Abstract
Little is still known about the effect of dietary patterns on left ventricular hypertrophy (LVH). Here, we derived dietary patterns by principal component analysis (PCA) and evaluated their association with LV structure, function, and remodelling. Our cross-sectional study included 438 members (aged 25–65 years; 59.1% women) of the Kardiovize Brno 2030 with no history of cardiovascular disease. Two dietary patterns were derived using PCA, namely prudent and western. Primary outcomes were echocardiographic parameters and LV geometric patterns, such as concentric LV remodelling (cLVR), concentric LVH (cLVH), and eccentric LVH (eLVH). Interestingly, participants with high adherence to the prudent dietary pattern had decreased odds of cLVH after adjustment for socio-demographic, clinical and behavioral covariates (OR = 0.24, 95% CI = 0.08–0.88; p = 0.031). By contrast, several echocardiographic parameters increased with increasing adherence to the western dietary pattern, which resulted in higher odds of cLVH among participants with high adherence (OR = 5.38, 95% CI = 1.17–23.58; p = 0.035). Although our findings may have an immediate relevance for public-health strategies, further large-size prospective studies should be encouraged to better understand the observed association and their causality.
Subject terms: Cardiac hypertrophy, Obesity, Epidemiology
Introduction
Several lines of evidence have shown that subclinical changes in left ventricular (LV) structure and function often precede symptomatic heart failure, and that LV remodelling (LVR) commonly follows cardiovascular events, such as myocardial infarction, idiopathic dilated cardiomyopathy, and volume or pressure overload1–3. The classification of LV geometry - proposed by Ganau and colleagues in 1992 - distinguished three abnormal LV geometric patterns based primarily on LV mass indexed to height2.7 (LVMI) and relative wall thickness (RWT) parameters4. Eccentric LV hypertrophy (eLVH) - characterized by the enlargement of cavity without changes in wall thickness - is mainly induced by volume overload. Instead, concentric LV remodelling (cLVR) and hypertrophy (cLVH) are characterized by an increase in thickness of the myocardium without a corresponding increase in LV size. cLVR and cLVH are traditionally considered as unfavorable LV adaptation to pressure overload, such as hypertension5,6. In general, these LV geometric patterns are associated with cardiovascular disease (CVD) and all-cause mortality4, while elevated LV mass (LVM) and systolic disfunction are risk factors for heart failure in asymptomatic individuals7. Obesity and hypertension - two of the major threats to public health that have been consistently and strongly associated with CVD incidence and mortality8,9 - are the main determinants of LVR, and there is consensus that their coexistence has an additive or even synergistic deleterious effect on LVR6,10,11. Thus, acting on risk factors for LVR might be helpful in reducing the burden of CVD worldwide.
In 2014, we established the Kardiovize Brno 2030 study, a prospective cohort recruited from the urban population of Brno (Czech Republic), which aims to investigate traditional and novel risk factors for CVD outcomes6,12–16. In this cohort, we have already demonstrated, on the one hand, the harmful effect of a western dietary pattern on blood pressure, fasting glucose and triglycerides, on the other hand, the protective effect of a diet rich in cereals, fish, fruit and vegetables against obesity12. Previous studies also demonstrated favorable effects of prudent dietary patterns, such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet, on LV structure and function17–19. By contrast, a dietary pattern rich in processed foods and poor in fruit and vegetables might be associated with metabolic dysfunction and aberrant LV function20. Although the identification of risk factors for LVR could have immediate relevance for public-health strategies, little is still known about the effect of dietary patterns in patients who are free of CVD. To our knowledge, no evidence exists about the association between a posteriori dietary patterns and LVH and/or remodelling. Here, we used the principal component analysis (PCA) to identify dietary patterns that maximally reflected dietary habits in a subsample of the Kardiovize cohort. This approach differs from traditional a priori methods that assess diet based on prior knowledge and scientific evidence21. By contrast, principal component analysis (PCA), together with cluster analysis, are the most commonly applied empirical methods, allowing to derive dietary patterns that maximally reflect dietary habits22. Next, we performed a cross-sectional analysis to evaluate the association of adherence to each dietary pattern with LV structure, function, and remodelling.
Methods
Study design and data collection
Kardiovize Brno 2030 cohort is a prospective study, which enrolled a random sample of 2160 adult residents of the city of Brno (Czech Republic) to assess traditional and novel risk factors for CVD12–16. The study protocol was approved by the Ethics Committee of St Anne’s University Hospital, Brno, Czech Republic (reference 2 G/2012) in accordance with the Declaration of Helsinki, and all participants signed an informed consent to participate in the study. To investigate the association of dietary patterns with LVH and remodelling, the current cross-sectional analysis used data from 438 participants with no history of CVD, and with complete assessment of anthropometric, biochemical, echocardiographic and dietary information. Socio-demographic (i.e., age, sex, educational level, marital and employment status) and behavioral (i.e., smoking status and physical activity) characteristics were collected by trained interviewers through structured questionnaires. Educational level was categorized as low (primary education), medium (secondary education), or high (tertiary education). Marital status was categorized into living alone (including single, divorced or widowed) or living in couple (including married and other relationships). Employment status was categorized into employed (including full-time or part-time employment) or unemployed (including retired). Smoking status was self-reported and categorized as current, former or never. Physical activity was assessed using the long form of the International Physical Activity Questionnaire (IPAQ-L) and reported as the Metabolic Equivalent of Task (MET-min/week)23.
Physical examination
Physical examination was performed by trained professionals according to standardized and validated protocols14–16,24. In brief, height and weight were measured to the nearest 1 cm and 1 kg, respectively, using a medical digital scale with meter (SECA 799; SECA, GmbH and Co. KG, Hamburg, Germany). BMI was calculated and categorized according to the WHO criteria25. Waist circumference was measured to the nearest 1 cm by manual tape measurement to define central obesity according to the WHO criteria26. Blood pressure was measured using a mercury sphygmomanometer (Baumanometer, W.A. Baum, Co., Inc., USA), and hypertension was defined as blood pressure ≥140/90 mmHg, or a prior diagnosis or taking antihypertensive drugs. As described elsewhere6, biochemical analyses were performed on 12-h fasting blood samples using a Modular SWA P800 analyzer (Roche, Basel, Switzerland). Specifically, total cholesterol, triglycerides and glucose concentrations were measured by the enzymatic colorimetric method (Roche Diagnostics GmbH, Germany), while HDL-cholesterol using the homogeneous method (Sekisui Medical, Japan). LDL-cholesterol concentrations were calculated using the homogeneous method or the Friedewald equation according to triglycerides levels. Hyperlipidemia was defined as having either total cholesterol of ≥5.0 mmol/L, or LDL cholesterol of ≥3 mmol/, or triglycerides of ≥1.7 mmol/L, or taking lipid-lowering drugs. Diabetes mellitus was defined as fasting glucose of ≥7 mmol/L, or a prior diagnosis or taking antidiabetic drugs.
