Abstract
Persons with severe persistent mental illness (SPMI)—which includes individuals with schizophrenia, bipolar disorder, and mood disorders such as major depression—are at high risk for poor health outcomes and premature death. Persons with SPMI are largely absent from research evaluating innovative health care models due to recruitment and retention barriers. This paper presents the protocol for a randomized control trial testing a reverse colocated integrated care model in an SPMI population receiving care at a mental health clinic at the U.S.-Mexico border. The study employs a randomized control trial design to determine whether reverse colocated integrated care improves physical and mental health of persons with SPMI. Participants will be randomized to receive the integrated primary care intervention or usual care (behavioral health only). All study participants will complete baseline, 6-, and 12-month assessments. Study outcomes included blood pressure, HbA1c, cholesterol, body mass index, depression, and adult functioning. Despite challenges in recruiting and retaining SPMI patients, co-locating primary care services within a local mental health authority has the potential to improve health and reduce health disparities experienced by persons with SPMI. The study will determine the impacts of this colocated integrated care model among SPMI patients in a socio-economically disadvantaged region.
Clinical Trials.gov Identifier: NCT03881657.
Keywords: Randomized controlled trial, Severe persistent mental illness, Primary care, Integrated health care systems, Health disparities
1. Introduction
Persons with serious and persistent mental illness (SPMI)—including schizophrenia, psychotic disorders, and mood disorders such as major depression and bipolar disorders—are among the most vulnerable populations in the world and experience elevated morbidity and mortality. Individuals with SPMI have a higher risk of premature death compared to persons without [[1], [2], [3]], dying on average one to ten years earlier than persons diagnosed with a non-SPMI mental illness [4]. Disparities in mortality rates by SPMI are attributed to higher prevalence of preventable conditions among this population, including cardiovascular disease [5], diabetes and its complications [6], respiratory disease including pneumonia and influenza [7], and infectious disease such as HIV/AIDS [8,9]. This population requires specialized medical and behavioral health care; however, persons with SPMI face various barriers in accessing care [10,11] including stigma [12,13] and racial/ethnic disparities in available medical care [14]. This results in delays in timely and effective delivery of medical services for persons with SPMI.
Prior research has shown that colocation and integration of primary care services within behavioral health settings improves access to routine primary care for persons with SPMI given their primary health care connection is through public-sector mental health programming [15]. Colocation also reduces the cost and inconvenience of traveling to multiple locations for behavioral and physical healthcare [[15], [16], [17]]. However, evidence is lacking on the effectiveness of colocation-integrated care approaches in improving health outcomes in an SPMI population, particularly in regions with known socio-economic disadvantages. Almost no empirical evidence is available to support the development of innovative interventions that might address the health conditions of this vulnerable population. Further challenging developments in this area of research is the difficulty of recruiting and retaining persons with severe mental illness into research studies [18], requiring diverse strategies at multiple levels [19]. Because of this gap in the literature, little information is available to support the development of interventions that might address the physical health comorbidities within this population.
As a subgrantee of the Sí Texas (Social Innovation for a Healthy South Texas) project funded by Social Innovation Fund grantee Methodist Healthcare Ministries, Tropical Texas Behavioral Health (TTBH) began implementing a reverse colocated integrated health care program model in November 2015. We hypothesized that delivery of integrated primary care services to adult clients with SPMI and co-morbid chronic illness from a clinic colocated within the outpatient behavioral health clinic where clients receive community-based behavioral health services would lead to improved physical and mental health. We sought to assess the impact of a reverse colocated model of integrated care on chronic disease (hypertension, diabetes, obesity, and hypercholesterolemia) severity, depression, and adult functioning and quality of life for individuals with SPMI. In this protocol paper, we present the rationale, study design, and methods of TTBH's colocated integrated care program.
2. Methods
2.1. Formative research
Tropical Texas Behavioral Health (TTBH) is the local mental health authority for the more than 1.2 million residents of Hidalgo, Cameron, and Willacy counties in Texas, a 3,100-square mile area along the Gulf-coast and South Texas border with Mexico. TTBH operates four outpatient clinics located in Edinburg, Harlingen, Brownsville, and Weslaco. Nearly all residents in this region are of Hispanic ethnicity (95%) [20].
TTBH clinical staff observed that a large number of their patients who were exclusively persons with SPMI self-reported not having a consistent or any source of primary care. Concerned about the high prevalence of physical comorbidities in this patient population, TTBH developed a working theory that their patients would feel more comfortable receiving primary care in a behavioral health setting than in a traditional primary care setting such as a federally qualified health center. To address this unmet need, TTBH staff began developing an intervention model based on the Wagner model for effective chronic illness care. The Wagner model features an organized delivery system linked with complementary community resources, sustained by productive interactions between multidisciplinary care teams and “activated” or educated patients and families [21].
Prior to designing the study, TTBH piloted their intervention model at two non-study sites (Edinburg and Harlingen clinics) using a quality improvement approach. Based on the feasibility, acceptability, and preliminary effectiveness of their pilot, TTBH concluded that the model would be feasible to implement system-wide. TTBH received grant funding from Methodist Healthcare Ministries of South Texas (MHM) through its Sí Texas program to rigorous evaluate this approach to integrated care and enable scaling of their model to their Brownsville clinic. TTBH built a new physical space to house primary care services at their Brownsville location. Health Resources in Action (HRiA) was hired by MHM as an external evaluator to work with TTBH in developing and implementing an evaluation study of its Sí Texas project at Brownsville.
