Migraine headache is a common, chronic disorder, and women comprise two thirds of patients with this disorder, that has negative effects on health and is costly for patients and society.1, 2 There is accumulating evidence linking migraine, particularly with aura, to increased risk for cardiovascular events.3 Given the high prevalence of this condition and the fact it affects younger women,1 it is important to educate cardiovascular disease (CVD) providers about the potential increased cardiovascular risk associated with migraine. In this review, an overview is provided of epidemiological studies linking migraine headache and cardiovascular events, with emphasis on women, and the possible pathophysiological mechanisms for this association. However, there are many important knowledge gaps on the sex‐related aspects of migraine that potentially impact advances in management. An additional purpose of this document is to summarize these areas and provide recommendations to address these gaps.
Briefly, migraine (ie, episodic migraine) is characterized by moderate to severe headache, with or without transient focal neurology symptoms (eg, aura) that persists ≈4 to 72 hours.4 Approximately 1 in 3 patients with migraine experience aura, which may be visual (eg, lights), auditory (eg, noises), somatosensory (eg, tingling and numbness), or motor (eg, jerking movements).5 Typically, aura precedes the onset of headache and persists for less than an hour.6 There is variation in the number of attacks between patients: some experience multiple attacks weekly while others experience <1 per year. There is also wide variation in the frequency of episodes within the same individual, and often the condition improves with advancing age. Patients who experience several attacks monthly (ie, ≥15 headache days a month) are classified as having chronic migraine.7, 8
Worldwide, migraine is the third most prevalent medical condition and second most disabling neurological disorder.9 In the United States, its annual prevalence is ≈1 in 8 adults.1, 2 Migraine usually affects young‐ to middle‐aged women (25–55 years old).1 Although the prevalence of migraine is generally low before puberty (≈2.5%) and is similar in boys and girls,10 the prevalence increases in girls at about 10 years of age versus boys.2, 10 The prevalence continues to rise in women versus men (18% versus 6%), peaking at about 30 years old.2 The prevalence decreases after age 42 years in both sexes, but remains 2 times higher in women.2
Pathophysiological Mechanisms for Increased Cardiovascular Risk
Underlying mechanisms for the increased risk of cardiovascular and cerebrovascular events in individuals with migraine are incompletely understood and are likely multifactorial. First, those with migraine have a higher prevalence of traditional cardiovascular risk factors such as hypertension and hyperlipidemia.11 However, many of the studies demonstrating an association between migraine and cardiovascular events have shown that the association remains after adjusting for the traditional CVD risk factors.3, 12, 13 Second, migraine, especially with aura, is a systemic illness associated with generalized endothelial dysfunction,14 and increased platelet aggregation.15 A similar clustering of endothelial dysfunction and increased platelet aggregation occurs in preeclampsia: also, some suggest that migraine is associated with a heightened prevalence of hypertensive disorders during pregnancy, including preeclampsia.16 Third, patent foramen ovale (PFO) is highly prevalent (up to 50% of migraineurs with aura)17; this might represent a substrate for paradoxical emboli. This hypothesis is supported by findings among migraine patients with cryptogenic stroke where the prevalence of PFO with right to left shunting approaches 80%.18 Fourth, the migraine attack might be a predisposing factor for venous thromboembolism because of prolonged immobility during the headache,13 however, some studies showed that this association is limited to migraine patients with aura.19 Finally, patients with migraine frequently use NSAIDs, which with chronic use are also associated with increased risk of cardiovascular events20, 21 (Figure).
Figure 1.
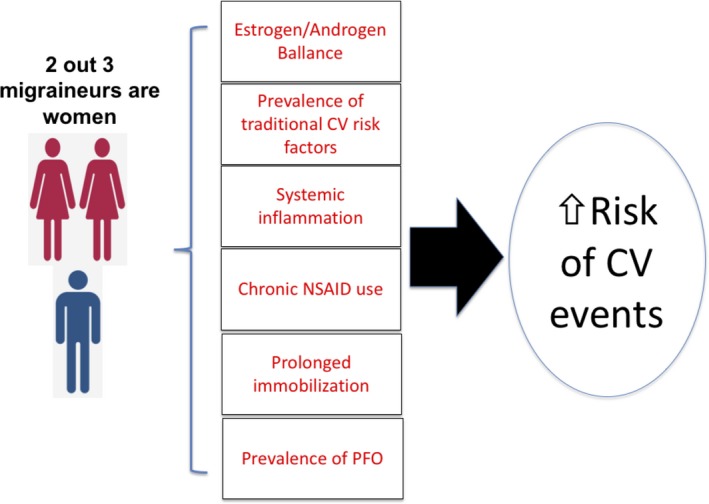
Summary for the pathophysiological mechanisms which have linked migraine with an increased risk of cardiovascular (CV) events. Migraine is more common in women (2 out of 3 migraineurs are women). Several pathophysiological mechanisms explain the link between migraine and the risk of cardiovascular events. NSAID indicates non‐steroidal anti‐inflammatory drugs; PFO, patent foramen ovale.
