Abstract
In this article, we share the raw protein and mRNA data obtained from basal and stimulated human peripheral blood mononuclear cells (PBMCs) derived from 15 individual treatment-naïve rheumatoid arthritis (RA) patients and synovial fluid mononuclear cells (SFMCs). In treatment-naïve RA patients, PBMCs were treated with a gradient of concentrations of As2O3 (0, 0.1, 0.5, 1.0, 2.0, 4.0 μM) for 48 hours. We found that 2.0 μM As2O3 promoted the apoptosis of PBMCs significantly, and 0.5 μM As2O3 was the lowest and effective concentration that contributed to Treg cell generation but it prevented Th17 cell differentiation, as assessed by flow cytometry. Furthermore, As2O3 decreased the transcription factor STAT3 mRNA expression of Th17 cells but increased the transcription factor Foxp3 of Treg cells. In synovial fluid from RA patients, consistent with PBMCs, As2O3 inhibited Th17 cell differentiation but promoted Treg cell generation. In an animal experiment, we analyzed the body-weight of mice as the indicator of As2O3 toxicity and calculated the spleen index. As2O3 significantly decreased the hematoxylin and eosin score in Type II collagen-induced arthritis in mice. Furthermore, As2O3 downregulated the frequency of Th1 but upregulated Th2 cells. For more insight please see Arsenic trioxide improves Treg and Th17 balance by modulating STAT3 in treatment-naive rheumatoid arthritis patients [1].
Keywords: Arsenic trioxide, Rheumatoid arthritis, Collagen induced arthritis, Regulatory T cell, T helper 17 cell, T helper 1 cell, T helper 2 cell
Specifications Table
| Subject area | Immunology |
|---|---|
| More specific subject area | Arthritis and Rheumatology |
| Type of data | Image, graph, figures, histogram. |
| How data was acquired | Flow cytometry (FC 500, Moflo XDP, Beckman Coulter); Real-time polymerase chain reaction (PCR) (Applied Biochemistry PCR system); Hematoxylin and eosin; immunofluorescence |
| Data format | Raw and Analysis |
| Experimental factors | Peripheral blood mononuclear cells (PBMCs) from treatment-naïve rheumatoid arthritis (RA) patients and synovial fluid mononuclear cells (SFMCs) from RA patients were freshly isolated and cultured in standard conditions in the presence of arsenic trioxide (As2O3); PBMCs and SFMCs were stained with fluorescent antibodies; RNA was extracted from PBMCs; spleen tissues were isolated from DBA/1J mice |