2.2. Intervention program logic model
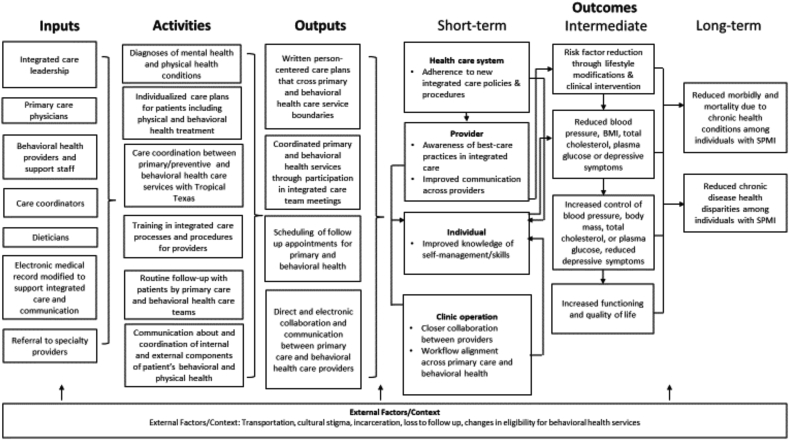
TTBH developed a program logic model for their Brownsville integrated care program based on the pilot test of their intervention (Fig. 1).
Fig. 1.
Reverse colocated integrated care program logic model.
3. Study design
A requirement of the Sí Texas grant included selecting a rigorous study design that would move the level of evidence ahead as defined by the Corporation for National and Community Service (CNCS). These design choices were limited to quasi-experimental (QE) and randomized controlled trial (RCT) designs.
HRiA interviewed key TTBH staff to evaluate the feasibility of implementing QE or RCT study designs at their Brownsville clinic location. Considerations included the following issues: TTBH's capacity to implement randomization, patient receptivity to a randomized approach and ability to provide informed consent given their SPMI diagnoses, and TTBH's capacity to prevent contamination. TTBH ultimately selected an RCT design because its organization had experience with sophisticated clinic workflows. Based on this operational expertise, it was thought feasible for TTBH to randomly assign patients into intervention and control groups and maintain separate workflow with minimal contamination. TTBH clinical staff agreed that randomization of SPMI individuals would be possible to achieve given precedents in the literature demonstrating successful enrollment [22].
TTBH's theory of change was that co-locating services would make patients more compliant with primary care treatment and improve their physical and mental health. Receiving care from an institution perceived as safe and trusted by patients with mental illness was thought to be a key factor in facilitating compliance with primary care plans. TTBH chose blood pressure as the primary outcome because of prior research identifying hypertension as susceptible to improvement in integrated care settings [23,24]. Other physical outcomes such as body mass index and total cholesterol as well as mental health measures (e.g., depression) were included as exploratory outcomes because there was limited literature on the effects of reverse colocation approaches on these outcomes.
3.1. Trial overview
The study was designed as a RCT of reverse colocated integrated care program delivered in a mental health clinic setting and conducted over a 12-month period. The intervention includes both primary care and behavioral health care or the usual care control group (only behavioral health care) provided within a behavioral health clinic, with evaluation focusing on blood pressure (primary outcome) and other physical or mental outcomes including HbA1c, body mass index (BMI), depression, total cholesterol, and quality of life. The study includes an implementation evaluation to assess early implementation (at the mid-point) and post-intervention implementation (summative) and to enhance impact evaluation. TTBH's research protocol was approved by the New England Independent Review Board (Protocol ID#120160441).
3.2. Study groups
3.2.1. Control group
Patients randomized to the usual care group (control group) will be referred to the nearest federally qualified health center (FQHC) or county health department for their primary care needs, as was the established policy of TTBH prior to the study. Each control group participant will be assigned to a behavioral health case manager that will keep in touch with them and refer them to external primary care services as needed. Over the study period, the control group patients will be requested to visit the clinic to provide baseline, 6-, and 12-month outcome measures.
3.3. Intervention group
Patients randomized to the study intervention group will receive the integrated primary care program. If the patient has diabetes, heart disease, hypertension, obesity, or hypercholesterolemia, the program provider will give appropriate treatment and refer patients to see the care coordinator and chronic care nurse/registered dietician as appropriate within seven days of the initial program visit. A care coordinator will then make a follow-up appointment for the patient depending on the participant's care plan. For patients assigned to the intervention group, this process will be repeated at every visit.
TTBH's model of integrated behavioral health (IBH) will be delivered by a collaborative team of health care providers including a primary care physician, licensed vocational nurses, a registered dietician, a chronic care nurse, and medical support staff, and coordinated by care coordinators at TTBH's Brownsville clinic. Program participants will be referred to specialists in the community as needed. TTBH's electronic medical record system is integrated across behavioral and primary care services. Primary care and behavioral health teams will meet periodically to discuss cases, share notes through the medical record, and refer patients as needed to primary care from behavioral health (and vice versa). Similar to control patients, all intervention patients will be asked to come into the TTBH clinic for reassessment of study measures at 6 and 12 months.
