | 2 (14.29%) | |||
| Time to (heart) transplantation | 123 [34, 339.5] | 147.5 [43, 460.5] | 0.1856 | 250 [64.5, 505.5] | 0.0252 | 75 [38, 247] | 0.4112 |
| Before cardiac surgery | <0.0001 | 0.0099 | 0.4878 | ||||
| Yes | 453 (64.71%) | 33 (39.29%) | 32 (88.89%) | 10 (71.43%) | |||
| No | 68 (9.71%) | 25 (29.76%) | 2 (5.56%) | 2 (14.29%) | |||
| Unknown | 179 (25.57%) | 26 (30.95%) | 2 (5.56%) | 2 (14.29%) | |||
| Symptomatic cerebrovascular disease | <0.0001 | 0.8394 | 1.0000 | ||||
| Yes | 35 (5%) | 3 (3.57%) | 2 (5.56%) | 0 (0%) | |||
| No | 653 (93.29%) | 70 (83.33%) | 34 (94.44%) | 14 (100%) | |||
| Unknown | 12 (1.71%) | 11 (13.1%) | 0 (0%) | 0 (0%) | |||
| Diabetes mellitus | 0.6527 | 0.0142 | 0.5624 | ||||
| Yes | 3 (0.43%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| No | 177 (25.29%) | 24 (28.57%) | 2 (5.56%) | 2 (14.29%) | |||
| Unknown | 520 (74.29%) | 60 (71.43%) | 34 (94.44%) | 12 (85.71%) | |||
| Dialysis | <0.0001 | 1.0000 | 0.2287 | ||||
| Yes | 12 (1.71%) | 2 (2.38%) | 36 (100.0%) | 1 (7.14%) | |||
| No | 688 (98.29%) | 73 (86.9%) | 0 (0%) | 13 (92.86%) | |||
| Unknown | 0 (0%) | 9 (10.71%) | 0 (0%) | 0 (0%) | |||
| Drug‐treated hypertension | 0.6050 | 0.1687 | 0.1972 | ||||
| Yes | 143 (20.43%) | 14 (16.67%) | 6 (16.67%) | 4 (28.57%) | |||
| No | 446 (63.71%) | 54 (64.29%) | 20 (55.56%) | 6 (42.86%) | |||
| Unknown | 111 (15.86%) | 16 (19.05%) | 10 (27.78%) | 4 (28.57%) | |||
| Functional limitationsb | 0.0208 | 0.4244 | 0.0304 | ||||
| None | 240 (34.29%) | 24 (28.57%) | 15 (41.67%) | 1 (7.14%) | |||
| Some | 160 (22.86%) | 30 (35.71%) | 5 (13.89%) | 2 (14.29%) | |||
| Severe | 209 (29.86%) | 27 (32.14%) | 13 (36.11%) | 9 (64.29%) | |||
| Unknown | 91 (13.00%) | 3 (3.657%) | 3 (8.33%) | 2 (14.29%) | |||
| Smoking history | 0.5803 | 0.0297 | 0.0904 | ||||
| Yes | 95 (13.57%) | 11 (13.1%) | 7 (19.44%) | 5 (35.71%) | |||
| No | 433 (61.86%) | 48 (57.14%) | 27 (75%) | 7 (50%) | |||
| Unknown | 172 (24.57%) | 25 (29.76%) | 2 (5.56%) | 2 (14.29%) | |||
| IABP | 10 (1.43%) | 1 (1.19%) | 1.0000 | 0 (0%) | 1.0000 | 0 (0%) | 1.0000 |
| ECMO | 8 (1.14%) | 1 (1.19%) | 1.0000 | 0 (0%) | 1.0000 | 1 (7.14%) | 0.1641 |
| LVAD | 0.2703 | 0.541 | 0.7276 | ||||
| Yes | 17 (2.43%) | 1 (1.19%) | 0 (0%) | 0 (0%) | |||
| No | 628 (89.71%) | 80 (95.24%) | 35 (97.22%) | 14 (100%) | |||
| Unknown | 55 (7.86%) | 3 (3.57%) | 1 (2.78%) | 0 (0%) | |||
| Implantable defibrillator | <0.0001 | 0.5562 | 0.8843 | ||||
| Yes | 291 (41.57%) | 5 (5.95%) | 13 (36.11%) | 6 (42.86%) | |||
| No | 397 (56.71%) | 78 (92.86%) | 23 (63.89%) | 8 (57.14%) | |||