In patients who suffer from migraine with aura, the underlying pathophysiological mechanism for the aura is thought to be related to cortical spreading depression, which is a slowly propagating wave of depolarization, resulting in the suppression of brain activity.22 Spreading depression is also the major mechanism for neuronal damage in the context of large vessel cerebrovascular occlusion (eg, middle cerebral artery). A wave of depolarization markedly increases interstitial glutamate and paves the way for excitotoxic neuronal death. If brain perfusion is normal, astrocytes are able to take up glutamate rapid enough to prevent neuronal injury. However, in the context of ischemia related to vascular occlusion by thrombus, this may not occur. It could be hypothesized that migraineurs with aura may be more likely to experience spreading depression with cortical vessel occlusion, hence infarction.
Role of Sex Hormones
As noted previously, the prevalence of migraine and frequency of attacks rises abruptly after puberty and declines about the time of menopause. Studies have consistently shown that women suffer more frequent, longer, and more severe episodes of headache.23, 24 Women are also less likely to have episodes of remission or migraine‐free periods.25 Moreover, women are more likely to experience more severe associated symptoms like photophobia, nausea, and vomiting compared with men.23, 26 In addition, some women experience more frequent episodes of migraine around the time of their menstrual cycle (ie, menstrual‐related migraine), or exclusively during the menstruation (ie, pure menstrual migraine). Another important observation is that women who are using combined oral contraceptive pills have a higher prevalence of headache, especially migraine.27 Notably, women are more likely to experience improvement in their symptoms during pregnancy.28 These observations suggest that sex hormones, particularly estrogen, play an important role in the sex‐related differences in migraine prevalence. It has been postulated that estrogen withdrawal triggers migraine episodes and/or lowers the pain threshold among women. One study found that women suffering from menstrual‐related migraine had a lower level of peak estradiol level compared with controls,29 and another study found that women with migraine had a faster late luteal phase estrogen decline compared with healthy controls.30 Stemming from this concept, small studies have suggested that testosterone therapy might improve migraine symptoms in women.31, 32 Further supporting this concept, one study showed that the prevalence of migraine among male to female transsexuals using anti‐androgens to suppress male sex characteristics and estrogens to induce female sex characteristics was comparable with that of genetic women, and was remarkably higher than that of men of the same population.33 Collectively, these findings support the concept that fluctuations in hormonal levels, especially estrogen, potentially explain the higher prevalence of migraine and severity of attacks in women.
Migraine and Cardiovascular Events
Considerable evidence links migraine, particularly with aura, to cardiovascular and cerebrovascular events (Tables 1 and 2).12, 13, 34, 35, 36, 37 These data have been studied more frequently among women. In a prospective cohort of >27 000 healthy women >45 years old with no prior ischemic event from the Women's Health Study, a self‐reported diagnosis of migraine with aura was associated with an increased risk of ischemic stroke, myocardial infarction, and a major cardiovascular event (defined as first instance of non‐fatal ischemic stroke, non‐fatal myocardial infarction, or death because of ischemic event) after adjusting for traditional CVD risk factors, oral contraceptive pill use, hormonal replacement therapy, and statins, but not for the use of NSAIDs, since women enrolled in the Women's Health Study had to be willing to forgo use of NSAIDs throughout the trial.12 These findings were further confirmed in another prospective cohort of ≈115 000 women aged 25 to 42 years at baseline from the Nurse's Health Study.34 Women with a physician's diagnosis of migraine had an increased risk of cardiovascular events including cardiovascular mortality over 20 years of follow‐up after adjusting for traditional CVD risk factors, oral contraceptive pill use, hormonal replacement therapy, and NSAID use.34 A similar observation was seen in middle‐aged women with signs and symptoms of ischemic heart disease, in which migraine was associated with an increased risk of major cardiac events driven by a 2‐fold increase in the risk of stroke adjusting for traditional CVD risk factors and for the severity of underlying coronary artery disease.35 In the largest cohort study to date (ie, >500K), of which 70% were women, migraine headache was associated with an increased adjusted risk of ischemic stroke, myocardial infarction, and venous thromboembolism after 19 years of follow‐up (adjusting for traditional risk factors and other comorbidities). In this study, the association between migraine and cardiovascular events was more pronounced in women than in men and an increased risk of peripheral artery disease was also observed in those with migraine.13 Despite the fact that these studies attempted to control for the traditional CVD risk factors, none compared the relative strength of different factors. Thus, the independent contribution of migraine to cardiovascular events relative to the traditional CVD risk factors remains an important knowledge gap. Table 1 summarizes cohort studies that evaluated the risk of cardiovascular outcomes and migraine exclusively among women, while Table 2 summarizes the outcomes among the subgroup of women who had migraine with aura. It is important to note that while these studies12, 13, 34, 35, 36, 37 mostly showed an association between migraine and risk of cardiovascular and cerebrovascular events even after adjusting for the traditional CVD risk factors (Table 3), only 1 study accounted for NSAID use,12 while 2 studies adjusted for oral contraceptive pill use.12, 34 Moreover, none of these studies adjusted for other important emerging non‐traditional risk factors among women such as hypertensive disorders of pregnancy and systemic autoimmune diseases.38, 39 Whether migraine confers an independent risk, beyond these non‐traditional and less studied risk factors, remains an important knowledge gap.
