Abstract
The impact of chronic exposure to fine particulate matter (particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5)) on respiratory disease and lung cancer mortality is poorly understood. In a cohort of 18.9 million Medicare beneficiaries (4.2 million deaths) living across the conterminous United States between 2000 and 2008, we examined the association between chronic PM2.5 exposure and cause-specific mortality. We evaluated confounding through adjustment for neighborhood behavioral covariates and decomposition of PM2.5 into 2 spatiotemporal scales. We found significantly positive associations of 12-month moving average PM2.5 exposures (per 10-μg/m3 increase) with respiratory, chronic obstructive pulmonary disease, and pneumonia mortality, with risk ratios ranging from 1.10 to 1.24. We also found significant PM2.5-associated elevated risks for cardiovascular and lung cancer mortality. Risk ratios generally increased with longer moving averages; for example, an elevation in 60-month moving average PM2.5 exposures was linked to 1.33 times the lung cancer mortality risk (95% confidence interval: 1.24, 1.40), as compared with 1.13 (95% confidence interval: 1.11, 1.15) for 12-month moving average exposures. Observed associations were robust in multivariable models, although evidence of unmeasured confounding remained. In this large cohort of US elderly, we provide important new evidence that long-term PM2.5 exposure is significantly related to increased mortality from respiratory disease, lung cancer, and cardiovascular disease.
Keywords: air pollution, cardiovascular disease mortality, chronic exposure, fine particles, lung cancer mortality, particulate matter, PM2.5, respiratory disease mortality
Long-term exposure to fine particulate matter (particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5)) has been associated with increased all-cause and cardiopulmonary mortality (1–13). Consistent with earlier studies such as the Harvard Six Cities and American Cancer Society cohort studies (1, 8), recent findings from the Rome Longitudinal Study (14) indicated a 4% (hazard ratio = 1.04, 95% confidence interval (CI): 1.03, 1.05) increased risk of all-cause mortality per 10-μg/m3 increase in PM2.5 exposure, with a higher associated risk for ischemic heart disease mortality (hazard ratio = 1.10, 95% CI: 1.06, 1.13). Investigators with the California Teachers Study and the Women's Health Initiative also reported similar findings (3, 15).
Examining the impact of long-term PM2.5 exposure on respiratory disease mortality, however, has been more challenging. Although chronic lower respiratory diseases are the third leading cause of death in the United States, they comprise only a small fraction of overall deaths (6% in 2014) as compared with cardiac disease (23%), which comprises the largest fraction (16). The relatively small number of deaths attributed to respiratory disease probably results in insufficient statistical power to test associations between air pollution and respiratory mortality. Additional challenges include the fact that respiratory disease in elderly persons is often accompanied by many comorbid conditions, which can result in variable cause-of-death determinations in individuals with respiratory disease. For example, the cause of death in persons with moderate chronic obstructive pulmonary disease (COPD) may be classified according to a more immediate cause of death (e.g., cardiovascular disease (CVD)), whereas the cause of death for persons with severe COPD is often listed as respiratory failure (17, 18). Since the impacts of air pollution have been shown to differ by underlying disease status (19), variable cause-of-death determinations may result in null (6, 14, 20) or inverse (21, 22) associations in studies based on small numbers of respiratory disease-related deaths. For example, while statistically significant positive associations between respiratory mortality and PM2.5 level were found in the Canadian Community Health Survey and the Japanese Three-Prefecture cohort study (with hazard ratios ranging from 1.11 to 1.54) (22, 23), other studies have shown null associations. Positive but statistically insignificant associations, for instance, were reported in the California Teachers Study (6, 15) and the National Institutes of Health's Diet and Health Study (24). While more evidence links PM2.5 exposure to increased risk of lung cancer mortality, this evidence is not definitive, as null associations have been reported in numerous US and European cohort studies (10, 14, 15, 25–28).
This limited and conflicting body of evidence demonstrates the need for larger studies that examine the impact of PM2.5 on respiratory and lung cancer mortality. To do so, we examined the association between chronic PM2.5 exposure and cause-specific mortality in a cohort of 18.9 million Medicare beneficiaries.
METHODS
Study population
This study was approved by the Institutional Review Board of Northeastern University. Through the Centers for Medicare and Medicaid Services, we compiled enrollment data from 52.9 million Medicare beneficiaries aged 65–120 years residing in the conterminous United States between December 2000 and the end of 2008. For each enrollee, we obtained beneficiary-specific information on date of birth, sex, race/ethnicity, zip code of residence, and survival. Using the International Classification of Diseases, Tenth Revision, codes from the National Death Index, we extracted information on mortality from nonaccidental and accidental causes and 3 major causes of death (and their subcategories) that together account for over 73% of all-cause mortality: CVD, respiratory disease, and cancer (Table 1).
Table 1.