3.4. Inclusion and exclusion criteria
Eligibility criteria are presented in Table 1.
Table 1.
Inclusion and exclusion criteria for the randomized control trial of a reverse colocated integrated care model.
| Inclusion criteria | Justification |
|---|---|
| Adults 18 and older | The intervention was designed for adults. While the study clinic had pediatric programs, the model has not been piloted in a pediatric population and would not be appropriate for study. |
| Residence in Cameron, Hidalgo, or Willacy Counties | These counties reflect a residential geography representative of the study clinic site's population. |
| Documented diagnosis by a licensed behavioral health care provider of at least one SPMI | Including only persons with clinical evidence of SPMI (schizophrenia, bipolar disorder, or major depression) ensures appropriate targeting of the designed intervention and that any effect observed is generalizable to an SPMI population. |
| Eligible to receive services at the study site | The study clinic site legally is only able to provide treatment to persons eligible to receive services from a local mental health authority. |
| Diagnosis of one or more of the following conditions: hypertension (blood pressure of 140/90 mmHg or higher), obesity (body mass index of 30.0 or higher), poorly controlled diabetes (HbA1c over 8.5%), or hypercholesterolemia (Total cholesterol level above 200). | The intervention was designed to improve chronic health conditions. |
| Capacity to provide informed consent as an individual or with caregiver consent | Informed consent was genuine and allows the study to collect valid data. Study personnel were trained not to obtain consent from potential participants if they appeared sedated or too emotionally distraught to give informed consent at the time of intake. As a local mental health authority, TTBH has established protocols to address patients in distress and/or suicidal. |
| No current source of primary care at the time of enrollment (per patient self-report) | Primary care was the intervention. |
| Exclusion criteria | |
| Suicidal at time of enrollment | There was a safety risk to the participant. |
| Pregnant at time of enrollment or during the study | TTBH's primary care model did not include services for pregnant women. |
4. Study operations and assessment
4.1. Recruitment and enrollment
TTBH will recruit existing behavioral health patients to participate in the study in several ways. First, TTBH will use electronic medical record (EMR) data to generate a list of patients who meet basic inclusion criteria (i.e., SPMI diagnosis, eligible to receive services, and residence). TTBH will examine this list against patients scheduled for behavioral health appointments at the Brownsville clinic, and send study recruitment letters by mail and approach them in-person on the day of their appointment to ascertain interest in learning more about the study. Second, TTBH will inform their behavioral health staff about the study and receive referrals to study staff. Finally, TTBH will print posters and brochures and made them available and prominent in their clinic waiting rooms. All materials were approved by IRB and available in English or Spanish.
When a patient potentially eligible for the study enters the clinic and expresses interest in participation, TTBH will verify eligibility for participation. This will be done through eligibility screening and assessment by a behavioral health care assistant who will ask the patient a series of questions.
If the prospective study participant meets study inclusion criteria, the patient (and if appropriate, his or her caregiver) will be asked to participate in the informed consent process. The process will be a modification of the Dunn et al. [25] informed consent process for schizophrenic individuals. Specifically, study personnel will read the consent form aloud in either English or Spanish to the prospective participants and assess their understanding of what the research participation entails, their rights as participants, and their chances of being randomized into the treatment group. Study personnel will emphasize the voluntary commitment required for participation, and that they do not know whether participating in the new program is any better than the standard of care. Caution will be exercised not to obtain consent from potential participants if they are sedated or too emotionally distraught to give informed consent at the time of intake. Potential participants who have a legal guardian or custodian will require consent by their guardian. In those cases, the guardian will be present during the informed consent process. A guardian will not be allowed to coerce a patient into agreeing to any condition or activity of the study. Study participants will be informed they could withdraw from the study or any aspect of the study at any time.
The informed consent process includes affirming participation in the randomization process, volunteering to take all baseline and follow-up surveys, volunteering to have vitals (e.g., blood pressure, height, weight) and blood work (to assess HbA1c and total cholesterol) taken during the study and affirming that they were part of a research study. Participants who do not consent to the study or who are unable to consent to the study will be referred to TTBH usual care behavioral health services. TTBH will ask study participants about their understanding of study conditions (e.g., voluntary, being able to withdraw, etc.) at each assessment following the Dunn protocol [25]. Enrollment will be conducted on a rolling basis between November 2015 and June 2016. The informed consent process will affirm participation in the randomization process, voluntary completion of all baseline and follow-up surveys, voluntary vitals (e.g., blood pressure, height, weight) and blood work (to assess HbA1c and total cholesterol) taken during the study, and affirming that they were part of a research study. Enrollment will be conducted on a rolling basis between November 2015 and June 2016.
We anticipated that SPMI patients may feel a range of negative emotions if not assigned to the intervention group even with explanation of random assignment. To address this particular challenge, an approach used by Gibbons et al. (2010), in which the usual care comparison group is assigned to a care coordinator to help control group participants get connected with care in the community, will be adopted [26]. Control group participants will have access to their care coordinator throughout the study.