| Unknown | 12 (1.71%) | 1 (1.19%) | 0 (0%) | 0 (0%) | |||
| Pulmonary artery mean pressure | <0.0001 | 0.0020 | 0.2877 | ||||
| ≥25 | 273 (39.00%) | 57 (67.86%) | 6 (16.67%) | 8 (57.14%) | |||
| <25 | 289 (41.29%) | 1 (1.19%) | 15 (41.67%) | 3 (21.43%) | |||
| Unknown | 138 (19.71%) | 26 (30.95%) | 15 (41.67%) | 3 (21.43%) | |||
| Albumin | 0.3497 | 0.4004 | 0.5143 | ||||
| ≥3.2 | 446 (63.71%) | 60 (71.43%) | 20 (55.56%) | 7 (50%) | |||
| <3.2 | 85 (12.14%) | 7 (8.33%) | 7 (19.44%) | 2 (14.29%) | |||
| Unknown | 169 (24.14%) | 17 (20.24%) | 9 (25%) | 5 (35.71%) | |||
| Drug‐treated COPD | 0.0710 | 0.0289 | 0.4531 | ||||
| Yes | 11 (1.57%) | 4 (4.76%) | 0 (0%) | 0 (0%) | |||
| No | 559 (79.86%) | 69 (82.14%) | 23 (63.89%) | 10 (71.43%) | |||
| Unknown | 130 (18.57%) | 11 (13.1%) | 13 (36.11%) | 4 (28.57%) | |||
| IV inotropes | 196 (28%) | 0 (0%) | <0.0001 | 9 (25%) | 0.6954 | 6 (42.86%) | 0.2365 |
| Life support | 217 (31%) | 16 (19.28%) | 0.0272 | 10 (27.78%) | 0.6831 | 7 (50%) | 0.1489 |
COPD indicates chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; LVAD, left ventricular assist device; SRTR, Scientific Registry of Transplant Recipients.
P values are from a test of the dual organ transplant column to the left vs. heart‐only transplant.
As defined in the SRTR database.
Heart‐Only Versus Heart+Kidney Transplant With ACHD
There were only 14 individuals with ACHD who underwent heart+kidney transplant. No deaths were reported during the time of observation; however, only 5 (35.7%) were followed for the full 5 years of interest. Because no heart+kidney transplant recipients perished, we were unable to perform survival analysis. However, rates of mortality did not differ significantly between heart‐alone and heart+kidney transplant (16.4% versus 0.0%, respectively; P=0.143).
Heart‐Only Versus Heart+Liver Transplant With ACHD
There were 36 individuals with ACHD who underwent heart+liver transplant. Rates of death at 5 years were similar between those who underwent heart alone versus heart+liver transplant (16.4% versus 13.9%, respectively; P=0.820). Similarly, heart+liver transplant was not significantly associated with 5‐year mortality risk (hazard ratio=1.59; 95% CI, 0.65–3.92; P=0.310).
Heart‐Only Versus Heart+Lung Transplant With ACHD
There were 84 individuals who underwent heart+lung transplant. Rates of death at 5 years were higher in the heart+lung group versus heart alone (35.7% versus 16.4%). Having a heart+lung transplant was associated with an elevated 5‐year mortality risk compared with having a heart‐only transplant (hazard ratio=1.80; 95% CI, 1.20–2.69; P=0.004; Figure 1).
Figure 1.