| Experimental features | The percentage of Treg, Th17, Th1 and Th2 in CD4+T cells in the presence of As2O3 was detected by flow cytometry; Treg and Th17 cell-related transcription factors were detected by quantitative real-time PCR. |
| Data source location | Department of Rheumatology, The First Affiliated Hospital, Harbin Medical University, Harbin, China |
| Data accessibility | Data are provided with this article |
| Related research article | Li. C, Zhang. J, Wang. W, Wang. H, Zhang. Y, Zhang. Z, Arsenic trioxide improves Treg and Th17 balance by modulating STAT3 in treatment-naive rheumatoid arthritis patients, Int Immunopharmacol, 2019,73:539–551. DOI:https://doi.org/10.1016/j.intimp.2019.05.001 |
Value of the Data
|
1. Data
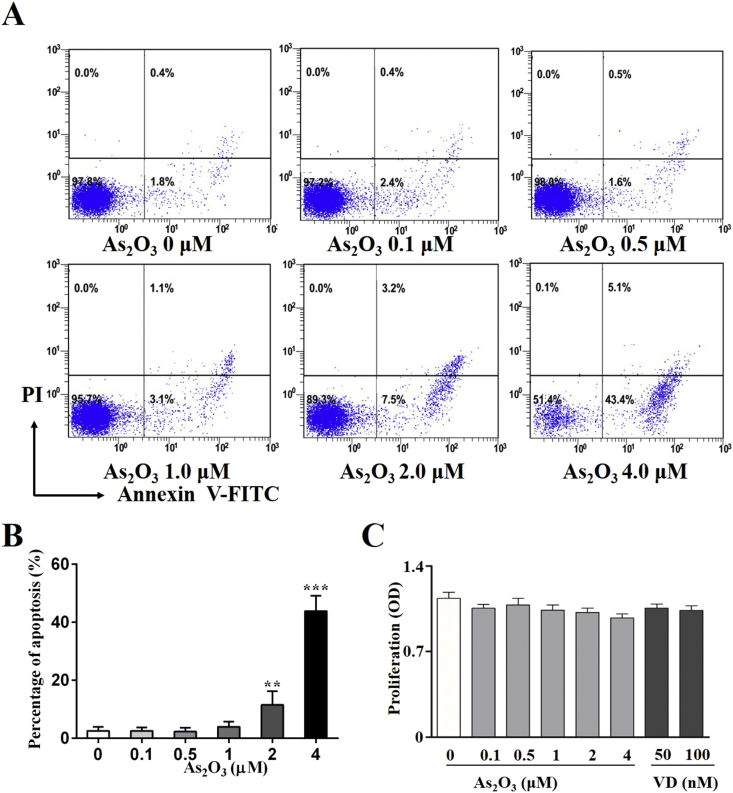
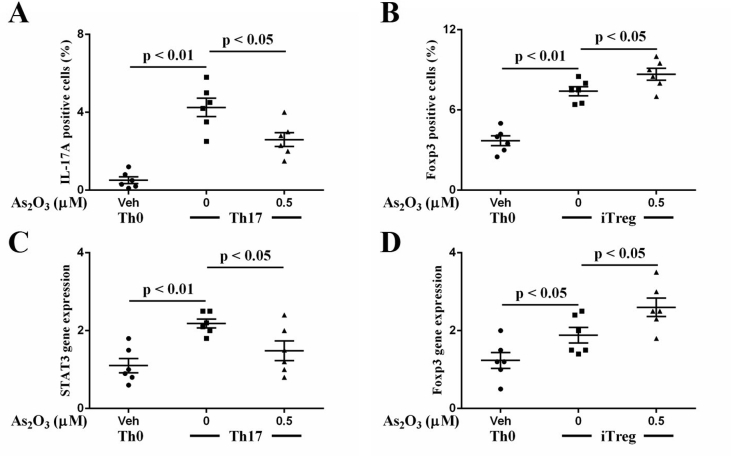
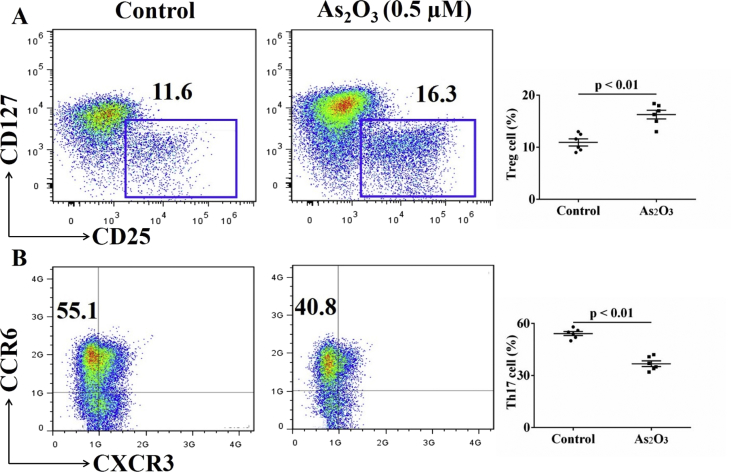
All treatment naïve-RA patients related clinical indictors were provided in Table 1 and flow cytometry panels can be referred in Table 2. As2O3 significantly promotes the apoptosis of CD4+T cells at a concentration of 2.0 μM in a dose-dependent manner (Fig. 1A, B, Table 3). We also detected the effect of As2O3 on rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLS) viability. The data showed that there is no significant change in cell viability (Fig. 1C) [2]. As2O3 at the lowest effective concentration of 0.5 μM selectively inhibited the differentiation of CD4+T cells into IL-17+CD4+T cells (Fig. 2A, Table 4) but contributed to Foxp3+CD4+T cell generation (Fig. 2B, Table 4) in the presence of special polarizing cytokines. Furthermore, in parallel with the previous results, As2O3 downregulated the STAT3 mRNA expression of Th17 cells (Fig. 2C, Table 4) but upregulated the Foxp3 mRNA expression of Treg cells (Fig. 2D, Table 4). Additionally, we also detected Treg and Th17 cells in synovial fluid from RA patients. The percentage of Treg (11.6%) and Th17 cells (55.1%) was higher in synovial fluid (SF) than that in peripheral blood (PB). We found that As2O3 at the concentration of 0.5 μM increased the frequency of Treg cells (Fig. 3A) but decreased Th17 cells (Fig. 3B, Table 5).
Table 1.