Echocardiography
Transthoracic echocardiography was performed with a GE-Vingmed Vivid E9 device (GE Vingmed Ultrasound AS, Horten, Norway) using a 1,5–4,6 MHz sector transducer, as described elsewhere6. In brief, images were obtained with the patient lying in a left lateral decubitus or supine position, and an ECG signal was recorded and displayed simultaneously. Analyses were performed using the EchoPAC PC software (version 113) and assessed according to the criteria of the American Society of Echocardiography (ASE)27–29. RWT was calculated as the ratio of the posterior wall thickness at end-diastole (LVPWd) doubled and left ventricle end-diastolic diameter (LVIDd). LVH was classified as LVMI (i.e. indexed to height2.7) >48 g/m2.7 for men or >44 g/m2.7 for women, according to ASE and the European Association of Cardiovascular Imaging (EACVI) recommendations30. LV geometry patterns were classified according to LVMI (i.e. normal or increased) and RWT (i.e. normal or altered). Normal LV geometry was described as RWT <0,42 and LVMI ≤48 g/m2.7 for men or ≤44 g/m2.7 for women; cLVH was described as RWT >0,42 and LVMI >48 g/m2.7 for men or >44 g/m2.7 for women; eLVH was described as RWT < 0,42 and LVMI >48 g/m2.7 for men or >44 g/m2.7 for women and cLVR was described as RWT >0,42 and LVMI ≤ 48 g/m2.7 for men or ≤48 g/m2.7 for women27,30.
Dietary assessment
Dietary data were collected through a 43-item Food Frequency Questionnaire (FFQ), using the previous week as reference period12. During the interview, participants were asked to indicate frequency of consumption classified (seven categories from “almost never” to “six or more times a day”). Standard portion size was attributed to each food item as the age- and sex-specific median food intake obtained from a dietary survey on the national level31, which involved age and gender representative sample of the Czech population32. Food intakes were calculated by multiplying frequency of consumption by standard serving size, and adjusted for total energy intake using the residual method33. To avoid the potential influence of outliers, subjects in the 5th and 95th percentiles of total energy intake were excluded from further analyses. PCA has been widely used in nutritional epidemiology to derive population-dependent dietary patterns. Thus, we classified food items into 31 predefined food groups based on the similarity of nutrient profiles or culinary usage12. Next, PCA followed by varimax rotation was performed on energy-adjusted intakes of each predefined food group to derive a posteriori dietary patterns. The number of dietary patterns to retain was defined based on eigenvalues >2.0, Scree plot examination, and interpretability of components. More details on PCA method are reported elsewhere12,34–36. Dietary patterns were described based on factor loadings with absolute value ≥0.25. Factor scores were calculated for each dietary pattern by summing the products between observed energy-adjusted food group intakes and their factor loadings, so that higher factor scores indicated higher adherence to dietary patterns and viceversa. To obtain a similar number of participants in each group of adherence, we categorized the adherence to each dietary pattern according to tertile distribution of factor scores as follows: low adherence (1st tertile of factor score), medium adherence (2nd tertile), or high adherence (3rd tertile). In a previous study on the Kardiovize cohort12, we confirmed the internal reproducibility of this method by performing separate PCA in two randomly selected subgroups.
Statistical analyses
All statistical analyses were conducted using SPSS software (version 22.0, SPSS, Chicago, IL). The Kolmogorov-Smirnov test was used to test the normality of continuous variables, and those underlying a skewed distribution were described using median and interquartile range (IQR) and compared using the Kruskal–Wallis test. Spearman’s correlation analysis was used to test correlation between continuous variables. Categorical variables were described using frequency (%) and compared using the Chi-square test. Logistic regression analysis was used to evaluate the association of adherence to dietary patterns with LVH, concentric remodelling and abnormal LV geometry patterns, using low adherence as reference group. Model 1 was adjusted for age, sex, BMI, and waist circumference, while model 2 further adjusted for smoking status, total energy intake, physical activity, diabetes and hypertension. All statistical tests were two-sided, and p values < 0.05 were considered statistically significant.
Results
Correlation between food intakes and echocardiographic parameters
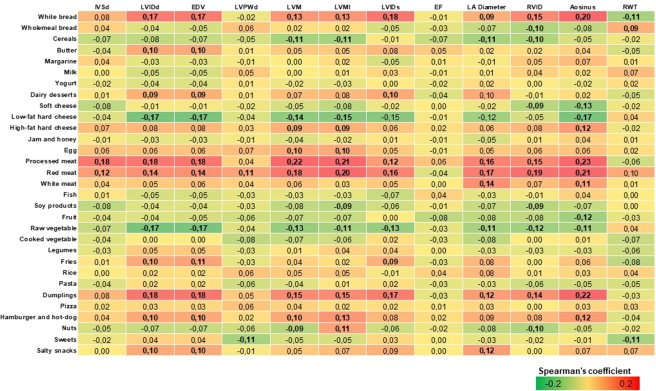
The Kardiovize Brno 2030 cohort included 438 participants (59.1% women), aged 25 to 65 years, who satisfied the selection criteria and could be included in the current analysis. In this subsample, we first tested correlations between food intakes and echocardiographic parameters (Fig. 1). In general, we observed that some food groups (e.g. cereals, low-fat cheese, fruit, raw vegetables, and nuts) negatively correlated with echocardiographic parameters associated with LV structure (IVSd, LVIDd, EDV, LVPWd, LVM, LVMI, LVIDs, LA Diameter, RVID, Aosinus, RWT) and function (EF). By contrast, other food groups (e.g. white bread, high-fat cheese, red and processed meat, dumplings, and salty snacks) positively correlated with the same echocardiographic parameters.