4.2. Randomization
Randomization will be conducted at the individual patient level, and participants will be randomized 1:1 into either the control or intervention groups. After providing written consent, the study participant will be assigned either the control or intervention group by selecting from among envelopes containing numbers randomly generated before the study begins. Each number corresponds to either the intervention or control group.
4.3. Measures
Quantitative measures employed aspects of the REACH evaluation framework and are presented in Table 2 [27].
Table 2.
Planned measures to be collected during the randomized controlled trial of the reverse colocated integrated care model.
| Measure | Description | Potential confounding or effect modifying measures | Months | ||
|---|---|---|---|---|---|
| Demographics | Age, gender, race, ethnicity, education | All | 0 | 6 | 12 |
| SPMI diagnosis | Assessed by behavioral health clinician | Type of SPMIa | |||
| Blood pressure | Manual measurement | At baseline | X | X | X |
| HbA1c | Blood test | At baseline | X | X | X |
| Serum lipid profile | Blood test | At baseline | X | X | X |
| Body Mass Index (BMI) | Height and weight; height only measured at baseline | At baseline | X | X | X |
| Depressive symptoms | PHQ-9 administered by a clinician | At baseline | X | X | X |
| Life functioning | ANSA administered by a clinician | At baseline | X | X | X |
| Dose of intervention | Percent of patients who completed at least one primary care visit and one dietician visit; percent of participants receiving a primary care visit/mean number of visits | At study completion | X | ||
Schizophrenia, bipolar disorder, or major depression.
4.3.1. Outcome measures
The impact measures assessed for the TTBH program include blood pressure, HbA1c, BMI, total cholesterol, depression, and quality of life. Patients with a blood pressure greater than or equal to 140/90 mmHg will be considered hypertensive. Blood tests will be conducted to measure HbA1c and levels of cholesterol. Patients with an HbA1c greater than or equal to 8.5% will be considered to have poorly controlled diabetes. Patients with a BMI greater than or equal to 30 will be considered obese. Patients with a total cholesterol of 200 mg/dL will be categorized as having high cholesterol. Using the PHQ-9 assessment tool [28], patients’ depressive symptoms will be categorized into severity categories as follows: 0–4 minimal, 5–9 mild, 10–14 moderate, 15–19 moderately severe, and 20–27 severe. The ANSA assessment tool, specifically the 14 constructs assessing life functioning, will be used to measure functioning and quality of life [29]. These constructs will be scored as either no evidence of problem (0), history/mild (1), moderate (2), or severe (3). For this study, a novel functioning score was created by the evaluation team as a way of describing the severity of this domain. To create the overall ANSA functioning score, the individual construct scores will be re-categorized as either no evidence or problem/history/mild (0) and moderate/severe (1). Next, the new domain scores (either 0 or 1) will be summed and a functioning score value generated for each participant. The total functioning ANSA score could range from 0 to 14, with 0 meaning the participant had no evidence of a problem for any of the domain constructs and 14 meaning the participant had moderate or severe problems for all the domain constructs.
4.3.2. Covariates to assess confounding and effect modification
Certain socio-demographic factors and morbidity conditions among patients likely influence physical and mental health outcomes at 6- and 12-months. These factors including age, gender, race/ethnicity, education, language, general health status, physical and mental health conditions will be examined to ascertain associations with assigned treatment status. Categorical age is operationally defined by the following categories: 18-24-year-olds, 25-34-year-olds, 35-44-year-olds, 45-54-year-olds, 55-64-year-olds, and those who are 65 years or older. Race was originally considered as a potential covariate; however, an ethnicity variable will be used in the final models, dichotomized into “Hispanic” and “non-Hispanic” categories due to field conditions and how the data collection procedures. Additional variables will be included in all models, including “education” and “primary language spoken.” Education will be dichotomized into “less than high school” and “high school or more.” Primary language will be treated as a categorical variable grouped as “English-speaking” or “Spanish-speaking.” To assess whether the IBH treatment may be particularly effective among certain patient subgroups, we will examine whether baseline comorbidity (hypertension, obesity, hypercholesterolemia, diabetes, and major depression) and age are potential effect modifiers of the treatment-outcome relationship. Age will be dichotomized (40 years and older/under 40 years old) when modelled as an effect modifier based on the average age of the overall study population of 40.9 years. Type of qualifying SPMI condition (schizophrenia, bipolar disorder, or major depression) and dose of the intervention (number of primary care and dietician visits received) will also be considered a potential effect modifier. Covariates will be included in all outcome analyses.
Other demographic factors such as marital status, insurance, income, employment as well as tobacco and other substance use information will not be systematically collected, nor will objective information about prescription medication utilization be available. The evaluation of the treatment effect may be susceptible to the residual confounding, in part due to the lack of some important patient-level factors.