Clinical characteristics of treatment-naïve RA patients who provided synovial fluid. PB, peripheral blood; SF, synovial fluid; RF, rheumatoid factor; CCP, citrullinated peptide; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; DAS28, disease activity score 28; TJC, tender joint count; SJC, swollen joint count; VAS, visual analog score.
| Patient identifier |
Sample | Diagnosis | Gender | Age (yrs) | Age at onset of RA (yrs) | Dis. RF (yrs) | Dur | CCP | ESR | CRP | DAS28 ESR | TJC (28) | SJC (28) | VAS score | Figures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PB | RA | F | 57 | 27 | 30 | pos | neg | pos | pos | 6.67 | 11 | 11 | 7 | 1A,B |
| 2 | PB | RA | M | 48 | 41 | 7 | pos | pos | pos | pos | 6.68 | 22 | 0 | 5 | Fig. 1, Fig. 2A–D |
| 3 | PB | RA | F | 55 | 54 | 1 | pos | pos | pos | pos | 8.19 | 24 | 24 | 7 | Fig. 1, Fig. 2 |
| 4 | PB | RA | F | 69 | 66 | 3 | pos | pos | pos | pos | 4.42 | 0 | 2 | 5 | Fig. 1, Fig. 2 |
| 5 | PB | RA | M | 41 | 41 | 0.5 | pos | pos | pos | neg | 3.91 | 2 | 2 | 5 | Fig. 1, Fig. 2 |
| 6 | PB | RA | F | 55 | 41 | 14 | neg | pos | pos | pos | 7.48 | 21 | 21 | 6 | Fig. 1, Fig. 2 |
| 7 | PB | RA | F | 46 | 39 | 7 | neg | pos | neg | neg | 2.16 | 0 | 0 | 5 | Fig. 1, Fig. 2 |
| 8 | PB | RA | F | 38 | 38 | 0.33 | pos | pos | pos | pos | 8.21 | 26 | 26 | 7 | Fig. 1, Fig. 2 |
| 9 | PB | RA | M | 63 | 47 | 16 | pos | pos | pos | pos | 5.87 | 10 | 1 | 6 | Fig. 1, Fig. 2 |
| 10 | SF | RA | F | 67 | 66 | 1 | pos | pos | pos | pos | 5.87 | 4 | 4 | 6 | 3A,B |
| 11 | SF | RA | M | 52 | 51 | 1 | pos | pos | pos | pos | 6.66 | 11 | 10 | 7 | 3A,B |
| 12 | SF | RA | F | 41 | 32 | 9 | pos | pos | neg | pos | 5.4 | 6 | 6 | 8 | 3A,B |
| 13 | SF | RA | F | 48 | 48 | 0.58 | pos | pos | neg | pos | 4.97 | 2 | 2 | 6 | 3A,B |
| 14 | SF | RA | F | 61 | 31 | 30 | pos | neg | pos | pos | 5.19 | 8 | 2 | 6 | 3A,B |
| 15 | SF | RA | F | 48 | 48 | 0.58 | neg | pos | pos | pos | 5.78 | 8 | 8 | 6 | 3A,B |
Table 2.
Antibodies and immunostaining panels used for flow cytometry. Detailed description of the fluorochrome-conjugated antibodies and immunostaining panels used to immunophenotype and flow sort the assessed CD4+T cell populations.
| Immunostaining Panel | Antibody | Fluorochrome | Clone | Manufacture |
|---|---|---|---|---|
| Human Treg Immunophenotyping (Freshly isolated PBMCs) | CD4 | FTTC | 13B8.2 | Beclr.r-n Coulter |
| CD25 | PE | Bl.49.9 | Beclr.r-n Coulter | |
| CD127 | PE-Cy5 | R34.34 | Beclr.r-n Coulter | |
| Foxp3 | BV421 | 206D | Biolezeai | |
| Human Thl7 Immunophenotyping (Freshly isolated PBMCs) | CD4 | FITC | 13B8.2 | 3eckir.in Coulter |
| IL-17 | PE | SCPL1362 | 3 eckman Coulter | |
| CXCR3 | PE | G025H7 | 3iolegend | |
| CCR6 | APC | G034E3 | 3iolegend | |
| Mouse Thl Immunophenotyping (Freshly isolated PBMCs) | CD4 | FTTC | RM4-5 | Biolegend |
| IFN-γ | PE | XMG1.2 | Biolegend | |
| IC | PE | RTK2071 | Biolegend | |
| Mouse Th2 Immunophenotyping (Freshly isolated PBMCs) | CD4 | FTTC | RM4-5 | 3iolegend |
| IL-4 | PE-Cy7 | 11B11 | 3iolegend | |
| IC | PE-Cy7 | RTK2071 | 3iolegend |
IC: Isotype control
Fig. 1.
