ABSTRACT
Background
Although preliminary evidence suggests that intermittent calorie restriction (ICR) exerts stronger effects on metabolic parameters, which may link obesity and major chronic diseases, compared with continuous calorie restriction (CCR), there is a lack of well-powered intervention studies.
Objective
We conducted a randomized controlled trial to test whether ICR, operationalized as the “5:2 diet,” has stronger effects on adipose tissue gene expression, anthropometric and body composition measures, and circulating metabolic biomarkers than CCR and a control regimen.
Design
One hundred and fifty overweight and obese nonsmokers [body mass index (kg/m2) ≥25 to <40, 50% women], aged 35–65 y, were randomly assigned to an ICR group (5 d without energy restriction and 2 d with 75% energy deficit, net weekly energy deficit ∼20%), a CCR group (daily energy deficit ∼20%), or a control group (no advice to restrict energy) and participated in a 12-wk intervention phase, a 12-wk maintenance phase, and a 26-wk follow-up phase.
Results
Loge relative weight change over the intervention phase was −7.1% ± 0.7% (mean ± SEM) with ICR, −5.2% ± 0.6% with CCR, and −3.3% ± 0.6% with the control regimen (Poverall < 0.001, PICR vs. CCR = 0.053). Despite slightly greater weight loss with ICR than with CCR, there were no significant differences between the groups in the expression of 82 preselected genes in adipose tissue implicated in pathways linking obesity to chronic diseases. At the final follow-up assessment (week 50), weight loss was −5.2% ± 1.2% with ICR, −4.9% ± 1.1% with CCR, and −1.7% ± 0.8% with the control regimen (Poverall = 0.01, PICR vs. CCR = 0.89). These effects were paralleled by proportional changes in visceral and subcutaneous adipose tissue volumes. There were no significant differences between ICR and CCR regarding various circulating metabolic biomarkers.
Conclusion
Our results on the effects of the “5:2 diet” indicate that ICR may be equivalent but not superior to CCR for weight reduction and prevention of metabolic diseases.
This trial was registered at clinicaltrials.gov as NCT02449148.
Keywords: obesity, weight loss, intermittent fasting, calorie restriction, adipose tissue gene expression
INTRODUCTION
Although continuous calorie restriction (CCR) is the most widely recommended calorie restriction regimen for weight loss and prevention of obesity-associated diseases, intermittent calorie restriction (ICR), comprising phases of severe energy restriction and regular energy intake, has gained attention as a novel dietary approach (1). With regard to circulating concentrations of glucose, insulin, insulin-like growth factor 1 (IGF-1), leptin, and adiponectin, evidence from mouse models suggests that the effects of ICR are stronger than those of CCR, even at similar net calorie intake (2–4). In humans, 2 studies among overweight and obese women suggested greater reductions in insulin concentrations and fat mass with ICR compared with CCR over 4 and 6 mo at similar amounts of net calorie intake and weight loss (5, 6). In these trials, ICR was operationalized as the so-called “5:2 diet,” with 5 d/wk at regular energy intake and 2 d/wk at an ∼75% energy deficit. Despite such promising first findings, it has been agreed that further randomized controlled trials (RCTs) are needed to assess whether ICR is superior to CCR at similar amounts of net energy intake with respect to a broader set of metabolic health indicators (7). In addition, analyses of longer-term maintenance of initial weight loss and metabolic outcomes with ICR are required to investigate its sustainability and practicability (8, 9), because a first systematic review of the few ICR studies which were longer than 6 mo did not point to greater benefits of ICR compared with CCR (10).
Here, we report results of the HELENA Trial (Healthy nutrition and energy restriction as cancer prevention strategies: a randomized controlled intervention trial) (NCT02449148), an RCT among 150 overweight or obese individuals, which was designed to investigate the effects of ICR (“5:2 diet”) and CCR at similar levels of net calorie restriction on human metabolism. The trial started with a 12-wk intervention (with comprehensive dietary counseling by trained dietitians at baseline and week 2, followed by biweekly phone calls by the dietitians). At the end of this intervention phase, participants returned to the study center for examinations and were motivated by the dietitians to continue with the prescribed regimen during the maintenance phase (weeks 13 to 24), but no further dietary counseling followed throughout the remaining weeks of the study, neither in person nor on the phone. At week 24, participants visited the study center again for examinations, and the study ended after the final follow-up assessment at week 50 (11). The effects of ICR and CCR were also compared with those of a control regimen without prescribed calorie restriction.
To test the hypothesis of potentially greater health benefits of ICR compared with CCR, we first assessed the metabolic effects of ICR in the more tightly monitored intervention phase (baseline to week 12), so as to find out if they were in fact stronger than those of CCR, even at a similar net energy intake. We chose differences in the expression of 82 preselected genes (11) that are central in pathophysiologic processes linking overweight and obesity to major chronic diseases (e.g., altered macronutrient metabolism, adipokine signaling, inflammation, steroid hormone production, and oxidative stress) as our biological model for diet-induced changes in metabolic function and as our primary outcome. The novel gene expression biomarkers were complemented with established metabolic, anthropometric, and imaging markers as secondary outcomes. We then evaluated whether ICR-induced changes in body weight, body composition, and metabolic biomarkers were sustainable under less supervised conditions across the maintenance phase and follow-up phase.
METHODS
Study design and participants
The HELENA Trial was approved by the ethics committee of the Heidelberg University Hospital (Heidelberg, Germany) and registered at clinicaltrials.gov before enrollment. Details on study design, power-calculation, recruitment procedures, study assessments, and intervention protocol have been published previously (11). Overall, 150 overweight or obese nonsmokers (50% women) were recruited for the study comprising a 12-wk intervention phase, a 12-wk maintenance phase, and a 26-wk follow-up phase.
The 1-y recruitment phase of the HELENA Trial (May 2015–May 2016) was based on flyer and poster campaigns as well as word of mouth. All data were collected at the German Cancer Research Center, Heidelberg, Germany. Eligibility for participation in accordance to the predefined inclusion [nonsmokers, women and men, BMI (in kg/m2) ≥25 and <40, age 35–65 y] and exclusion criteria (see Supplemental Table 1) was approved at the study center and all participants provided a written informed consent before study start (11). Participants received monetary incentives after completing the assessments at week 12 (260€) and week 50 (140€). The lower age limit of 35 y was chosen to recruit a similar number of pre- and postmenopausal women (assumed average age at menopause: 50 y). The upper age limit was set at 65 y because changes to body composition may show different patterns and have different causes among elderly persons, compared with younger ones. Participants were assigned to 1 of the 3 study arms (ICR, CCR, or Control) in a ratio of 1:1:1 by stratified block randomization (sex and age <50 compared with age ≥50 y) with a fixed block size of 6. The web-based software RANDI2 (12) was used to generate the random allocation sequence. Because the HELENA Trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. However, the technical staff was blinded for downstream laboratory work and data management.
As the primary endpoint, we defined differences in the expression of 82 preselected genes (Supplemental Table 2) in subcutaneous adipose tissue (SAT) between ICR and CCR assessed at baseline and week 12. Secondary outcomes included differences in anthropometric measures (body weight, BMI, and waist circumference as assessed at baseline, week 12, week 24, and week 50), body fat composition [visceral adipose tissue (VAT) and SAT volumes as well as liver fat content at baseline, week 12, and week 50], circulating biomarker concentrations (baseline, week 12, week 24, and week 50), blood pressure (baseline, week 12, and week 50), and health-related quality of life (HR-QoL) (baseline, week 12, and week 50). The majority of the HELENA Trial participants (n = 145) provided an additional informed consent for magnetic resonance tomography imaging (MR-imaging) to assess abdominal body composition (baseline, week 12, and week 50). All participants were requested to contact the study center to report adverse events at any time.
