Abstract
Deficiencies in current embryo culture media likely contribute to the poor blastocyst development rates and pregnancy retention rates for in vitro produced (IVP) bovine embryos. Of special concern is the lack of micronutrients in these media formulations. One micronutrient of interest is zinc, an essential trace element involved with various enzyme and transcription factor activities. The objective of this work was to describe whether zinc sulfate supplementation during in vitro embryo culture affects bovine embryo development and blastomere numbers. Either 0, 2, 20, or 40 µM zinc sulfate was supplemented to presumptive zygotes cultured in synthetic oviductal fluid containing AAs and bovine serum albumin for 8 d. None of the treatments affected cleavage rates. Percentage of blastocysts on days 7 and 8 postfertilization was not affected by supplementing 2 or 20 µM zinc but were reduced (P < 0.05) with 40 µM zinc. In blastocysts harvested on day 8, inner cell mass (ICM) and total cell number were increased (P < 0.05) with 2 µM zinc supplementation but not with the other zinc concentrations. Numbers of trophectoderm cells were not affected by zinc treatment. In conclusion, supplementing zinc during bovine embryo culture did not impact blastocyst development but improved ICM cell numbers. This improvement in ICM cell number may have implications for improved pregnancy retention rates after IVP embryo transfer as smaller ICM sizes are associated with poor pregnancy success in cattle.
Keywords: blastocyst, bovine, embryo, inner cell mass, zinc
Introduction
The in vitro production (IVP) of bovine embryos has become a popular way to generate a large number of embryos from genetically elite cows and heifers (van Wagtendonk-de Leeuw et al., 2000). These embryos may also be used for impregnating cows with poor fertilization rates and for circumventing the detrimental effects of heat stress and other environmental stresses (Ealy et al., 2019). In 2016, ~1-million IVP embryo transfers were completed worldwide, with 400,000 IVP embryos transferred to cattle in North America (IETS Data Retrieval Committee Report, December 2017).
Despite the increasing use of IVP, the ability of transferred IVP embryos to maintain pregnancies (i.e., embryo competency) remains inferior to that of embryos produced in vivo (Peterson and Lee, 2003; Ealy et al., 2019). Culture media used for maturing and fertilizing oocytes and developing embryos are undoubtedly a major contributor to this reduced posttransfer competency. In recent years, bovine embryos have primarily been cultured in media formulated specifically for mammalian embryos. Synthetic oviduct fluid (SOF)-based media formulations are popular in bovine IVP systems because its formulation is based on a biochemical analysis of energy sources, AAs, pH buffers, and various salts found within ovine oviducts (Tervit et al., 1972). Several modifications to the original SOF formulation (e.g., AAs, citrate, and adjusted glucose concentrations) allow for bovine blastocyst development in the absence of serum (Takahashi and First, 1992; Gardner et al., 1994; Fields et al., 2011).
Unfortunately, several micronutrients (e.g., vitamins and trace minerals) that exist in oviductal and uterine secretions are not present in SOF and other embryo media formations. Trace mineral salt supplementation is a well-established component to optimizing fertility and improving oocyte quality and embryo and fetal development (Apgar, 1985; Wilde, 2006; Tian and Diaz, 2013). Trace mineral supplementation can also improve ovum pickup success in cattle (Dantas et al., 2019). The omission of these micronutrients in embryo media formulations likely reflects that these media originally were developed to be used in combination with fetal bovine serum (FBS), which contains many of these micronutrients. The absence of serum may also reduce the incidence and severity of large offspring syndrome in calves produced from IVP embryos, although other factors also influence the occurrence of large offspring (Farin et al., 2006; Ealy et al., 2019). Many present-day embryo culture media use bovine serum albumin (BSA) in place of serum to provide a protein source that is semi-defined, but BSA, unlike serum, lacks zinc.