Characteristics of Air Pollution Monitoring Stations and PM2.5 Exposure, Numbers of Participants, and Numbers of Deaths, Overall and by Cause, Among Medicare Enrollees Aged 65–120 Years, United States, 2000–2008
| Characteristic | ICD-10 Code | Mediana (IQRb) | No. | % |
|---|---|---|---|---|
| No. of monitoring stations | 988 | |||
| PM2.5 exposure, μg/m3 | 12.5 (10.3–14.3) | |||
| No. of Medicare enrollees | 14,630 (6,777–27,142) | 18,937,461 | ||
| Monthly no. and % of deaths per monitor | ||||
| All causes | 2,845 (1,154–5,520) | 4,190,595 | 100.0 | |
| Nonaccidental | A–R | 2,774 (1,130–5,381) | 4,097,110 | 98.0 |
| Accidental | V–Y | 70 (30–128) | 93,467 | 2.2 |
| All cardiovascular disease | I00–I99 | 1,069 (442–2,161) | 1,683,577 | 40.2 |
| Ischemic heart disease | I20–I25 | 508 (208–1,048) | 890,806 | 21.3 |
| Cerebrovascular disease | I60–I69 | 207 (87–396) | 293,786 | 7.0 |
| Congestive heart failure | I50 | 85 (34–170) | 119,631 | 2.9 |
| All respiratory disease | J00–J99 | 329 (138–633) | 460,725 | 11.0 |
| COPD | J40–J44 | 178 (73–337) | 238,214 | 5.7 |
| Pneumonia | J12–J18 | 78 (33–156) | 126,635 | 3.0 |
| All cancer | C–D | 631 (256–1,230) | 925,632 | 22.1 |
| Lung cancer | C34 | 182 (73–346) | 255,544 | 6.1 |
Abbreviation: COPD, chronic obstructive pulmonary disease; ICD-10, International Classification of Diseases, Tenth Revision; IQR, interquartile range; PM2.5, particulate matter less than or equal to 2.5 μm in aerodynamic diameter.
a Median value among locations and months.
b 25th–75th percentiles.
PM2.5 data
We obtained data on daily PM2.5 concentrations from the Environmental Protection Agency's Air Quality System. We considered 988 air quality monitors that had daily measurements for 4 or more calendar years, with each year having ≥9 months with ≥4 daily measurements between 2000 and 2008, and used centered PM2.5 levels, calculated as the monthly PM2.5 concentration at a monitor minus its monitor-specific overall mean, as our primary exposure measure (see the Web Appendix, available at https://academic.oup.com/aje, for detailed methodology). We assessed the impact of chronic PM2.5 exposure based on 12- to 60-month moving averages for all examined outcomes.
Statistical analyses
For each month during the study period, we matched the PM2.5 moving averages for a given monitor to eligible Medicare beneficiaries (and their data) who lived in zip codes with a geographic centroid within a 6-mile (9.6-km) radius of that monitor. The closest monitor was selected if a zip code centroid was located within the buffer zones of 2 or more valid monitors. We computed the numbers of Medicare beneficiaries and cause-specific deaths for each 5-year age interval, monitor, and study month. To avoid excessive zero counts, we collapsed ages at or above 90 years into 1 interval.
We applied log-linear regression models to examine the association between long-term PM2.5 exposure and cause-specific mortality nationwide, as well as by 4 US Census regions (Web Appendix and Web Figure 1) (29). All results are expressed as risk ratios for dying in a given month per 10-μg/m3 increase in moving average PM2.5 concentration. Statistical analyses were conducted using SAS 9.4 software (SAS Institute, Inc., Cary, North Carolina) and R 3.3.1 software (R Foundation for Statistical Computing, Vienna, Austria).
For sensitivity analyses, we assessed the impact of exposure error by defining PM2.5 exposure based on 3-mile (4.8-km) and 12-mile (19.2-km) buffer zones, as compared with the 6-mile (9.6-km) buffer zone used in our primary analysis. We also conducted multivariable regression analyses to assess the association with and without adjusting for potential confounding by behavioral covariates chosen from the Selected Metropolitan/Micropolitan Area Risk Trends of the Behavioral Risk Factor Surveillance System (BRFSS), which first became available in 2002 (30). We specifically controlled for monthly county-level prevalences of nonwhites, current smokers, persons with diabetes, heavy alcohol drinkers (i.e., >2 drinks/day), and persons with asthma, average median income, and body mass index; these variables were selected a priori based on their previous associations with either mortality or PM2.5. Note that data on BRFSS factors were available for only a subset of our cohort, as only 534 of the 988 PM2.5 monitors were located in a county with BRFSS data. The average PM2.5 level from monitors included in the BRFSS analysis was 12.29 μg/m3, whereas it was 12.04 μg/m3 from excluded monitors. The spatial distribution of the monitors with BRFSS data was similar to that for those in our primary analysis, though with slightly fewer monitors in the Midwest (24% vs. 27% overall) and more in the Northeast (20% vs. 15% overall).
In addition, we examined the extent to which our findings remained affected by unmeasured confounding in BRFSS-adjusted models, following a method described by Greven et al. (29). We decomposed PM2.5 data into 2 orthogonal component measures, “temporal” PM2.5 and “spatiotemporal” PM2.5 (previously referred to as “global” and “local” PM2.5, respectively). We estimated their associations with cause-specific mortality simultaneously in nonadjusted and BRFSS-adjusted models using a data subset for which BRFSS data were available (Web Appendix). “Temporal” PM2.5 represents the national temporal trends in monthly PM2.5 concentrations centered by the average concentrations for all monitors and across the study period; “spatiotemporal” PM2.5 refers to the monitor-specific temporal trends in monthly PM2.5 concentrations compared with the national “temporal” trends. As described in detail by Greven et al. (29), estimates of “temporal” and “spatiotemporal” PM2.5 should be similar in the absence of confounding or model misspecification, since risks associated with the same unit change in PM2.5 level should be the same, irrespective of whether they are estimated using “temporal” or “spatiotemporal” measures of PM2.5.