Calorie restriction intervention
Study participants were randomly assigned to 1 of 3 study groups: ICC, CCR, or Control. After random assignment, participants underwent baseline examinations, and the nutrition intervention started with the first dietary consultation session run by 2 trained dietitians (see the Supplemental Methods and Supplemental Table 3 for details). Individuals’ resting energy expenditure (for the ICR and CCR groups) was calculated based on the Harris-Benedict equation (13), whereas total energy expenditure was estimated by multiplication with Physical Activity Level factors, projected from questionnaire data on physical activity, individuals’ profession, and working time. Participants in all groups (ICR, CCR, or Control) were informed about the guidelines of the German Nutrition Society for a healthy balanced diet (14, 15) by the dietitians and advised to maintain habitual physical activity levels throughout the study. The number of personal contacts and counseling sessions was the same for all study participants overall, but individuals in the ICR and CCR arms received longer and more comprehensive counseling sessions with personalized dietary plans, specific for the ICR or CCR regimens (details follow; also see the Supplemental Methods). The second personal dietary consultation took place in week 2 of the intervention phase, when individual questions were answered, knowledge about the dietary regimen was deepened, and participants were motivated by the dietitians. All participants were then contacted with biweekly phone calls during the intervention phase (at weeks 4, 6, 8, and 10) by the dietitians to assess possible side effects. For the ICR group and the CCR group, the biweekly phone calls further included participant motivation to promote continuation in the study and monitoring of self-reported compliance. At the postintervention assessment (week 12), which was also the starting point of the maintenance phase, all participants discussed their experiences with a dietician and were motivated to maintain the diet across the following 12 wk (weeks 13–24). Unlike in the intervention phase (biweekly phone contacts in addition to 2 personal dietary consultations at baseline and during week 2), however, participants did not receive further dietetic support throughout the maintenance phase. At week 24, participants returned to the study center for examinations. At this point no further dietary advice was given by the study personnel and the last phase of the study was merely an observational follow-up phase (weeks 25–50). The results presented in this paper are based on data collected at baseline as well as at weeks 12, 24, and 50 (see the Supplemental Methods and Supplemental Table 3).
Participants in the ICR group were advised to restrict their energy intake on 2 self-selected nonconsecutive days per week to 25% of the individual energy requirement. The remaining 5 d of the week were based on a eucaloric balanced diet [according to the guidelines of the German Society for Nutrition (14, 15)]. Thus, the weekly average calorie intake corresponded to ∼80% of the normal energy requirement. For calorie-restricted days, detailed personalized meal plans were created, in which possible choices for meal components arranged by food groups were given; participants had to select 4 food items out of the vegetable group, 2 out of the low-fat dairy product group, and 1 food item out of each of the meat/fish, carbohydrate, and fruits groups, in combination with a minimum intake of 2 L of low-energy drinks. Digital kitchen scales were provided to facilitate exact weighing of food quantities and participants were asked to mark the performed calorie-restricted days in a diary across the 12-wk intervention phase.
In the CCR group, participants were requested to consume ∼80% of the individual energy requirement daily. Personalized diet plans aiming at implementing the recommendations of the German Society for Nutrition and to reduce energy-dense foods were provided by the dietitians. Meal planning, portion size choices, and strategies to reduce energy intake were discussed with the participants. Further details on the assessment of compliance and HR-QoL are given in the Supplemental Methods.
Biospecimen collection and laboratory analyses
Details on the collection and processing of biospecimens (SAT, blood) and all laboratory analyses are provided in the Supplemental Methods.
Statistical analyses
The sample size calculation for the primary endpoint of the HELENA Trial (differential expression of 82 preselected genes between ICR and CCR) was published previously (11). In brief, we projected that a difference of 1.5-fold in gene expression levels between the ICR and CCR groups could be detected with 80% power at group sizes of n = 42 adjusting for multiple testing by the Bonferroni method. To estimate the variance in gene expression markers, we used data from a previous trial of 2 of our co-investigators (16). Given that gene expression profiles are not used as routine biomarkers of metabolism, we further tested that the sample size was sufficient to detect differences in established secondary endpoints (i.e., weight, BMI, blood pressure, circulating biomarkers) at 80% power before commencement of the trial (11).
Microarray-derived expression levels were preprocessed in Chipster 3.8 (CSC) including log2 transformation, imputation of missing values with the K-nearest neighbor method (17), and application of ComBat to handle possible batch effects (18). For intention-to-treat-analyses on the primary endpoint of our study (expression of 82 prespecified genes), linear mixed models for repeat measurements with age and sex adjustment as well as Bonferroni adjustment of P values to account for multiple comparisons were carried out with the use of SAS version 9.4 (SAS Institute). Mean ± SE log2 fold changes (FC) within groups were calculated as differences between the expression levels at baseline and the expression levels at week 12: log2(FC) = log2(expression at week 12/expression at baseline).
For analyses on secondary endpoints, linear mixed models for repeat measurements, again implemented as intention-to-treat-analyses with age and sex adjustment, were carried out in SAS version 9.4 (SAS Institute). No adjustment for multiple comparisons was made for any of the secondary outcome measures.
The SAS syntax for the fitted linear mixed model that we used for analyses on the primary and the secondary endpoints is shown in the Supplemental Methods. In the MIXED procedure, we set all primary or secondary endpoints as the dependent variables (assigning values from baseline and week 12, or baseline and week 24, or baseline and week 50). Age and sex were used as fixed effects, time was set in the “repeat” statement with the participants identifier set as “subject,” and the covariance structure was set to “unstructured.” Based on the assumption that the repeated measurements of 1 individual are not independent, we decided to treat the participant identifier as a random effect in the model (equivalent to having time as “repeat” and “subject” as participant identifier). The results of the linear mixed models provided time, treatment, and time-by-treatment interaction effects for every outcome, but only P values for time-by-treatment interaction are reported here. In case of significant overall differences in the dependent variables across all 3 study groups according to the time-by-treatment P value, we carried out post hoc 2-group comparisons (ICR compared with CCR, ICR compared with Control, and CCR compared with Control) by linear mixed models adjusted for age and sex, again obtaining P values for time-by-treatment interactions. Our linear mixed model estimated the study effects under a missing-at-random assumption, including a maximum likelihood estimation of missing values (19). An overview of the frequency of missing values by parameter, dietary intervention group, and time point is provided in Supplemental Table 4.
Data on secondary endpoint measures were shown as mean values ± SDs (with normal 95% CIs) whereas relative changes (with baseline values as the reference) were expressed as means ± SEMs of loge relative changes (20). The loge relative changes were calculated as ln(value at week 12/value at baseline) 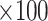 ; corresponding loge relative changes between baseline and week 24 or baseline and week 50 were calculated by the same formula, with the baseline value as the reference.
; corresponding loge relative changes between baseline and week 24 or baseline and week 50 were calculated by the same formula, with the baseline value as the reference.
RESULTS
Baseline characteristics
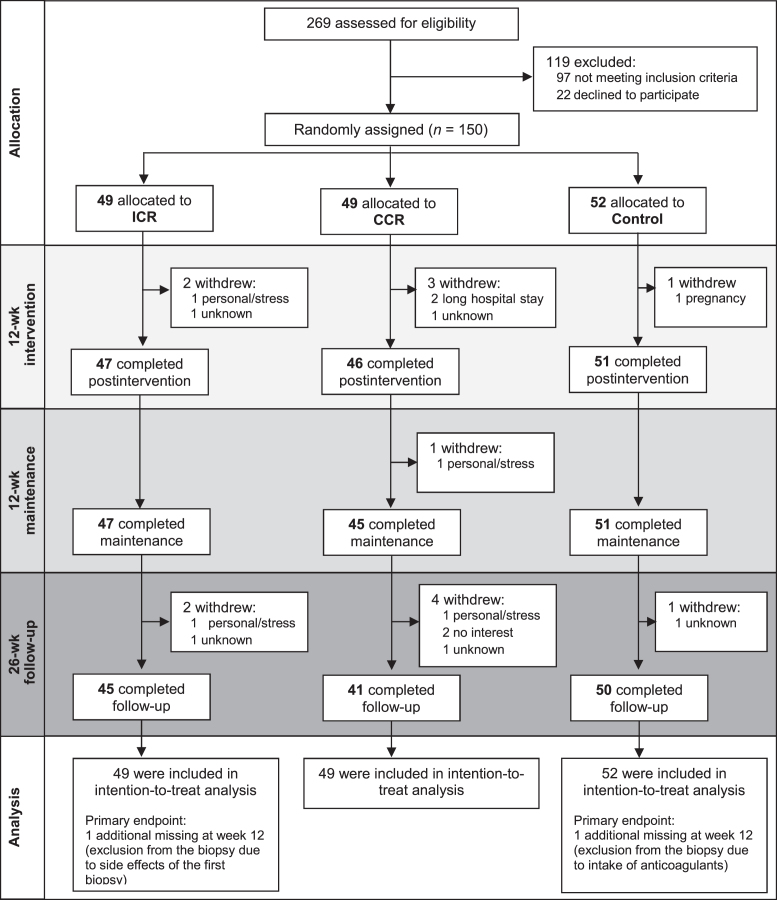
The 150 participants were randomly assigned to either ICR (n = 49), CCR (n = 49), or Control group (n = 52). At enrollment, the participants in the 3 study groups were comparable with regard to baseline characteristics, including sex, age, BMI, and educational level (Table 1). Overall, 144 participants (96.0%) completed the 12-wk intervention phase, 143 (95.3%) the 12-wk maintenance phase, and 136 (90.7%) the 26-wk follow-up phase (Figure 1). Across the entire study period of 50 wk there were 4 dropouts in the ICR, 7 in the CCR, and 2 in the Control group (for reasons for dropout see Supplemental Table 5).