Zinc is a trace mineral that is essential for various physiological actions. Over 300 metalloproteins and >2,000 transcription factors depend on zinc for chemical reactions or protein stability for numerous cellular activities, including cell proliferation, DNA methylation, DNA repair, and apoptosis (McCall et al., 2000; Dreosti, 2001; Yamasaki et al., 2007). Zinc is present at concentrations of 0.8 to 0.9 µg/mL (12 to 14 µM) in bovine serum and 0.16 to 4.3 µg/mL (2.4 to 65.0 µM) in bovine uterine lumen fluid, depending on the method of fluid capture (Wiebold, 1988; Alavi-Shoushtari et al., 2012).
We propose that the absence of zinc in bovine embryo media formations has deleterious consequences on IVP embryo development and competency. Zinc supplementation is beneficial during in vitro maturation (Picco et al., 2010; Anchordoquy et al., 2011, 2014), but no reports exist that describe whether supplementing zinc after fertilization influences bovine embryo development. The objective of this work was to examine the effects of zinc supplementation to BSA-containing, FBS-lacking SOF (SOF-BE1) on IVP bovine embryo development and on total, inner cell mass (ICM), and trophectoderm (TE) cell numbers in blastocysts.
Materials and Methods
No animals were used for this work. All studies were completed on slaughterhouse-derived materials from a commercial slaughterhouse that followed humane slaughter practices according to USDA guidelines. Unless specified otherwise, reagents were purchased from ThermoFisher Chemical Company (Waltham, MA).
In Vitro Embryo Production
Bovine embryos were produced by in vitro maturation, fertilization, and culture procedures described previously (Rivera and Hansen, 2001; Wooldridge and Ealy, 2019). In brief, cumulus-oocyte complexes (COCs) obtained from beef and dairy cow ovaries purchased from Brown Packing Co. (Gaffney, SC) were matured for 21 to 24 h in tissue culture medium 199 containing Earle’s salts supplemented as described previously (Wooldridge and Ealy, 2019). Matured COCs and isolated sperm (four Holstein bulls, donation from Select Sires, Plain City, OH) were co-incubated in SOF-based fertilization medium (Denicol et al., 2014). The time of fertilization was designated as day 0. After 16 h, presumptive zygotes were denuded by gentle pipetting and placed in 50 µL drops of SOF-BE1 (23 to 26 zygotes/drop) (Fields et al., 2011).
Zinc Supplementation
On day 1 postfertilization, zinc sulfate solution (2 M in water; Sigma-Aldrich, St. Louis, MO) was added to SOF-BE1 at 0, 2, 20, or 40 µM. Embryos were cultured until day 8 postfertilization (1 to 3 drops/replicate; seven replicates, 325 to 401 total zygotes/treatment). Cleavage was assessed on day 3 (percentage of zygotes that formed ≥2-cell stage embryos), and blastocyst formation was determined on days 7 and 8 (percentage of cleaved embryos that formed blastocysts). Regular blastocysts were defined as embryos containing a blastocoel cavity that did not exhibit expansion in diameter or zona pellucida thinning. Advanced blastocysts included expanded blastocysts (expansion in diameter with zona pellucida thinning), hatching blastocysts (zona pellucida hatching), and hatched blastocysts (expanded blastocysts lacking a zona pellucida).
Differential Cell Labeling in Blastocysts
In five of the seven replicates, all blastocysts were collected from each treatment (total 22 to 57 blastocysts/treatment) and fixed and immunostained as described previously (Wooldridge and Ealy, 2019). In brief, immunostaining involved incubation with an anti-caudal-type homeobox-2 (CDX2) antibody (Biogenex, San Ramon, CA; sold ready-to-use) followed by washing and incubation with donkey anti-mouse Alexa Fluor 488 or 647. Nuclear DNA was stained with 4′,6-diamidino-2-phenylindole (1 µg/mL). After washing, embryos were flattened between a glass slide and cover slip. Fluorescent markers were visualized by using an Eclipse Ti-E inverted microscope equipped with an X-Cite 120 epifluorescence illumination system. Images were captured with DS-L3 digital camera and assembled with NIS-Elements Software (Nikon Instruments, Melville, NY). Cell counting was completed by using the cell counter plugin in the program FIJI (ImageJ) to label and record individual nuclei stained with CDX2, a TE-specific marker, or that lacked CDX2 staining (ICM cells) (Schindelin et al., 2012).