As2O3 shows an apoptotic effect on CD4+T cells from 2.0 μM. (A). Apoptosis of CD4+ T cells from treatment-naïve RA patients was detected by flow cytometry. (B). Quantification of the apoptosis percentage. (C). The effect of As2O3 and vitamin D on RA FLS viability, as evaluated by cell count kit 8 (CCK8) assay. **p<0.05 vs non-treatment group, ***p<0.001 vs non-treatment group by unpaired Student t-test. Additionally, mean values, S.E.M and Tukey's multiple comparisons statistics (post hoc) are shown in tabular from below their respective graphs.
Table 3.
The effects of As2O3 on apoptosis of CD4+T cells.
| Patent identifier | Sample | As2O3 (μM) |
|||||
|---|---|---|---|---|---|---|---|
| 0 |
0.1 |
0.5 |
1.0 |
2.0 |
4.0 |
||
| Apoptosis(%) | |||||||
| 1 | PB | 2.4 | 1.8 | 1.6 | 3.1 | 7.5 | 43.4 |
| 2 | PB | 1.6 | 1.2 | 1.4 | 3.8 | 7.3 | 48.0 |
| 3 | PB | 3.2 | 23 | 2.2 | 2.5 | 8.8 | 41.5 |
| 4 | PB | 2.5 | 3.0 | 3.5 | 5.1 | 103 | 50.5 |
| 5 | PB | 5.5 | 4.5 | 4.2 | 7.2 | 12.1 | 45.3 |
| 6 | PB | 1.0 | 2.0 | 1.2 | 2.3 | 15.4 | 35.7 |
Fig. 2.
As2O3 prevented the differentiation of CD4+T cells into Th17 cells (A) but promoted Treg cell generation (B). Furthermore, As2O3 downregulated the STAT3 mRNA expression of Th17 cells (C) but upregulated the Foxp3 mRNA expression of Treg cells (D). GAPDH was used as an internal control for normalization real time analysis. The results shown are representative of six independent experiments (n = 6), and the values are expressed as mean ± SEM.
Table 4.
The effects of As2O3 on the differentiation of CD4+T cells under special polarizing cytokines. Th17 cells: Anti-CD3 (2 μg/mL), Anti-CD28 (4 μg/mL), IL-1β (10 ng/mL), IL-6 (20 ng/mL), IL-23 (100 ng/mL), TGF-β (1 ng/mL). iTreg cells: Anti-CD3 (2 μg/mL), Anti-CD28 (4 μg/mL), IL-2 (20 U/mL), TGF-β1 (2 ng/mL).
| As2O3 (μM) | Th17 cells (%) | STAT3 gene expression | Treg cells (%) | Foxp3 gene expression |
|---|---|---|---|---|
| veh | 0.2 | 0.9 | 4.2 | 1.2 |
| 0.5 | 1.5 | 2.5 | 1.5 | |
| 0.8 | 1.0 | 3.5 | 1.0 | |
| 1.2 | 0.6 | 5.0 | 2.0 | |
| 0.1 | 0.8 | 3.0 | 1.2 | |
| 0.3 | 1.8 | 4.0 | 0.5 | |
| 0 | 4.5 | 2.2 | 7.5 | 1.5 |
| 4.3 | 2.5 | 6.4 | 2.0 | |
| 3.5 | 2.1 | 8.5 | 2.4 | |
| 5.8 | 2.5 | 6.5 | 2.5 | |
| 13 | 2.0 | 7.5 | 1.5 | |
| 5.0 | 1.8 | 8.0 | 1.4 | |
| 0.5 | 23 | 2.0 | 9.0 | 1.8 |
| 4.0 | 2.4 | 9.8 | 2.3 | |
| 2.8 | 1.5 | 7.0 | 2.5 | |
| 3.0 | 1.0 | 8.0 | 3.5 | |
| 1.5 | 12 | 8.5 | 3.0 | |
| 2.0 | 0.8 | 9.5 | 2.5 |
Fig. 3.
As2O3 increased the frequency of Treg cells (A) but decreased Th17 cells (B) in synovial fluid from RA patients, as assessed by flow cytometry. The results shown are representative of six independent experiments (n = 6).
Table 5.