TABLE 1.
Baseline characteristics of the study population1
| ICR | CCR | Control | |
|---|---|---|---|
| (n = 49; 49% women) | (n = 49; 49% women) | (n = 52; 52% women) | |
| Age, y | 49.4 ± 9.0 | 50.5 ± 8.0 | 50.7 ± 7.1 |
| Weight, kg | 96.4 ± 15.8 | 92.5 ± 15.7 | 93.3 ± 13.3 |
| Height, cm | 173.3 ± 9.7 | 171.9 ± 9.9 | 173.1 ± 9.9 |
| BMI, kg/m2 | 32.0 ± 3.8 | 31.2 ± 4.0 | 31.1 ± 3.6 |
| Visceral adipose tissue volume,2 cm³ | 4817.8 ± 1889.1 | 4894.5 ± 2178.3 | 4943.1 ± 2267.4 |
| Subcutaneous adipose tissue volume,2 cm³ | 12,821.6 ± 4267.2 | 12,193.1 ± 3996.6 | 11,944.8 ± 3845.2 |
| Education level,3n (%) | |||
| Primary school | 7 (14.3) | 5 (10.6) | 3 (3.9) |
| Secondary school certificate | 11 (22.5) | 14 (29.8) | 17 (32.7) |
| Higher education entrance qualification | 31 (62.3) | 28 (59.6) | 33 (63.5) |
| Systolic blood pressure, mm Hg | 139.4 ± 18.7 | 136.0 ± 16.7 | 136.0 ± 12.5 |
| Diastolic blood pressure, mm Hg | 87.2 ± 9.9 | 87.3 ± 8.7 | 87.8 ± 7.3 |
| Glucose, mg/dL | 92.7 ± 7.5 | 93.9 ± 7.5 | 93.5 ± 7.4 |
| Insulin, mU/L | 11.6 ± 5.4 | 12.6 ± 6.9 | 12.7 ± 7.3 |
| HOMA-IR | 2.7 ± 1.3 | 3.0 ± 1.7 | 3.0 ± 1.8 |
| HDL cholesterol, mg/dL | 54.1 ± 14.4 | 56.2 ± 16.3 | 51.8 ± 11.8 |
| LDL cholesterol, mg/dL | 124.5 ± 22.4 | 122.5 ± 31.5 | 130.4 ± 27.3 |
1Values are means ± SDs unless otherwise indicated,n = 150. CCR, continuous calorie restriction; ICR, intermittent calorie restriction.
2Abdominal adipose tissue phenotyping with magnetic resonance tomography imaging was initiated for 145 individuals who gave their additional informed consent to participate in the magnetic resonance-imaging module of the HELENA Trial.
3Two participants did not state their educational level.
FIGURE 1.
CONSORT diagram on the HELENA Trial. CCR, continuous calorie restriction; CONSORT, Consolidated Standards of Reporting Trials; ICR, intermittent calorie restriction.
Compliance to the calorie restriction
Compliance to ICR or CCR across the intervention phase was assessed by 7-d dietary records at baseline and during week 12 (Supplemental Table 6). The ICR group reported a loge relative change of −34.7% ± 4.5% in energy intake between baseline and week 12, followed by −25.4% ± 2.9% and −9.8% ± 3.5% in the CCR and Control groups, respectively (Poverall = 0.03, PICR vs. CCR = 0.10). Regarding macronutrient composition, there was an overall increase in protein and carbohydrate intake relative to total energy intake, paralleled by reductions in fat. Simultaneously, there was an increase in fiber intake and a decrease of alcohol consumption in all groups. To analyze possible changes in physical activity (which were not intended by design), energy expenditure was measured by accelerometer devices at baseline and in week 12. In contrast to the CCR and Control groups, where physical activity–associated energy expenditure showed almost no changes (CCR: 0.2% ± 4.4%; Controls: −2.9% ± 3.7%), there was a decrease by −13.4% ± 4.6% over the course of the intervention in the ICR group. However, no significant between-group differences in physical activity–associated energy expenditure were observed.
The average number of days at ∼25% energy intake over the 12-wk intervention phase was 1.8 d/wk in the ICR group. As reported in the dietary records, compliance was best between week 2 and week 7, when the majority of participants (>40 participants) performed 2 energy-restricted days per week (Supplemental Figure 1). Retrospective questionnaires at week 24 (n = 46) and week 50 (n = 42) showed that the number of participants who performed 2 energy-restricted days per week decreased across the maintenance and follow-up phases, from 15 (32.6%) in week 24 to 9 (21.4%) in week 50 (Supplemental Figure 1).
Intervention effects on body weight
The 12-wk intervention phase of the HELENA Trial was used as a biological model to first compare metabolic effects of ICR and CCR with regard to differences in adipose tissue gene expression levels and circulating biomarkers. Because it was our goal to investigate differences in gene expression and other metabolic markers between ICR and CCR at a similar net calorie intake and a projected weight loss of ∼5% over 12 wk, we first report the intervention effects on body weight, before outlining the effects on adipose tissue gene expression and circulating metabolic markers. Subsequently, we present our findings on the sustainability of intervention effects on anthropometric, body composition, and metabolic parameters after the maintenance phase (week 24) and follow-up phase (week 50).
Over the course of the 12-wk intervention phase, the greatest loge relative changes in weight (mean ± SEM) of −7.1% ± 0.7% were observed in the ICR group, followed by the CCR group (−5.2% ± 0.6%) and the Control group (−3.3% ± 0.6%) (Figure 2). Whereas statistical analyses showed a significant time-by-treatment interaction across all 3 study groups (P < 0.001) and for the 2-group comparisons of ICR with Control (P < 0.01) and CCR with Control (P < 0.01), the difference between ICR and CCR was borderline significant (P = 0.053).
FIGURE 2.
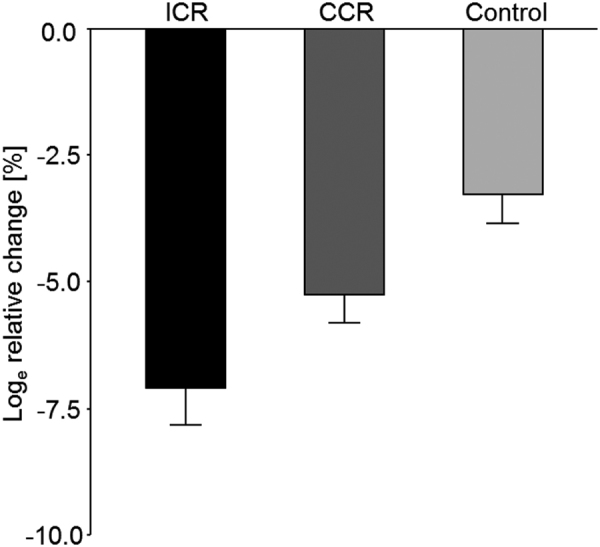
Changes in body weight by study group across the 12-wk intervention phase. Data are shown as mean ± SEM loge relative changes, with baseline values as the reference for the ICR group, CCR group, and control group (n = 150). Linear mixed models showed a significant time-by-treatment interaction across all 3 study groups (P < 0.001) and for the 2-group comparisons of ICR with Control (P < 0.01) and CCR with Control (P < 0.01); the difference between ICR and CCR was borderline significant (P = 0.053). CCR, continuous calorie restriction; ICR, intermittent calorie restriction.