Statistical Analysis
All analyses were completed by least-squares ANOVA using the general linear model of the Statistical Analysis System (SAS for Windows, version 9.4; SAS Institute Inc., Cary, NC). Main effects tested were treatment (zinc concentration) and replicate. The Dunnett’s test was used to contrast each zinc treatment group with the control. Replicate was used as the experimental unit for embryo development studies. Percentage data were arcsine transformed before analysis but are presented in figures as nontransformed means and SEM. Embryo was used as the experiment unit for cell number data. In all studies, statistical significance was determined at P ≤ 0.05.
Results
Effects of Zinc Supplementation on Embryo Development
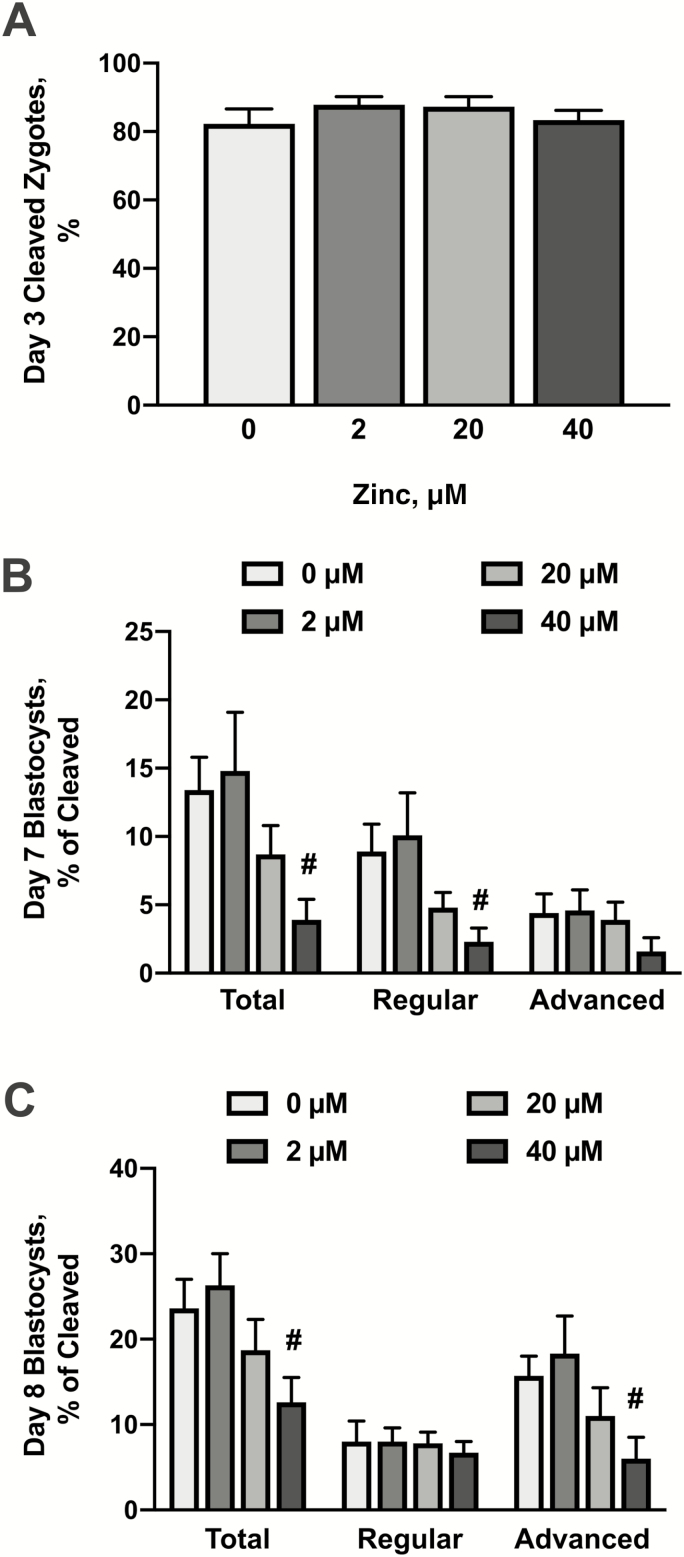
Supplementation with zinc began immediately after fertilization (day 1; 16 h after initial exposure to spermatozoa). On day 3 postfertilization, zinc supplementation did not affect cleavage rates (Fig. 1A). On days 7 and 8 (Fig. 1B and C), supplementation with 2 or 20 μM zinc did not affect total, regular, or advanced blastocyst formation. However, exposure to 40 μM zinc reduced (P < 0.05) the percentage of total blastocysts on both days 7 and 8. This treatment also reduced (P < 0.05) the percentage of regular blastocysts on day 7 and the percentage of advanced blastocysts on day 8.