The effects of As2O3 on the Treg and Th17 cells from synovial fluid.
| As2O3 (μM) | Percentage (%) (n = 6) |
|
|---|---|---|
| Treg | Th17 | |
| 0 | 11.6 | 55.1 |
| 12.5 | 58.2 | |
| 13.4 | 52.8 | |
| 10.5 | 56.5 | |
| 9.5 | 50.3 | |
| 9.4 | 54.6 | |
| 0.5 | 16.3 | 40.8 |
| 18.5 | 35.3 | |
| 15.4 | 37.5 | |
| 13.3 | 34.8 | |
| 17.5 | 42.7 | |
| 18.4 | 32.9 | |
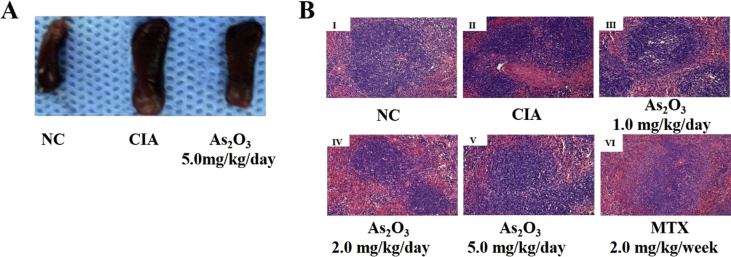
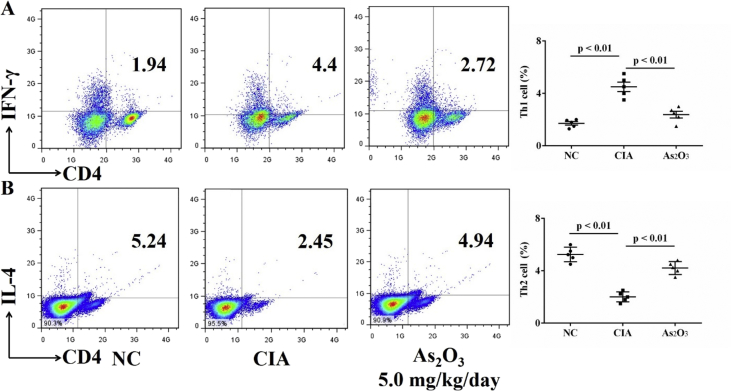
To further study the effects of As2O3 on CD4+T cell proliferation and differentiation, a Type II collagen-induced arthritis (CIA) mouse model was constructed and the groups were divided as described previously [3]. First, we assessed the severity of arthritis from Day 19 after primary immunization according to a standard score system [1]. Second, we used body-weight as the indicator of As2O3 toxicity. As2O3 (1.0, 2.0 or 5.0 mg/kg/day) did not markedly affect the body-weight of CIA mice compared with CIA control mice (Table 6). Interestingly, As2O3 reduced the size of spleen tissues and also decreased the spleen index (spleen weight/mice weight) (Fig. 4A, Table 7). Hematoxylin and eosin (H&E) staining revealed that As2O3 (2.0 or 5.0 mg/kg/day) significantly decreased splenic corpuscle proliferation and inflammatory infiltration (Fig. 4B, Table 7). We also found that As2O3 upregulated the percentage of Th2 cells but downregulated Th1 cells in the spleen (Fig. 5A, B, Table 7).
Table 6.
The effects of As2O3 on body-weight. The body-weight was detected from Day 34 to Day 40. MTX: methotrexate.
| Body weight (g) As2O3 (mg kg day) (n = 5) |
Days from first immunization |
||||||
|---|---|---|---|---|---|---|---|
| 34 | 35 | 36 | 37 | 38 | 39 | 40 | |
| NC | 20.5 | 20.6 | 20.7 | 21.0 | 21.3 | 21.7 | 21.7 |
| NC | 19.6 | 19.7 | 19.8 | 19.9 | 20.1 | 20.3 | 20.6 |
| NC | 20.3 | 20.3 | 21.3 | 21.3 | 21.3 | 21.7 | 21.7 |
| NC | 16.4 | 16.7 | 16.9 | 17.2 | 17.8 | 17.9 | 17.9 |
| NC | 17.9 | 18.0 | 18.2 | 18.4 | 18.5 | 18.7 | 18.8 |
| CIA | 15.3 | 15.9 | 15.7 | 15.7 | 16.1 | 17.0 | 16.9 |
| CIA | 16.3 | 16.3 | 15.4 | 15.5 | 15.6 | 16.0 | 16.4 |
| CIA | 16.0 | 15.9 | 15.7 | 15.5 | 15.6 | 15.8 | 16.8 |
| CIA | 19.0 | 18.8 | 18.2 | 18.0 | 18.2 | 18.1 | 18.0 |
| CIA | 16.8 | 16.5 | 16.3 | 16.3 | 16.3 | 16.9 | 17.3 |
| 1.0 | 20.0 | 20.1 | 19.5 | 19.9 | 20.2 | 20.6 | 21.2 |
| 1.0 | 20.9 | 20.6 | 20.4 | 20.5 | 21.1 | 21.4 | 21.7 |
| 1.0 | 20.8 | 21.2 | 20.7 | 21.2 | 21.4 | 21.7 | 21.8 |
| 1.0 | 17.8 | 17.7 | 17.3 | 18.0 | 18.5 | 19.2 | 19.2 |
| 1.0 | 19.5 | 19.4 | 19.4 | 19.2 | 19.2 | 19.0 | 19.1 |
| 2.0 | 16.6 | 16.1 | 16.4 | 16.2 | 17.4 | 17.4 | 18.1 |
| 2.0 | 20.6 | 20.7 | 19.9 | 20.1 | 20.3 | 20.7 | 20.5 |