Intervention effects on changes in SAT gene expression
Linear mixed models did not show significant differences in the adipose tissue expression of the 82 preselected genes across the 3 study groups after prespecified Bonferroni correction for multiple testing. Differences in gene expression over time and between groups were marginal for most genes. Without Bonferroni correction, expression levels of 13 of the preselected genes were different at P values <0.05 (see Table 2 for the 13 genes and Supplemental Table 2 for all 82 preselected genes). Of these, interleukin 8 (IL8) showed the greatest differences in FC between ICR and CCR (0.61 ± 0.23, Puncorrected = 0.02). However, the difference in FC between ICR and Control was lower (0.09 ± 0.24). The difference in FC between CCR and Control for IL8 was −0.51 ± 0.24. An additional post hoc screen across all transcripts of the microarray did not show any genes to be differentially expressed between the groups.
TABLE 2.
Preselected genes with significant differences (uncorrected Poverall < 0.05) in expression levels1
| Gene symbol | Mean FC2 | FC differences | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Address ID | ICR | CCR | Control | ICR-CCR | ICR-Control | CCR-Control | Uncorrected3 | Corrected4 | ||
| 7330035 | SFRP4 | secreted frizzled-related protein 4 | −0.13 ± 0.03 | −0.05 ± 0.03 | 0.01 ± 0.03 | −0.08 ± 0.04 | −0.15 ± 0.05 | −0.06 ± 0.04 | 0.003 | 0.24 |
| 6450672 | VEGFA | vascular endothelial growth factor A | 0.01 ± 0.01 | −0.05 ± 0.01 | 0.01 ± 0.02 | 0.06 ± 0.02 | 0.00 ± 0.02 | −0.06 ± 0.02 | 0.01 | 1 |
| 3940017 | LOX | lysyl oxidase | −0.23 ± 0.05 | −0.08 ± 0.05 | −0.04 ± 0.05 | −0.15 ± 0.07 | −0.19 ± 0.07 | −0.04 ± 0.07 | 0.02 | 1 |
| 3610129 | HSD17B1 | hydroxysteroid(17-β)dehydrogenase1 | −0.09 ± 0.02 | 0.02 ± 0.03 | −0.06 ± 0.03 | −0.10 ± 0.03 | −0.03 ± 0.04 | 0.07 ± 0.04 | 0.02 | 1 |
| 1980309 | IL8 | interleukin 8 | 0.12 ± 0.16 | −0.49 ± 0.16 | 0.03 ± 0.18 | 0.61 ± 0.23 | 0.09 ± 0.24 | −0.51 ± 0.24 | 0.02 | 1 |
| 2640543 | SFRP4 | secreted frizzled-related protein 4 | −0.22 ± 0.07 | −0.10 ± 0.06 | 0.02 ± 0.07 | −0.12 ± 0.09 | −0.24 ± 0.10 | −0.12 ± 0.09 | 0.03 | 1 |
| 4050022 | TSHR | thyroid stimulating hormone receptor | 0.03 ± 0.02 | −0.03 ± 0.02 | −0.01 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.02 | −0.03 ± 0.02 | 0.03 | 1 |
| 2900129 | FTO | fat mass and obesity associated | −0.05 ± 0.03 | 0.05 ± 0.02 | 0.00 ± 0.02 | −0.10 ± 0.04 | −0.04 ± 0.04 | 0.05 ± 0.03 | 0.03 | 1 |
| 2680056 | CES1 | carboxylesterase 1 | −0.43 ± 0.07 | −0.19 ± 0.08 | −0.32 ± 0.07 | −0.25 ± 0.10 | −0.12 ± 0.10 | 0.13 ± 0.10 | 0.03 | 1 |
| 7330544 | ALDOC | aldolase C, fructose-bisphosphate | −0.40 ± 0.07 | −0.31 ± 0.09 | −0.16 ± 0.06 | −0.09 ± 0.11 | −0.24 ± 0.09 | −0.15 ± 0.11 | 0.04 | 1 |
| 3710523 | CYP19A1 | cytochrome P450, family 19, subfamily A, polypeptide 1 | −0.05 ± 0.02 | −0.01 ± 0.02 | 0.02 ± 0.02 | −0.04 ± 0.03 | −0.07 ± 0.03 | −0.03 ± 0.03 | 0.04 | 1 |
| 1570553 | IL8 | interleukin 8 | −0.02 ± 0.08 | −0.25 ± 0.08 | 0.00 ± 0.08 | 0.23 ± 0.11 | −0.01 ± 0.12 | −0.24 ± 0.11 | 0.04 | 1 |
| 840309 | LEP | leptin | −0.23 ± 0.06 | −0.05 ± 0.05 | −0.14 ± 0.04 | −0.18 ± 0.07 | −0.09 ± 0.07 | 0.08 ± 0.06 | 0.04 | 1 |
1Data were included from 150 participants; statistical analyses were performed with the use of an intention-to-treat approach. CCR, continuous calorie restriction; FC, fold change; ICR, intermittent calorie restriction.
2Mean log FCs ± SEs within groups were calculated as differences between the expression levels at baseline (T0) and the expression levels at week 12 (T1): log2(FC) = log2(T1/T0).
3 Pvalues for time-by-treatment interactions across all 3 study groups were calculated with linear mixed models adjusted for age and sex. No adjustment was made for multiple comparisons. P values for post hoc pairwise comparisons between individual study groups are shown in Supplemental Table 2.
4 P values for time-by-treatment interactions across all 3 study groups were calculated with linear mixed models adjusted for age and sex including Bonferroni correction for multiple comparisons (P values were multiplied by the number of genes, i.e., by 82).
Intervention effects on circulating biomarkers
With the exception of fasting glucose, there were no differential effects of the 3 interventions on circulating biomarkers (Table 3). All study groups experienced reductions in fasting serum lipids (LDL, HDL, cholesterol, triglycerides), insulin concentrations, HOMA-IR levels, adipokines (adiponectin, leptin), liver function parameters (gamma-glutamyl transpeptidase, alanine transaminase, aspartame transaminase), and brain-derived neurotropic factor, and increases in resistin and IGF-1 concentrations, without significant between-group differences (Table 3). Further inflammatory markers (C-reactive protein, interferon-γ, IL-6, IL-8, TNF-α) showed no significant between-group differences, either. The only measured parameter for which we observed a nominally significant difference across all 3 groups (P = 0.04) and between ICR and CCR (P < 0.01) was fasting glucose, with loge relative changes of −2.9% ± 1.2% (ICR) and −7.6% ± 1.2% (CCR). However, it should be noted that the effects of neither ICR nor CCR were statistically different from those of the control regimen (−5.2% ± 1.4%), and that no differences in glucose concentrations between ICR and CCR were observed at later time points.
TABLE 3.