Figure 1.
Effects of zinc supplementation during in vitro bovine embryo culture on cleavage rate and blastocyst development. Bovine zygotes were cultured in SOF-BE1 containing 0, 2, 20, or 40 µM zinc sulfate from days 1 to 8 postfertilization (23 to 26 zygotes/drop, 1 to 3 drops/replicate, seven replicates). Panel A: Percentage of zygotes that cleaved to at least the two-cell stage. Panels B and C: The percentage cleaved embryos that generated blastocysts at day 7, Panel B and day 8, Panel C. Blastocysts are categorized as total (all blastocysts), regular (the presence of a blastocoel cavity but no expansion or zona pellucida thinning), or advanced (expansion and zona pellucida thinning with or without hatching). Means and SEMs are shown. The asterisks indicate increases in responses relative to the control value and the hashtag indicates decreases in responses relative to the control (P < 0.05).
Zinc Supplementation Increases ICM and Total Cell Numbers in Blastocysts
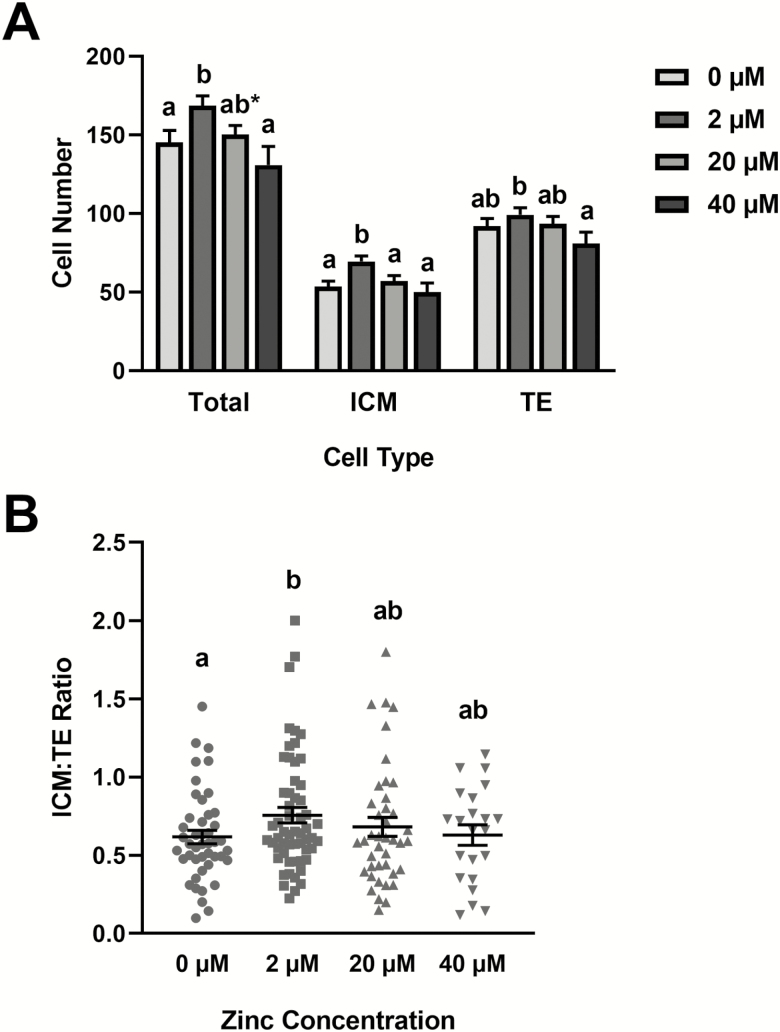
Differential cell staining was used to examine TE, ICM, and total cell numbers in day 8 blastocysts exposed to the zinc treatments (Fig. 2). Supplementation with 2 μM zinc increased (P < 0.05) total and ICM cell numbers but had no effect on TE cell numbers (Fig. 2A). No effects on cell numbers were detected in embryos supplemented with 20 or 40 μM zinc. No differences in the ICM:TE ratio were observed in any of the treatment groups (Fig. 2B).