| 2.0 | 19.0 | 18.7 | 19.3 | 18.5 | 18.7 | 19.1 | 19.2 |
| 2.0 | 18.8 | 18.2 | 18.0 | 18.3 | 18.8 | 19.5 | 19.2 |
| 2.0 | 19.5 | 19.2 | 18.9 | 19.0 | 20.2 | 20.0 | 20.1 |
| 5.0 | 16.9 | 17.3 | 16.7 | 17.6 | 17.4 | 18.0 | 18.5 |
| 5.0 | 20.6 | 17.7 | 17.6 | 16.9 | 17.6 | 17.7 | 17.5 |
| 5.0 | 18.7 | 20.8 | 20.8 | 21.2 | 20.8 | 21.2 | 21.5 |
| 5.0 | 16.8 | 17.3 | 17.2 | 17.8 | 18.0 | 18.2 | 19.3 |
| 5.0 | 19.2 | 20.7 | 20.7 | 21.5 | 21.4 | 22.0 | 21.9 |
| MTX | 16.9 | 17.1 | 17.4 | 18.1 | 18.5 | 19.4 | 19.6 |
| MTX | 17.9 | 20.5 | 21.4 | 21.9 | 22.0 | 22.0 | 21.7 |
| MTX | 21. | 18.8 | 19.1 | 19.0 | 19.6 | 20.1 | 20.2 |
| MTX | 17.6 | 16.8 | 16.6 | 17.1 | 17.2 | 17.9 | 17.9 |
| MTX | 20.5 | 19.1 | 19.3 | 19.5 | 19.4 | 19.2 | 19.3 |
Fig. 4.
The effects of As2O3 on spleen tissues. (A). As2O3 decreased the volume of the spleen. (B). The spleen index in the CIA control group increased significantly compared with the normal control group. However, the spleen index of groups under As2O3 (2.0 or 5.0 mg/kg/day) and MTX treatment decreased significantly compared with the CIA control group. Splenic corpuscle proliferation and splenic sinus congestion in the CIA group increased significantly compared with the normal control group. However, splenic corpuscle proliferation and splenic sinus congestion in the As2O3 and MTX treatment groups decreased significantly compared with the CIA control group.
Table 7.
The effects of As2O3 on spleen tissues. Percentage of Th1 and Th2 cells in spleen lymphocytes. Were detected by flow cytometry.
| Group (n = 5) As.Oj (mg kg day) |
Index of spleen | Spleen Histological score | Thl (%) | Th2 (%) |
|---|---|---|---|---|
| NC | 2.1 | 0.8 | 1.9 | 5.2 |
| NC | 2.2 | 1.1 | 1.5 | 6.0 |
| NC | 1.9 | 1.2 | 2.0 | 5.5 |
| NC | 1.8 | 1.0 | 1.8 | 4.5 |
| NC | 2.0 | 1.3 | 1.3 | 5.0 |
| CIA | 4.8 | 2.7 | 4.5 | 2.2 |
| CIA | 4.6 | 2.6 | 5.0 | 2.0 |
| CIA | 5.1 | 2.5 | 4.0 | 1.5 |
| CIA | 5.2 | 3.2 | 5.5 | 2.5 |
| CIA | 4.5 | 3.5 | 3.5 | 1.8 |
| 1.0 | 4.0 | 2.3 | 4.3 | 2.8 |
| 1.0 | 4.3 | 2.4 | 5.0 | 2.3 |
| 1.0 | 4.5 | 2.1 | 3.5 | 2.0 |
| 1.0 | 4 2 | 2.8 | 4.8 | 3.5 |
| 1.0 | 3.9 | 3.0 | 3.0 | 3.2 |
| 2.0 | 3.6 | 1.8 | 3.5 | 3.8 |
| 2.0 | 3.9 | 2.0 | 4.0 | 2.9 |
| 2.0 | 4.0 | 1.7 | 3.0 | 3.0 |
| 2.0 | 4.2 | 2.0 | 4.2 | 3.5 |
| 2.0 | 3.2 | 2.8 | 3.0 | 4.0 |
| 5.0 | 3.4 | 1.4 | 2.7 | 4 2 |
| 5.0 | 3.2 | 1.1 | 2.5 | 4.5 |
| 5.0 | 3.0 | 1.7 | 3.0 | 4.0 |
| 5.0 | 3.5 | 1.2 | 1.5 | 3.5 |
| 5.0 | 2.9 | 2.0 | 2.2 | 4.8 |
| MTX | 2.6 | 1.2 | 2.5 | 4.8 |
| MTX | 3.0 | 1.3 | 2.2 | 4.3 |
| MTX | 2.8 | 1.5 | 3.0 | 3.9 |
| MTX | 3.2 | 1.8 | 1.4 | 5.2 |
| MTX | 2.7 | 1.0 | 2.1 | 5.0 |
Fig. 5.