Table 1.
Cohort Studies Evaluating Risk of Cardiovascular Events Among Women With Migraine
| Study (Ref.) | Diagnosis of Migraine | Migraine, n/No Migraine, n | Age, y Meana | Women, %a | Follow‐Up, y | Outcomes (Hazard Ratio, 95% CI) |
|---|---|---|---|---|---|---|
| Prospective | ||||||
| Kurth et al12 | Self‐reported questionnaire | 5125/22 715 | 53 | 100 | 10 |
MACCE (1.42, 1.16–1.74) Ischemic stroke (1.22, 0.88–1.68) MI (1.41, 1.03–1.91) Cardiac death (1.63, 1.07–2.50) |
| Kurth et al34 | Self‐reported physician diagnosis | 17 531/98 010 | 35 | 100 | 20 |
MACCE (1.50, 1.33–1.69) MI (1.39, 1.18–1.64) Any stroke (1.62, 1.37–1.83) |
| Rambarat et al35 | Self‐reported questionnaire | 224/693 | 54 | 100 | 6.5 |
MACCE (1.83, 1.22–2.75) Any stroke (2.33, 1.16–4.68) |
| Adelborg et al13, b | ICD‐8‐10 codes | 51 032/510 320 | 35 | 71 | 19 |
MI (1.49, 1.36–1.64) Ischemic stroke (2.26, 2.21–2.41) |
| Retrospective | ||||||
| Peng et al36, b | ICD‐9 codes | 119 017/119 107 | 41 | 72 | 3.6 | Ischemic stroke (1.28, 1.11–1.47) |
ICD‐8 indicates International Classification of Diseases, Eighth Edition; ICD‐9, International Classification of Diseases, Ninth Edition; ICD‐10, International Classification of Diseases, Tenth Edition; MACCE, major adverse cardiovascular and cerebrovascular events; MI, myocardial infarction.
These data are for the migraine group.
The reported summary estimates are for the women subgroup.
Table 2.
Cohort Studies Reporting the Cardiovascular Events for the Subgroup of Migraine With Aura Among Women
| Study | Outcome | Adjusted Hazards Ratio (95% CI) |
|---|---|---|
| Kurth et al12 | Ischemic stroke | 1.91 (1.17–3.10) |
| Myocardial infarction | 2.08 (1.30–3.31) | |
| Cardiac mortality | 2.33 (1.21–4.51) | |
| Peng et al36 | Ischemic stroke | 1.60 (1.08–2.38) |
| Gudmundsson et al37 | All‐cause mortality | 1.21 (1.09–1.33) |
| Cardiac mortality | 1.18 (1.00–1.40) |
Table 3.
List of Covariates Adjusted for by Individual Studies
| Study | Covariates |
|---|---|
| Kurth et al12 | Age, hypertension, DM, BMI, smoking, alcohol consumption, menopause, HRT, OCP, FH of premature MI, LDL, and HDL levels, statin use |
| Kurth et al34 | Age, HDL, DM, hypertension, BMI smoking, alcohol consumption, physical activity, HRT, menopausal state, OCP, aspirin, NSAID use, FH of premature MI |
| Rambarat et al35 | Age, race, hypertension, BMI, DM, dyslipidemia, smoking, FH of CAD, CAD severity, aspirin use |
| Adelborg et al13 | DM, obesity, HDL, hypertension, valvular heart disease, COPD, renal failure, liver disease, cancer, alcoholism, thyroid disease |
| Peng et al36 | Age, Charlson Comorbidity Index, DM, hyperlipidemia, hypertension, valvular heart disease, COPD, renal failure, liver disease |
| Gudmundsson et al37 | Age, BMI, smoking, education, hypertension |
BMI indicates body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; FH, family history; HDL, high‐density lipoprotein; HRT, hormonal replacement therapy; LDL, low‐density lipoprotein; MI, myocardial infarction; NSAID, non‐steroidal anti‐inflammatory; OCP, oral contraceptive therapy.