Figure 1.
Correlation matrix between food intakes and echocardiographic parameters. Results are reported as Spearman’s correlation coefficient and those with p-value < 0.05 are indicated in bold. Abbreviations: interventricular septum thickness at end-diastole, IVSd; end-diastolic volume, EDV; posterior wall thickness at end-diastole, LVPWd; left ventricle mass, LVM; left ventricle mass indexed to height2.7, LVMI; left ventricle end-systolic diameter, LVIDs; ejection fraction, EF; left atrial diameter, LA Diameter; right ventricle diameter, RVID; aortic diameter at the sinus of Valsalva, Aosinus; relative wall thickness, RWT.
Identification of dietary patterns
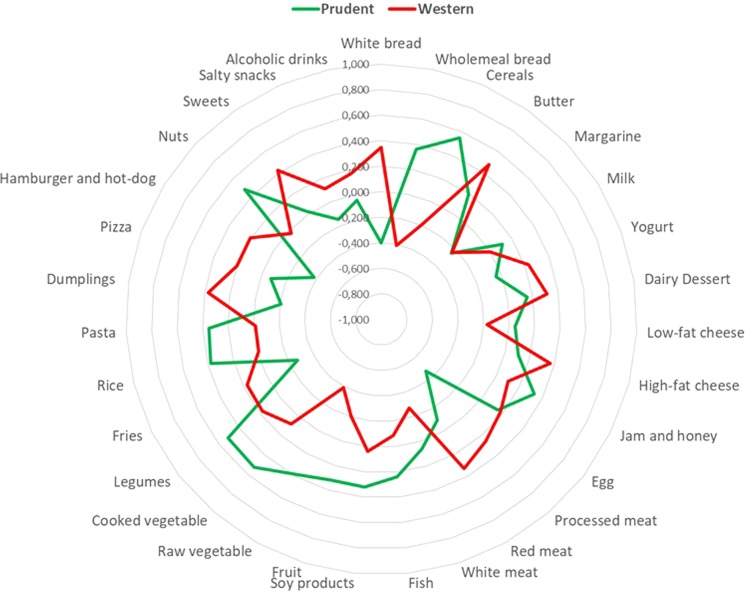
To address to what extent dietary patters, rather than specific food groups, may affect LV remodelling, we first derived two dietary patterns with eigenvalues ≥2.0, which explained 13.8% of total variance among 31 predefined food groups. Figure 2 shows factor loadings, which can be viewed as the correlation between each food group and dietary pattern. Accordingly, the first dietary pattern - characterized by high intake of whole-meal bread, cereals, jam and honey, soy products, fruit, raw and cooked vegetables, legumes, rice and pasta, and low intake of white bread, processed meat, fries, hamburger and hot-dog - was defined prudent, which was consistent with the well-accepted term used in this field of research12,34. Participants with high adherence to the prudent dietary pattern were older, less frequently men, less likely to live alone, and more frequently unemployed and physical active than those with low adherence (Table 1). With respect to cardio-metabolic parameters, participants with high adherence to the prudent dietary pattern exhibited lower waist circumference and triglycerides levels, higher HDL-cholesterol levels, and lower prevalence of obesity and hypertension (Table 1). By contrast, the second dietary pattern - characterized by high intake of white bread, butter, sweets, high-fat cheese, red and processed meat, and dumplings, and low intake of whole-meal bread, low-fat cheese, white meat, and raw vegetables - was named western. Participants with high adherence to the western dietary pattern were younger, more frequently men, and had more total energy intake than those with low adherence (Table 1). With respect to cardio-metabolic parameters, participants with high adherence to the western dietary pattern exhibited higher waist circumference and triglycerides levels, and lower HDL-cholesterol levels (Table 1).
Figure 2.
Radar graph of factor loadings characterizing dietary patterns. Red line indicates factor loadings related to the western dietary pattern. Green line indicates factor loadings related to the prudent dietary pattern. Dietary patterns are described based on factor loadings with absolute value ≥0.25.
Table 1.
Characteristics of study population by adherence to dietary patterns.
| Characteristics | Prudent | Western | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| High adherence (1st tertile) | Medium adherence (2nd tertile) | High adherence (3rd tertile) | p-value | High adherence (1st tertile) | Medium adherence (2nd tertile) | High adherence (3rd tertile) | p-value | ||
| Age, years | 45.0 (17.0) | 47.0 (18.0) | 48.0 (18.0) | 0.014 | 50.0 (18.0) | 45.5 (17.0) | 43.0 (16.0) | <0.001 | 47.0 (19.8) |
| Sex (% male) | 56.4% | 54.1% | 40.4% | 0.007 | 35.7% | 48.8% | 66.5% | <0.001 | 40.9% |
| Educational level (% low) | 11.9% | 8.5% | 7.4% | 0.560 | 9.1% | 6.8% | 11.9% | 0.283 | 10.2% |
| Marital status (% living alone) | 42.6% | 35.4% | 27.7% | 0.013 | 37.7% | 39.5% | 28.4% | 0.064 | 39.3% |
| Employment (% unemployed) | 4.7% | 15.0% | 20.2% | <0.001 | 17.4% | 12.9% | 9.9% | 0.117 | 16.9% |
| Smoking (% current smokers) | 22.2% | 22.0% | 14.7% | 0.213 | 17.6% | 18.1% | 23.2% | 0.341 | 20.1% |
| Total energy intake, kcal | 2064 (924) | 2108 (882) | 2051 (933) | 0.193 | 1910 (836) | 2119 (930) | 2234 (941) | <0.001 | 1995 (946) |
| Physical activity, MET-min/week | 2570 (3796) | 3169 (4483) | 3585 (4489) | 0.040 | 3205 (4028) | 3162 (3515) | 2758 (4536) | 0.694 | 3267 (4696) |
| BMI, Kg/m2 | 25.3 (6.7) | 25.0 (5.0) | 24.6 (5.7) | 0.072 | 24.7 (6.2) | 24.7 (5.8) | 25.6 (4.6) | 0.243 | 24.5 (6.0) |
| Obesity (%) | 22.2% | 10.2% | 8.5% | 0.002 | 13.1% | 13.6% | 14.1% | 0.213 | 13.5% |