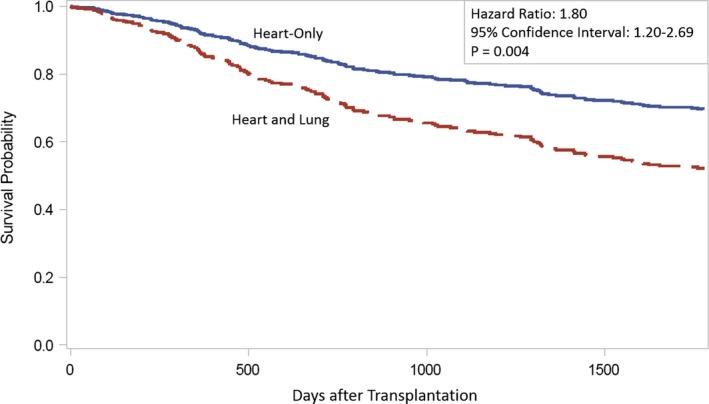
Five‐year survival curves for heart‐only vs heart and lung transplant recipients with congenital heart disease.
Multivariable Analysis: Heart‐Only Versus Heart+Lung Transplant in ACHD at 1 and 5 Years
The following variables were considered for the multivariable analysis: transplant type (heart‐only versus heart+lung), sex, previous cardiac surgery, cerebrovascular disease, dialysis, functional limitation, implantable defibrillator, body mass index, time to transplant, working for income, extracorporeal membrane oxygenation, ventricular assist device, and intra‐aortic balloon pump. Using step‐wise selection, the optimal model included transplant type, previous cardiac surgery, and functional limitation. After adjustment, only being on life support pretransplant was associated with increased risk of mortality at 1 year (adjusted hazard ratio, 1.84; 95% CI, 1.04–3.37; P=0.0370). At 5 years, heart+lung transplant was associated with a significantly increased risk of 5‐year mortality (adjusted hazard ratio, 1.75; 95% CI, 1.15–2.65; P=0.009; Table 2). Having previous cardiac surgery compared with having an unknown cardiac surgery history significantly increased 5‐year mortality (adjusted hazard ratio=1.83; 95% CI, 1.20–2.79; P=0.005); however, there was no significant association in mortality when comparing patients with a known surgical history who did not have previous cardiac surgery (P=0.266). Presence of severe pretransplant functional limitation was associated with a 2‐fold increase in 5‐year mortality compared with recipients without functional limitation (adjusted hazard ratio=2.03; 95% CI, 1.32–3.13; P=0.001).
Table 2.
Risk Factors for 5‐Year Transplantation Mortality
| Comparison | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Transplant type (heart and lung vs heart‐only) | 1.75 | 1.15–2.65 | 0.009 |
| Previous cardiac surgery vs no previous cardiac surgery | 1.34 | 0.8–2.25 | 0.266 |
| Previous cardiac surgery vs unknown previous cardiac surgery | 1.83 | 1.2–2.79 | 0.005 |
| Severe functional limitations vs no functional limitations | 2.03 | 1.32–3.13 | 0.001 |
| Some functional limitations vs no functional limitations | 1.27 | 0.79–2.04 | 0.324 |
| Unknown functional limitations vs no functional limitations | 1.35 | 0.75–2.42 | 0.312 |
Multiorgan Transplant Recipients With Versus Without ACHD
There were 955 patients who received heart+kidney transplant, 14 of whom had ACHD. There were no differences in mortality at 30 days (0 [0%] versus 3 [0.32%]; P=1.000); 1‐year (0 [0%] versus 64 [6.8%]; P=0.9131), or 5 years (0 [0%] versus 138 [14.67%]; P=0.2252) for ACHD and non‐ACHD patients, respectively. There were 169 patients who received heart+liver transplant, 36 of whom had ACHD. There were no differences in mortality at 30 days (1 [2.78%] versus 0 [0%] P=0.2130), 1 year (3 [8.33%] versus 11 [8.27%]; P=0.9735), or 5 years (5 [13.89%] versus 16 [12.03%]; P=0.6772) for ACHD and non‐ACHD patients, respectively. There were 342 patients who received heart+lung transplant, 84 (24.6%) of whom had ACHD. There were no differences in mortality at 30 days (0% mortality in both cohorts), 1 year (8 [9.52%] versus 26 [10.08%]; P=0.742), or 5 years (30 [35.7%] versus 93 [36.1%]; P=0.77) for ACHD and non‐ACHD patients, respectively (Figure 2; depicts only heart+lung because event rates are too low in the other 2 groups to permit survival analysis).