RESULTS
Our study population included 18.9 million elderly Medicare enrollees residing in 7,788 zip codes across the United States, with an average of 10 million individuals enrolled in any given month (Table 1). This population comprised 36% of all Medicare enrollees (Web Table 1). During the study period, 4.2 million deaths were reported, with 98% and 2% of deaths being from nonaccidental causes and accidental causes (e.g., accidents, drug overdoses), respectively. CVD accounted for 40% of all mortality, followed by cancer (22%) and respiratory disease (11%). Over half of all CVD deaths were caused by ischemic heart disease, with a median of 508 deaths per month and monitor. Cerebrovascular diseases (e.g., stroke) and congestive heart failure also were significant causes of CVD mortality. Fifty-two percent of respiratory deaths were caused by COPD and 27% by pneumonia; 28% of cancer deaths were attributable to lung cancer. The annual average PM2.5 concentration nationwide was 12.5 μg/m3 (Web Figure 2).
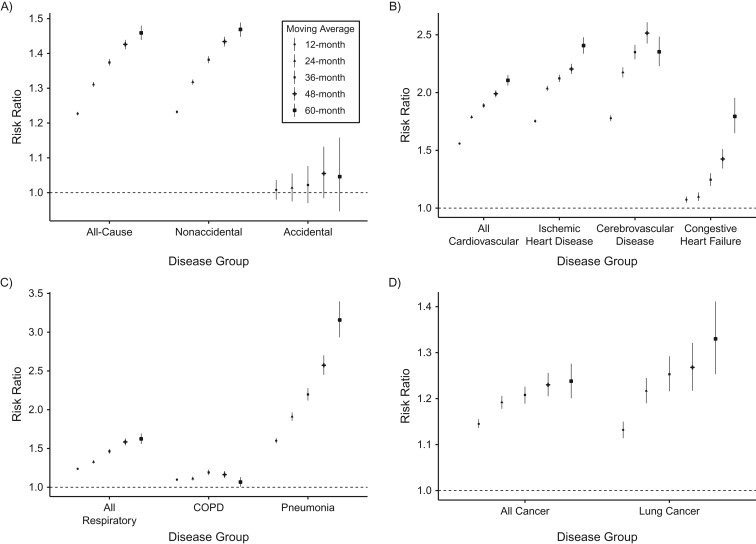
Figure 1 shows the PM2.5-associated risk ratios for total mortality and mortality from 11 causes for multiple exposure windows. We found a 10-μg/m3 increase in 12-month moving average PM2.5 level to be significantly associated with elevated risk of dying from all causes (risk ratio (RR) = 1.23, 95% CI: 1.22, 1.23) and nonaccidental causes (RR = 1.23, 95% CI: 1.23, 1.24) but not from accidental causes (Figure 1A). Risk ratios were greatest for CVD causes (RR = 1.56, 95% CI: 1.55, 1.57); among these, PM2.5 exposure was linked to a 1.78-fold increase in the risk of cerebrovascular disease death (95% CI: 1.75, 1.80) and a 1.75-fold increase in the risk of ischemic heart disease death (95% CI: 1.74, 1.77) (Figure 1B). PM2.5-associated risk of respiratory mortality (RR = 1.24, 95% CI: 1.23, 1.25) was similar to that observed for all-cause mortality (Figure 1C). An increase in PM2.5 was significantly associated with 1.60-fold (95% CI: 1.57, 1.63) and 1.10-fold (95% CI: 1.08, 1.12) increases in the risks of pneumonia and COPD mortality, respectively. PM2.5-associated risk ratios equaled 1.15 (95% CI: 1.14, 1.16) and 1.13 (95% CI: 1.11, 1.15) for all causes of cancer and lung cancer, respectively (Figure 1D), and were of similar magnitude as those for all-cause and respiratory mortality. PM2.5-associated mortality risks remained significant and positive for most causes of death when longer moving averages were examined. Risk ratios increased with longer moving averages for most causes of death, except COPD mortality, for which the risk ratio associated with 60-month moving average PM2.5 exposure was lower than the risk ratios for other moving averages. Web Table 2 shows that PM2.5-associated mortality risks differed by region, with the highest risk ratios being found in the Northeast and the lowest in the West and South.
Figure 1.
Risk ratios for all-cause (A), cardiovascular disease (B), respiratory disease (C), and cancer (D) mortality associated with 10-μg/m3 increases in 12- to 60-month moving average exposure to particulate matter less than or equal to 2.5 μm in aerodynamic diameter (PM2.5) nationwide, United States, 2000–2008. Bars, 95% confidence intervals. COPD, chronic obstructive pulmonary disease.