| Waist circumference | 90.0 (20.0) | 88.0 (16.0) | 86.0 (19.0) | 0.035 | 86.0 (19.0) | 86.0 (19.0) | 90.0 (18.0) | 0.009 | 85.0 (20.0) |
| Central Obesity (%) | 25.8% | 20.3% | 22.3% | 0.490 | 25.6% | 18.1% | 24.6% | 0.207 | 22.2% |
| Systolic Blood Pressure, mmHg | 117.5 (20.0) | 117.0 (18.9) | 116.8 (19.0) | 0.734 | 118.5 (23.5) | 117.3 (16.3) | 114.0 (17.0) | 0.188 | 115.5 (18.6) |
| Diastolic Blood Pressure, mmHg | 79.8 (13.1) | 79.8 (15.4) | 79.0 (11.4) | 0.591 | 80.0 (12.5) | 79.8 (14.0) | 78.0 (13.0) | 0.623 | 78.8 (13.0) |
| Hypertension (%) | 42.0% | 35.6% | 26.6% | 0.009 | 39.8% | 36.2% | 28.2% | 0.067 | 35.0% |
| Fasting Glucose, nmol/l | 4.9 (0.6) | 4.8 (0.6) | 4.9 (0.7) | 0.355 | 4.8 (0.7) | 4.8 (0.7) | 4.9 (0.7) | 0.050 | 4.8 (0.5) |
| Diabetes (%) | 2.3% | 1.1% | 0.6% | 0.359 | 1.1% | 1.7% | 1.1% | 0.867 | 1.3% |
| Triglycerides, nmol/l | 1.1 (0.8) | 1.0 (0.8) | 0.9 (0.7) | 0.003 | 0.9 (0.5) | 1.0 (0.9) | 1.1 (0.9) | 0.001 | 1.0 (0.8) |
| Total Cholesterol, nmol/l | 5.3 (1.5) | 5.0 (1.4) | 5.0 (1.3) | 0.186 | 5.2 (1.4) | 5.1 (1.3) | 5.0 (1.5) | 0.177 | 5.1 (1.4) |
| HDL Cholesterol, nmol/l | 1.4 (0.5) | 1.5 (0.4) | 1.6 (0.5) | <0.001 | 1.6 (0.5) | 1.5 (0.5) | 1.4 (0.5) | <0.001 | 1.6 (0.6) |
| LDL Cholesterol, nmol/l | 3.1 (1.3) | 3.0 (1.2) | 3.1 (1.1) | 0.360 | 3.1 (1.3) | 3.1 (1.3) | 3.2 (1.0) | 0.164 | 3.0 (1.3) |
| Hyperlipidaemia (%) | 42.0% | 32.2% | 27.1% | 0.051 | 28.4% | 33.9% | 39.3% | 0.110 | 36.0% |
Results are reported as median (Interquartile range), or percentage. Statistical analyses were performed using Chi-square test for bivariate or categorical variables, and Kruskal–Wallis test for continuous variables.
Abbreviations: metabolic equivalent task, MET; body mass index, BMI; high-density lipoprotein, HDL; low-density lipoprotein, LDL.
Association of dietary patterns with echocardiographic parameters
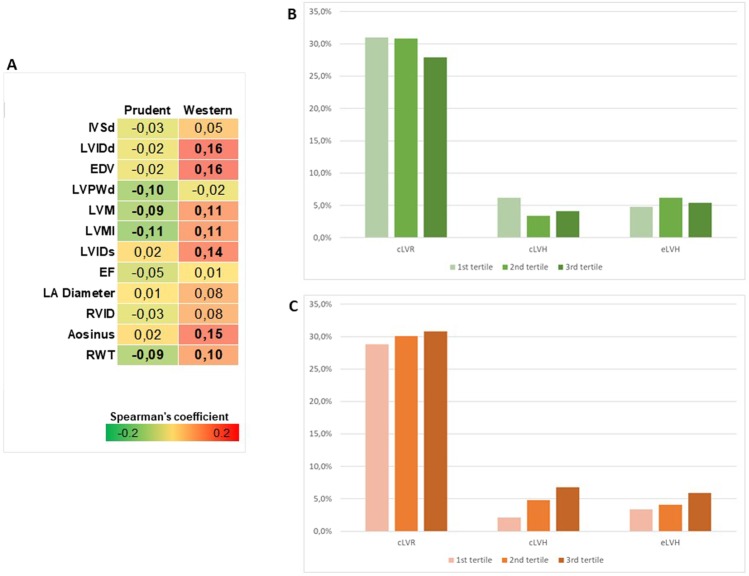
We next compared echocardiographic parameters across tertiles of adherence to each dietary pattern (Table 2). While no differences with respect to the prudent dietary pattern were evident, we observed increasing trend of LVID in diastole, EDV, LVMI, LVID in systole, Aosinus, and prevalence of LVH from the bottom to the top tertile of adherence to the western dietary pattern. This was consistent with weak but significant positive correlations between factor score of western dietary pattern and several echocardiographic parameters, including LVM, LVMI and RWT (Fig. 3A). By contrast, factor score of prudent dietary pattern was weakly but negatively correlated with LVM, LVMI and RWT. Since LVMI and RWT allowed to discriminate patients with LV remodelling, we next assessed the relative variations in LV geometry according to adherence to each dietary pattern. Overall, cLVR was the most prevalent abnormal pattern in the whole cohort (29.9%), while either eLVH or cLVH were less frequent (5.5% and 4.6%, respectively). Participants with high adherence to the prudent dietary pattern exhibited lower but not significant prevalence of cLVR and cLVH than those with low adherence (Fig. 3B). By contrast, participants with high adherence to the western pattern exhibited higher but not significant prevalence of cLVR, cLVH, and eLVH than those with low adherence (Fig. 3C). We finally performed logistic regression analyses to determine the association of dietary patterns with LVH, concentric remodelling and specific LV geometry patterns. Although adherence to the prudent dietary pattern did not seem to affect LVH or concentric remodelling in general, it was associated with cLVH. Indeed, compared to low adherence to the prudent pattern, high adherence significantly decreased the odds of cLVH after adjusting for age, sex, BMI, and waist circumference (OR = 0.28, 95% CI = 0.10–0.94; p = 0.030), and further adjusting for physical activity, smoking status, total energy intake, diabetes and hypertension (OR = 0.24, 95% CI = 0.08–0.88; p = 0.031) (Table 3). By contrast, compared to participants with low adherence to the western dietary pattern, those with high adherence were more likely to exhibit LVH (OR = 2.54, 95% CI = 1.09–5.89; p = 0.030) and specifically cLVH (OR = 5.38, 95% CI = 1.17–23.58; p = 0.035; Table 4), after adjusting for age, sex, BMI, waist circumference, physical activity, smoking status, total energy intake, diabetes and hypertension.