Figure 2.
Effects of zinc supplementation during in vitro bovine embryo culture on total, ICM, and TE cell numbers in day 8 blastocysts. Bovine zygotes were cultured in SOF-BE1 containing 0, 2, 20, or 40 µM zinc sulfate from days 1 to 8 postfertilization (23 to 26 zygotes/drop, 1 to 3 drops/replicate, five replicate studies). On day 8, differential fluorescence staining was completed on blastocysts to identify TE and ICM cells. Panel A: Cell counts in each treatment (means and SEMs). Panel B: Treatment mean and SEMs (bars) and individual (dots) ICM:TE ratios for blastocysts. The asterisks indicate increases in responses relative to the control value and the hashtag indicates decreases in responses relative to the control (P < 0.05).
Discussion
Zinc plays essential roles in DNA repair and oxidative stress responses, so its absence in bovine embryo media may be detrimental to IVP bovine embryos. This is the first report we are aware of that describes the effects of physiological concentrations of zinc during bovine embryo culture. A previous report examining zinc and bovine embryo development supplemented nonphysiological amounts of zinc (≥150 µM zinc) and found it to be cytotoxic (Stephenson and Brackett, 1999). The zinc concentration range examined here covered the range found in bovine serum (12 to 14 µM) and uterine fluid (2.4 to 65.0 µM) (Wiebold, 1988; Alavi-Shoushtari et al., 2012). This work identified that a much lower concentration of 40 µM zinc was cytotoxic to bovine embryos, which is below some reported concentrations in uterine fluid. The SOF medium formation used for this work contained BSA, which is a prominent zinc carrier in the bloodstream (Handing et al., 2016), but uterine fluid likely contains more zinc binding factors to prevent high zinc concentrations from being cytotoxic to an embryo. The lowest zinc concentration tested (2 µM) was included in the present work in part because it represented the lower range of zinc concentrations found in the bovine uterus. Also, this concentration approximated the amount of zinc supplied in medium containing 10% FBS (various serum supplements contain 12 to 14 µM zinc before dilution).
The principal highlight of this work was observing improvements in ICM and total cell numbers with 2 µM zinc supplementation. The improvement in total cell numbers was caused primarily by the increase in ICM numbers, although a small, nonstatistically significant improvement in TE numbers also was observed. No differences in the ratio of ICM:TE cells were observed, likely because of the broad distribution of ratios observed in all treatment groups. Greater zinc concentrations did not affect total, ICM, or TE cell numbers. We did not examine why this biphasic response in ICM numbers was observed but predict that the greater zinc concentrations could have low-level cytotoxic effects on embryonic cells (Borovansky and Riley, 1989). This idea is consistent with the poor development of embryos exposed to 40 µM zinc.
The increase in ICM cell numbers observed in this work is noteworthy because a small ICM size is commonly associated with poor posttransfer embryo competency. The ICM develops into the embryonic disk, and this structure will produce the fetus as well as the yolk sac and allantois (Maddox-Hyttel et al., 2003). Reductions in embryonic disk size and the absence of embryonic disks have been noted in IVP bovine conceptuses (Bertolini et al., 2002a, 2002b; Fischer-Brown et al., 2002, 2004; Block et al., 2007; Loureiro et al., 2011). The roles that zinc play in mediating bovine ICM and embryonic disk development are not clear, but there is evidence in the mouse embryo that zinc plays a role in yolk sac development. Culturing mouse embryos in the absence of zinc does not affect in vitro development rates, but pregnancy losses after transfer were observed and linked to impaired yolk sac development and egg cylinder formation (Hanna et al., 2003).