Intervention effects on blood-based biomarkers, HR-QoL, liver fat, and liver function parameters1
| Baseline | Week 12 | ||||
|---|---|---|---|---|---|
| Mean ± SD (95% CI) | Mean ± SD (95% CI) | Loge relative change2 | P value3 overall | ||
| Blood-based biomarkers | |||||
| LDL cholesterol, mg/dL | ICR | 124.5 ± 22.4 (119.0, 130.0) | 115.3 ± 28.3 (108.4, 122.2) | −7.5 ± 2.6 | 0.74 |
| CCR | 122.5 ± 31.5 (114.9, 130.0) | 116.1 ± 26.3 (109.5, 122.7) | −7.9 ± 2.1 | ||
| Control | 130.4 ± 27.3 (124.0, 136.8) | 119.8 ± 23.9 (114.1, 125.5) | −8.7 ± 2.4 | ||
| HDL cholesterol, mg/dL | ICR | 54.1 ± 14.4 (50.7, 57.6) | 49.6 ± 13.8 (46.2, 53.0) | −8.7 ± 2.5 | 0.39 |
| CCR | 56.2 ± 16.3 (52.3, 60.1) | 50.4 ± 14.7 (46.7, 54.1) | −12.3 ± 1.8 | ||
| Control | 51.8 ± 11.8 (49.1, 54.5) | 47.2 ± 10.5 (44.7, 49.7) | −9.2 ± 1.9 | ||
| Cholesterol, mg/dL | ICR | 205.0 ± 30.8 (197.6, 212.4) | 185.1 ± 36.8 (176.1, 194.1) | −10.9 ± 2.3 | 1.00 |
| CCR | 202.9 ± 39.3 (193.5, 212.4) | 186.6 ± 31.8 (178.6, 194.6) | −10.6 ± 1.4 | ||
| Control | 211.8 ± 36.1 (203.4, 220.2) | 191.1 ± 31.3 (183.7, 198.5) | −9.7 ± 1.7 | ||
| Triglycerides, mg/dL | ICR | 130.0 ± 83.8 (110.0, 150.1) | 100.6 ± 47.5 (89.0, 112.3) | −20.7 ± 6.0 | 0.68 |
| CCR | 121.2 ± 66.3 (105.3, 137.1) | 100.8 ± 48.4 (88.7, 112.9) | −19.2 ± 3.4 | ||
| Control | 145.0 ± 85.5 (125.1, 164.8) | 120.5 ± 59.2 (106.5, 134.5) | −13.2 ± 4.9 | ||
| Insulin, mU/L | ICR | 11.6 ± 5.4 (10.3, 12.9) | 10.8 ± 5.6 (9.4, 12.2) | −6.3 ± 6.6 | 0.19 |
| CCR | 12.6 ± 6.9 (11.0, 14.3) | 9.7 ± 5.1 (8.4, 11.0) | −19.3 ± 5.2 | ||
| Control | 12.7 ± 7.3 (11.0, 14.4) | 11.7 ± 7.3 (10.0, 13.5) | −9.1 ± 5.7 | ||
| HOMA-IR | ICR | 2.7 ± 1.3 (2.4, 3.0) | 2.4 ± 1.3 (2.1, 2.7) | −9.0 ± 7.2 | 0.11 |
| CCR | 3.0 ± 1.7 (2.5, 3.4) | 2.1 ± 1.2 (1.8, 2.4) | −27.1 ± 5.9 | ||
| Control | 3.0 ± 1.8 (2.6, 3.4) | 2.6 ± 1.8 (2.2, 3.1) | −14.3 ± 6.3 | ||
| IGF-1, ng/mL | ICR | 108.9 ± 31.3 (101.3, 116.6) | 120.1 ± 38.4 (110.7, 129.5) | 11.7 ± 4.7 | 0.50 |
| CCR | 115.1 ± 36.6 (105.9, 124.9) | 115.4 ± 37.6 (105.9, 124.9) | 3.4 ± 5.5 | ||
| Control | 121.4 ± 32.1 (114.0, 128.9) | 125.9 ± 44.9 (115.3, 136.6) | 2.5 ± 4.2 | ||
| Glucose, mg/dL | ICR | 92.7 ± 7.5 (90.9, 94.5) | 89.9 ± 7.2 (88.1, 91.6) | −2.9 ± 1.2 | 0.04 |
| CCR | 93.9 ± 7.5 (92.1, 95.7) | 87.2 ± 7.8 (85.2, 89.2) | −7.6 ± 1.2 | ICR vs. CCR P = 0.01 CCR vs. Control P = 0.16 ICR vs. Control P = 0.24 | |
| Control | 93.5 ± 7.4 (91.8, 95.3) | 89.0 ± 9.5 (86.8, 91.3) | −5.2 ± 1.4 | ||
| Adiponectin, ng/mL | ICR | 18.3 ± 12.5 (15.3, 21.3) | 17.5 ± 11.0 (14.8, 20.2) | −5.2 ± 3.9 | 0.94 |
| CCR | 17.1 ± 10.3 (14.6, 19.6) | 16.2 ± 9.3 (13.9, 18.5) | −7.5 ± 3.6 | ||
| Control | 16.6 ± 10.2 (14.2, 19.0) | 15.5 ± 9.8 (13.2, 17.9) | −7.9 ± 4.1 | ||
| Leptin, ng/mL | ICR | 25.2 ± 24.0 (19.4, 31.0) | 16.8 ± 16.2 (12.8, 20.7) | −47.9 ± 9.2 | 0.78 |
| CCR | 23.3 ± 19.8 (18.6, 28.1) | 15.9 ± 16.9 (11.6, 20.2) | −48.4 ± 6.5 | ||
| Control | 25.9 ± 25.0 (20.1, 31.7) | 18.9 ± 22.0 (13.7, 24.1) | −44.3 ± 8.2 | ||
| Resistin, ng/mL | ICR | 5.7 ± 2.5 (5.1, 6.3) | 6.5 ± 2.7 (5.9, 7.2) | 14.4 ± 5.3 | 0.55 |
| CCR | 5.8 ± 2.3 (5.3, 6.4) | 6.7 ± 2.4 (6.1, 7.3) | 16.7 ± 4.5 | ||
| Control | 5.6 ± 2.4 (5.0, 6.2) | 6.9 ± 2.8 (6.2, 7.6) | 20.8 ± 4.3 | ||
| CRP, ng/pL | ICR | 4.2 ± 4.0 (3.2, 5.2) | 4.2 ± 5.0 (3.0, 5.4) | −17.0 ± 8.5 | 0.45 |
| CCR | 4.1 ± 3.8 (3.2, 5.0) | 3.2 ± 3.2 (2.4, 4.0) | −24.5 ± 10.8 | ||
| Control | 5.4 ± 7.9 (3.6, 7.2) | 4.0 ± 4.2 (3.0, 5.0) | −25.1 ± 13.3 | ||
| IL-6, ng/µL | ICR | 1.3 ± 1.0 (1.1, 1.5) | 1.4 ± 1.1 (1.1, 1.7) | 5.6 ± 8.7 | 0.10 |
| CCR | 1.5 ± 1.4 (1.2, 1.8) | 1.7 ± 1.7 (1.3, 2.1) | 12.1 ± 7.7 | ||
| Control | 1.9 ± 3.0 (1.2, 2.6) | 1.6 ± 1.8 (1.2, 2.0) | −8.0 ± 8.1 | ||
| IL-8, ng/µL | ICR | 11.3 ± 5.1 (10.1, 12.6) | 10.6 ± 5.0 (9.3, 11.8) | −8.8 ± 3.9 | 0.51 |
| CCR | 9.8 ± 4.3 (8.8, 10.9) | 10.4 ± 4.5 (9.2, 11.5) | −0.3 ± 3.9 | ||
| Control | 12.2 ± 19.4 (7.7, 16.7) | 12.4 ± 13.6 (9.2, 15.6) | 6.6 ± 5.5 | ||
| IFN-γ, ng/µL | ICR | 13.1 ± 10.0 (10.7, 15.4) | 23.4 ± 77.4 (4.5, 42.4) | 4.8 ± 12.3 | 0.90 |
| CCR | 16.3 ± 14.4 (12.8, 19.7) | 21.1 ± 38.5 (11.5, 30.8) | 1.1 ± 10.0 | ||
| Control | 17.7 ± 23.8 (12.2, 23.2) | 24.9 ± 53.8 (12.2, 37.7) | 1.5 ± 12.7 | ||
| TNF-α, ng/µL | ICR | 4.6 ± 2.7 (3.9, 5.2) | 4.4 ± 2.7 (3.8, 5.1) | −0.5 ± 2.7 | 0.72 |
| CCR | 4.7 ± 2.8 (4.0, 5.3) | 4.3 ± 2.7 (3.6, 5.0) | −2.5 ± 2.3 | ||
| Control | 4.2 ± 2.6 (3.6, 4.8) | 4.4 ± 2.6 (3.8, 5.0) | 1.5 ± 2.7 | ||
| BDNF, ng/mL | ICR | 2.1 ± 0.6 (1.9, 2.2) | 1.9 ± 0.5 (1.8, 2.0) | −10.2 ± 2.1 | 0.22 |
| CCR | 2.2 ± 0.8 (2.1, 2.4) | 2.1 ± 0.4 (2.0, 2.2) | −6.7 ± 3.2 | ||
| Control | 2.1 ± 0.6 (1.9, 2.2) | 2.0 ± 0.6 (1.8, 2.1) | −4.5 ± 2.6 | ||
| SHBG, nM | ICR | 36.4 ± 24.3 (30.5, 42.3) | 42.0 ± 27.1 (35.4, 48.6) | 15.0 ± 5.1 | 0.68 |
| CCR | 40.7 ± 38.0 (31.5, 49.9) | 44.4 ± 28.3 (37.0, 51.7) | 11.9 ± 6.2 | ||
| Control | 35.2 ± 18.6 (30.8, 39.6) | 39.2 ± 25.1 (33.3, 45.2) | 6.8 ± 6.1 | ||
| Women only (n = 75) | |||||
| Estrone, pg/mL | |||||
| Premenopausal | ICR | 42.6 ± 12.1 (34.5, 50.7) | 38.3 ± 16.6 (27.2, 49.4) | −21.8 ± 16.2 | 0.72 |
| CCR | 39.4 ± 22.3 (26.5, 52.4) | 44.7 ± 33.5 (22.2, 67.1) | 10.1 ± 27.6 | ||
| Control | 56.0 ± 37.0 (36.8, 75.2) | 51.2 ± 32.6 (33.4, 69.0) | −8.2 ± 16.3 | ||