Table 2.
Echocardiographic parameters by adherence to dietary patterns.
| Echo parameters | Prudent | Western | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | p-value | 1st tertile | 2nd tertile | 3rd tertile | p-value | |
| IVSd | 0.9 (0.3) | 0.9 (0.1) | 1.0 (0.2) | 0.829 | 0.9 (0.3) | 1.0 (0.2) | 0.9 (0.2) | 0.447 |
| LVIDd | 4.7 (0.68 | 4.7 (0.8) | 4.7 (0.5) | 0.534 | 4.6 (0.6) | 4.7 (0.7) | 4.8 (0.7) | 0.002 |
| EDV | 103.5 (39.8) | 101.0 (35.5) | 101.0 (28.0) | 0.568 | 99.0 (30.0) | 103.0 (34.0) | 106.5 (36.5) | 0.001 |
| LVPWd | 0.9 (0.1) | 0.9 (0.2) | 0.9 (0.2) | 0.128 | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.802 |
| LVM | 157.9 (59.7) | 156.4 (56.0) | 146.9 (55.2) | 0.121 | 147.7 (59.2) | 157.5 (58.1) | 157.9 (54.4) | 0.073 |
| LVMI | 37.2 (19.7) | 33.0 (17.4) | 32.4 (19.9) | 0.246 | 32.5 (18.7) | 35.4 (18.2) | 38.1 (22.4) | 0.018 |
| LVIDs | 3.1 (0.6) | 3.1 (0.5) | 3.1(0.5) | 0.977 | 3.0 (0.5) | 3.1 (0.5) | 3.2 (0.4) | 0.004 |
| EF | 63.0 (8.0) | 66.0 (7.0) | 63.0 (7.0) | 0.148 | 63.0 (7.0) | 64.0 (8.0) | 64.0 (7.8) | 0.893 |
| LA diameter | 3.4 (0.7) | 3.6 (0.6) | 3.5 (0.7) | 0.994 | 3.6 (0.7) | 3.5 (0.7) | 3.5 (0.6) | 0.131 |
| RVID | 3.2 (0.5) | 3.2 (0.7) | 3.1 (0.6) | 0.627 | 3.1 (0.6) | 3.1 (0.6) | 3.2 (0.6) | 0.227 |
| Aosinus | 3.1 (0.7) | 3.3 (0.5) | 3.2 (0.5) | 0.087 | 3.1 (0.7) | 3.2 (0.6) | 3.3 (0.4) | <0.001 |
| RWT | 0.40 (0.10) | 0.39 (0.08) | 0.39 (0.10) | 0.280 | 0.38 (0.09) | 0.40 (0.09) | 0.41 (0.09) | 0.064 |
| LVH (%)a | 11.0% | 9.6% | 9.5% | 0.889 | 6.2% | 10.3% | 13.7% | 0.039 |
| Concentric remodelling (%)b | 37.2% | 34.2% | 32.0% | 0.637 | 32.2% | 35.6% | 35.6% | 0.777 |
Results are reported as median (Interquartile range) or percentage. Statistical analysis was performed using Kruskal–Wallis test for continuous variables and Chi-squared test for bivariate variables.
aDefined as LVMI >48 g/m2.7 for men or >44 g/m2.7 for women.
bDefined as RWT >0.42.
Abbreviations: interventricular septum thickness at end-diastole, IVSd; end-diastolic volume, EDV; posterior wall thickness at end-diastole, LVPWd; left ventricle mass, LVM; left ventricle mass indexed to height2.7, LVMI; left ventricle end-systolic diameter, LVIDs; ejection fraction, EF; left atrial diameter, LA Diameter; right ventricle diameter, RVID; aortic diameter at the sinus of Valsalva, Aosinus; relative wall thickness, RWT; left ventricular hypertrophy, LVH.
Figure 3.
Association of dietary patterns with echocardiographic parameters and left ventricular remodelling. (A) Correlation matrix between factor scores and echocardiographic parameters; results are reported as Spearman’s correlation coefficient and those with p-value < 0.05 are indicated in bold. (B) Distribution of left ventricular remodelling patterns by adherence to the prudent dietary pattern. (C) Distribution of left ventricular remodelling patterns by adherence to the western dietary pattern. Abbreviations: interventricular septum thickness at end-diastole, IVSd; end-diastolic volume, EDV; posterior wall thickness at end-diastole, LVPWd; left ventricle mass, LVM; left ventricle mass indexed to height2.7, LVMI; left ventricle end-systolic diameter, LVIDs; ejection fraction, EF; left atrial diameter, LA Diameter; right ventricle diameter, RVID; aortic diameter at the sinus of Valsalva, Aosinus; relative wall thickness, RWT.
Table 3.