4.4. Data collection
The TTBH program will use valid and reliable measures and instruments to answer the evaluation questions. The intervention and control groups will have all measures collected at baseline, 6 and 12 months, with consistent data collection within the two groups. Clinical data taken during the vitalization process (e.g., blood pressure, height, weight) will be entered by a nurse directly into the patient's health record. Blood tests for HbA1c and total cholesterol will be done on-site, and results will be input to the EMR by lab technicians. The ANSA and PHQ-9 questionnaires are integrated within the EMR via a data entry form for each questionnaire and are available in both English and Spanish. The clinician conducting the patient interview for ANSA and PHQ-9 will enter participant responses directly into the EMR data entry form, designed with built in validation checks for out of range answers.
4.5. Strategies to minimize attrition
Three main strategies will be employed to minimize participant attrition over the course of the study. The first is to collect thorough and detailed contact information from all participants during the enrollment process. The next is the utilization of care managers who will maintain communication with participants using the provided contact information while leveraging their relationships with their patients to remind them about their upcoming appointments. Care managers will also be able to schedule study follow-ups on the same day as scheduled primary and behavioral health care appointments to limit the number of trips to the clinic for participants. Lastly, TTBH will offered financial incentives to study participants who complete study assessments: study participants will receive a Walmart or HEB gift card for completing an assessment with a value of $10 for a baseline assessment, $20 for a 6-month assessment, and $30 for a 12-month assessment. In addition, the study team at TTBH will hold regular meetings and discussions aimed to improve their study processes to improve participant retention.
4.6. Implementation evaluation
An implementation evaluation was developed by HRiA to assess the fidelity of the intervention model. HRiA evaluators will conduct interviews with program staff at implementation mid-point of implementation and close of study. Focus groups will be conducted with intervention and control group participants after the study has ended. Across the two time points, a total of 30 staff members will be interviewed, and approximately 50 study participants will take part in focus groups. Qualitative data will be collected using interview and focus group guides developed using the RE-AIM evaluation framework [27]. Questions about recruitment and attrition will be included within the mid-point implementation evaluation to obtain formative information about the effectiveness of the study recruitment strategy and support quality improvement. Quantitative data will also be collected from the clinic's EMR system to assess the implementation of TTBH's intervention. These data will include information on intervention and control participants' behavioral health and primary care visits such as number of completed and missed visits.
4.7. Sample size estimation
Blood pressure is the primary outcome for the study, and hypertension has the highest prevalence rate (22%) among the major physical health outcomes among patients with severe persistent mental illness (SPMI) at the TTBH Brownsville clinic. The sample size calculation was based on the continuous measure of hypertension as represented by systolic blood pressure (SBP). Prior research on integrated care and hypertension informed our sample size calculation. Scharf et al. (2014) [24] evaluated a SAMHSA primary and behavioral health care integration (PBHCI) model (a model with core components similar to those of the Wagner model (1998) upon which TTBH's intervention is based), and found reductions in SBP between persons served at all participating PBHCI and control site pairs: -4.95 mm/Hg for site pair 1, -2.43 mm/Hg for site pair 2, and -0.63 mm/Hg for site pair 3. A randomized controlled trial testing the effects of integrated hypertension and depression pharmacotherapy in a sample of older patients, identified a substantial treatment effect on SBP of 14 mmHg lower [23]. Based on these estimates, we applied a relatively conservative estimate of −5 mm/Hg for the treatment effect with a series of sensitivity analyses to assess the range of sample sizes needed. The formula for the sample size calculation was based on Diggle et al., 2002 [30] comparing time-averaged differences for continuous outcomes in repeated measurement studies.
The study was powered to account for a 20% potential loss to follow-up of participants during the 12-month follow-up period. With an estimated effective sample size of 145 per study arm, there would be 80% power to detect a reduction of 5 mmHg from a baseline measure of 135 mmHg, with a two-sided alpha of 0.05 and correlation of 0.15. Assuming 20% potential loss to follow-up of participants during a 12-month period, 182 participants per study group (364 total sample size) were targeted for recruitment. The attrition threshold was selected based on TTBH's own experience recruiting SPMI patients for new programs across their facilities (ranging from 15% to 30%).
Consistent with literature on evaluating brief behavioral health interventions [31,32] and collaborative care models for patients with one or more physical and/or mental health illnesses [33], the analytic sample will include all enrolled participants rather than a subset of participants who reach a certain clinical threshold (e.g., 140/90 mmHg or above).
5. Statistical analysis
The unit of analysis is at the individual patient level. We will conduct descriptive analyses of all variables. For continuous variables we will examine means, standard deviations, and ranges, and for categorical variables, we will report proportions. Descriptive statistics will be examined for the intervention and comparison group. These statistics will include patients’ sociodemographic and other key covariates such as baseline chronic disease status. Because random assignment is employed, baseline difference by group status will be examined and if the baseline impact measures are determined to be significantly different by treatment status, baseline differences in comparative analysis on follow-up impact measures will be included as covariates in multivariate models. Because patients may receive varying level (e.g., doses) of intervention, we will also adjust models for the number of and types of services and level of participation in treatment services.
To determine if the IBH model achieves greater decrease in outcome variables of interest (e.g., blood pressure) at 12-month follow-up compared to control patients, we will compare groups on the outcome variable and note differences that are statistically significant at the p < 0.05. Analyses will use an intent-to-treat approach.