Sensitivity analyses
We reexamined exposures using monitors located within 3 miles (4.8 km) or 12 miles (19.2 km) of the centroid of enrollee zip codes. We found that the associations between 12-month moving average PM2.5 level and all-cause mortality were statistically significant for buffer zones of all sizes, and further were of similar magnitude as those for the 6-mile (9.6-km) buffer zone (Web Table 3). Table 2 compares the PM2.5-associated risks for all-cause mortality and mortality from 3 major causes, estimated with and without adjustment for BRFSS covariates nationwide and by region using the smaller PM2.5 monitor subset. We observed that most risk ratios were similar or slightly attenuated in models adjusting for BRFSS covariates, as compared with those from nonadjusted models. The largest attenuation, 3.8%, was seen for mortality from congestive heart failure (RR = 1.16 vs. RR = 1.11 in adjusted model), followed by pneumonia mortality, with a 3.1% decrease in risk ratio (RR = 1.49 vs. RR = 1.45 in adjusted model). Risk estimates for cancer and lung cancer mortality increased by 1.4% and 1.9% in the BRFSS-adjusted models, respectively. The consistently small changes in the risk ratios for adjusted models suggest little, if any, confounding of PM-mortality associations by the examined BRFSS covariates.
Table 2.
Mortality Risk Associated With a 10-μg/m3 Increase in 12-Month Moving Average PM2.5 Concentration, Nationwide and by Region, United States, 2000–2008
| Cause of Death and Region | Nonadjusted Model | BRFSS-Adjusted Modela | ||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| All causes | ||||
| United States | 1.223 | 1.215, 1.232 | 1.206 | 1.197, 1.214 |
| West | 1.163 | 1.152, 1.174 | 1.167 | 1.156, 1.178 |
| Midwest | 1.210 | 1.193, 1.228 | 1.172 | 1.155, 1.190 |
| South | 1.215 | 1.193, 1.239 | 1.151 | 1.129, 1.174 |
| Northeast | 1.471 | 1.447, 1.496 | 1.397 | 1.372, 1.422 |
| Accidents | ||||
| United States | 1.119 | 1.066, 1.174 | 1.146 | 1.091, 1.205 |
| West | 1.179 | 1.098, 1.265 | 1.237 | 1.147, 1.335 |
| Midwest | 1.034 | 0.936, 1.141 | 1.025 | 0.926, 1.134 |
| South | 1.137 | 1.004, 1.287 | 1.110 | 0.977, 1.261 |
| Northeast | 1.064 | 0.942, 1.201 | 1.115 | 0.981, 1.269 |
| All cardiovascular disease | ||||
| United States | 1.502 | 1.487, 1.517 | 1.475 | 1.459, 1.492 |
| West | 1.388 | 1.368, 1.407 | 1.420 | 1.399, 1.442 |
| Midwest | 1.447 | 1.414, 1.481 | 1.344 | 1.313, 1.377 |
| South | 1.606 | 1.557, 1.656 | 1.457 | 1.411, 1.505 |
| Northeast | 1.993 | 1.941, 2.046 | 1.772 | 1.723, 1.822 |
| Ischemic heart disease | ||||
| United States | 1.644 | 1.621, 1.666 | 1.639 | 1.615, 1.663 |
| West | 1.485 | 1.458, 1.512 | 1.546 | 1.516, 1.577 |
| Midwest | 1.569 | 1.519, 1.619 | 1.427 | 1.381, 1.476 |
| South | 1.845 | 1.765, 1.928 | 1.618 | 1.544, 1.695 |
| Northeast | 2.338 | 2.259, 2.420 | 2.056 | 1.983, 2.132 |
| Cerebrovascular disease | ||||
| United States | 1.724 | 1.681, 1.767 | 1.729 | 1.683, 1.777 |
| West | 1.642 | 1.587, 1.698 | 1.802 | 1.734, 1.872 |
| Midwest | 1.622 | 1.533, 1.715 | 1.508 | 1.423, 1.598 |
| South | 1.828 | 1.700, 1.965 | 1.619 | 1.502, 1.746 |
| Northeast | 2.215 | 2.065, 2.377 | 1.927 | 1.787, 2.077 |
| Congestive heart failure | ||||
| United States | 1.158 | 1.110, 1.207 | 1.114 | 1.066, 1.163 |
| West | 0.838 | 0.785, 0.894 | 0.848 | 0.795, 0.904 |
| Midwest | 1.382 | 1.273, 1.500 | 1.352 | 1.241, 1.474 |
| South | 1.405 | 1.253, 1.576 | 1.371 | 1.219, 1.543 |
| Northeast | 1.665 | 1.507, 1.839 | 1.476 | 1.327, 1.642 |
| All respiratory disease | ||||
| United States | 1.264 | 1.239, 1.290 | 1.248 | 1.222, 1.274 |
| West | 1.194 | 1.162, 1.228 | 1.186 | 1.152, 1.220 |
| Midwest | 1.244 | 1.190, 1.302 | 1.229 | 1.173, 1.287 |
| South | 1.201 | 1.132, 1.274 | 1.113 | 1.047, 1.183 |
| Northeast | 1.648 | 1.565, 1.736 | 1.528 | 1.446, 1.616 |
| COPD | ||||
| United States | 1.169 | 1.136, 1.203 | 1.174 | 1.139, 1.209 |
| West | 1.146 | 1.102, 1.193 | 1.139 | 1.094, 1.186 |
| Midwest | 1.248 | 1.173, 1.328 | 1.265 | 1.186, 1.350 |
| South | 0.967 | 0.891, 1.049 | 0.919 | 0.845, 0.999 |
| Northeast | 1.357 | 1.254, 1.468 | 1.323 | 1.217, 1.438 |
| Pneumonia | ||||
| United States | 1.491 | 1.441, 1.543 | 1.445 | 1.394, 1.498 |
| West | 1.340 | 1.283, 1.398 | 1.313 | 1.256, 1.374 |