| Postmenopausal | ICR | 33.5 ± 13.7 (27.5, 39.5) | 39.2 ± 18.1 (31.3, 47.1) | 11.7 ± 8.2 | 0.82 |
| CCR | 34.0 ± 17.2 (25.9, 42.1) | 36.5 ± 18.6 (27.6, 45.3) | 9.1 ± 5.6 | ||
| Control | 30.1 ± 12.0 (24.7, 35.6) | 33.4 ± 16.3 (25.4, 41.5) | 13.2 ± 15.7 | ||
| Testosterone, ng/mL | ICR | 0.5 ± 0.2 (0.4, 0.6) | 0.5 ± 0.2 (0.4, 0.6) | 0.7 ± 9.1 | 0.50 |
| CCR | 0.5 ± 0.4 (0.4, 0.6) | 0.4 ± 0.2 (0.3, 0.5) | 1.8 ± 27.0 | ||
| Control | 0.6 ± 0.2 (0.5, 0.6) | 0.5 ± 0.2 (0.4, 0.6) | −12.7 ± 6.4 | ||
| Men only (n = 75) | |||||
| Estrone, pg/mL | ICR | 39.6 ± 13.1 (35.0, 44.2) | 36.9 ± 11.2 (32.8, 41.0) | −10.3 ± 5.4 | 0.93 |
| CCR | 39.0 ± 14.5 (34.0, 43.9) | 35.6 ± 12.3 (31.2, 40.0) | −10.7 ± 5.2 | ||
| Control | 35.7 ± 11.9 (31.7, 39.8) | 31.4 ± 9.1 (28.3, 34.5) | −11.6 ± 4.5 | ||
| Testosterone, ng/mL | ICR | 4.3 ± 1.8 (3.7, 5.0) | 4.1 ± 1.7 (3.5, 4.7) | −4.4 ± 9.5 | 0.37 |
| CCR | 3.6 ± 1.4 (3.1, 4.1) | 4.2 ± 2.5 (3.3, 5.1) | 4.9 ± 11.0 | ||
| Control | 3.7 ± 2.0 (3.0, 4.4) | 3.6 ± 1.7 (3.0, 4.2) | −5.2 ± 12.6 | ||
| HR-QoL | |||||
| PCS | ICR | 53.6 ± 6.8 (51.9, 55.4) | 53.3 ± 8.1 (51.3, 55.4) | −2.2 ± 3.1 | 0.37 |
| CCR | 52.7 ± 7.0 (51.0, 54.5) | 54.4 ± 6.1 (52.8, 55.9) | 3.6 ± 1.6 | ||
| Control | 51.2 ± 8.1 (49.2, 53.2) | 51.9 ± 7.5 (50.1, 53.7) | 1.6 ± 2.7 | ||
| MCS | ICR | 49.7 ± 11.2 (46.8, 52.6) | 51.7 ± 11.4 (48.8, 54.6) | 3.8 ± 5.1 | 0.82 |
| CCR | 49.3 ± 9.5 (46.9, 51.6) | 50.8 ± 11.3 (47.9, 53.7) | 0.9 ± 3.7 | ||
| Control | 50.3 ± 9.3 (48.0, 52.5) | 53.1 ± 7.0 (51.4, 54.8) | 6.3 ± 2.6 | ||
| Liver fat and function parameters | |||||
| Liver fat, % | ICR | 7.7 ± 4.6 (6.5, 8.8) | 5.2 ± 3.4 (4.3, 6.0) | −37.0 ± 8.6 | 0.11 |
| CCR | 8.4 ± 8.0 (6.4, 10.3) | 6.1 ± 5.6 (4.7, 7.5) | −34.6 ± 5.1 | ||
| Control | 7.1 ± 4.7 (5.9, 8.2) | 5.7 ± 4.4 (4.6, 6.8) | −24.8 ± 6.2 | ||
| GGT, U/L | ICR | 27.0 ± 14.4 (23.5, 30.4) | 19.4 ± 11.1 (16.6, 22.1) | −34.6 ± 5.5 | 0.20 |
| CCR | 25.6 ± 14.4 (22.1, 29.0) | 20.9 ± 10.5 (19.9, 26.4) | −21.9 ± 4.3 | ||
| Control | 28.9 ± 18.1 (24.6, 33.0) | 23.8 ± 15.5 (20.1, 27.5) | −21.4 ± 3.3 | ||
| AST, U/L | ICR | 23.4 ± 5.2 (22.2, 24.7) | 19.6 ± 4.7 (18.5, 20.7) | −16.9 ± 3.0 | 0.15 |
| CCR | 23.3 ± 6.0 (21.9, 24.7) | 21.5 ± 5.0 (20.2, 22.8) | −8.5 ± 3.6 | ||
| Control | 22.7 ± 4.9 (21.5, 23.8) | 21.0 ± 4.6 (19.9, 22.0) | −8.4 ± 3.3 | ||
| ALT, U/L | ICR | 27.4 ± 10.4 (24.9, 29.9) | 20.8 ± 11.1 (18.1, 23.5) | −30.4 ± 4.2 | 0.08 |
| CCR | 26.6 ± 12.3 (23.7, 29.6) | 23.9 ± 10.8 (21.6, 26.9) | −9.8 ± 5.6 | ||
| Control | 26.5 ± 10.9 (24.0, 29.1) | 22.1 ± 8.1 (20.2, 24.1) | −17.0 ± 3.3 | ||
1Data were included from 150 participants; statistical analyses were performed with the use of an intention-to-treat approach. ALT, alanine transaminase; AST, aspartame transaminase; BDNF, brain-derived neurotropic factor; CCR, continuous calorie restriction; CRP, C-reactive protein; GGT, gamma-glutamyl transpeptidase; HR-QoL, health-related quality of life; ICR, intermittent calorie restriction; IFN-γ, interferon gamma; IGF-1, insulin-like growth factor 1; MCS, mental component summary score; PCS, physical component summary score; SHBG, sex hormone-binding globulin.
2Means ± SEMs of individual loge relative changes between baseline and postintervention; calculated as ln(value week 12/value baseline) x 100.
3 P values for time-by-treatment interactions across all 3 study groups (overall) were calculated with linear mixed models adjusted for age and sex. No adjustment was made for multiple comparisons. If the “P value overall” was significant (P < 0.05), P values for time-by-treatment interactions (also from linear mixed models adjusted for age and sex) of post hoc 2-group comparisons, i.e., ICR compared with CCR, ICR compared with Control, and CCR compared with Control, are shown.
Sensitivity analyses
Because 2 previous trials on ICR and metabolic biomarkers, which were carried out only among overweight and obese women, had indicated that ICR, operationalized as the “5:2 diet,” had more pronounced effects on insulin concentrations and HOMA-IR than CCR (5, 6), we further stratified our analyses on differences in biomarker concentrations (shown in Table 3) by sex. However, there were no significant differences in the effects of ICR and CCR on glucose, insulin, HOMA-IR, or any other endpoints among either female or male participants of our trial. There was no indication for heterogeneity of findings between study participants who were overweight (BMI ≥25 to <30), and participants who were obese (BMI ≥30 to <40) at baseline. Finally, sensitivity analyses excluding the 14 participants of the ICR group who had an energy-restricted day before the blood draw at week 12 had no substantial effect on the study results.
Sustainability of intervention effects across the maintenance and follow-up phases
The investigated outcomes across the maintenance and follow-up phases included changes in anthropometric measures (body weight, BMI, and waist circumference), body composition (SAT and VAT volumes), liver fat content, blood pressure values, and circulating biomarkers. With regard to circulating biomarker concentrations, we only quantified those serum markers across all study assessments for which at least within-group changes were evident at week 12.