Logistic regression analysis of the association of adherence to the prudent dietary pattern with left ventricular remodelling and hypertrophy.
| Regression Model | Prudent pattern | cLVR | cLVH | eLVH | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Model 1 | 1st tertile | Ref | Ref | ref | |||
| 2nd tertile |
0.94 (0.54–1.54) |
0.868 |
0.48 (0.16–1.42) |
0.178 |
0.85 (0.32–2.26) |
0.718 | |
| 3rd tertile |
0.78 (0.47–1.31) |
0.330 |
0.28 (0.10–0.94) |
0.030 |
0.58 (0.24–1.58) |
0.236 | |
| Trenda | 0.578 | 0.034 | 0.509 | ||||
| Model 2 | 1st tertile | Ref | Ref | ref | |||
| 2nd tertile |
0.99 (0.68–1.73) |
0.987 |
0.58 (0.26–1.12) |
0.308 |
0.92 (0.40–2.44) |
0.853 | |
| 3rd tertile |
0.87 (0.55–1.38) |
0.364 |
0.24 (0.08–0.88) |
0.031 |
0.58 (0.27–1.55) |
0.231 | |
| Trenda | 0.601 | 0.040 | 0.482 | ||||
ap-value for trend. Results are expressed as multivariable-adjusted odds ratio (OR) and 95% confidence interval (CI). Statistical analysis was performed using logistic regression adjusting for age, sex, BMI and waist circumference (Model 1), and further adjusting for physical activity (MET), smoking status, total energy intake, diabetes and hypertension (Model 2).
Abbreviations: concentric left ventricular remodelling, cLVR; concentric left ventricular hypertrophy, cLVH; eccentric left ventricular hypertrophy; reference group, Ref.
Table 4.
Logistic regression analysis of the association to the western dietary pattern with left ventricular remodelling and hypertrophy.
| Regression Model | Western pattern | cLVR | cLVH | eLVH | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Model 1 | 1st tertile | Ref | Ref | Ref | |||
| 2nd tertile |
0.87 (0.1–1.45) |
0.599 |
3.04 (0.76–12.31) |
0.112 |
0.65 (0.20–2.00) |
0.437 | |
| 3rd tertile |
0.84 (0.48–1.43) |
0.512 |
3.70 (0.92–14.87) |
0.062 |
1.73 (0.65–4.69) |
0.300 | |
| Trenda | 0.838 | 0.168 | 0.216 | ||||
| Model 2 | 1st tertile | Ref | Ref | Ref | |||
| 2nd tertile |
0.94 (0.56–1.54) |
0.683 |
4.63 (0.95–20.65) |
0.057 |
0.71 (0.28–2.03) |
0.513 | |
| 3rd tertile |
0.93 (0.57–1.53) |
0.718 |
5.38 (1.17–23.58) |
0.035 |
1.45 (0.57–4.34) |
0.499 | |
| Trenda | 0.912 | 0.041 | 0.387 | ||||
ap-value for trend. Results are expressed as multivariable-adjusted odds ratio (OR) and 95% confidence interval (CI). Statistical analysis was performed using logistic regression adjusting for age, sex, BMI and waist circumference (Model 1), and further adjusting for physical activity (MET), smoking status, total energy intake, diabetes and hypertension (Model 2).
Abbreviations: concentric left ventricular remodelling, cLVR; concentric left ventricular hypertrophy, cLVH; eccentric left ventricular hypertrophy; reference group, Ref.
Discussion
Our study showed associations between dietary patterns and LV structure, function and remodelling in participants with no history of CVD from the Kardiovize cohort, a randomly selected sample of urban residents of Brno, Czech Republic. Ours, to the best of our knowledge, is the first study to examine the effects of dietary patterns derived using PCA. Indeed, using a dietary pattern approach, rather than the more traditional focus on single nutrients or foods, may be more effective in identifying risk factors for LVR with an immediate relevance for public-health strategies. In this context, factor analysis and PCA, similar in their mathematical basis, have been largely applied to construct a linear function that maximally explains the variation in food intakes of the study population37,38. In the present study, we derived a prudent dietary pattern, which was characterized by high intake of whole-meal bread, cereals, jam and honey, soy products, fruit, raw and cooked vegetables, legumes, rice and pasta, and low intake of white bread, processed meat, fries, and hamburger and hot-dog. We demonstrated that, compared with low adherence, high adherence to this dietary pattern was not only favorably associated with low prevalence of obesity and hypertension, but also decreased the odds of cLVH, after adjusting for demographic, behavioral, and clinical covariates. These findings partially confirmed previous evidence that adherence to the Mediterranean diet improved LV structure and function in participants from the Multi-Ethnic Study of Atherosclerosis (MESA) study and the Northern Manhattan study17,18. In line with this evidence, in the MESA cohort, the DASH diet - which emphasized consumption of fruits, vegetables, whole grains, poultry, fish, nuts, and low-fat dairy products and minimized consumption of red meat, sweets, and sugar-sweetened beverages - was favorably associated with LV volume, stroke volume, and ejection fraction19. Indeed, these dietary patterns improved ventricular filling, which in turn increased end-diastolic filling, LV volumes, stroke volume, and ejection fraction17. However, in the MESA study, these findings were not indicative of LVR because there was no association of Mediterranean diet with LV mass-to-volume ratio and LVH17, defined as LVM above the population-specific 95th percentile39. The choice of method to define LVH for risk prediction and reduction has long been debated reaching no general consensus11,40. As previously proposed, the best methods could be LVM normalization to free fat mass or to height41. Since free fat mass was not routinely measured in the Kardiovize cohort, we adopted the method based on normalization of LVM to height2.7, which is the main factor to contribute to the magnitude of free fat deposition. Differences in the choice of method to define LVH might partially explain controversy between our results and those reported by previous studies. Using PCA, we also derived a western dietary pattern characterized by high intake of white bread, butter, sweets, high-fat cheese, red and processed meat, and dumplings, and low intake of whole-meal bread, low-fat cheese, white meat, and raw vegetables. Adherence to this dietary pattern was associated not only with unhealthy metabolic profile, but also with echocardiographic parameters, including LVM, LVMI and RWT. Accordingly, participants with high adherence to the western dietary pattern were more likely to exhibit LVH, specifically cLVH, than those with low adherence, after adjusting for demographic, behavioral, and clinical covariates. This is consistent with a research on the MESA cohort, which used the reduced rank regression to derive a dietary pattern that maximally explained the variation in metabolic syndrome components. This dietary pattern - characterized by intake of foods with a high glycemic index, high-fat meats, cheeses, processed foods and low intake of vegetables, soy, fruit, green and black tea, low-fat dairy desserts, seeds and nuts, and fish - was unfavorably associated with LV mass and systolic function.