We will employ generalized regression analysis following a modeling sequence from bivariate models to multiple regression models adjusting for key substantive covariates (e.g., age, gender) and other covariates found to be nonequivalent between the two groups at baseline. For each outcome measure of interest, we will conduct endpoint analysis where each outcome at 12-month is regressed on treatment status, with key covariates including outcome measured at the baseline. This endpoint analysis approach is a conventional approach to analyze clinical trial data collected from individuals with both baseline data and end-point data of primary interest [34]. We will report regression coefficients (e.g., beta) with p-values to determinate the relative magnitude of the adjusted association for each independent variable. Independent variables with high correlations may result in collinearity. To address collinearity, we will review correlations among these independent variables and assess its potential impact on the standard error estimates for the regression coefficients in the model by examining the variance inflation factor. We will check the distributional assumption of the outcomes and the residuals of the fitted models to ascertain that assumptions for the statistical models are satisfied. Additionally, because multiple follow-up impact measures form individual trajectories, we will also employ longitudinal analysis assessing whether the impact measure trajectories differ by intervention status [35].
For missing data due to dropouts, we will assess whether missing data are correlated with patient characteristics. While data may not be missing completely at random, it may be reasonable to assume missing at random conditional on covariates [36]. Multiple imputation will be applied to assessing the treatment effect accounting for patterns of missing data [37]. Similar analytic procedures will be used for secondary outcomes. We will construct treatment by covariates interactions to assess the effect of potential moderators and further conduct stratified analyses by levels of these moderators (e.g., age, patient physical and mental health status at baseline). We will use SAS version 9.4 (SAS Institute Inc., Cary, NC). Effect sizes (e.g., Cohen's d) will be calculated for all outcomes [38].
6. Research capacity building
One of the challenges of implementing an RCT approach is the prospective learning curve for staff at TTBH to implement study procedures with fidelity. TTBH had limited experience in implementing a research study protocol, and staff needed to be hired and/or trained to implement recruitment, informed consent, randomization, and data collection procedures. TTBH developed and implemented an orientation for existing and new hires. This training included human subjects training through the NIH online certification program and on-site training on implementation of IRB-approved procedures. TTBH will periodically conduct refresher trainings as new staff came on board to ensure consistent study protocol implementation. TTBH study leadership will participate in quarterly, in-person Evaluation Learning Collaborative trainings sponsored by MHM as part of the Sí Texas project grant. These trainings will include troubleshooting common implementation challenges (e.g., recruitment) and peer-to-peer consultation from other Sí Texas subgrantees that are also implementing integrated care programs.
7. Discussion
Behavioral health care leaders need innovative approaches to address the high levels of unmet physical and mental health needs of persons with SPMI. The gap in the existing literature around the effectiveness of interventions to improve chronic diseases in the SPMI population provides little direction to behavioral health care delivery systems that handle the impact of unaddressed medical needs of their patients within the safety net of a behavioral health setting. TTBH is one such system that creatively adapted and piloted an integrated care approach developed for the general population to a vulnerable population of persons with SPMI receiving care at their mental health clinics. Designing a RCT of persons with SPMI residing in a U.S.-Mexico border region in Texas and testing an adaptation of a well-established integrated care model is an innovative approach to addressing the complex and high-cost medical needs of this SPMI population.
The colocation approach has strong face validity, but can such a trial demonstrate improvements in physical health for a population that is hard to reach, retain, and to adhere to a care plan? A first step towards building this evidence base includes assessment of recruitment and retention of study participants with SPMI in a randomized control trial, particularly by an organization with limited research-related experience. The strategy of having incremental incentives may play a role in minimizing study attrition. It should be noted, however, that incentive approaches as planned within this trial may not be feasible in all settings (e.g., funding constraints). Evidence from the process evaluation study participant focus groups may also yield important qualitative information about the role of TTBH's established patient relationships in promoting retention.
The process evaluation will also gather important information about intervention fidelity and the execution of the trial. Since this trial is being conducted in a setting with a challenging study population situated in a region with limited socioeconomic resources, the process evaluation has the potential to produce valuable lessons about how to conduct similar studies within such settings. TTBH program staff implementation experiences will also reveal potential facilitators and barriers to adopting and implementing integrated care interventions that have applicability in similar, resource-constrained contexts.
In summary, TTBH's randomized control trial of reverse colocation integrated care will be implemented among SPMI patients from a socioeconomically disadvantaged region. If the trial demonstrates improvement in any of the outcome measures, it will not only advance our understanding of valuable enhancement of conducting trials involving persons with SPMI but will be major progress towards developing effective interventions aimed at improving the physical and mental health of SPMI populations. Additional research will be required to understand the role of social determinants of health as related to health service utilization and economic implications of promoting reverse colocation integrated care strategies for SPMI populations served by health care systems operating in socioeconomically disadvantaged regions.
Funding source
This material is based upon work supported by the Corporation for National and Community Service under Social Innovation Fund Grant Number 14SIHTX001. Opinions or points of view expressed in this document are those of the authors and do not necessarily reflect the official position of, or a position that is endorsed by, the Corporation for National and Community Service.
Declaration of competing interest
The authors declare they have no conflict of interest to report.