| Midwest | 1.554 | 1.419, 1.702 | 1.452 | 1.321, 1.596 |
| South | 1.816 | 1.611, 2.047 | 1.559 | 1.376, 1.766 |
| Northeast | 2.053 | 1.876, 2.247 | 1.829 | 1.661, 2.013 |
| All cancer | ||||
| United States | 1.120 | 1.104, 1.136 | 1.107 | 1.091, 1.124 |
| West | 1.067 | 1.046, 1.089 | 1.065 | 1.043, 1.088 |
| Midwest | 1.087 | 1.055, 1.121 | 1.075 | 1.041, 1.109 |
| South | 1.122 | 1.078, 1.168 | 1.082 | 1.037, 1.127 |
| Northeast | 1.342 | 1.295, 1.390 | 1.321 | 1.272, 1.371 |
| Lung cancer | ||||
| United States | 1.146 | 1.115, 1.179 | 1.149 | 1.116, 1.183 |
| West | 1.132 | 1.086, 1.180 | 1.157 | 1.107, 1.209 |
| Midwest | 1.037 | 0.979, 1.099 | 1.041 | 0.981, 1.105 |
| South | 1.205 | 1.118, 1.298 | 1.172 | 1.085, 1.265 |
| Northeast | 1.311 | 1.223, 1.405 | 1.329 | 1.235, 1.431 |
Abbreviations: BRFSS, Behavioral Risk Factor Surveillance System; CI, confidence interval; PM2.5, particulate matter less than or equal to 2.5 μm in aerodynamic diameter; RR, risk ratio.
a Results were adjusted for county-level race (being nonwhite), smoking, diabetes, body mass index, alcohol consumption (>2 drinks/day), asthma, and median income. Data on BRFSS factors were available for only a subset of the cohort, since only 534 of the 988 PM2.5 monitors were located in a county with BRFSS data.
We further examined the ability of BRFSS-adjusted models to control for potential confounding by decomposing PM2.5 into 2 components describing “temporal” and “spatiotemporal” variation, per the approach of Greven et al. (29) (Web Table 4). In nonadjusted models, we observed positive, statistically significant associations for an increase in “temporal” PM2.5 levels, irrespective of the cause of death. In contrast, risk ratios associated with “spatiotemporal” PM2.5 levels were approximately centered around 1 and were often statistically insignificant. The difference in the “temporal” and “spatiotemporal” coefficients suggested the presence of unmeasured confounding; this was most evident for pneumonia mortality, where the difference was the largest. In contrast, the 2 coefficients were similar for COPD mortality, suggesting little unmeasured confounding. In BRFSS-adjusted models, most risk ratios associated with “temporal” PM2.5 levels were greatly attenuated, as compared with those for corresponding base models, especially for CVD and pneumonia deaths. Risks associated with “spatiotemporal” PM2.5 did not change substantially upon adjustment for BRFSS covariates, and risk ratios remained centered around 1. We also found both “temporal” and “spatiotemporal” risk ratios for cancer and lung cancer mortality to be unaffected upon adjustment for BRFSS covariates. Overall, BRFSS adjustment reduced the magnitude of “temporal” risk ratios for all-cause and cardiorespiratory mortality, resulting in smaller differences between “temporal” and “spatiotemporal” risk ratios. This suggests that BRFSS covariates accounted for a portion of the unmeasured confounding but not all of it.
DISCUSSION
We assessed the impacts of long-term PM2.5 exposure on total and cause-specific mortality in a large cohort of almost 19 million Medicare beneficiaries with 4.2 million deaths—to our knowledge, the largest cohort examined to date. By virtue of its large size, we were able to examine PM2.5-associated impacts on multiple causes of death, most notably respiratory disease mortality, for which current evidence is sparse and conflicting. We showed that a 10-μg/m3 elevation in 12-month moving average PM2.5 exposure was associated with 24%, 60%, and 10% greater risks of respiratory disease, pneumonia, and COPD mortality, respectively, in an elderly population living in the United States. Elevated risk (13%) of lung cancer mortality associated with PM2.5 was also found, as were strong and positive associations with all-cause and CVD-related mortality. Notably, the magnitudes of the associations increased as exposure windows increased from 12 months to 60 months. All associations were robust when results were adjusted for BRFSS covariates. This adjustment controlled for some unmeasured confounding but not all, as suggested by the smaller but still persistent differences between “spatiotemporal” and “temporal” risk ratios in the BRFSS-adjusted models.