The observed effects of dietary pattern on LV structure, function and remodelling might be attributed to intakes of individual foods. Indeed, we observed that healthy foods, such as cereals, low-fat cheese, fruit, raw vegetables, and nuts, positively affected echocardiographic parameters. By contrast, the same parameters were exacerbated by high-fat, hypercaloric, refined and processed foods. However, from a public health perspective, the identification of protective or deleterious dietary patterns remains the most relevant approach towards development of novel strategies against LVR and LVH. The observed relationships might be also mediated by associations between dietary patterns and several CVD risk factors, especially those related to LVR and LVH. As we previously demonstrated in the whole Kardiovize cohort12 and now reaffirmed in current subsample, the western dietary pattern had a deleterious effect on several cardio-metabolic risk factors, while the consumption of a diet rich in cereals, fish, fruit and vegetables was associated with a healthier cardio-metabolic profile. Consistently, previous epidemiological studies demonstrated that intake of healthy foods was associated with more favorable blood pressure42,43, insulin sensitivity (38), fasting glucose42,44, and lipid profile42. More recently, Lara and colleagues demonstrated that adherence to a dietary pattern rich in fried food, organ meats, processed meats, eggs, added fats, and sugar-sweetened beverages was associated with increased risk of heart failure, whereas a plant-based dietary pattern was inversely associated with incident heart failure risk45.
Limitations of our study included its cross-sectional design, which did not allow us to understand causality of observed relationships. Moreover, one of the main weaknesses of FFQ is that it relies on memory and on the skills of interviewer. However, using the previous week as reference period could reduce reporting biases due to memory. Additionally, we used a FFQ with standard portion sizes - which did not preclude potential measurement errors and may suffer from inaccuracies - and total variance explained by the PCA-derived dietary patterns was relatively low. To manage potential errors, standard portion sizes were obtained from an individual dietary survey on the national level, and subjects in the 5th and 95th percentiles of total energy intake were excluded. Despite these limitations, food compositions of our data-derived dietary patterns were consistent with those identified by previous studies in different European countries34,46–49. However, this approach does not allow to compare the effects of different dietary patterns. Accordingly, we compared and reported findings on the effects of different degrees of adherence to each dietary pattern. Finally, we cannot rule out the possibility of bias from residual confounders that might affect the association of dietary patterns with LV structure, function, and remodelling.
To our knowledge, our study is the first examining associations of PCA-derived dietary patterns with LV structure, function and remodelling. This approach may be more effective in identifying risk factors for LVR, rather than more traditional focus on single foods or a priori dietary pattern. Notably, participants with adherence to a prudent dietary pattern exhibited lower prevalence of obesity, hypertension, and cLVH. In contrast, the adherence to a western dietary pattern worsened their cardio-metabolic profile, which translated into higher prevalence of cLVH. Although our findings may have an immediate relevance for public-health strategies, further large-size prospective studies should be encouraged to better understand the observed association and their causality.
Acknowledgements
This work was supported by the National Program of Sustainability II (MEYS CR) (no. LQ1605) to MV. AM, MB and AA were partially funded by the Department of Medical and Surgical Sciences and Advanced Technologies “GF Ingrassia”, University of Catania (Piano Triennale di Sviluppo delle Attività di Ricerca Scientifica del Dipartimento–2016–2018).
Author contributions
Conceptualization, A.M., M.B., M.V. and A.A.; formal analysis, J.H., J.J. and O.H.; data curation and analysis, A.M.; original draft preparation, A.M. and M.V.; review and editing, A.M., J.H., J.M.I., J.J., M.B., M.V. and A.A.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonella Agodi, Email: agodia@unict.it.
Manlio Vinciguerra, Email: manlio.vinciguerra@fnusa.cz.
References
- 1.Hunt SA, et al. focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/S0735-1097(01)01110-X. [DOI] [PubMed] [Google Scholar]
- 3.Schocken DD, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 4.Ganau A, et al. Patterns of left ventricular hypertrophy and geometric remodelling in essential hypertension. Journal of the American College of Cardiology. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-V. [DOI] [PubMed] [Google Scholar]
- 5.de Simone G, et al. Evaluation of concentric left ventricular geometry in humans: evidence for age-related systematic underestimation. Hypertension. 2005;45:64–68. doi: 10.1161/01.HYP.0000150108.37527.57. [DOI] [PubMed] [Google Scholar]
- 6.Maugeri, A. et al. Independent effects of hypertension and obesity on left ventricular mass and geometry: evidence from the Cardiovision 2030 study. J. Clin. Med. (2019). [DOI] [PMC free article] [PubMed]
- 7.Fung TT, et al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circulation research. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 9.Kjeldsen SE. Hypertension and cardiovascular risk: General aspects. Pharmacological research. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Avelar E, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49:34–39. doi: 10.1161/01.HYP.0000251711.92482.14. [DOI] [PubMed] [Google Scholar]
- 11.Rodilla E, et al. Impact of abdominal obesity and ambulatory blood pressure in the diagnosis of left ventricular hypertrophy in never treated hypertensives. Medicina clinica. 2014;142:235–242. doi: 10.1016/j.medcli.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Agodi Antonella, Maugeri Andrea, Kunzova Sarka, Sochor Ondrej, Bauerova Hana, Kiacova Nikola, Barchitta Martina, Vinciguerra Manlio. Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients. 2018;10(7):898. doi: 10.3390/nu10070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maugeri A., Kunzova S., Medina-Inojosa J.R., Agodi A., Barchitta M., Homolka M., Kiacova N., Bauerova H., Sochor O., Lopez-Jimenez F., Vinciguerra M. Association between eating time interval and frequency with ideal cardiovascular health: Results from a random sample Czech urban population. Nutrition, Metabolism and Cardiovascular Diseases. 2018;28(8):847–855. doi: 10.1016/j.numecd.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Hruskova Jana, Maugeri Andrea, Podroužková Helena, Štípalová Tatiana, Jakubík Juraj, Barchitta Martina, Medina-Inojosa Jose, Homolka Martin, Agodi Antonella, Kunzova Sarka, Sochor Ondrej, Lopez-Jimenez Francisco, Vinciguerra Manlio. Association of Cardiovascular Health with Epicardial Adipose Tissue and Intima Media Thickness: The Kardiovize Study. Journal of Clinical Medicine. 2018;7(5):113. doi: 10.3390/jcm7050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Movsisyan Narine K, Vinciguerra Manlio, Lopez-Jimenez Francisco, Kunzová Šárka, Homolka Martin, Jaresova Jana, Cífková Renata, Sochor Ondřej. Kardiovize Brno 2030, a prospective cardiovascular health study in Central Europe: Methods, baseline findings and future directions. European Journal of Preventive Cardiology. 2017;25(1):54–64. doi: 10.1177/2047487317726623. [DOI] [PubMed] [Google Scholar]