Multiple meta‐analyses have also confirmed these associations. In 3 meta‐analyses that focused on ischemic stroke, the consistent finding was that migraine was associated with ≈2‐fold increase in the adjusted risk of ischemic stroke.40, 41, 42 This association was observed only in women but not in men.40, 42 In 2 of these meta‐analyses only migraine with aura was associated with ischemic stroke,41, 42 but the earlier meta‐analysis demonstrated an association between migraine and ischemic stroke irrespective of aura.40 One meta‐analysis of 15 studies evaluating the adjusted risk of ischemic heart disease with migraine showed that migraine, particularly with aura, was associated with angina and myocardial infarction.43 In the largest meta‐analysis of cohort studies (>1 million individuals), migraine was associated with an increased adjusted risk of major cardiovascular and cerebrovascular events, driven by an increased risk of myocardial infarction and stroke.3 This effect was more pronounced among those with aura and among women. While these observational studies and meta‐analyses have consistently demonstrated an association between migraine and cardiac events, particularly stroke, age might play a role in this association. For example, in 1 study of older patients with migraine (mean age 69 years), migraine with and without aura was not associated with risk of stroke at a mean follow‐up of 11 years.44 Nevertheless, young women provide an opportunity to understand the relationship of migraine to vascular events without masking with the traditional causes of stroke (ie, atherosclerotic disease or cardio‐embolic).
Role of Patent Foramen Ovale Closure
Studies have suggested a higher prevalence of PFO among migraineurs, particularly those with aura.45 Interestingly, it was noted that patients who underwent PFO closure for other reasons (ie, cryptogenic stroke or decompression illness) had improvement or complete relief from migraine symptoms.46 These findings encouraged subsequent observational studies, however, the findings were not replicated in 3 randomized trials.47, 48, 49 Although the 2 most recent randomized trials of PFO closure in migraine failed to meet their primary end point,48, 49 aggregate data from these randomized trials suggest that a subset of patients with more frequent migraine attacks might experience reduction in average migraine days and the total number of attacks.50 Further, the PREMIUM (Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects With Migraine and PFO Using the AMPLATZER PFO Occluder to Medical Management) trial showed that the subgroup of patients with aura might benefit from PFO closure.49 Nevertheless, these were secondary end points; thus, routine PFO closure for migraine should not be recommended before adequately powered sham‐controlled trials are conducted. Stemming from the microemboli PFO‐mediated hypothesis, the TRACTOR (Ticagrelor Therapy for Refractory Migraine Study) trial examined whether ticagrelor would reduce migraine attack frequency in patients with right to left shunting. However, that single arm small study showed that ticagrelor was associated with only modest reduction in migraine attack frequency and a large number of participants experienced side effects.51
Clinical Implications and Future Directions
The link between migraine and increased risk of cardiovascular and cerebrovascular events has important implications. Despite abundant data suggesting links between migraine, particularly with aura, and cardiovascular events, especially ischemic stroke, it is important to emphasize that the rate of events in all of these studies was low. For example, in the largest cohort study to date,13 the cumulative incidence of myocardial infarction per 1000 people for the migraine cohort compared with the general population was 25 versus 17, and for ischemic stroke was 45 versus 25, respectively, over 19 years of follow up. The diagnosis of migraine was either based on self‐reported questionnaires or administrative database, therefore there is some risk of bias in assessment of the exposure (ie, ascertainment bias). However, this issue might only result in a bias towards the null and therefore would result in an underestimate of the true strength of the association between migraine and cardiovascular events. Further, most prospective studies did not evaluate the outcomes for migraine with aura. Even in the studies that did, the ascertainment of aura might not reliable. Another consideration is that aura could be over‐represented in these studies as even those with migraine without aura still suffer some visual changes during the prodromal phase.52 Moreover, patients who have migraine with aura complicated with hemiparesis might be misclassified as having a stroke, which might complicate the relationship between migraine and stroke. Nevertheless, even if the diagnosis of aura was overestimated in these studies, the association between migraine with aura and cardiovascular events, particularly myocardial infarction and cardiac mortality, is likely valid.
Although the rate of the cardiovascular events is low, this still translates into a substantial increase in population risk given the high prevalence of migraine worldwide. Therefore, it is important first to recognize migraine as an established risk for cardiovascular and cerebrovascular events. Most physicians in practice do not recognize migraine as a cardiovascular risk factor. The updated QRISK3 algorithm has recently introduced migraine as a cardiovascular risk factor for individuals 25 to 84 years old,53 yet none of the US‐based cardiovascular risk assessment tools have migraine listed as a risk factor. Moreover, a recent study has shown that migraine, particularly with aura, was associated with a higher risk of postoperative ischemic stroke and 30‐day readmissions after surgery,54 and this effect was observed irrespective of sex. These findings suggest that migraine history would be useful if incorporated in the perioperative surgical risk assessment.