None of the zinc concentrations tested in this work influenced cleavage or blastocyst rates. This is in contrast to work in the pig, where zinc supplementation during embryo culture improved both cleavage and blastocyst rates (Jeon et al., 2015). That study noted improvements in both parthenogenetically activated and fertilized embryos in a narrow window of zinc concentrations, where 12 µM zinc (0.8 µg/mL) increased cleavage and blastocyst rates but supplementing ≤6 or ≥18 µM did not affect either parameter (Jeon et al., 2015). It is not clear if the lack of positive effects on cleavage rates and blastocyst development in this study reflect species differences in embryonic responses to zinc or if a smaller range of zinc concentrations than tested here is needed to detect changes. It is interesting to note that zinc supplementation during bovine COC maturation improved subsequent blastocyst development (Picco et al., 2010; Anchordoquy et al., 2011; Anchordoquy et al., 2014). Perhaps the timing of zinc supplementation is an important contributor to observing positive effects of zinc on cleavage and blastocyst rates. More work is needed to explore how zinc and potentially other trace minerals may be used to improve the quantity and quality of blastocysts from IVP systems.
In summary, this work provides evidence that the addition of 2 µM zinc sulfate but not 20 or 40 µM zinc improves ICM and total cell numbers in IVP bovine blastocysts. This could lead to improved bovine embryo media formulations for greater posttransfer embryo competency.
Conflict of interest statement
None declared.
Acknowledgments
The authors thank Dr. Bo Harstine and Select Sires, Inc. (Plain City, OH) for donating the bovine semen used for this work.
Footnotes
This project was supported by Agriculture and Food Research Initiative (AFRI) Competitive (grant nos. 2017-67015-26461 and 2018-67030-28727) from the USDA National Institute of Food and Agriculture (NIFA) and by the National Institutes of Health (NIH) (grant no. R21-OD026516-01). Fellowship support for LKW was provided by the Institute for Critical Technology and Applied Science (ICTAS) Doctoral Scholars Program at Virginia Tech and by Agriculture and Food Research Initiative Competitive (grant no. 2018-67011-27993) from the USDA National Institute of Food and Agriculture.
Literature Cited
- Alavi-Shoushtari S. M., Asri Rezaie S., Pak M., Alizadeh S., Abedizadeh R., and Khaki A.. . 2012. Copper and zinc concentrations in the uterine fluid and blood serum during the bovine estrous cycle. Vet. Res. Forum 3:199–203. [PMC free article] [PubMed] [Google Scholar]
- Anchordoquy J. M., Anchordoquy J. P., Sirini M. A., Picco S. J., Peral-García P., and Furnus C. C.. . 2014. The importance of having zinc during in vitro maturation of cattle cumulus-oocyte complex: role of cumulus cells. Reprod. Domest. Anim. 49:865–874. doi: 10.1111/rda.12385 [DOI] [PubMed] [Google Scholar]
- Anchordoquy J. M., Picco S. J., Seoane A., Anchordoquy J. P., Ponzinibbio M. V., Mattioli G. A., Peral García P., and Furnus C. C.. . 2011. Analysis of apoptosis and DNA damage in bovine cumulus cells after exposure in vitro to different zinc concentrations. Cell Biol. Int. 35:593–597. doi: 10.1042/CBI20100507 [DOI] [PubMed] [Google Scholar]
- Apgar J. 1985. Zinc and reproduction. Annu. Rev. Nutr. 5:43–68. doi: 10.1146/annurev.nu.05.070185.000355 [DOI] [PubMed] [Google Scholar]
- Bertolini M., Beam S. W., Shim H., Bertolini L. R., Moyer A. L., Famula T. R., and Anderson G. B.. . 2002a. Growth, development, and gene expression by in vivo- and in vitro-produced day 7 and 16 bovine embryos. Mol. Reprod. Dev. 63:318–328. doi: 10.1002/mrd.90015 [DOI] [PubMed] [Google Scholar]