To our knowledge, this is the first study to have found positive and statistically significant associations between PM2.5 exposures and respiratory disease-related mortality in a US population. Previously, Pinault et al. (23) and Katanoda et al. (22) reported significant and elevated PM2.5-associated risks of respiratory disease mortality using data on adult participants in the Canadian Community Health Survey and a Japanese cohort, respectively. Our findings related to COPD and pneumonia mortality are consistent with those seen in cohorts of older Norwegian men (31) and older Japanese adults (22), respectively. Results from other studies differ, however. Pope et al. (21) reported statistically significant inverse associations of PM2.5 with respiratory (relative risk = 0.92, 95% CI: 0.86, 0.98) and COPD (relative risk = 0.84, 95% CI: 0.77, 0.93) mortality in the American Cancer Society cohort. Gan et al. (32) found an insignificant positive association between PM2.5 and COPD mortality after adjustment for confounders in a Canadian cohort, while investigators in other studies observed no evidence of a relationship between PM2.5 and COPD mortality (2, 8, 22, 24). Consistent with these findings, in a meta-analysis of 17 studies, Hoek et al. (9) found an insignificant pooled excess risk (relative risk = 1.03, 95% CI: −0.94, 1.13) of respiratory disease mortality per 10-μg/m3 increase in PM2.5, which they attributed to possibly stronger associations between respiratory mortality and primary traffic-related indicators (i.e., nitrogen dioxide, traffic intensity) as compared with long-range transported PM2.5 (31–33). Alternatively or in addition, our results suggest that the sample sizes of previous studies were too small to detect an association between PM2.5 and respiratory mortality, since our findings of significant positive associations were based on 460,000 respiratory disease deaths, 240,000 COPD deaths, and 120,000 pneumonia deaths—numbers substantially larger than those in the earlier studies (e.g., 8,397 respiratory deaths in a Diet and Health Study cohort (24); 541 COPD deaths in a Canadian cohort (32)) (10). Other factors that may explain our findings could include differences in population, exposure assessment, pollution mixture, study period, outcome assessment, and confounder control (e.g., smoking status) between studies (34).
Our findings for lung cancer are consistent with conclusions of the International Agency for Research on Cancer stating that exposure to ambient air pollution and particulate matter is associated with lung cancer risk (35). This conclusion was based in large part on evidence from a meta-analysis that showed a PM2.5-associated mortality risk for lung cancer of 1.09 (95% CI: 1.04, 1.14), reflecting results from several large-scale cohort studies carried out in North America, Europe, and Asia that showed positive relationships with lung cancer death (11, 14, 22, 36), although no association was found in other US and European cohort studies (10, 25–28). Likewise, our findings of PM2.5-associated risks for all-cause and CVD-related deaths were consistent with, though larger in magnitude than, many of the PM2.5-associated mortality estimates reported in previous large-scale cohort studies (2, 3, 9, 21), perhaps because of our larger sample size and longer study period.
Importantly, our findings were robust to adjustment for neighborhood behavioral characteristics, as evidenced by similar risk ratios from nonadjusted and BRFSS-adjusted models. However, when decomposing PM2.5 into its “temporal” and “spatiotemporal” components, as in the study by Greven et al. (29), who also used a Medicare cohort and similar statistical modeling methods, we found that risk ratios associated with “temporal” PM2.5 levels were greatly attenuated in the adjusted models as compared with nonadjusted models, while those associated with “spatiotemporal” PM2.5 remained unchanged. Although BRFSS adjustment resulted in smaller differences between “temporal” and “spatiotemporal” risk ratios, observable differences persisted, suggesting insufficient adjustment of unmeasured confounding by BRFSS data alone (29). Other possible sources of confounding include (but are not limited to) PM2.5 composition, correlated gaseous pollutants, and long-term time trends in both PM2.5 concentrations and mortality. Using the same Medicare cohort for an extended study period (2000–2012), members of our research group (Eum et al., Tufts University, unpublished manuscript, 2016) observed an even smaller difference between “temporal” (RR = 1.47, 95% CI: 1.43, 1.52) and “spatiotemporal” (RR = 1.13, 95% CI: 1.12, 1.14) risk ratios upon removal of the long-term temporal trends, while both risk ratios remained statistically significant. This suggests that temporal trends may account for a substantial portion of the unmeasured confounding in the chronic PM2.5-associated mortality risks. Notably, the “temporal” coefficients but generally not the “spatiotemporal” coefficients were statistically significant; the meaning of each coefficient, absent comparison with the other, is not clear but warrants further study.
The positive associations of PM2.5 with cardiorespiratory and lung cancer mortality are consistent with biological pathways through which PM2.5 may influence health. PM2.5 has been shown to deposit in the alveolar region of the lung and to move into interstitial spaces between cells and towards other organs (e.g., the heart and brain). Particulate matter is hypothesized to induce pulmonary and systemic inflammation, the release of potentially harmful cytokines (e.g., interleukin-6), hypercoagulability, and enhanced thrombosis and to alter cardiac autonomic function, thereby causing or accelerating the development of atherosclerosis and CVD-related diseases (21, 37, 38). The mechanisms through which chronic PM2.5 exposure may increase risk of respiratory disease mortality are poorly defined but may involve systemic inflammation and oxidative stress in the lung epithelial cells (39–41). Other pathways may include decrements in lung function and airway hyperreactivity, thereby causing respiratory symptoms/disease (e.g., COPD exacerbation) and even death (21, 42). Furthermore, it has been suggested that in target cells, particulate matter induces reactive oxygen and nitrogen species, acting as both DNA-damaging species and inflammation-signaling moieties involved in pathways such as genotoxicity and cell and tissue proliferation, ultimately promoting lung cancer (43).