The average achieved weight loss over the intervention phase was maintained in all study groups up to week 24, followed by a slight trend for weight regain between weeks 24 and 50, especially in the ICR and control groups (Figure 3 and Supplemental Table 7). Overall, mean loge relative changes in weight between baseline and week 50 were highest for the ICR group (−5.2% ± 1.2%), followed by the CCR (−4.9% ± 1.1%) and control groups (−1.7% ± 0.8%). Although there were significant time-by-treatment interactions (P < 0.05) for differences in body weight across the 3 study groups and for the pairwise comparison of ICR with Control and CCR with Control at weeks 24 and 50, there were no significant differences between ICR and CCR.
FIGURE 3.
Change in anthropometric measures and abdominal body composition by study group (n = 150). Data are shown as means ± SEM of loge relative changes for (A) body weight, (B) waist circumference, (C) VAT, and (D) SAT with baseline values as the reference. See Supplemental Table 6 for mean values (SD and 95% CI) and statistical analyses for differences between the study groups based on linear mixed models. CCR, continuous calorie restriction; ICR, intermittent calorie restriction; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
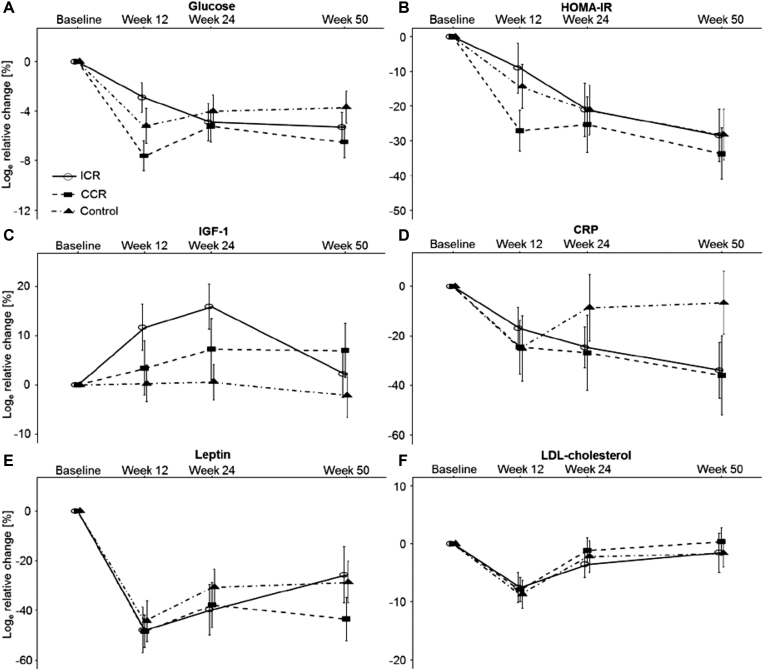
The loge relative changes in waist circumference and abdominal VAT and SAT volumes showed a pattern similar to the one observed in body weight over time (Figure 3). There were no significant differences for the observed decreases in systolic and diastolic blood pressure values between the groups across the 12-wk intervention phase and neither were there any differences at the 50-wk follow-up assessment (Supplemental Figure 2). Loge relative changes in circulating biomarkers over the 50-wk study course are depicted in Figure 4 and Supplemental Table 7. Overall, there were no significant between-group differences in LDL cholesterol, HDL cholesterol, cholesterol, triglycerides, insulin, HOMA-IR, glucose, C-reactive protein, IGF-1, leptin, and resistin.
FIGURE 4.
Changes in circulating biomarkers by study group (n = 150). Data are shown as means ± SEM of loge relative changes for (A) glucose, (B) HOMA-IR, (C) IGF-1, (D) CRP, (E) leptin, and (F) LDL-cholesterol with baseline values as the reference. See Supplemental Table 6 for mean values (SD and 95% CI) and statistical analyses for differences between the study groups based on linear mixed models. CRP, C-reactive protein; IGF-1, insulin-like growth factor 1.
HR-QoL and side effects
HR-QoL, reported as physical and mental component summary scores, was assessed with the standardized Short Form Health Survey-12 (SF-12) at baseline, week 12, and week 50. There were no significant between-group differences regarding HR-QoL (Supplemental Figure 3). No major adverse effects of the interventions were reported. In the ICR group, 5 individuals reported minor adverse physical symptoms (Supplemental Table 8), including feeling cold, tired, and having mild headaches, on energy-restricted days. Two participants reported mild cognitive impairments, i.e., lack of concentration, on energy-restricted days. Moreover, 3 participants in the ICR group reported mild adverse physical effects (dizziness, cramps) on non–energy-restricted days. In the CCR group, cognitive (lack of concentration) or physical (appearance of skin rash with increased intake of whole-grain bread) adverse effects were each reported by 1 individual. There were no side effects in the control group. Adverse effects were only reported in the intervention phase, but not in the maintenance or follow-up phases.
DISCUSSION
In this randomized trial, ICR did not exert stronger effects on the adipose tissue transcriptome, circulating biomarkers (of glucose metabolism, lipid metabolism, and inflammation as well as adipokines and steroid hormones), body weight, or VAT and SAT volumes than CCR among overweight or obese adults, neither during the 12-wk intervention phase nor over the 12-wk maintenance phase or 26-wk follow-up phase. The only exception was glucose, for which a significantly stronger decrease at week 12 was observed for CCR compared with ICR, despite a borderline significant trend for greater weight loss with ICR. However, there was no significant difference in glucose concentrations between either CCR or ICR and the control regimen, and no differential effects of the dietary regimes on glucose concentrations were observed at weeks 24 and 50. Thus, considering the high number of tested endpoints, our findings of differential changes in glucose concentrations over the intervention phase could also be due to chance.
The impact of timing of calorie restriction (intermittent compared with continuous) on differences in the expression of genes in adipose tissue was investigated, to our knowledge, for the first time in this trial. With correction for multiple comparisons, there were no significant differences in the expression of preselected genes implicated in energy and macronutrient metabolism, insulin signaling, inflammation, and growth factor signaling between the study arms. Transcriptome-wide analyses beyond the preselected genes did not reveal differences, either, which indicates that timing of energy restriction may not induce differential transcriptional regulation of adipose tissue metabolism at similar levels of net energy intake. This finding is in agreement with transcriptomics studies, in which adipose tissue transcriptome regulation was a function of overall calorie restriction and weight loss, rather than the underlying dieting approach (low-fat or low-carbohydrate diet) to achieve calorie restriction (16, 21, 22).
To date ICR, operationalized as the “5:2 diet,” has been compared with CCR at an equal net calorie intake in 4 studies (5, 6, 23, 24). A 12-wk pilot trial by Carter et al. (23) among type 2 diabetics (n = 63) revealed comparable effects of ICR and CCR with respect to weight loss, fat mass, fat-free mass, and glycated hemoglobin concentrations. Another pilot trial by Conley et al. (24) showed that the “5:2 diet” and CCR were similarly effective to induce weight loss and changes in biomarkers of lipid metabolism among 24 male war veterans over 6 mo. In 2 RCTs among nondiabetic overweight or obese women (n = 107 and n = 115) by Harvie et al. (5, 6), no significant differences regarding weight loss or metabolic biomarkers were observed between ICR and CCR, which is consistent with our results. However, Harvie et al. reported significantly greater decreases in insulin concentrations (5, 6), HOMA-IR (5, 6), and total fat mass (6) with ICR than with CCR. In our trial, by contrast, there were no differences in insulin concentrations, HOMA-IR, and fat volumes. Although the reason for the inconsistency of findings on ICR and insulin sensitivity remains elusive, one possible explanation may be that the 2 energy-restricted days were consecutive in the Harvie et al. trials, but nonconsecutive in ours. Nevertheless, results from the Harvie et al. trials, ours, and other controlled human studies (10) are consistent in that they suggest similar effects of ICR and CCR regarding other cardiometabolic outcomes.
The present study was the first trial, to our knowledge, on the “5:2 diet” to include an extended 6-mo follow-up phase (after a 3-mo intervention phase and a 3-mo maintenance phase) to investigate if participants would maintain the dieting regimen for long-term weight control. We observed a slight trend for weight regain after initial weight loss in the ICR group, which was not observed for the CCR group. Interestingly, the weight regain in the ICR group was paralleled by a trend for re-increase in SAT rather than VAT volume. However, there were no significant differences in body weight, waist circumference, and VAT or SAT volume between ICR and CCR at any study time point. Despite the slight tendency of weight regain in the ICR group, the low dropout rates and maintenance of HR-QoL scores in all study groups of the present trial indicate that the “5:2 diet” and CCR are similarly practicable and beneficial for overweight and obese individuals. The reported decrease in compliance to the 2 energy-restricted days per week as the trial progressed may suggest that the “5:2 diet” is a feasible approach for initial weight loss, whereas longer-term adherence is restricted to a subgroup of overweight and obese individuals. At the same time, a "6:1" scheme after successful initial weight loss may be sufficient for weight maintenance so that adherence to 2 energy-restricted days over longer durations may not be needed. Overall, the results suggest that the “5:2 diet” may be an appropriate alternative to CCR for individuals who tolerate it well.