To date, there are no established recommendations to reduce the cardiovascular risk among patients with migraine. Guideline recommendations do not recommend routine anti‐platelet therapy, mostly because of the lack of sufficient evidence.55 Unfortunately, randomized trials to establish a role for anti‐platelet therapy for primary prevention of cardiovascular events in patients with migraine might not be feasible, as these would necessitate large patient samples followed for long duration. Similarly, evidence on potential benefit of chronic statin therapy in this population is lacking, despite the well‐recognized stroke preventative effects in those at relatively low risk of major vascular events.56 Growing evidence indicates that some lipid‐independent effects of statins are mediated by improved endothelial function and vasomotor reactivity, and reduction of oxidative stress,57 all of which provide the hypothetical basis to study their use in migraine. In fact, 1 small randomized trial provided some evidence that the combination of simvastatin and vitamin D3 reduces the frequency of migraine attacks,58 however; a similar study has yet to be replicated.
Also, a knowledge gap exists about whether controlling migraine symptoms and use of long‐term combination cardiovascular preventive therapy (eg, beta‐blockers, statins, aspirin, etc) might reduce the risk of cardiovascular events, particularly stroke. Triptans are commonly used to abort moderate to severe attacks of migraine. Although triptans exert vasoconstrictive effects, studies indicate that triptan use does not increase cardiovascular risk in patients with migraine.59 Use of NSAIDs should be limited to acute migraine attacks, to mitigate long‐term risks of cardiovascular events associated with chronic NSAID therapy. Moreover, both agents (ie, NSAIDs and triptans) should be avoided in pregnant women and those with cardiovascular risk factors such as hypertension and focus on prevention of attacks and using other analgesic agents should be entertained.
Patients with migraine should be counseled about a healthy lifestyle such as regular physical activity, smoking cessation, and modification of other traditional CVD risks (eg, blood pressure control and weight loss). For example, a study of elderly patients with migraine found significant interaction between migraine and smoking for the risk of stroke and combined vascular events, such that migraine was associated with an increased risk of stroke and vascular events among active smokers but not among non‐smokers.44 These findings highlight the importance of smoking cessation. As population‐based studies have demonstrated that patients with migraine are more likely to smoke, have high blood pressure, unfavorable cholesterol profile, and report a parental history of premature myocardial infarction,60 it is important to identify those patients who might benefit from traditional risk factor modification. A recent European position document has suggested that hormonal contraceptive use, particularly combined therapy, might further increase ischemic stroke risk in those with migraine, especially those with aura.61 This recommendation is consistent with American Heart Association guidelines for stroke in women, which cautions against use of hormonal contraceptives, but notes that the evidence is not conclusive.62
Similar to evolution of PFO closure for cryptogenic stroke, where earlier trials showed lack of benefit from PFO closure but, with careful patient selection, recent trials have shown benefit,63, 64 future randomized trials should focus on the particular subgroups that might derive potential benefit from PFO closure (ie, those with aura and/or frequent attacks). However, based on findings of earlier observational studies, patients with migraine who undergo PFO closure for another indication (ie, cryptogenic stroke) might experience improvement in migraine symptoms. It is important to note that none of the 3 randomized trials for PFO closure for migraine examined the interaction of sex on outcomes.48, 49, 50 It also remains unknown if routine PFO closure might mitigate the risk of future cardiovascular and cerebrovascular events. Moreover, none of the studies assessing stroke risk with migraine evaluated the concomitant presence of PFO, and whether the risk of stroke with migraine would persist after adjusting for the presence of PFO.
Conclusions
Migraine is a prevalent, chronic condition that mostly affects young to middle‐aged women, followed by gradual decline with aging. Migraine carries a considerable physical, social, and economic burden. Shortly after puberty, the prevalence of migraine sharply rises in women and remains much higher compared with men. Women are also more likely to experience more frequent and worse migraine attacks. These observations suggest that sex hormones, particularly estrogen, play an important role in the pathophysiology of migraine. However, it remains unknown if hormonal modulation might help alleviate this condition.
There is a large body of studies linking migraine with cardiovascular and cerebrovascular events, particularly ischemic stroke, in women. This association is more robust for migraine with aura. Although the numbers of events in these studies were small, the high prevalence of migraine worldwide and the young age of the women who suffer this condition should direct attention of future research to evaluate therapies and/or interventions to mitigate this risk. To date, there has been no established therapy to lower cardiovascular risk among women with migraine. Physicians should inquire about migraine history when assessing the cardiovascular risk of women.