Our study had several limitations. First, we did not have information on beneficiaries’ activity and mobility patterns, which may have contributed to exposure misclassification. While we used ambient, nearest-monitor PM2.5 concentrations, which are imperfect proxies of personal PM2.5 exposure, previous studies have shown chronic health risks to be underestimated using nearest-monitor exposures, which lends support to our finding of significant positive associations (44). We linked PM2.5 exposure to beneficiary information by year in order to account for residential moves and zip code boundary changes, and we used exposure values that were geographically close to residences, capturing exposures that resulted from nearby PM2.5 emission sources, and thus reducing the potential for exposure misclassification. This is supported by results from our sensitivity analyses, which showed similar risk ratios associated with PM2.5 exposures based on 3-mile (4.8-km) buffer zones as compared with 6-mile (9.6-km) buffer zones. Second, the number of associations between PM2.5 exposure and cause-specific mortality that were examined raises concerns regarding multiple comparisons; however, given the consistency of our findings across outcomes and their high level of significance, we believe that our findings are nevertheless robust. Third, although we did not have data on personal-level characteristics, we adjusted for county-level BRFSS variables, including those related to smoking and comorbidity. We found that adjustment for BRFSS variables did not eliminate the observed significant, positive PM2.5-mortality associations. Confounding by personal-level characteristics is unlikely to explain our findings, since, in a reanalysis of the American Cancer Society and Six Cities studies, Krewski et al. (45) reported little change in the risk estimates with adjustment for individual-level characteristics. Nonetheless, residual confounding by unmeasured covariates, such as long-term temporal trends, remains. Lastly, our findings may not be generalizable to younger age groups or to beneficiaries living away from air pollution monitors.
These limitations are balanced by the substantial strengths of our study. With nearly 19 million Medicare beneficiaries and 4.2 million deaths over the study period, our study was well-powered to detect meaningful associations, allowing us to provide valuable new information on the relationship between PM2.5 exposures and specific CVD, respiratory, and cancer-related deaths, in addition to all-cause mortality. Our findings imply that a decrease of 1 μg/m3 in population-averaged PM2.5 exposure would result in 38,403 fewer all-cause deaths, 21,503 fewer CVD deaths, and 1,150 fewer COPD deaths per year nationwide, respectively, given the mean PM2.5 concentration of 12.5 μg/m3 and the 1,858,081 all-cause, 477,840 CVD, and 122,375 COPD deaths that occurred in the elderly (ages ≥65 years) US population in 2012, respectively (46).
In conclusion, we found new evidence of positive associations between chronic PM2.5 exposure and respiratory and pneumonia mortality in a large US elderly cohort. We also found adverse associations of PM2.5 with lung cancer and CVD-related mortality. Findings were statistically robust upon adjustment for neighborhood behavioral covariates, though unmeasured confounding remained.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Health Sciences, Bouvé College of Health Sciences, Northeastern University, Boston, Massachusetts (Vivian C. Pun, Fatemeh Kazemiparkouhi, Justin Manjourides, Helen H. Suh); Division of Occupational and Environmental Health, Jockey Club School of Public Health and Primary Care, Chinese University of Hong Kong, Hong Kong, China (Vivian C. Pun); and Department of Civil and Environmental Engineering, School of Engineering, Tufts University, Medford, Massachusetts (Helen H. Suh).
This work was supported by Electric Power Research Institute grant 00-10003095.
We acknowledge Dr. Jeffrey Yanosky of Pennsylvania State University (State College, Pennsylvania) for providing daily PM2.5 grid data. We also thank our Advisory Committee—Drs. A. Rappold at the Environmental Protection Agency (Washington, DC), D. Rich at the University of Rochester (Rochester, New York), G. Wellenius at Brown University School of Public Health (Providence, Rhode Island), H. Chang at Emory University's Rollins School of Public Health (Atlanta, Georgia), and J. Wendt Hess at Shell Oil Company (Houston, Texas)—for their thoughtful review of and comments on our work.
Conflict of interest: none declared.