Of note, results of a 1-y RCT on another popular form of ICR, alternate day fasting (ADF), have recently been published (25). In this trial, effects of ADF with alternating “fast days” (25% of baseline energy intake) and “feast days” (125% energy intake) were compared with those of CCR. Both ADF and CCR showed equal benefits with regard to changes in body weight and metabolic biomarkers over time and there were no significant differences between the regimens (25). Hence, our results and the findings by Trepanowski et al. (25) suggest that ICR and CCR regimens are similarly effective to induce weight loss and to improve metabolic biomarkers.
Our trial made possible in-depth analyses on the effects of ICR based on a wide range of metabolic biomarkers over 50 wk, to our knowledge for the first time. The open-label design of the study guaranteed higher external validity compared with a feeding trial, and facilitated an assessment of the practicability of ICR in a real-world setting. However, because the recommended foods and beverages had to be purchased and prepared by the study participants and were not administered in a controlled setup, we could not directly monitor participants’ compliance. Nevertheless, our data on weight loss and loge relative changes in dietary intakes do point to very good overall compliance. With respect to the comparison of ICR and CCR with the control regimen, it has to be considered that the control group also showed moderate initial weight loss, although energy restriction was not explicitly recommended. This may be a trial effect, i.e., a change of behavior under observation in a study. In addition, our approach to standardize dietary composition in all 3 groups by advising participants to follow the guidelines for a healthy balanced diet by the German Nutrition Society may have led to moderate weight loss in the control group. One further limitation of our trial is that we could not use VAT samples for gene expression analyses, because taking VAT biopsies in a dietary intervention trial such as ours is not possible for ethical and practical reasons. Two prespecified secondary endpoints—differences in circulating bile acids and telomere lengths—could not be assessed owing to technical and financial reasons. Finally, the generalizability of the findings to other populations may be limited considering the enrollment of predominantly metabolically healthy overweight and obese individuals of European ancestry.
In summary, this study indicated that ICR and CCR are alternative energy restriction regimens for weight loss with comparable improvements to obesity-associated metabolic profiles, at least over 50 wk. Both regimens were well tolerated by the majority of participants and may be equivalent weight management approaches. Further investigations are needed on the effectiveness, practicability, and safety of ICR for patients with chronic diseases, such as type 2 diabetes, cardiovascular diseases, or cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our study team (Marie-Luise Groß, Laura Gruner, Erna Motsch, and Karin Nischwitz), the laboratory team (Muhabbet Celik, Bettina Ehret, Christine Niesik, Renate Skatula, and Marita Wenzel), and the operational team (Waltraud Kröner, Ilona Krüger-Friedemann, Yvonne Küster, and Christoph Neumann). Biomaterial samples were processed by the Biobank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the Biobank. We also thank all members of the Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg, for performing the MR-imaging. We thank the microarray unit of the DKFZ Genomics and Proteomics Core Facility for providing the Illumina Whole, Genome Expression Beadchips and related services.
The authors’ responsibilities were as follows—RS, CMU, MK, H-UK, R Kaaks, and TK: designed the research; RS, MEG, TJ, R Kirsten, and TK: conducted the research; DS, TN, DN, and RS: analyzed the data or performed the statistical analysis; JN, OvS, CLS, and H-UK: organized and supervised the MR component of the trial; RS and TK: wrote the manuscript and had primary responsibility for final content; and all authors: discussed the results, critically revised the manuscript, and approved its final content. None of the authors reported a conflict of interest related to the study.
Notes
The HELENA Trial was funded by the Helmholtz Association of German Research Centers (Cross Program Topic: Metabolic Dysfunction). MRI examinations were performed in and financed by the Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg. The financing of the MRI was also supported by the Stiftung zur Förderung der Erforschung der Zivilisationserkrankungen, Baden-Baden, Germany. CMU was funded by the Huntsman Cancer Foundation, Salt Lake City, UT and by NIH grant U01 CA 206110.
Supplemental Tables 1–8, Supplemental Figures 1–3, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ADF, alternate day fasting; CCR, continuous calorie restriction; FC, fold change; HR-QoL, health-related quality of life; ICR, intermittent calorie restriction; IGF-1, insulin-like growth factor 1; MR-imaging, magnetic resonance tomography imaging; RCT, randomized controlled trial; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
REFERENCES
- 1. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. PNAS. 2003;100(10):6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48(10):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Ling L, Su G, Han M, Fan X, Xun P, Xu G. Effect of intermittent versus chronic calorie restriction on tumor incidence: a systematic review and meta-analysis of animal studies. Sci Rep. 2016;6:33739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG et al.. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S et al.. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—a narrative review of human and animal evidence. Behav Sci (Basel). 2017;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend?. Int J Obes (Lond). 2015;39(5):727–33. [DOI] [PubMed] [Google Scholar]
- 9. Thom G, Lean M. Is there an optimal diet for weight management and metabolic health?. Gastroenterology. 2017;152(7):1739–51. [DOI] [PubMed] [Google Scholar]
- 10. Headland M, Clifton PM, Carter S, Keogh JB. Weight-loss outcomes: a systematic review and meta-analysis of intermittent energy restriction trials lasting a minimum of 6 months. Nutrients. 2016;8(6):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schübel R, Graf ME, Nattenmuller J, Nabers D, Sookthai D, Gruner LF, Johnson T, Schlett CL, von Stackelberg O, Kirsten R et al.. The effects of intermittent calorie restriction on metabolic health: rationale and study design of the HELENA Trial. Contemp Clin Trials. 2016;51:28–33. [DOI] [PubMed] [Google Scholar]
- 12. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465–7. [DOI] [PubMed] [Google Scholar]
- 13. Harris JA, Benedict FG. A biometric study of human basal metabolism. PNAS. 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oberritter H, Schabethal K, von Ruesten A, Boeing H. The DGE nutrition circle – presentation and basis of the food-related recommendations from the German Nutrition Society (DGE). ErnahrungsUmschau. 2013;60(2):24–9. [Google Scholar]
- 15. Jungvogel A, Wendt I, Schabethal K, Leschik-Bonnet E, Oberritter H. Revised: the 10 DGE rules. ErnahrungsUmschau. 2013;60(11):M644–M5. [Google Scholar]
- 16. Campbell KL, Foster-Schubert KE, Makar KW, Kratz M, Hagman D, Schur EA, Habermann N, Horton M, Abbenhardt C, Kuan LY et al.. Gene expression changes in adipose tissue with diet- and/or exercise-induced weight loss. Cancer Prev Res (Phila). 2013;6(3):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17(6):520–5. [DOI] [PubMed] [Google Scholar]
- 18. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. [DOI] [PubMed] [Google Scholar]
- 19. Baraldi AN, Enders CK. An introduction to modern missing data analyses. J School Psychol. 2010;48(1):5–37. [DOI] [PubMed] [Google Scholar]
- 20. Tornqvist L, Vartia P, Vartia YO. How should relative changes be measured?. Am Stat. 1985;39(1):43. [Google Scholar]
- 21. Capel F, Viguerie N, Vega N, Dejean S, Arner P, Klimcakova E, Martinez JA, Saris WH, Holst C, Taylor M et al.. Contribution of energy restriction and macronutrient composition to changes in adipose tissue gene expression during dietary weight-loss programs in obese women. J Clin Endocrinol Metab. 2008;93(11):4315–22. [DOI] [PubMed] [Google Scholar]
- 22. Dahlman I, Linder K, Arvidsson Nordstrom E, Andersson I, Liden J, Verdich C, Sorensen TI, Arner P. Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am J Clin Nutr. 2005;81(6):1275–85. [DOI] [PubMed] [Google Scholar]
- 23. Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract. 2016;122:106–12. [DOI] [PubMed] [Google Scholar]
- 24. Conley M, Le Fevre L, Haywood C, Proietto J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr Diet. 2018;75(1):65–72. [DOI] [PubMed] [Google Scholar]
- 25. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J et al.. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.