As2O3 decreased the frequency of Th1 cells (A) but increased the proportion of Th2 cells (B) in the spleen of DBA/1J mice, as assessed by flow cytometry. The results shown are representative five independent experiments, and the values are expressed as mean ± SEM.
2. Experimental design, materials and methods
2.1. Treatment-naïve RA patients
Treatment-naïve RA patients who fulfilled the American College of Rheumatology 1987 revised criteria [4] were enrolled in the study. Treatment-naïve RA patients were recruited from the Department of Rheumatology of the First Affiliated Hospital of Harbin Medical University. Some patients were taking nonsteroidal anti-inflammatory drugs; none of the patients had been receiving disease-modifying antirheumatic drugs or corticosteroids. The study was conducted with formal approval from the Ethical Committee of Harbin Medical University.
2.2. Peripheral blood and synovial fluid mononuclear cell isolation of naïve CD4+T cells
PBMCs were isolated from treatment-naïve RA patients and healthy volunteers. Synovial fluid mononuclear cells were isolated from synovial fluid from RA patients, then separated by density gradient on a Ficoll-Hypaque apparatus (MD Pacific Biotechnology, Tianjin, China). Naïve CD4+T lymphocytes were purified on a high-speed cell sorter system (Moflo XDP, Beckman Coulter, Indianapolis, IN, USA). The naïve CD4+T cells had a purity of >95%, which was confirmed by flow cytometry (FC500, Beckman Coulter, Indianapolis, IN, USA).
2.3. Apoptosis assay
Sorted live CD4+T cells from treatment-naïve RA patients were cultured in the presence of different concentrations of As2O3 for 48 hours. Apoptosis was determined by Annexin V and propidium iodide staining according to the manufacture's protocol (BD Biosciences, San Diego, CA, USA). When gated on total cells, Annexin V- and propidium iodide-negative cells were considered as living. No significant changes in total cell numbers were observed. Samples were analyzed by flow cytometry (FC 500, Beckman Coulter, Indianapolis, IN, USA).
2.4. In vitro stimulation polarization of CD4+T cells
CD4+T cells were cultured with anti-CD3 (2 μg/mL)/anti-CD28 (4 μg/mL) (Biolegend, San Diego, CA). IL-1 β (10 ng/mL), IL-6 (20 ng/mL), TGF-β (1 ng/mL) (Biolegend, San Diego, CA), IL-23 (100 ng/mL) (R&D Systems, Minneapolis, USA) were added for Th17 cell polarization; TGF-β (2 ng/mL) and IL-2 (20 U/mL) (Biolegend, San Diego, CA) were added for Treg cell polarization. At the same time, As2O3 (0.5 μM) (Yitaida Pharmaceutical Factory, Harbin, Heilongjiang, China) was added once a day for 3 days.
2.5. In vitro proliferation assay
The cell proliferation assay was evaluated with a Cell Counting Kit-8 (Sigma, St Louis, MO, USA) following the procedures described earlier with minor modifications [5]. Briefly, 104 cells were seeded in a 96-well plate. After 24 h, different concentrations of drugs or vehicles were added with fresh medium. Cells were incubated at 37 °C for 48 h before detected at 450 nm immediately. The experiments were repeated three times.