REFERENCES
- 1. Pope CA 3rd, Burnett RT, Thun MM, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities Study. Am J Respir Crit Care Med. 2006;173(6):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller KA, Siscovick DS. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23(4):243–297. [DOI] [PubMed] [Google Scholar]
- 5. Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117(11):1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostro B, Lipsett M, Reynolds P, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118(3):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crouse DL, Peters PA, van Donkelaar A, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120(5):708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lepeule J, Laden F, Dockery D, et al. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities Study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beelen R, Hoek G, van den Brandt PA, et al. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR Study). Environ Health Perspect. 2008;116(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao J, Yang C, Li J, et al. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater. 2011;186(2–3):1594–1600. [DOI] [PubMed] [Google Scholar]
- 12. Filleul L, Rondeau V, Vandentorren S, et al. Twenty five year mortality and air pollution: results from the French PAARC survey. Occup Environ Med. 2005;62(7):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gehring U, Heinrich J, Krämer U, et al. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 2006;17(5):545–551. [DOI] [PubMed] [Google Scholar]
- 14. Cesaroni G, Badaloni C, Gariazzo C, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121(3):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipsett MJ, Ostro BD, Reynolds P, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California Teachers Study cohort. Am J Respir Crit Care Med. 2011;184(7):828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Center for Health Statistics Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Table 19. Leading Causes of Death and Numbers of Deaths, by Sex, Race, and Hispanic Origin: United States, 1980 and 2014 Hyattsville, MD: National Center for Health Statistics; 2015. http://www.cdc.gov/nchs/data/hus/2015/019.pdf. Updated April 27, 2016. Accessed June 19, 2016. [PubMed]
- 17. Zielinski J, MacNee W, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis. 1997;52(1):43–47. [PubMed] [Google Scholar]
- 18. Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. [DOI] [PubMed] [Google Scholar]
- 19. Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostro B, Hu J, Goldberg D, et al. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study cohort. Environ Health Perspect. 2015;123(6):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pope CA 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. [DOI] [PubMed] [Google Scholar]
- 22. Katanoda K, Sobue T, Satoh H, et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol. 2011;21(2):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinault L, Tjepkema M, Crouse DL, et al. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian Community Health Survey cohort. Environ Health. 2016;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thurston GD, Ahn J, Cromar KR, et al. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP Diet and Health cohort. Environ Health Perspect. 2016;124(4):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDonnell WF, Nishino-Ishikawa N, Petersen FF, et al. Relationships of mortality with the fine and coarse fractions of long-term ambient PM10 concentrations in nonsmokers. J Expo Anal Environ Epidemiol. 2000;10(5):427–436. [DOI] [PubMed] [Google Scholar]
- 26. Hart JE, Garshick E, Dockery DW, et al. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jerrett M, Burnett RT, Beckerman BS, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188(5):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carey IM, Atkinson RW, Kent AJ, et al. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187(11):1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greven S, Dominici F, Zeger S, et al. An approach to the estimation of chronic air pollution effects using spatio-temporal information. J Am Stat Assoc. 2011;106(494):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention SMART: BRFSS city and county data and documentation. 2014. http://www.cdc.gov/brfss/smart/smart_data.htm. Updated September 21, 2016. Accessed June 20, 2016.
- 31. Næss Ø, Nafstad P, Aamodt G, et al. Relation between concentration of air pollution and cause-specific mortality: four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, Norway. Am J Epidemiol. 2007;165(4):435–443. [DOI] [PubMed] [Google Scholar]
- 32. Gan WQ, FitzGerald JM, Carlsten C, et al. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–727. [DOI] [PubMed] [Google Scholar]
- 33. Nafstad P, Håheim LL, Wisløff T, et al. Urban air pollution and mortality in a cohort of Norwegian men. Environ Health Perspect. 2004;112(5):610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeger SL, Dominici F, McDermott A, et al. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000–2005). Environ Health Perspect. 2008;116(12):1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. International Agency for Research on Cancer Outdoor Air Pollution (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 109). Lyon, France: International Agency for Research on Cancer; 2015:454.
- 36. Krewski D, Jerrett M, Burnett RT, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;(140):5–114. [PubMed] [Google Scholar]
- 37. Pun VC, Hart JE, Kabrhel C, et al. Prospective study of ambient particulate matter exposure and risk of pulmonary embolism in the Nurses’ Health Study cohort. Environ Health Perspect. 2015;123(12):1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suwa T, Hogg JC, Quinlan KB, et al. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39(6):935–942. [DOI] [PubMed] [Google Scholar]
- 39. Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27(5):611–618. [DOI] [PubMed] [Google Scholar]
- 40. Hodgson MJ, Bracker A, Yang C, et al. Hypersensitivity pneumonitis in a metal-working environment. Am J Ind Med. 2001;39(6):616–628. [DOI] [PubMed] [Google Scholar]
- 41. Becker S, Mundandhara S, Devlin RB, et al. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol. 2005;207(2 suppl):269–275. [DOI] [PubMed] [Google Scholar]
- 42. Pirozzi C, Sturrock A, Carey P, et al. Respiratory effects of particulate air pollution episodes in former smokers with and without chronic obstructive pulmonary disease: a panel study. COPD Res Pract. 2015;1:1. [Google Scholar]
- 43. Knaapen AM, Borm PJA, Albrecht C, et al. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109(6):799–809. [DOI] [PubMed] [Google Scholar]
- 44. Yanosky JD, Paciorek CJ, Laden F, et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krewski D, Burnett R, Glodberg M, et al. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of Particulate Air Pollution and Mortality Cambridge, MA: Health Effects Institute; 2001. http://pubs.healtheffects.org/view.php?id=6. Accessed December 19, 2015.
- 46. Heron M. Deaths: leading causes for 2012. Natl Vital Stat Rep. 2015;64(10):1–93. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.