Appendix
American College of Cardiology Cardiovascular Disease in Women Committee
Carl J. Pepine, MD; Kathryn Lindley, MD; Harmony Reynolds MD; Annabelle Volgman, MD; Anita Asgar, MD; Leslee J. Shaw, PHD; Margo Minissian, PhD; Andrea M. Russo, MD; Jerome Fleg, MD; Islam Elgendy, MD; Uri Elkayam, MD; Cindy Giullian, ACNP; Michele A. Hamilton, MD; Puja K. Mehta, MD; Odayme Quesada, MD; Malissa Wood, MD; Kelly Epps, MD; Leslie Cho, MD; Melinda Baughman Davis, MD; Sharonne Hayes, MD.
Sources of Funding
This work was supported by contracts from the National Heart, Lung, and Blood Institute, nos. N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164, grants U01 64829, U01 HL649141, U01 HL649241, T32 HL69751, 1R03 AG032631 from the National Institute on Aging, K12 HD051959 Building Interdisciplinary Research Careers in Women's Health (Taqueti), GCRC grant MO1‐RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women's Heart Research Fellowship, Cedars‐Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars‐Sinai Medical Center, Los Angeles. Dr Pepine was also supported by National Institutes of Health grants HL33610, HL56921, UM1 HL087366; the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine; National Institutes of Health NCATS (National Center for Advancing Translational Sciences)—University of Florida Clinical and Translational Science UL1TR001427; and PCORnet‐OneFlorida Clinical Research Consortium CDRN‐1501‐26692.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e014546 DOI: 10.1161/JAHA.119.014546.)
Contributor Information
Carl J. Pepine, Email: carl.pepine@medicine.ufl.edu.
the American College of Cardiology Cardiovascular Disease in Women Committee:
Kathryn Lindley, Kathryn Lindley, Harmony Reynolds, Harmony MD, Annabelle Volgman, Anita Asgar, Leslee J. Shaw, Margo Minissian, Andrea M. Russo, Jerome Fleg, Uri Elkayam, Cindy Giullian, Michele A. Hamilton, Puja K. Mehta, Odayme Quesada, Malissa Wood, Kelly Epps, Leslie Cho, Melinda Baughman Davis, and Sharonne Hayes
References
- 1. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 2. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia. 2010;30:1065–1072. [DOI] [PubMed] [Google Scholar]
- 3. Mahmoud AN, Mentias A, Elgendy AY, Qazi A, Barakat AF, Saad M, Mohsen A, Abuzaid A, Mansoor H, Mojadidi MK, Elgendy IY. Migraine and the risk of cardiovascular and cerebrovascular events: a meta‐analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodick DW. Migraine. Lancet. 2018;391:1315–1330. [DOI] [PubMed] [Google Scholar]
- 5. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. [DOI] [PubMed] [Google Scholar]
- 6. Hansen JM, Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Goadsby PJ, Charles A. Migraine headache is present in the aura phase: a prospective study. Neurology. 2012;79:2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72:S3–S7. [DOI] [PubMed] [Google Scholar]
- 8. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population based study. Headache. 2008;48:1157–1168. [DOI] [PubMed] [Google Scholar]
- 9. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bille BS. Migraine in school children. A study of the incidence and short‐term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta Paediatr Suppl. 1962;136:1–151. [PubMed] [Google Scholar]
- 11. Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. [DOI] [PubMed] [Google Scholar]
- 13. Adelborg K, Szepligeti SK, Holland‐Bill L, Ehrenstein V, Horváth‐Puhó E, Henderson VW, Sørensen HT. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, Kim JH, Im WS, Kang L, Park JE, Park HJ, Park HK, Song EC, Lee SK, Kim M, Roh JK. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–1517. [DOI] [PubMed] [Google Scholar]
- 15. D'Andrea G, Toldo M, Cortelazzo S, Milone FF. Platelet activity in migraine. Headache. 1982;22:207–212. [DOI] [PubMed] [Google Scholar]
- 16. Facchinetti F, Allais G, Nappi RE, D'Amico R, Marozio L, Bertozzi L, Ornati A, Benedetto C. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia. 2009;29:286–292. [DOI] [PubMed] [Google Scholar]
- 17. Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia. 2008;28:531–540. [DOI] [PubMed] [Google Scholar]