Acknowledgements
The authors wish to acknowledge the contributions of several individuals. We thank Lisa Wolff, Jim Banks, Michelle Brodesky, and Stephanie Tapia for their support in the study design phase; Erika Gaitan for supporting the implementation of the evaluation study; and Elizabeth Showalter for cleaning and analyzing TTBH's study data. We would also like to thank Alison El Ayadi for editing this manuscript.
Contributor Information
Karen Sautter Errichetti, Email: kerrichetti@bridgew.edu.
M. Marlen Ramirez, Email: maramirez@ttbh.org.
Amy Flynn, Email: aflynn@hria.org.
Ziming Xuan, Email: zxuan@bu.edu.
References
- 1.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br. J. Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. http://www.ncbi.nlm.nih.gov/pubmed/9519087 [DOI] [PubMed] [Google Scholar]
- 2.Saha S., Chant D., McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch. Gen. Psychiatr. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 3.Harris E.C., Barraclough B. Excess mortality of mental disorder. Br. J. Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. http://www.ncbi.nlm.nih.gov/pubmed/9850203 [DOI] [PubMed] [Google Scholar]
- 4.DE Hert M., Correll C.U., Bobes J., Cetkovich-Bakmas M., Cohen D., Asai I., Detraux J., Gautam S., Möller H.-J., Ndetei D.M., Newcomer J.W., Uwakwe R., Leucht S. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. http://www.ncbi.nlm.nih.gov/pubmed/21379357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott K.M., de Jonge P., Alonso J., Viana M.C., Liu Z., O'Neill S., Aguilar-Gaxiola S., Bruffaerts R., Caldas-de-Almeida J.M., Stein D.J., de Girolamo G., Florescu S.E., Hu C., Taib N.I., Lépine J.-P., Levinson D., Matschinger H., Medina-Mora M.E., Piazza M., Posada-Villa J.A., Uda H., Wojtyniak B.J., Lim C.C.W., Kessler R.C. Associations between DSM-IV mental disorders and subsequent heart disease onset: beyond depression. Int. J. Cardiol. 2013;168:5293–5299. doi: 10.1016/j.ijcard.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chwastiak L.A., Davydow D.S., McKibbin C.L., Schur E., Burley M., McDonell M.G., Roll J., Daratha K.B. The effect of serious mental illness on the risk of rehospitalization among patients with diabetes. Psychosomatics. 2014;55:134–143. doi: 10.1016/j.psym.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinschmidt K.M., Ingram M., Schachter K., Sabo S., Verdugo L., Carvajal S. The impact of integrating community advocacy into community health worker roles on health-focused organizations and community health workers in Southern Arizona. J. Ambul. Care Manag. 2015;38:244–253. doi: 10.1097/JAC.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 8.Lagios K., Deane F.P. Severe mental illness is a new risk marker for blood-borne viruses and sexually transmitted infections. Aust. N. Z. J. Public Health. 2007;31:562–566. doi: 10.1111/j.1753-6405.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes E., Bassi S., Gilbody S., Bland M., Martin F. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3:40–48. doi: 10.1016/S2215-0366(15)00357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohn R., Saxena S., Levav I., Saraceno B. The treatment gap in mental health care. Bull. World Health Organ. 2004;82:858–866. doi:/S0042-96862004001100011. [PMC free article] [PubMed] [Google Scholar]
- 11.Levinson Miller C., Druss B.G., Dombrowski E.A., Rosenheck R.A. Barriers to primary medical care among patients at a community mental health center. Psychiatr. Serv. 2003;54:1158–1160. doi: 10.1176/appi.ps.54.8.1158. [DOI] [PubMed] [Google Scholar]
- 12.Happell B., Scott D., Platania-Phung C. Perceptions of barriers to physical health care for people with serious mental illness: a review of the international literature, issues ment. Health Nurs. 2012;33:752–761. doi: 10.3109/01612840.2012.708099. [DOI] [PubMed] [Google Scholar]
- 13.Stefanovics E., He H., Ofori-Atta A., Cavalcanti M.T., Neto H.R., Makanjuola V., Ighodaro A., Leddy M., Rosenheck R. Cross-national analysis of beliefs and attitude toward mental illness among medical professionals from five countries. Psychiatr. Q. 2016;87:63–73. doi: 10.1007/s11126-015-9363-5. [DOI] [PubMed] [Google Scholar]
- 14.Maura J., Weisman de Mamani A. Mental health disparities, treatment engagement, and attrition among racial/ethnic minorities with severe mental illness: a review. J. Clin. Psychol. Med. Settings. 2017;24:187–210. doi: 10.1007/s10880-017-9510-2. [DOI] [PubMed] [Google Scholar]
- 15.Druss B.G., Rohrbaugh R.M., Levinson C.M., Rosenheck R.A. Integrated medical care for patients with serious psychiatric illness: a randomized trial. Arch. Gen. Psychiatr. 2001;58:861–868. doi: 10.1001/archpsyc.58.9.861. http://www.ncbi.nlm.nih.gov/pubmed/11545670 [DOI] [PubMed] [Google Scholar]
- 16.Boardman J.B. Health access and integration for adults with serious and persistent mental illness. Fam. Syst. Health. 2006;24:3–18. [Google Scholar]
- 17.Shackelford J.R., Sirna M., Mangurian C., Dilley J.W., Shumway M. Descriptive analysis of a novel health care approach: reverse colocation-primary care in a community mental health "home". Prim. Care Companion CNS Disord. 2013;15 doi: 10.4088/PCC.13m01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanuch S.W., Cassidy K.A., V Dawson N., Athey M., Fuentes-Casiano E., Sajatovic M. Recruiting and retaining individuals with serious mental illness and diabetes in clinical research: lessons learned from a randomized, controlled trial. J. Health Dispar. Res. Pract. 2016;9:115–126. http://www.ncbi.nlm.nih.gov/pubmed/28533944 [PMC free article] [PubMed] [Google Scholar]
- 19.Loue S., Sajatovic M. Research with severely mentally ill latinas: successful recruitment and retention strategies. J. Immigr. Minority Health. 2008;10:145–153. doi: 10.1007/s10903-007-9063-9. [DOI] [PubMed] [Google Scholar]
- 20.Bureau U.S.C. American FactFinder - results. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_17_1YR_B03001&prodType=table (n.d.)