- Bertolini M., Mason J. B., Beam S. W., Carneiro G. F., Sween M. L., Kominek D. J., Moyer A. L., Famula T. R., Sainz R. D., and Anderson G. B.. . 2002b. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 58(5):973–994. doi: 10.1002/mrd.90015 [DOI] [PubMed] [Google Scholar]
- Block J., Fischer-Brown A. E., Rodina T. M., Ealy A. D., and Hansen P. J.. . 2007. The effect of in vitro treatment of bovine embryos with IGF-1 on subsequent development in utero to day 14 of gestation. Theriogenology 68(2):153–161. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.) doi: 10.1016/j.theriogenology.2007.04.045 [DOI] [PubMed] [Google Scholar]
- Borovansky J., and Riley P. A.. . 1989. Cytotoxicity of zinc in vitro. Chem. Biol. Interact. 69(2–3):279–291. doi: 10.1016/0009-2797(89)90085-9 [DOI] [PubMed] [Google Scholar]
- Dantas F. G., Reese S. T., Filho R. V. O., Carvalho R. S., Franco G. A., Abbott C. R., Payton R. R., Edwards J. L., Russell J. R., Smith J. K., and Pohler K. G.. . 2019. Effect of complexed trace minerals on cumulus-oocyte complex recovery and in vitro embryo production in beef cattle1,2. J. Anim. Sci. 97(4):1478–1490. doi: 10.1093/jas/skz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicol A. C., Block J., Kelley D. E., Pohler K. G., Dobbs K. B., Mortensen C. J., Ortega M. S., and Hansen P. J.. . 2014. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 28:3975–3986. doi: 10.1096/fj.14-253112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti I. E. 2001. Zinc and the gene. Mutat. Res. 475(1–2):161–167. doi: 10.1016/s0027-5107(01)00067-7 [DOI] [PubMed] [Google Scholar]
- Ealy A. D., Wooldridge L. K., and McCoski S. R.. . 2019. Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 97:2555–2568. doi: 10.1093/jas/skz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin P. W., Piedrahita J. A., and Farin C. E.. . 2006. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology 65:178–191. doi: 10.1016/j.theriogenology.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Fields S. D., Hansen P. J., and Ealy A. D.. . 2011. Fibroblast growth factor requirements for in vitro development of bovine embryos. Theriogenology 75(8):1466–1475. doi:S0093-691X(10)00646-1 [pii] 10.1016/j.theriogenology.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Fischer-Brown A. E., Lindsey B. R., Ireland F. A., Northey D. L., Monson R. L., Clark S. G., Wheeler M. B., Kesler D. J., Lane S. J., Weigel K. A., . et al. 2004. Embryonic disc development and subsequent viability of cattle embryos following culture in two media under two oxygen concentrations. Reprod. Fertil. Dev. 16(8):787–793. doi:RD04026 [pii] [DOI] [PubMed] [Google Scholar]
- Fischer-Brown A., Monson R., Parrish J., and Rutledge J.. . 2002. Cell allocation in bovine embryos cultured in two media under two oxygen concentrations. Zygote 10:341–348. doi: 10.1017/s0967199402004082 [DOI] [PubMed] [Google Scholar]
- Gardner D. K., Lane M., Spitzer A., and Batt P. A.. . 1994. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol. Reprod. 50(2):390–400. doi: 10.1095/biolreprod50.2.390 [DOI] [PubMed] [Google Scholar]
- Handing K. B., Shabalin I. G., Kassaar O., Khazaipoul S., Blindauer C. A., Stewart A. J., Chruszcz M., and Minor W.. . 2016. Circulatory zinc transport is controlled by distinct interdomain sites on mammalian albumins. Chem. Sci. 7:6635–6648. doi: 10.1039/c6sc02267g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L. A., Clegg M. S., Momma T. Y., Daston G. P., Rogers J. M., and Keen C. L.. . 2003. Zinc influences the in vitro development of peri-implantation mouse embryos. Birth Defects Res. A 67(6):414–420. doi: 10.1002/bdra.10046 [DOI] [PubMed] [Google Scholar]