- 16.Maugeri Andrea, Medina-Inojosa Jose, Kunzova Sarka, Agodi Antonella, Barchitta Martina, Sochor Ondrej, Lopez-Jimenez Francisco, Geda Yonas, Vinciguerra Manlio. Sleep Duration and Excessive Daytime Sleepiness Are Associated with Obesity Independent of Diet and Physical Activity. Nutrients. 2018;10(9):1219. doi: 10.3390/nu10091219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitan EB, et al. Mediterranean diet score and left ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2016;104:595–602. doi: 10.3945/ajcn.115.128579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardener H, et al. A Mediterranean-style diet and left ventricular mass (from the Northern Manhattan Study) Am J Cardiol. 2015;115:510–514. doi: 10.1016/j.amjcard.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen HT, et al. DASH eating pattern is associated with favorable left ventricular function in the multi-ethnic study of atherosclerosis. J Am Coll Nutr. 2012;31:401–407. doi: 10.1080/07315724.2012.10720466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, et al. Dietary pattern, the metabolic syndrome, and left ventricular mass and systolic function: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:362–368. doi: 10.3945/ajcn.2009.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waijers PM, Feskens EJ, Ocké MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97:219–231. doi: 10.1017/S0007114507250421. [DOI] [PubMed] [Google Scholar]
- 22.Newby P, Tucker K. Empirically Derived Eating Patterns Using Factor or Cluster Analysis: A Review. Nutr Rev. 2004;62(5):177–203. doi: 10.1111/j.1753-4887.2004.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Maugeri A, et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. 2019;131:274–281. doi: 10.1016/j.freeradbiomed.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser854, 1-452 (1995). [PubMed]
- 26.WHO. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. (World Health Organization, Geneva, 8-11 December, 2008).
- 27.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Zoghbi WA, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Nagueh SF, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Brussaard JH, et al. A European food consumption survey method–conclusions and recommendations. Eur J Clin Nutr. 2002;56(Suppl 2):S89–94. doi: 10.1038/sj.ejcn.1601432. [DOI] [PubMed] [Google Scholar]
- 32.Ruprich, J., Dofkova, M., Rehurkova, I., Slamenikova, E. & Resova, D. Individual food consumption - the national study SISP04. CHFCH NIPH in Prague, http://www.chpr.szu.cz/spotrebapotravin.htm (2006).
- 33.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 34.Barchitta Martina, Maugeri Andrea, Quattrocchi Annalisa, Agrifoglio Ottavia, Scalisi Aurora, Agodi Antonella. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients. 2018;10(4):469. doi: 10.3390/nu10040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barchitta Martina, Maugeri Andrea, Magnano San Lio Roberta, Favara Giuliana, La Rosa Maria Clara, La Mastra Claudia, Quattrocchi Annalisa, Agodi Antonella. Dietary Patterns are Associated with Leukocyte LINE-1 Methylation in Women: A Cross-Sectional Study in Southern Italy. Nutrients. 2019;11(8):1843. doi: 10.3390/nu11081843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maugeri Andrea, Barchitta Martina, Favara Giuliana, La Rosa Maria Clara, La Mastra Claudia, Magnano San Lio Roberta, Agodi Antonella. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients. 2019;11(6):1308. doi: 10.3390/nu11061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pala V, et al. Associations between dietary pattern and lifestyle, anthropometry and other health indicators in the elderly participants of the EPIC-Italy cohort. Nutr Metab Cardiovasc Dis. 2006;16:186–201. doi: 10.1016/j.numecd.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235–2240. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 39.Bluemke DA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodiwiss AJ, Norton GR. Obesity and left ventricular hypertrophy: the hypertension connection. Current hypertension reports. 2015;17:539. doi: 10.1007/s11906-015-0539-z. [DOI] [PubMed] [Google Scholar]
- 41.Alessandro Gondoni L, Titon AM, Montano M, Nibbio F, Bertone G. The importance of a shared definition of left ventricular hypertrophy: The case of obese women. Int J Cardiol. 2017;227:404–406. doi: 10.1016/j.ijcard.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Estruch R, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 43.Psaltopoulou T, et al. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 44.Buscemi S, et al. Association of dietary patterns with insulin resistance and clinically silent carotid atherosclerosis in apparently healthy people. Eur J Clin Nutr. 2013;67:1284–1290. doi: 10.1038/ejcn.2013.172. [DOI] [PubMed] [Google Scholar]
- 45.Lara KM, et al. Dietary Patterns and Incident Heart Failure in USAdults Without Known Coronary Disease. J Am Coll Cardiol. 2019;73:2036–2045. doi: 10.1016/j.jacc.2019.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heidemann C, Scheidt-Nave C, Richter A, Mensink GB. Dietary patterns are associated with cardiometabolic risk factors in a representative study population of German adults. Br J Nutr. 2011;106:1253–1262. doi: 10.1017/S0007114511001504. [DOI] [PubMed] [Google Scholar]
- 47.Barbaresko J, et al. Comparison of two exploratory dietary patterns in association with the metabolic syndrome in a Northern German population. Br J Nutr. 2014;112:1364–1372. doi: 10.1017/S0007114514002098. [DOI] [PubMed] [Google Scholar]
- 48.Suliga Edyta, Kozieł Dorota, Cieśla Elżbieta, Rębak Dorota, Głuszek Stanisław. Dietary Patterns in Relation to Metabolic Syndrome among Adults in Poland: A Cross-Sectional Study. Nutrients. 2017;9(12):1366. doi: 10.3390/nu9121366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner A, et al. Sedentary behaviour, physical activity and dietary patterns are independently associated with the metabolic syndrome. Diabetes Metab. 2012;38:428–435. doi: 10.1016/j.diabet.2012.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.