Figure 2.
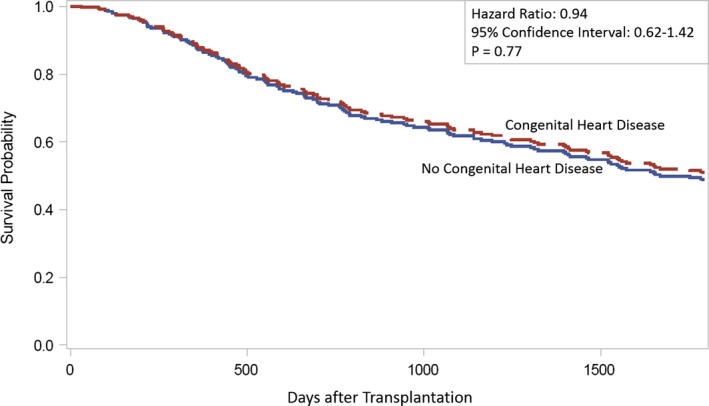
Five‐year survival curves for heart and lung transplant recipients with vs without congenital heart disease.
Discussion
In the present study, we investigated outcomes after multiorgan transplant in ACHD patients with end‐stage heart failure requiring heart transplant. We found that 5‐year mortality was significantly higher in multiorgan compared with heart‐only transplant in ACHD patients. On further analysis, the increased risk was completely attributable to recipients of combined heart‐lung transplant. Multivariable analysis confirmed that undergoing heart+lung transplant, having unknown previous cardiac surgery status, and severe pretransplant functional limitation were responsible for the majority of the observed increased risk. Nevertheless, we found that outcomes after multiorgan transplant in ACHD were no different from those in non‐ACHD patients. These results, in combination with previous data, suggest that neither need for multiorgan transplant nor presence of ACHD should be impediments to transplant candidacy in ACHD patients with end‐stage heart failure.
There are very few studies that have evaluated multiorgan transplant outcomes in the ACHD population. One small retrospective study in 2014 by Robinson et al reported on 3 ACHD patients who received multiorgan transplants, all of whom had good outcomes. The researchers speculated that when indicated, multiorgan transplant in this population may be reasonable.7 In a more‐recent retrospective study from 2017 by Menachem et al, 9 patients underwent heart‐liver and 3 patients underwent heart‐lung transplants with good outcomes.8 The present analysis adds to this body of literature by including a much larger population.
In our analysis of heart+kidney transplant in the ACHD population, we found no difference in 5‐year mortality as compared with heart‐only transplants. We also found no difference in mortality overall compared with heart+kidney transplant in non‐ACHD patients. Although the prevalence of heart+kidney transplantation has increased over the past 10 years in the general population, there remain very few ACHD patients who undergo heart‐kidney transplant, and the present cohort was too small to permit further analysis or to be particularly informative.5 Larger future studies would be of benefit to help guide heart‐kidney transplant decision making and management in ACHD.
We similarly found no difference in 5‐year mortality among ACHD patients undergoing heart+liver transplant as compared with heart‐only. We also found no difference in overall mortality after heart+liver transplant in ACHD versus non‐ACHD. This is similar to findings that Bradley et al reported in 2017.9 A later analysis of the United Network for Organ Sharing database from 1987 to 2015 by Bryant et al suggested that ACHD patients who undergo combined heart+liver transplant may have better outcomes than ACHD patients who undergo heart‐alone transplantation.10 As was the case with combined heart+kidney transplant, however, numbers remain very small and further studies are needed to confirm these and the present findings. With aging of the Fontan‐palliated population, it is likely that the numbers of combined heart+liver transplants in ACHD will increase.