- 21.Wagner E.H. Chronic disease management: what will it take to improve care for chronic illness? Effect Clin. Pract. 1998;1:2–4. http://www.ncbi.nlm.nih.gov/pubmed/10345255 [PubMed] [Google Scholar]
- 22.Scharf D.M., Eberhart N.K., Schmidt Hackbarth N., Horvitz-Lennon M., Beckman R.L., Han B., Lovejoy S.L., Pincus H.A., Burnam M.A. Evaluation of the SAMHSA primary and behavioral health care integration (PBHCI) grant program. 2014. https://www.rand.org/pubs/research_reports/RR546.html [PMC free article] [PubMed]
- 23.Bogner H.R., de Vries H.F. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann. Fam. Med. 2008;6:295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharf D.M., Eberhart N.K., Hackbarth N.S., Horvitz-Lennon M., Beckman R., Han B., Lovejoy S.L., Pincus H.A., Burnam M.A. Evaluation of the SAMHSA primary and behavioral health care integration (PBHCI) grant program: final report. Rand Heal. Q. 2014;4:6. http://www.ncbi.nlm.nih.gov/pubmed/28560076 [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn L.B., Lindamer L.A., Palmer B.W., Golshan S., Schneiderman L.J., Jeste D.V. Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am. J. Geriatr. Psychiatry. 2002;10:142–150. [PubMed] [Google Scholar]
- 26.Gibbons C.J., Stirman S.W., Brown G.K., Beck A.T. Engagement and retention of suicide attempters in clinical research: challenges and solutions. Crisis. 2010;31:62–68. doi: 10.1027/0227-5910/a000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. http://www.ncbi.nlm.nih.gov/pubmed/10474547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson C., Johnston M. Adult needs and strengths assessment–abbreviated referral version to specify psychiatric care needed for incoming patients: exploratory analysis. Psychol. Rep. 2008;102:131–143. doi: 10.2466/pr0.102.1.131-143. [DOI] [PubMed] [Google Scholar]
- 30.Diggle P. Oxford University Press; 2013. Analysis of Longitudinal Data. [Google Scholar]
- 31.Ray-Sannerud B.N., Dolan D.C., Morrow C.E., Corso K.A., Kanzler K.E., Corso M.L., Bryan C.J. Longitudinal outcomes after brief behavioral health intervention in an integrated primary care clinic. - ProQuest. Fam. Syst. Health. 2012;30:60–71. doi: 10.1037/a0027029. [DOI] [PubMed] [Google Scholar]
- 32.Bryan C.J., Morrow C., Appolonio K.K. Impact of behavioral health consultant interventions on patient symptoms and functioning in an integrated family medicine clinic. J. Clin. Psychol. 2009;65:281–293. doi: 10.1002/jclp.20539. [DOI] [PubMed] [Google Scholar]
- 33.Katon W.J., Lin E.H.B., Von Korff M., Ciechanowski P., Ludman E.J., Young B., Peterson D., Rutter C.M., McGregor M., McCulloch D. Collaborative care for patients with depression and chronic illnesses. N. Engl. J. Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liebschutz J.M., Xuan Z., Shanahan C.W., LaRochelle M., Keosaian J., Beers D., Guara G., O'Connor K., Alford D.P., Parker V., Weiss R.D., Samet J.H., Crosson J., Cushman P.A., Lasser K.E. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care. JAMA intern. Med. 2017;177:1265. doi: 10.1001/jamainternmed.2017.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzmaurice G.M., Laird N.M., Ware J.H. Wiley; 2011. Applied Longitudinal Analysis. [Google Scholar]
- 36.Rubin D.B. Wiley-Interscience; 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 37.Crawford S.L., Tennstedt S.L., McKinlay J.B. A comparison of anlaytic methods for non-random missingness of outcome data., J. Clin. Epidemiol. 1995;48:209–219. doi: 10.1016/0895-4356(94)00124-9. http://www.ncbi.nlm.nih.gov/pubmed/7869067 [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]