- 18. West BH, Noureddin N, Mamzhi Y, Low CG, Coluzzi AC, Shih EJ, Gevorgyan Fleming R, Saver JL, Liebeskind DS, Charles A, Tobis JM. Frequency of patent foramen ovale and migraine in patients with cryptogenic stroke. Stroke. 2018;49:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng KP, Chen YT, Fuh JL, Tang CH, Wang SJ. Association between migraine and risk of venous thromboembolism: a nationwide cohort study. Headache. 2016;56:1290–1299. [DOI] [PubMed] [Google Scholar]
- 20. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644. [DOI] [PubMed] [Google Scholar]
- 21. Ross SJ, Elgendy IY, Bavry AA. Cardiovascular safety and bleeding risk associated with nonsteroidal anti‐inflammatory medications in patients with cardiovascular disease. Curr Cardiol Rep. 2017;19:8. [DOI] [PubMed] [Google Scholar]
- 22. Charles A. Advances in the basic and clinical science of migraine. Ann Neurol. 2009;65:491–498. [DOI] [PubMed] [Google Scholar]
- 23. Bolay H, Ozge A, Saginc P, Orekici G, Uludüz D, Yalın O, Siva A, Bıçakçi Ş, Karakurum B, Öztürk M. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia. 2015;35:792–800. [DOI] [PubMed] [Google Scholar]
- 24. Celentano DD, Linet MS, Stewart WF. Gender differences in the experience of headache. Soc Sci Med. 1990;30:1289–1295. [DOI] [PubMed] [Google Scholar]
- 25. Bille B. A 40‐year follow‐up of school children with migraine. Cephalalgia. 1997;17:488–491; discussion 487. [DOI] [PubMed] [Google Scholar]
- 26. Steiner TJ, Scher AI, Stewart WF, Kolodner K, Liberman J, Lipton RB. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia. 2003;23:519–527. [DOI] [PubMed] [Google Scholar]
- 27. Aegidius K, Zwart JA, Hagen K, Schei B, Stovner LJ. Oral contraceptives and increased headache prevalence: the Head‐HUNT Study. Neurology. 2006;66:349–353. [DOI] [PubMed] [Google Scholar]
- 28. Macgregor EA. Headache in pregnancy. Continuum (Minneap Minn). 2014;20:128–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ibrahimi K, van Oosterhout WP, van Dorp W, Danser AH, Garrelds IM, Kushner SA, Lesaffre EM, Terwindt GM, Ferrari MD, van den Meiracker AH, MaassenVanDenBrink A. Reduced trigeminovascular cyclicity in patients with menstrually related migraine. Neurology. 2015;84:125–131. [DOI] [PubMed] [Google Scholar]
- 30. Pavlovic JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal‐Perry GS, Lipton RB, Derby CA. Sex hormones in women with and without migraine: evidence of migraine‐specific hormone profiles. Neurology. 2016;87:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glaser R, Dimitrakakis C, Trimble N, Martin V. Testosterone pellet implants and migraine headaches: a pilot study. Maturitas. 2012;71:385–388. [DOI] [PubMed] [Google Scholar]
- 32. Lichten EM, Bennett RS, Whitty AJ, Daoud Y. Efficacy of danazol in the control of hormonal migraine. J Reprod Med. 1991;36:419–424. [PubMed] [Google Scholar]
- 33. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593–594. [DOI] [PubMed] [Google Scholar]
- 34. Kurth T, Winter AC, Eliassen AH, Dushkes R, Mukamal KJ, Rimm EB, Willett WC, Manson JE, Rexrode KM. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rambarat CA, Elgendy IY, Johnson BD, Reis SE, Thompson DV, Sharaf BL, Bittner V, Sopko G, Bairey Merz CN, Pepine CJ, Ahmed B. Migraine headache and long‐term cardiovascular outcomes: an extended follow‐up of the Women's Ischemia Syndrome Evaluation. Am J Med. 2017;130:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng KP, Chen YT, Fuh JL, Tang CH, Wang SJ. Migraine and incidence of ischemic stroke: a nationwide population‐based study. Cephalalgia. 2017;37:327–335. [DOI] [PubMed] [Google Scholar]
- 37. Gudmundsson LS, Scher AI, Aspelund T, Eliasson JH, Johannsson M, Thorgeirsson G, Launer L, Gudnason V. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ. 2010;341:c3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre‐eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63:1815–1822. [DOI] [PubMed] [Google Scholar]
- 39. Crowson CS, Liao KP, Davis JM III, Solomon DH, Matteson EL, Knutson KL, Hlatky MA, Gabriel SE. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta‐analysis of observational studies. BMJ. 2005;330:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu X, Zhou Y, Zhao H, Peng C. Migraine and the risk of stroke: an updated metaanalysis of prospective cohort studies. Neurol Sci. 2017;38:33–40. [DOI] [PubMed] [Google Scholar]