2.6. Flow cytometry analysis
For intracellular cytokines detection, cells were stimulated with the corresponding cell activation cocktail (with Brefeldin A) (Biolegend, San Diego, CA) for 6 h. After surface staining for 15 min, the cells were resuspended in a fixation buffer, and washed three times with a permeabilization solution (Biolegend, San Diego, CA) for 5 mins each at 1500 rpm. Intracellular cytokine staining was performed according to the manufacturer's protocol. Isotype control staining consistently resulted in 0.1% positive cells throughout the experiments. The following reagents were used for human experiments: fluorescein isothiocyanate-conjugated CD4 (clone: 13B8.2), biotinylated and phycoerythrin-conjugated CD25 (clone: B1.49.9), peridinin chlorophyll A protein-Cy5-conjugated CD127 (clone: R34.34), allophycocyanin-conjugated CCR6 (clone: G034E3) and phycoerythrin-conjugated CXCR3 (clone: G025H7). All these antibodies were purchased from Beckman Coulter (San Diego, CA, USA). The following reagents were used for mouse assays: anti-CD4-FITC (clone: RM4-5), anti–IFN–γ phycoerythrin (clone: XMG1.2) and anti-IL-4-PC7 (clone: 11B11). All these antibodies were purchased from Biolegend (San Diego, CA, USA).
2.7. Real-time quantitative polymerase chain reaction analysis
The methods used for RNA extraction and cDNA synthesis have been described previously [3]. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase and β-actin, and were calculated via the 2−ΔΔCt method.
2.8. Animal model and experimental protocol
Male DBA/1 J mice (5–8 weeks old, 20 g ± 2 g in weight; Charles River, Beijing, China) were housed under specific pathogen-free conditions at the veterinary institute of Harbin Medical University and were fed with standard mouse chow and water. All experimental procedures were examined and approved by the Institutional Animal Care and Use Committee of Harbin Medical University. The method for the CIA mouse model was performed in our laboratory as described previously [3]. The scoring system was used according to a protocol described previously [6]. The mice were carefully monitored for toxicity symptoms and weighed every 3 days. The mean body-weight and spleen index of each group were calculated and used as a parameter of toxicity.
2.9. Histology analysis
Mice spleens were collected after sacrifice. The procedures of fixation and embedding have been described previously [3]. In brief, the spleens were embedded in paraffin, cut into 5-μm sections and stained with H&E. Histology scores were assessed as previously described [3].
2.10. Spleen lymphocyte preparation
Mice were sacrificed, then spleens were drained and by passing through a 200 μm wire mesh to obtain single-cell suspensions. PBMCs were isolated by Ficoll density gradient isolation (Haoyang, Tianjin, China) according to the manufacturer's instructions. Mononuclear cells were obtained and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL of penicillin, 100 μg/ml of streptomycin, nonessential amino acids, 1 μM sodium pyruvate and 2 mM l-glutamine. Subsequently, flow cytometry detected the percentage of Th1 and Th2 cells.
2.11. Statistical analysis
The data were analyzed by using GraphPad Prism Software (Version 6 for Windows; Graphpad Prism, San Diego, CA, USA). Simple comparisons were conducted with unpaired, two-tailed Students's t-test for parametric data. Values of P < 0.05 were considered statistically significant. All data were expressed as mean ± S.E.M.
Acknowledgments
We are grateful to Ms. Yanli Wang for technical assistance with flow cytometry and Yu Wang for providing synovial fluid. This work is supported by National Science Foundation of China, Grant No. 81273291, No. 81771749 and No. 81771748.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Li C., Zhang J., Wang W., Wang H., Zhang Y., Zhang Z. Arsenic trioxide improves Treg and Th17 balance by modulating STAT3 in treatment-naive rheumatoid arthritis patients. Int. Immunopharmacol. 2019;73:539–551. doi: 10.1016/j.intimp.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang W., Li C., Zhang Y., Zhang Z. Arsenic trioxide synergistic with vitamin D rescues the defective VDR-PPARγ autophagy functional module in rheumatoid arthritis. PPAR Res. 2019;2019 doi: 10.1155/2019/6403504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Li C., Zhang Y., Lin Z., Zhang Y., Zhang Z. Inhibition of angiogenesis by arsenic trioxide via TSP-1-TGF-β1-CTGF-VEGF functional module in rheumatoid arthritis. OncoTargets. 2017;8(43):73529–73546. doi: 10.18632/oncotarget.19867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnett F., Edworthy S., Bloch D., McShane D., Fries J., Cooper N. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Zhang Y., Han Z., He K. Malignancy of cancers and synthetic lethal interactions associated with mutations of cancer driver genes. Medicine (Baltim.) 2016;95(8):e2697. doi: 10.1097/MD.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand D., Latham K., Rosloniec E. Collagen-induced arthritis. Nat. Protoc. 2007;2(5):1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]