Our findings suggest that, similar to what is observed in non‐ACHD patients, ACHD patients with heart+lung transplants have worse outcomes than those receiving heart transplant alone. Recently, Dimopoulos et al examined heart alone versus heart+lung transplants in all CHD patients in England from 1997 to 2015. Our analysis differs from theirs in that ours includes exclusively adult patients from the United States. Similar to findings in the present study, Dimopoulos et al found that heart‐lung transplant significantly increased mortality.11 In the general non‐ACHD adult US population, existing analyses are similarly consistent with the present findings and suggest a median survival for heart+lung transplant at of 5.8 years in the era between 2004 and 2016.4 Despite the increased mortality for ACHD patients with combined heart+lung transplant, mortality is no different than in non‐ACHD patients undergoing the same procedure. This is a particularly important finding given that pulmonary arterial hypertension is a common sequelae of congenital heart disease with a prevalence in the ACHD population estimated at 4% to 10%, far exceeding that in the non‐ACHD population.12 This difference is likely responsible for a significantly greater proportion of ACHD patients undergoing combined heart+lung transplant as opposed to heart‐alone compared with their non‐ACHD counterparts. This speculation is supported by the difference in mean pulmonary arterial pressure in heart+lung versus heart‐alone transplant recipients in the present cohort.
Multivariable analysis identified 2 additional variables associated with increased 5‐year mortality in the ACHD multiorgan transplant population. Both unknown previous cardiac surgery status and functional limitation were associated with a nearly 2‐fold increase in risk. Given that the association with previous cardiac surgery is driven by comparison with individuals in whom previous cardiac surgery status was listed as “unknown” and the fact that the great majority of patients with ACHD who undergo transplant because of complications related to their cardiac disease have had previous surgery, it is difficult to know what to make of this association. The SRTR database does not collect information on the specific lesion present in adult CHD patients precluding further investigation. In contrast, the association between greater functional limitation and increased risk is not surprising and indicates that sicker patients do worse after transplant. This finding is consistent with existing data in the non‐ACHD population.4 Such patients may have been referred for listing too late in the course of their illness or have spent too long on the waiting list, as previous researchers have suggested.1, 13 Along these lines, Rudasill et al have shown that working status at the time of transplant is independently associated in outcomes after both heart‐alone and lung‐alone transplant. Although their study excluded multiorgan transplant, they concluded that working status may encompass factors beyond functional limitation (such as resilience) that may improve a patient's ability to survive critical illness and organ transplant.14 Our study did not show that pretransplant work status had a significant impact on posttransplant mortality, which may reflect that work status is not as useful a prognosticator of outcomes in ACHD patients undergoing mutliorgan transplant. Further studies exploring work status in multiorgan transplant in both the ACHD and non‐ACHD population with a particular focus on psychological resilience would be of interest.
Limitations
This is a retrospective study that utilizes the United Network for Organ Sharing/SRTR database and is subject to all limitations typically associated with retrospective analysis of this type, including the dependence on participating sites for entry of accurate data. The United Network for Organ Sharing has the advantage of being an obligatory database, and therefore the present analysis is likely to be an exhaustive representation of multiorgan transplant in ACHD. Nevertheless, it can only represent patient outcomes that have been reported. It is possible that there are outcomes that were not documented within the database. Given that this database is not primarily dedicated to congenital heart disease, we were unable to further clarify the anatomical complexity among ACHD patients; more‐complicated patients would likely prove more‐complicated surgeries, which may impact outcome. Small patient numbers are an impediment to this data set. As an example, we were unable to perform survival models for heart+liver and heart+kidney groups because of small event counts (eg, none of the heart+kidney recipients perished). These analyses should therefore be interpreted with some caution. Given that this is a retrospective study based on existing data from the United Network for Organ Sharing/SRTR, the candidate selection for multiorgan transplant was controlled by individual transplant centers. There may have been a bias in favor of more‐optimal patients for multiorgan transplant as compared with heart‐alone, which would bias survival in favor of the multiorgan group. Finally, availability of organs may have precluded or significantly delayed transplantation, which would skew results toward poorer outcomes in the multiorgan group, although this does not appear to be the case based on analysis of waiting‐list times.
Conclusions
Multiorgan transplant is a reasonable option for ACHD patients with multiorgan failure. ACHD patients requiring multiorgan transplant listing should not be considered to represent a greater risk than non‐ACHD patients.
Sources of Funding
This work was supported by institutional funding from the University of Texas Southwestern Medical Center.
Disclosures
None.