- 42. Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta‐analysis. Am J Med. 2010;123:612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sacco S, Ornello R, Ripa P, Tiseo C, Degan D, Pistoia F, Carolei A. Migraine and risk of ischaemic heart disease: a systematic review and meta‐analysis of observational studies. Eur J Neurol. 2015;22:1001–1011. [DOI] [PubMed] [Google Scholar]
- 44. Monteith TS, Gardener H, Rundek T, Elkind MS, Sacco RL. Migraine and risk of stroke in older adults: Northern Manhattan Study. Neurology. 2015;85:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takagi H, Umemoto T; Group A . A meta‐analysis of case‐control studies of the association of migraine and patent foramen ovale. J Cardiol. 2016;67:493–503. [DOI] [PubMed] [Google Scholar]
- 46. Wilmshurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right‐to‐left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet. 2000;356:1648–1651. [DOI] [PubMed] [Google Scholar]
- 47. Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, Lipscombe SL, Rees T, De Giovanni JV, Morrison WL, Hildick‐Smith D, Elrington G, Hillis WS, Malik IS, Rickards A. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double‐blind, sham‐controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117:1397–1404. [DOI] [PubMed] [Google Scholar]
- 48. Mattle HP, Evers S, Hildick‐Smith D, Becker WJ, Baumgartner H, Chataway J, Gawel M, Göbel H, Heinze A, Horlick E, Malik I, Ray S, Zermansky A, Findling O, Windecker S, Meier B. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur Heart J. 2016;37:2029–2036. [DOI] [PubMed] [Google Scholar]
- 49. Tobis JM, Charles A, Silberstein SD, Sorensen S, Maini B, Horwitz PA, Gurley JC. Percutaneous closure of patent foramen ovale in patients with migraine: the PREMIUM trial. J Am Coll Cardiol. 2017;70:2766–2774. [DOI] [PubMed] [Google Scholar]
- 50. Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G, Bhatt DL. Percutaneous closure of patent foramen ovale in migraine: a meta‐analysis of randomized clinical trials. JACC Cardiovasc Interv. 2018;11:816–818. [DOI] [PubMed] [Google Scholar]
- 51. Reisman AM, Robbins BT, Chou DE, Yugrakh MS, Gross GJ, Privitera L, Nazif T, Sommer RJ. Ticagrelor for Refractory Migraine/Patent Foramen Ovale (TRACTOR): An open‐label pilot study. Neurology. 2018;91:1010–1017. [DOI] [PubMed] [Google Scholar]
- 52. Laurell K, Artto V, Bendtsen L, Hagen K, Häggström J, Linde M, Söderström L, Tronvik E, Wessman M, Zwart JA, Kallela M. Premonitory symptoms in migraine: a crosssectional study in 2714 persons. Cephalalgia. 2016;36:951–959. [DOI] [PubMed] [Google Scholar]
- 53. Hippisley‐Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Timm FP, Houle TT, Grabitz SD, Lihn AL, Stokholm JB, Eikermann‐Haerter K, Nozari A, Kurth T, Eikermann M. Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1–18. [DOI] [PubMed] [Google Scholar]
- 56. Cholesterol Treatment Trialists’ (CTT) Collaborators , Mihaylova B, Emberson J, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhao J, Zhang X, Dong L, Wen Y, Cui L. The many roles of statins in ischemic stroke. Curr Neuropharmacol. 2014;12:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Buettner C, Nir RR, Bertisch SM, Bernstein C, Schain A, Mittleman MA, Burstein R. Simvastatin and vitamin D for migraine prevention: a randomized, controlled trial. Ann Neurol. 2015;78:970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roberto G, Raschi E, Piccinni C, Conti V, Vignatelli L, D'Alessandro R, De Ponti F, Poluzzi E. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia. 2015;35:118–131. [DOI] [PubMed] [Google Scholar]
- 60. Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population‐based study. Neurology. 2005;64:614–620. [DOI] [PubMed] [Google Scholar]
- 61. Sacco S, Merki‐Feld GS, KL AE, Bitzer J, Canonico M, Kurth T, Lampl C, Lidegaard Ø, Anne MacGregor E, MaassenVanDenBrink A, Mitsikostas DD, Nappi RE, Ntaios G, Sandset PM, Martelletti P. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mojadidi MK, Zaman MO, Elgendy IY, Mahmoud AN, Patel NK, Agarwal N, Tobis JM, Meier B. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol. 2018;71:1035–1043. [DOI] [PubMed] [Google Scholar]
- 64. Mojadidi MK, Elgendy AY, Elgendy IY, Mahmoud AN, Elbadawi A, Eshtehardi P, Patel NK, Wayangankar S, Tobis JM, Meier B. Transcatheter patent foramen ovale closure after cryptogenic stroke: an updated meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:2228–2230. [DOI] [PubMed] [Google Scholar]