- Jeon Y., Yoon J. D., Cai L., Hwang S. U., Kim E., Lee E., Jeung E. B., and Hyun S. H.. . 2015. Effect of zinc on in vitro development of porcine embryos. Theriogenology 84(4):531–537. doi: 10.1016/j.theriogenology.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Loureiro B., Block J., Favoreto M. G., Carambula S., Pennington K. A., Ealy A. D., and Hansen P. J.. . 2011. Consequences of conceptus exposure to colony-stimulating factor 2 on survival, elongation, interferon-{tau} secretion, and gene expression. Reproduction 141(5):617–624. doi: 10.1530/REP-10-0511 [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel P., Alexopoulos N. I., Vajta G., Lewis I., Rogers P., Cann L., Callesen H., Tveden-Nyborg P., and Trounson A.. . 2003. Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction 125(4):607–623. doi: 10.1530/rep.0.1250607 [DOI] [PubMed] [Google Scholar]
- McCall K. A., Huang C., and Fierke C. A.. . 2000. Function and mechanism of zinc metalloenzymes. J. Nutr. 130(5S Suppl.):1437S–1446S. doi: 10.1093/jn/130.5.1437S [DOI] [PubMed] [Google Scholar]
- Peterson A. J., and Lee R. S.. . 2003. Improving successful pregnancies after embryo transfer. Theriogenology 59(2):687–697. doi:S0093691X02012487 [pii] [DOI] [PubMed] [Google Scholar]
- Picco S. J., Anchordoquy J. M., de Matos D. G., Anchordoquy J. P., Seoane A., Mattioli G. A., Errecalde A. L., and Furnus C. C.. . 2010. Effect of increasing zinc sulphate concentration during in vitro maturation of bovine oocytes. Theriogenology 74:1141–1148. doi: 10.1016/j.theriogenology.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Rivera R. M., and Hansen P. J.. . 2001. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 121:107–115. doi:10.1530/rep.0.1210107 [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., . et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9(7):676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. L., and Brackett B. G.. . 1999. Influences of zinc on fertilisation and development of bovine oocytes in vitro. Zygote 7:195–201. doi: 10.1017/s096719949900057x [DOI] [PubMed] [Google Scholar]
- Takahashi Y., and First N. L.. . 1992. In vitro development of bovine one-cell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37:963–978. doi: 10.1016/0093-691x(92)90096-a [DOI] [PubMed] [Google Scholar]
- Tervit H. R., Whittingham D. G., and Rowson L. E.. . 1972. Successful culture in vitro of sheep and cattle ova. J. Reprod. Fertil. 30:493–497. doi: 10.1530/jrf.0.0300493 [DOI] [PubMed] [Google Scholar]
- Tian X., and Diaz F. J.. . 2013. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev. Biol. 376(1):51–61. doi: 10.1016/j.ydbio.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wagtendonk-de Leeuw A. M., Mullaart E., de Roos A. P., Merton J. S., den Daas J. H., Kemp B., and de Ruigh L.. . 2000. Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring. Theriogenology 53(2):575–597. doi: 10.1016/S0093-691X(99)00259-9 [DOI] [PubMed] [Google Scholar]
- Wiebold J. L. 1988. Embryonic mortality and the uterine environment in first-service lactating dairy cows. J. Reprod. Fertil. 84:393–399. doi: 10.1530/jrf.0.0840393 [DOI] [PubMed] [Google Scholar]
- Wilde D. 2006. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 96(3–4):240–249. doi: 10.1016/j.anireprosci.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Wooldridge L. K., and Ealy A. D.. . 2019. Interleukin-6 increases inner cell mass numbers in bovine embryos. BMC Dev. Biol. 19:2. doi: 10.1186/s12861-019-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Sakata-Sogawa K., Hasegawa A., Suzuki T., Kabu K., Sato E., Kurosaki T., Yamashita S., Tokunaga M., Nishida K., . et al. 2007. Zinc is a novel intracellular second messenger. J. Cell Biol. 177(4):637–645. doi: 10.1083/jcb.200702081 [DOI] [PMC free article] [PubMed] [Google Scholar]