Acknowledgments
The data reported here have been supplied by the Hennepin Health Research Institute as the contractor for the SRTR. The interpretation and reporting of these data re the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US government.
(J Am Heart Assoc. 2019;8:e014088 DOI: 10.1161/JAHA.119.014088.)
References
- 1. Burchill LJ. Heart transplantation in adult congenital heart disease. Heart. 2016;102:1871–1877. [DOI] [PubMed] [Google Scholar]
- 2. Khan A, Gurvitz M. Epidemiology of ACHD: what has changed and what is changing? Prog Cardiovasc Dis. 2018;61:275–281. [DOI] [PubMed] [Google Scholar]
- 3. Opina AD, Franklin WJ. Management of heart failure in adult congenital heart disease. Prog Cardiovasc Dis. 2018;61:308–313. [DOI] [PubMed] [Google Scholar]
- 4. Chambers DC, Cherikh WS, Goldfarb SB, Hayes D Jr, Kucheryavaya AY, Toll AE, Khush KK, Levvey BJ, Meiser B, Rossano JW, Stehlik J. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty‐fifth adult lung and heart‐lung transplant report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37:1169–1183. [DOI] [PubMed] [Google Scholar]
- 5. Colvin M, Smith JM, Hadley N, Skeans MA, Carrico R, Uccellini K, Lehman R, Robinson A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 annual data report: heart. Am J Transplant. 2018;18(suppl 1):291–362. [DOI] [PubMed] [Google Scholar]
- 6. Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D Jr, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Stehlik J. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty‐fifth adult heart transplantation report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37:1155–1168. [DOI] [PubMed] [Google Scholar]
- 7. Robinson JA, Driscoll DJ, O'Leary PW, Burkhart HM, Dearani JA, Daly RC, Edwards BS, Dahl SH, Johnson JN. Cardiac and multiorgan transplantation for end‐stage congenital heart disease. Mayo Clin Proc. 2014;89:478–483. [DOI] [PubMed] [Google Scholar]
- 8. Menachem JN, Golbus JR, Molina M, Mazurek JA, Hornsby N, Atluri P, Fuller S, Birati EY, Kim YY, Goldberg LR, Wald JW. Successful cardiac transplantation outcomes in patients with adult congenital heart disease. Heart. 2017;103:1449–1454. [DOI] [PubMed] [Google Scholar]
- 9. Bradley EA, Pinyoluksana KO, Moore‐Clingenpeel M, Miao Y, Daniels C. Isolated heart transplant and combined heart‐liver transplant in adult congenital heart disease patients: insights from the united network of organ sharing. Int J Cardiol. 2017;228:790–795. [DOI] [PubMed] [Google Scholar]
- 10. Bryant R III, Rizwan R, Zafar F, Shah SA, Chin C, Tweddell JS, Morales DL. Contemporary outcomes of combined heart‐liver transplant in patients with congenital heart disease. Transplantation. 2018;102:e67–e73. [DOI] [PubMed] [Google Scholar]
- 11. Dimopoulos K, Muthiah K, Alonso‐Gonzalez R, Banner NR, Wort SJ, Swan L, Constantine AH, Gatzoulis MA, Diller GP, Kempny A. Heart or heart‐lung transplantation for patients with congenital heart disease in England. Heart. 2019;105:596–602. [DOI] [PubMed] [Google Scholar]
- 12. Ntiloudi D, Zanos S, Gatzoulis MA, Karvounis H, Giannakoulas G. How to evaluate patients with congenital heart disease‐related pulmonary arterial hypertension. Expert Rev Cardiovasc Ther. 2019;17:11–18. [DOI] [PubMed] [Google Scholar]
- 13. Davies RR, Russo MJ, Yang J, Quaegebeur JM, Mosca RS, Chen JM. Listing and transplanting adults with congenital heart disease. Circulation. 2011;123:759–767. [DOI] [PubMed] [Google Scholar]
- 14. Rudasill SE, Iyengar A, Kwon OJ, Sanaiha Y, Dobaria V, Benharash P. Recipient working status is independently associated with outcomes in heart and lung transplantation. Clin Transplant. 2019;33:e13462. [DOI] [PubMed] [Google Scholar]


